Abstract
Vascular calcification is highly prevalent in patients with chronic kidney disease (CKD) and increases mortality in those patients. Impaired calcium and phosphate homeostasis, increased oxidative stress, and loss of calcification inhibitors have been linked to vascular calcification in CKD. Additionally, impaired bone may perturb serum calcium/phosphate and their key regulator, parathyroid hormone, thus contributing to increased vascular calcification in CKD. Therapeutic approaches for CKD, such as phosphate binders and bisphosphonates, have been shown to ameliorate bone loss as well as vascular calcification. The precise mechanisms responsible for vascular calcification in CKD and the contribution of bone metabolism to vascular calcification have not been elucidated. This review discusses the role of systemic uremic factors and impaired bone metabolism in the pathogenesis of vascular calcification in CKD. The regulation of the key osteogenic transcription factor Runt-related transcription factor 2 (Runx2) and the emerging role of Runx2-dependent receptor activator of nuclear factor kappa-B ligand (RANKL) in vascular calcification of CKD are emphasized.
Keywords: Chronic kidney disease, Vascular calcification, Bone loss, PTH, Runx2, RANKL
Introduction
Cardiovascular complications are major clinical problems in patients with chronic kidney disease (CKD), which accounts for 50 % of deaths in end-stage renal disease patients [1, 2]. The combination of vascular calcification and impaired bone metabolism in CKD has been identified as the chronic kidney disease mineral bone disorder (CKD-MBD) by the Kidney Disease: Improving Global Outcomes Foundation [3]. Increased vascular calcification in CKD patients predicts a poor prognosis in terms of overall survival and cardiovascular morbidity and mortality [2, 4, 5]. Previously regarded as a passive calcium and phosphate hydroxyapatite deposition, vascular calcification has now been recognized as a cell-regulated process whereby vascular smooth muscle cells (VSMC) undergo molecular and phenotypic changes resembling bone formation during embryogenesis. Paradoxically, reduced bone mass occurs simultaneously with increased vascular calcification in CKD patients and animal models of CKD [6•], suggesting that different intrinsic signaling in tissue-specific microenvironments may govern mineralization in both bone and the vasculature. The precise mechanism responsible for vascular calcification in CKD and the contribution of impaired bone metabolism to vascular calcification have not been fully elucidated. This review discusses the contributions of systemic uremic factors and impaired bone metabolism on the pathogenesis of vascular calcification in CKD. The regulation of the key osteogenic transcription factor, Runx2 and Runx2-dependent RANKL in vascular calcification of CKD, and the effects of current therapies are emphasized.
Vascular Calcification in CKD
Vascular calcification is a prominent feature of atherosclerosis, diabetes, and CKD. It occurs either at the tunica intima or the tunica media depending on the underlying pathological disorders. Medial arterial calcification (MAC) is most common in patients with CKD, which locates mainly in the tunica media that contains VSMC and elastic tissues [6•]. Pathologically, MAC develops along elastic laminae as sheet-like deposits in its earliest form and as thick hydroxyapatite crystals in the media at its most severe stage. It occurs independent of hypercholesterolemia and atherosclerosis [7]. In clinical studies, MAC was observed in young and middle-aged patients without conventional atherosclerotic risk factors [5]. In dialysis patients, MAC was closely associated with the duration of hemodialysis and calcium–phosphate disorders [5]. Medial calcification often results in arterial stiffness and reduced vascular compliance, with an increased risk of cardiovascular mortality [5, 8].
Atherosclerotic intimal calcification is also found in patients with CKD [5]. Patchy VSMC calcification of the intimal layer is associated with subintimal deposition of lipids and lipoproteins, which may stimulate innate and adaptive immune responses that induce endothelial cells and VSMC to express inflammatory molecules, which stimulates monocyte/macrophage infiltration [9]. As a result, increased inflammation, oxidized lipids, and fibrous matrix secretion in the atherosclerotic lesions further accelerate vascular calcification [10], which eventually leads to atherosclerotic plaque rupture [11]. In CKD patients, both atherosclerotic intimal calcification and atherosclerosis-independent MAC are associated with increased cardiovascular mortality compared to that of CKD patients without vascular calcification [5].
Calcification Paradox–Bone Loss and Vascular Calcification
The adverse calcification of the physiologically non-mineralized blood vessel walls in the CKD patients has been associated with decreased bone mass assessed by bone mineral density (BMD). Emerging clinical studies have demonstrated that the development of vascular calcification is inversely correlated with the decreased BMD and strongly associated with the incidence of bone fractures and mortality in CKD patients [12, 13]. In a 2-year cohort study, arterial calcification was correlated with the prevalence of vertebral fractures in hemodialysis patients, which was positively associated with increased mortality [12]. Similarly, the highest degree of aortic calcification was associated with the highest bone loss in a population-based longitudinal study of postmenopausal women [13]. In animal models of CKD, decreased bone density and increased risk of bone fracture are strongly correlated with the degree of vascular calcification [6•]. The molecular mechanisms underlying the opposing regulation of mineralization of bone and vascular cells in CKD and the cross talk between these two systems are poorly understood. However, this relationship has provided rationale for the study of many factors thought important in the pathogenesis of arterial calcification.
Regulation of Vascular Calcification in CKD
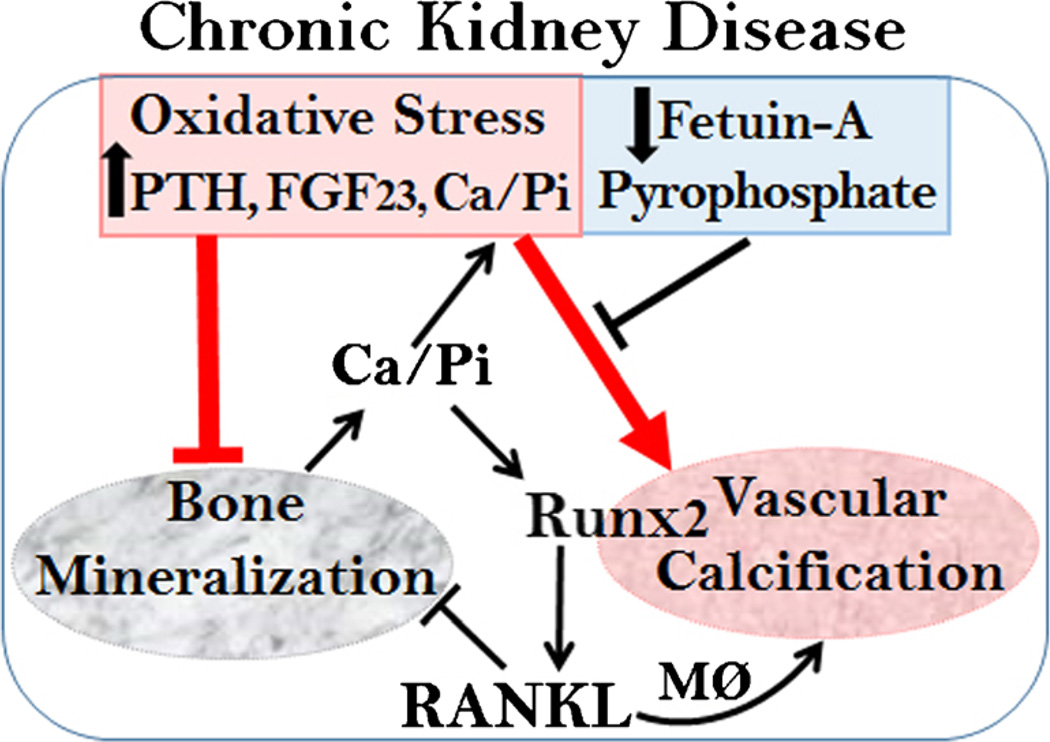
The molecular mechanisms underlying the pathogenesis of vascular calcification in CKD have not been fully understood. Patients with CKD with impaired calcium and phosphate homeostasis are manifested by hyperphosphatemia, hyperparathyroidism, elevated fibroblast growth factor 23 (FGF23), increased oxidative stress, and decreased calcification inhibitors such as fetuin-A and pyrophosphates [14–16]. At the molecular level, we and others have demonstrated that the Runt-related transcription factor 2 (Runx2), the key transcription factor of the Runx family that regulates osteoblast differentiation and chondrocyte maturation [17], is an essential and sufficient regulator for vascular calcification [18, 19•, 20]. The expression of the Runx2 is identified in arteries from CKD patients but not in normal vessels [21, 22], suggesting an important role of Runx2 in the pathogenesis of vascular calcification associated in CKD. Previous studies demonstrated that pooled uremic serum directly increased Runx2 expression and enhanced calcification of VSMC [22, 23]. Runx2 is regulated at multiple levels by a complex signal cascade. The regulation of Runx2 by the systemic uremic factors in CKD, including high levels of calcium/phosphate and oxidative stress, is emphasized below, and the contributions of the key calcification inhibitors are discussed (Fig. 1).
Fig. 1.
Regulation of vascular calcification in CKD. Impaired calcium/phosphate homeostasis (Ca/Pi), increased oxidative stress, and loss of calcification inhibitors promote vascular calcification in CKD. In addition, increased PTH induces calcium/phosphate release by bone in CKD patients, resulting in severe hyperphosphatemia. High calcium/phosphate, PTH, FGF23, and increased oxidative stress induce the expression of osteogenic transcription factor Runx2, which promotes osteogenic differentiation of VSMC that leads to vascular calcification. Loss of calcification inhibitors, including fetuin-A and pyrophosphate, further enhances VSMC calcification. Upregulation of Runx2 in VSMC induces the expression of RANKL, which in turn may directly enhance VSMC calcification and contribute to bone loss via bone-resorbing osteoclasts. Additionally, VSMC-expressed RANKL promotes migration, cytokine production, and osteoclast formation of macrophages (MØ), which may further accelerate vascular calcification
Altered Bone Turnover and Parathyroid Hormone
Impaired mineral metabolism has been shown to be associated with increased risk of cardiovascular mortality. As a primary reservoir for calcium and phosphate, bone is critical in maintaining systemic mineral homeostasis [1]. Increased serum phosphorous or dietary phosphate intake was associated with enhanced serum parathyroid hormone (PTH), which further promoted renal phosphate excretion [24]. Hyperparathyroidism-induced phosphate release from bone and impaired phosphate excretion led to advanced nephron loss in CKD patients [25] and thus resulting in severe hyperphosphatemia. Accrued high levels of serum phosphate then further stimulated the secretion of PTH, forming a vicious cycle. In hemodialysis patients, increased PTH was associated with vascular calcification [26]. Similarly, increased aortic calcification and decreased bone mass were associated with increased serum phosphorous and PTH levels in CKD rats [27]. In addition, the effects of uremic serum on VSMC calcification were positively correlated with serum PTH level [28]. Therefore, it is likely that impaired bone mineralization in CKD patients may contribute to the systemic mineral metabolic disorder by regulating PTH concentration and impairing calcium phosphate homeostasis, which may lead to the progression of vascular calcification. Hyperphosphatemia also increases FGF23, which, together with its co-receptor klotho, may also be a pathogenic factor in arterial calcification.
Impaired bone metabolism in CKD patients includes high-turnover and adynamic bone disease based on the bone remodeling rates. In adynamic bone disease, a low rate of bone formation results in inability to incorporate an excessive calcium load [29]. Reduced bone formation rates were associated with hyperphosphatemia and vascular calcification in the low-density lipoprotein receptor (LDLR)-deficient mice with CKD, whereas enhancing skeletal phosphate deposition by BMP-7 treatment reduced serum phosphate and inhibited vascular calcification [30]. High turnover is the most common form of metabolic bone disorder in CKD, which is characterized by accelerated bone remodeling in response to high levels of PTH [31]. PTH activates bone resorption through enhancing osteoclast formation by increased osteoblastic production of the receptor activator of nuclear factor kappa-B ligand (RANKL), a master regulator of osteoclastogenesis [32]. PTH-accelerated bone remodeling in CKD results in increased release of serum phosphate and calcium from bone [33]. Inhibiting bone resorption by using bisphosphonates or RANKL antibody has been shown to block vascular calcification [34–36], supporting the association of high bone turnover with the development of vascular calcification.
Elevated Calcium/Phosphate Levels
Hyperphosphatemia is one of the major factors associated with the development of vascular calcification in patients with CKD. The opposite effects of hyperphosphatemia on bone and vascular calcification have been well documented. High phosphorous diet enhanced vascular calcification and reduced bone mass in CKD rats [27]. In a community-based study, elevated serum calcium and phosphate levels were correlated with cardiovascular mortality as well as all-cause mortality in the general population [37••]. Hyperphosphatemia or higher serum phosphate levels even within the normal range have been shown to be associated with higher risk of cardiovascular events and mortality [38••]. In a prospective cohort analysis, higher levels of serum phosphorus independently predicted the incidence and prevalence for vascular calcification, comparable to traditional cardiovascular disease risk factors [39]. In patients with CKD, calcium supplementation in the form of calcium-based phosphate binders led to the development of vascular calcification and increased risk of cardiovascular events [40]. High phosphate diet promoted MAC in CKD mice, compared with normal phosphate diet (6). In a uremic mouse model of CKD, high phosphate diet-induced MAC was accelerated by osteogenic differentiation of VSMC [41]. Using an ex vivo rat aortic ring assay, Lomashvili et al. demonstrated that high calcium and/or high phosphate treatments induced medial VSMC calcification [42]. In vitro studies further demonstrated that high phosphate levels comparable to those seen in hyperphosphatemic individuals directly promoted osteogenic differentiation of VSMC, as indicated by increased expression of bone-related markers and loss of SMC marker genes [23, 43–45]. Similarly, extracellularly added calcium comparable to the levels observed in hypercalcemic subjects with or without addition of high phosphate induced VSMC calcification in vitro [45, 46•]. The molecular mechanism underlying high calcium and phosphate on vascular calcification is not fully understood. Hyperphosphatemia and hypercalcemia increased the secretion of matrix vesicles in human VSMC, which may initiate mineralization of extracellular matrix as seen in bone [45]. In addition, high phosphate induced apoptosis, which is associated with VSMC calcification induced by extracellular phosphate [45]. However, other studies found that phosphate-induced VSMC calcification was not associated with apoptosis or cell-derived vesicles [44]. Increased expression of Runx2 was associated with phosphorus-induced calcification of bovine calcifying vascular cells [43]. Furthermore, Li et al. demonstrated that high phosphate induced VSMC calcification by the upregulation of Runx2 via activation of type III sodium-dependent phosphate co-transporter [44]. We have demonstrated that upregulation of Runx2 is sufficient to induce VSMC calcification [18]. Thus, high calcium and phosphate-induced Runx2 upregulation in VSMC promotes vascular calcification in CKD.
Increased Oxidative Stress
Increases in both aortic and systemic oxidative stress were shown to be a key feature of the uremic state in CKD patients and uremic rats [47]. Enhanced oxidative stress is closely associated with the development of vascular calcification in vitro and in vivo in CKD [18, 47, 48]. In cell cultures, oxidative stress was found to modulate osteogenic differentiation of vascular cells and bone cells in an opposite manner [18, 48, 49]. We demonstrated that hydrogen peroxide induces VSMC calcification [18]. In contrast, oxidative stress inhibited phosphate-induced osteogenic differentiation of rat osteoblasts [49]. A recent prospective study revealed that a systemic increase in oxidative stress was associated with a higher risk of hip bone fracture in postmenopausal women [50], further demonstrating an inverse correlation between oxidative stress and BMD in vivo. Tempol, an antioxidant, inhibits the progression of vascular calcification in uremic CKD animals, suggesting that uremia-induced oxidative stress promotes the development of vascular calcification in CKD [47]. Hydrogen peroxide or xanthine/xanthine oxidase-induced oxidative stress was found to induce osteogenic differentiation of calcifying bovine vascular cells [48]. In contrast, minimally oxidized low-density lipoprotein (OxLDL) and hydrogen peroxide increased intracellular oxidative stress and inhibited osteogenic differentiation of pre-osteoblasts or bone marrow stromal cells [48]. In addition, OxLDL inhibited phosphate-induced mineralization of rat osteoblasts by generation of oxidative stress [49]. In animal models, statins were shown to decrease oxidative stress through enhancing antioxidant systems and suppressing the activity of NADPH oxidase, which inhibited osteoporosis in aged and ovariectomized rats [51]. In VSMC, increased oxidative stress converted LDL into OxLDL, which promoted VSMC calcification [52•]. Consistently, we demonstrated that oxidative stress induced VSMC calcification by upregulation of Runx2, which is mediated by the activation of the AKT/FOXO1/3 signaling axis via inhibiting Runx2 ubiquitination that leads to increased Runx2 transactivity [18, 53].
Altogether, studies from our lab and others have demonstrated that uremic serum as well as uremia-associated elevation of calcium/phosphate and oxidative stress induces Runx2 expression. We have demonstrated that SMC-expressed Runx2 is essential for osteogenic differentiation of VSMC that leads to atherosclerotic calcification in the ApoE−/− mice [20]. Further characterization of the SMC-specific Runx2 deletion mice in a CKD model should uncover a definitive role of uremia-induced Runx2 in regulating vascular calcification in CKD.
Decreased Calcification Inhibitors
Along with increased levels of calcium and phosphate, decreased calcification inhibitors, including fetuin-A and pyrophosphate, are found in serum from CKD patients, which may contribute to increased vascular calcification in CKD. The modes of action of fetuin-A and pyrophosphate may be directly linked to their functions in modulating calcium and phosphate. Therefore, decreased serum fetuin-A or pyrophosphate in CKD patients results in loss of inhibition on calcium and phosphate-induced signals, thus impairing bone metabolism and promoting VSMC calcification.
Low concentrations of fetuin-A were found in serum of dialysis patients, which were associated with increased inflammation and vascular calcification [15]. Fetuin-A is a serum glycoprotein that binds to calcium and forms stable colloidal calciprotein particles [54]. Accordingly, the plasma fetuin-A is a systemic inhibitor of mineralization [55]. Fetuin-A can be taken up by VSMC and secreted via release of matrix vesicles, thus inhibiting matrix vesicles-initiated nucleation of calcium phosphate that leads to the development of vascular calcification [56]. Consistently, fetuin-A was found to inhibit hyperphosphatemia-induced nanocrystals and block osteogenic differentiation of VSMC [57]. In a cross-sectional study, lower serum fetuin-A level was associated with decreased BMD in postmenopausal women compared to control group [58•]. Therefore, increased fetuin-A may prevent bone loss and inhibit vascular calcification in CKD.
Plasma pyrophosphate is reduced in hemodialysis patients compared to healthy control subjects, supporting a role of pyrophosphate in vascular calcification in CKD [16]. Pyrophosphate strongly binds to hydroxyapatite and counteracts the crystalline complex formation of phosphate with calcium to form hydroxyapatite [59]. High inorganic phosphate-induced osteogenic differentiation and calcification of VSMC is prevented in the presence of pyrophosphate, possibly through reducing the formation of hydroxyapatite nanocrystals [57]. Mice deficient in ectonucleotide pyrophosphatase phosphodiesterase, an enzyme that synthesizes extracellular pyrophosphate, exhibited low plasma pyrophosphate concentrations that lead to rapid development of severe aortic calcification [60]. Accordingly, bisphosphonates, non-hydrolyzable analogs of pyrophosphates, are broadly used to treat vascular calcification as well as osteoporosis.
Increased RANKL Expression
Emerging clinical studies have demonstrated a positive correlation of serum RANKL and its decoy receptor, osteoprotegerin (OPG), with vascular calcification in CKD [61–63]. In elderly hemodialysis CKD patients, serum OPG is increased along with increased PTH and phosphorus and decreased fetuin-A [61]. A recent study demonstrated increased circulating OPG in predialysis, dialysis, and transplant CKD patients and suggested a role of OPG in predicting the progression of vascular calcification in CKD [62]. In patients with CKD stage 5, higher OPG levels are positively correlated with RANKL and PTH and negatively correlated with the bone resorption marker, TRAP5b [63]. The function of RANKL and OPG in regulating vascular calcification in CKD is unknown. The RANKL/RANK system is critical for the formation of osteoclasts, the bone-resorbing cells [64]. Nonetheless, accumulating data have begun to connect this key osteoclast regulatory system with cardiovascular pathology. The first evidence supporting the role of OPG in linking vascular calcification with osteoporosis derives from the use of OPG-deficient mice. Bucay et al. demonstrated that mice deficient in OPG developed vascular calcification, while decreased BMD and increased bone fracture rates [65]. Furthermore, recombinant OPG treatment was found to significantly reduce vascular calcification in LDL receptor-deficient mice [66]. On the other hand, increased expression of RANKL was determined in calcified atherosclerotic lesions [19•, 67–69]. In animal models, increased RANKL mediated angiotensin II-induced bone loss [70] and vascular calcification [71]. Inhibition of RANKL by estrogen reversed osteoporosis and vascular calcification in the ovariectomized ApoE−/− mice [67]. Rinotas et al. recently demonstrated that increased RANKL expression in transgenic mice carrying human RANKL promoted bone loss and soft tissue calcification [72]. RANKL antibody, denosumab, effectively reduced osteoporosis and arterial calcification in these transgenic mice [36]. In clinical studies, denosumab effectively increased bone density and reduced fracture risk in postmenopausal women with osteoporosis (average 74 years old) [73•]. Although denosumab showed no overall effects on the 3-year progression of aortic calcification in these women, it reduced calcification in patients with aortic calcification scores (AC) <6 while increased calcification in patients with AC≥6 (severe) [74], indicating the disease stages at the time of denosumab treatment may affect the outcome. Additionally, it is likely that the sources of RANKL in the dynamic tissue environment, vasculature and bone, may also contribute to the different effects of denosumab on bone and vasculature in the postmenopausal osteoporotic women. Further investigations are warranted to understand the distinct molecular mechanisms underlying RANKL/OPG in regulating bone formation and vascular calcification.
In VSMC, increased Runx2 induces the expression of RANKL via direct binding to the promoter regions of RANKL [19•]. We found that RANKL deficiency did not affect VSMC calcification in vitro [19•]; however, increased RANKL enhanced VSMC calcification [75••]. In a coculture of VSMC with bone marrow-derived macrophages, RANKL was also found to promote VSMC calcification indirectly through increasing the expression of pro-calcific cytokines by macrophages [76]. In addition, we identified a novel role of VSMC-expressed RANKL in promoting macrophage infiltration and osteoclast formation [19•, 20]. Furthermore, a positive correlation between VSMC calcification and vascular osteoclasts was demonstrated in the atherosclerotic lesions [20], supporting a novel paradigm that RANKL may function on osteoclasts to regulate vascular calcification as it does in bone. This notion is further supported by the observations that anti-resorbing bisphosphonates also inhibit vascular calcification in humans and animals [34, 35]. Nonetheless, further studies are necessary to determine the precise function and mechanisms of RANKL in regulating vascular calcification in CKD and/or other vasculopathy (Fig. 1).
Therapeutic Approaches to Vascular Calcification in CKD
In light of the key regulators of vascular calcification in CKD reviewed above, three main current therapeutic approaches for vascular calcification in CKD are discussed below, including phosphate binders, calcimimetics, and pyrophosphate analogs. Although the efficacies of these drugs are inconsistent, beneficial effects have been demonstrated in preclinical and clinical studies.
Phosphate Binders
High phosphate is one of the major factors that induce vascular calcification. Accordingly, phosphate binders that lower serum phosphorous levels have been tested in multiple clinical trials to determine their effect on vascular calcification in CKD. Sevelamer was the first non-calcium or aluminum-containing phosphate binder marketed to treat hyperphosphatemia in CKD patients. Long-term follow-up study demonstrated that sevelamer decreased serum calcium and phosphate in hemodialysis patients, which was associated with increased expression of Klotho, an inhibitor of vascular calcification [77•]. In high phosphate diet-fed CKD rats, sevelamer treatment inhibited renal dysfunction and secondary hyperparathyroidism and reduced vascular calcification [78]. In uremic ApoE−/− mice, sevelamer inhibited both intimal and medial calcification, which was associated with reduced oxidative damage [79]. Similarly, lanthanum, a non-calcium phosphate inhibitor, blocked vascular calcification in uremic ApoE−/− mice and decreased expression of type I collagen in aortic plaques [80]. In vitro studies also demonstrated a direct effect of lanthanum on inhibiting high phosphate-induced VSMC calcification [81•]. In a small pilot study, lanthanum was more effective in inhibiting progression of coronary artery calcification in patients on hemodialysis, compared to a calcium-based phosphate binder, calcium carbonate [82]. Consistently, sevelamer was superior to calcium-based phosphate binders in reducing serum calcium and LDL in patients on renal replacement therapy [83]. In some studies, calcium-containing phosphate binders were also found to increase vascular calcification [1, 5] and decreased BMD in hemodialysis patients [84]. In general, non-calcium-containing phosphate binders are more advantageous than calcium-containing phosphate binders in reducing vascular calcification and improving survival of hemodialysis patients [85, 86], although sevelamer did not reduce overall mortality of hemodialysis patients in the largest study to date [87].
Calcimimetics
Calcimimetics are drugs that mimic the action of calcium on cells and tissues by allosteric activation of calcium-sensing receptor (CaR). Loss of functional CaR by overexpressing dominant-negative CaR increased VSMC calcification in culture [88]. In contrast, high calcium-induced VSMC calcification was inhibited by a calcimimetic, R-568 [88]. Similarly, calcimimetics, R-568 or AMG 641, inhibited calcification of human VSMC, which was associated with an increased expression of CaR [89]. In addition, calcimimetic calindol inhibited high phosphate-induced VSMC calcification, as a single agent or combining with phosphate binder lanthanum [90]. The effects of these calciminetics on vascular calcification were also demonstrated in preclinical animal models of CKD. R-568 reduced parathyroid hyperplasia and inhibited the development of atherosclerosis and aortic calcification in ApoE−/− mice with CKD [91] as well as in a slowly progressive model of CKD [92]. In uremic rats, AMG 641 reduced vascular calcification and inhibited the augmentation of parathyroid gland size associated with secondary hyperparathyroidism [93]. Long-term administration of cinacalcet, the only clinically approved calcimimetic, decreased serum calcium, phosphate, and calcium phosphate products and suppressed the progression of abdominal aortic calcification in patients on hemodialysis [94]. In a randomized study, coronary artery calcification scores were less in the aorta and aortic valve in hemodialysis patients treated with cinacalcet combined with vitamin D sterol compared to vitamin D sterol alone [95]. Unfortunately, this did not translate to reduced mortality or major cardiovascular events in the large EVOLVE study in dialysis patients with moderate to severe secondary hyperparathyroidism [96].
Pyrophosphate Analogs
As pyrophosphate analogs, bisphosphonates are most frequently prescribed drugs to treat osteoporosis because of their inhibitory effect on bone resorption. The anti-resorbing bis-phosphonate drugs that increase bone formation are also found to inhibit artery calcification in humans and animals [34, 35]. The mechanisms of bisphosphonates responsible for inhibiting vascular calcification are not fully understood. In animal studies, bisphosphonates did not affect serum calcium and phosphate [35] but inhibited vascular calcification induced by warfarin [35]. The beneficial effects of bisphosphates on vascular calcification appear to be limited to disease status and study populations in the clinical studies. Etidronate significantly reduced aortic calcification in hemodialysis patients without changing serum calcium and phosphate [97]. In the multiethnic study of atherosclerosis, bisphosphonates reduce vascular calcification in older women but increased calcification in younger ones [34]. A retrospective cohort study of female patients with CKD stages 3–4 revealed that bisphosphonates therapy was not associated with lower risk of cardiovascular events although it was associated with diminished risk of all-cause mortality [98]. In addition, ibandronate did not improve aortic calcification in osteoporotic elderly women [99], and alendronate did not decrease the progression of vascular calcification in patients with CKD stages 3–4 [100]. Accordingly, the effects of bisphosphonates therapy on vascular calcification in CKD patients are inconsistent; more basic and clinic studies are warranted to understand the mechanisms whereby bisphosphonates regulate vascular calcification. Furthermore, there are concerns over the use of bisphosphonates in patients with advanced kidney disease.
Conclusion
CKD-MBD is an important clinical problem, and the severity of vascular calcification predicts cardiovascular mortality of CKD patients. Decreased bone and increased vascular calcification in CKD-MBD suggest that distinct intrinsic signaling in the tissue-specific microenvironment may govern the mineralization in bone and in the vasculature. Clinical and experimental studies have linked many key common factors that contribute to bone and vascular defects in the CKD-BMD, including hyperphosphatemia, hyperparathyroidism, elevated FGF23, and oxidative stress, which inversely regulate bone and vascular calcification (Fig. 1). Phosphate binders and antioxidants have been shown to improve bone loss and prevent progression of vascular calcification in CKD patients or animal models. The impaired bone metabolism in CKD may perturb serum calcium/phosphate and PTH signaling, which contribute to the pathogenesis of vascular calcification. In addition, the loss of calcium/phosphate inhibitory factors, such as fetuin-A and pyrophosphate, further enhances vascular calcification. In VSMC, uremic signals, such as high levels of calcium/phosphate and oxidative stress, converge at Runx2 to induce osteogenic differentiation and calcification. We have found that Runx2 induces the expression of RANKL, the key regulator of the bone-resorbing osteoclasts. As anti-resorbing bisphosphonate drugs and RANKL antibody inhibit bone loss as well as vascular calcification in humans and animal models, RANKL is emerging as a novel regulator for vascular calcification. Further investigations are warranted to understand the distinct function and the underlying molecular mechanisms of RANKL in the bone and the vasculature in CKD.
Acknowledgments
Due to the scope and limitation, we apologize for not being able to include all the important work in the field. The authors thank Jay M. McDonald, MD (University of Alabama at Birmingham, UAB) for critical review. The original research programs of the authors are supported by grants from the National Institutes of Health, HL092215 and DK100847, and Veterans Administration BX000369 and BX001591 (project 2) to YC.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest CH Byon and Y Chen both declare no conflicts of interest.
Human and Animal Rights and Informed Consent All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Contributor Information
Chang Hyun Byon, Email: chbyon@uab.edu.
Yabing Chen, Email: ybchen@uab.edu.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Moe SM. Vascular calcification and renal osteodystrophy relationship in chronic kidney disease. European journal of clinical investigation. 2006:51–62. doi: 10.1111/j.1365-2362.2006.01665.x. 2006/08/04. [DOI] [PubMed] [Google Scholar]
- 2.Massy ZA, Maziere C, Kamel S, Brazier M, Choukroun G, Tribouilloy C, et al. Impact of inflammation and oxidative stress on vascular calcifications in chronic kidney disease. Pediatr Nephrol. 2005;20:380–382. doi: 10.1007/s00467-004-1623-9. [DOI] [PubMed] [Google Scholar]
- 3.Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. doi: 10.1038/sj.ki.5000414. [DOI] [PubMed] [Google Scholar]
- 4.Goodman WG, London G, Amann K, Block GA, Giachelli C, Hruska KA, et al. Vascular calcification in chronic kidney disease. Am J Kidney Dis. 2004;43:572–579. doi: 10.1053/j.ajkd.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 5.London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2003:1731–1740. doi: 10.1093/ndt/gfg414. 2003/08/26. [DOI] [PubMed] [Google Scholar]
- 6. Lau WL, Linnes M, Chu EY, Foster BL, Bartley BA, Somerman MJ, et al. High phosphate feeding promotes mineral and bone abnormalities in mice with chronic kidney disease. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association - European Renal Association. 2013:62–69. doi: 10.1093/ndt/gfs333. 2012/10/10.. Recapitulate full aspects of bone mineral disorders in chornic kidney diseases using high phosphate diet-fed mouse model.
- 7.Shanahan CM, Cary NR, Salisbury JR, Proudfoot D, Weissberg PL, Edmonds ME. Medial localization of mineralization-regulating proteins in association with Monckeberg’s sclerosis: evidence for smooth muscle cell-mediated vascular calcification. Circulation. 1999;100:2168–2176. doi: 10.1161/01.cir.100.21.2168. [DOI] [PubMed] [Google Scholar]
- 8.Giachelli CM. Mechanisms of vascular calcification in uremia. Seminars in nephrology. 2004:401–402. doi: 10.1016/j.semnephrol.2004.06.005. 2004/10/19. [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011:317–325. doi: 10.1038/nature10146. 2011/05/20. [DOI] [PubMed] [Google Scholar]
- 10.Otsuka F, Sakakura K, Yahagi K, Joner M, Virmani R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arteriosclerosis, thrombosis, and vascular biology. 2014:724–736. doi: 10.1161/ATVBAHA.113.302642. 2014/02/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kelly-Arnold A, Maldonado N, Laudier D, Aikawa E, Cardoso L, Weinbaum S. tRevised microcalcification hypothesis for fibrous cap rupture in human coronary arteries. Proceedings of the National Academy of Sciences of the United States of America. 2013:10741–10746. doi: 10.1073/pnas.1308814110. 2013/06/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Garcia M, Gomez-Alonso C, Naves-Diaz M, Diaz-Lopez JB, Diaz-Corte C, Cannata-Andia JB. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrology, dialysis, transplantation: official publication of the European Dialysis and Transplant Association—European Renal Association. 2009:239–216. doi: 10.1093/ndt/gfn466. 2008/08/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. The Journal of clinical endocrinology and metabolism. 2004:4246–4253. doi: 10.1210/jc.2003-030964. 2004/09/10. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds JL, Skepper JN, McNair R, Kasama T, Gupta K, Weissberg PL, et al. Multifunctional roles for serum protein fetuin-a in inhibition of human vascular smooth muscle cell calcification. J Am Soc Nephrol. 2005;16:2920–2930. doi: 10.1681/ASN.2004100895. [DOI] [PubMed] [Google Scholar]
- 15.Roman-Garcia P, Carrillo-Lopez N, Fernandez-Martin JL, Naves-Diaz M, Ruiz-Torres MP, Cannata-Andia JB. High phosphorus diet induces vascular calcification, a related decrease in bone mass and changes in the aortic gene expression. Bone. 2010:121–128. doi: 10.1016/j.bone.2009.09.006. 2009/09/24. [DOI] [PubMed] [Google Scholar]
- 16.Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radical Biol Med. 2001;31:509–519. doi: 10.1016/s0891-5849(01)00610-4. [DOI] [PubMed] [Google Scholar]
- 17.Byon CH, Javed A, Dai Q, Kappes JC, Clemens TL, Darley-Usmar VM, et al. Oxidative stress induces vascular calcification through modulation of the osteogenic transcription factor Runx2 by AKT signaling. J Biol Chem. 2008;283:15319–15327. doi: 10.1074/jbc.M800021200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maziere C, Savitsky V, Galmiche A, Gomila C, Massy Z, Maziere JC. Oxidized low density lipoprotein inhibits phosphate signaling and phosphate-induced mineralization in osteoblasts. Involvement of oxidative stress. Biochim Biophys Acta. 2010;1802:1013–1019. doi: 10.1016/j.bbadis.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 19. Yang S, Feskanich D, Willett WC, Eliassen AH, Wu T. Association between global biomarkers of oxidative stress and hip fracture in postmenopausal women: a prospective study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2014:2577–2583. doi: 10.1002/jbmr.2302. 2014/06/25.. Reports epidemiologic study showing an association of oxidative stress with bone fracture in postmenopausal women using newly developed biomarkers of oxidative stress.
- 20.Antoniucci DM, Yamashita T, Portale AA. Dietary phosphorus regulates serum fibroblast growth factor-23 concentrations in healthy men. The Journal of clinical endocrinology and metabolism. 2006:3144–3149. doi: 10.1210/jc.2006-0021. 2006/06/01. [DOI] [PubMed] [Google Scholar]
- 21.Slatopolsky E, Caglar S, Gradowska L, Canterbury J, Reiss E, Bricker NS. On the prevention of secondary hyperparathyroidism in experimental chronic renal disease using “proportional reduction” of dietary phosphorus intake. Kidney international. 1972:147–151. doi: 10.1038/ki.1972.84. 1972/09/01. [DOI] [PubMed] [Google Scholar]
- 22.Estepa JC, Aguilera-Tejero E, Lopez I, Almaden Y, Rodriguez M, Felsenfeld AJ. Effect of phosphate on parathyroid hormone secretion in vivo. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1999:1848–1854. doi: 10.1359/jbmr.1999.14.11.1848. 1999/11/26. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Fu P, Fan W, Gou R, Huang Y, Qiu H, et al. Involvement of parathyroid hormone-related protein in vascular calcification of chronic haemodialysis patients. Nephrology (Carlton, Vic) 2012:552–560. doi: 10.1111/j.1440-1797.2012.01601.x. 2012/03/28. [DOI] [PubMed] [Google Scholar]
- 24.Patidar A, Singh DK, Winocour P, Farrington K, Baydoun AR. Human uraemic serum displays calcific potential in vitro that increases with advancing chronic kidney disease. Clinical science (London, England : 1979) 2013:237–215. doi: 10.1042/CS20120638. 2013/03/08. [DOI] [PubMed] [Google Scholar]
- 25.Kurz P, Monier-Faugere MC, Bognar B, Werner E, Roth P, Vlachojannis J, et al. Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney international. 1994:855–861. doi: 10.1038/ki.1994.342. 1994/09/01. [DOI] [PubMed] [Google Scholar]
- 26.Mathew S, Davies M, Lund R, Saab G, Hruska KA. Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. European journal of clinical investigation. 2006:43–50. doi: 10.1111/j.1365-2362.2006.01663.x. 2006/08/04. [DOI] [PubMed] [Google Scholar]
- 27.Elder G. Pathophysiology and recent advances in the management of renal osteodystrophy. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2002:2094–2105. doi: 10.1359/jbmr.2002.17.12.2094. 2002/12/10. [DOI] [PubMed] [Google Scholar]
- 28.Huang JC, Sakata T, Pfleger LL, Bencsik M, Halloran BP, Bikle DD, et al. PTH differentially regulates expression of RANKL and OPG. J Bone Miner Res. 2004;19:235–214. doi: 10.1359/JBMR.0301226. [DOI] [PubMed] [Google Scholar]
- 29.Hruska KA, Saab G, Mathew S, Lund R. Renal osteodystrophy, phosphate homeostasis, and vascular calcification. Seminars in dialysis. 2007:309–315. doi: 10.1111/j.1525-139X.2007.00300.x. 2007/07/20. [DOI] [PubMed] [Google Scholar]
- 30.Elmariah S, Delaney JA, O’Brien KD, Budoff MJ, Vogel-Claussen J, Fuster V, et al. Bisphosphonate use and prevalence of valvular and vascular calcification in women MESA (The Multi-Ethnic Study of Atherosclerosis) Journal of the American College of Cardiology. 2010:1752–1759. doi: 10.1016/j.jacc.2010.05.050. 2010/11/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Price PA, Faus SA, Williamson MK. Bisphosphonates alendronate and ibandronate inhibit artery calcification at doses comparable to those that inhibit bone resorption. Arteriosclerosis, thrombosis, and vascular biology. 2001:817–824. doi: 10.1161/01.atv.21.5.817. 2001/05/23. [DOI] [PubMed] [Google Scholar]
- 32.Helas S, Goettsch C, Schoppet M, Zeitz U, Hempel U, Morawietz H, et al. Inhibition of receptor activator of NF-kappaB ligand by denosumab attenuates vascular calcium deposition in mice. Am J Pathol. 2009;175:473–478. doi: 10.2353/ajpath.2009.080957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishimura E, Taniwaki H, Tabata T, Tsujimoto Y, Jono S, Emoto M, et al. Cross-sectional association of serum phosphate with carotid intima-medial thickness in hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005:859–865. doi: 10.1053/j.ajkd.2005.02.008. 2005/04/30. [DOI] [PubMed] [Google Scholar]
- 34.Ketteler M, Bongartz P, Westenfeld R, Wildberger JE, Mahnken AH, Bohm R, et al. Association of low fetuin-A (AHSG) concentrations in serum with cardiovascular mortality in patients on dialysis: a cross-sectional study. Lancet. 2003:827–833. doi: 10.1016/S0140-6736(03)12710-9. 2003/03/19. [DOI] [PubMed] [Google Scholar]
- 35.Lomashvili KA, Khawandi W, O’Neill WC. Reduced plasma pyrophosphate levels in hemodialysis patients. Journal of the American Society of Nephrology : JASN. 2005:2495–2500. doi: 10.1681/ASN.2004080694. 2005/06/17. [DOI] [PubMed] [Google Scholar]
- 36.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 37. Byon CH, Sun Y, Chen J, Yuan K, Mao X, Heath JM, et al. Runx2-upregulated receptor activator of nuclear factor kappaB ligand in calcifying smooth muscle cells promotes migration and osteoclastic differentiation of macrophages. Arteriosclerosis, thrombosis, and vascular biology. 2011:1387–1396. doi: 10.1161/ATVBAHA.110.222547. 2011/04/02.. Report a direct regulation of RANKL by Runx2 in smooth muscle cells which promotes the migration and osteoclastic differentiation of macrophages.
- 38. Sun Y, Byon CH, Yuan K, Chen J, Mao X, Heath JM, et al. Smooth muscle cell-specific runx2 deficiency inhibits vascular calcification. Circ Res. 2012;111:543–552. doi: 10.1161/CIRCRESAHA.112.267237.. Determine that smooth muscle-specific deletion of Runx2 inhibited vascular calcification in mice and uncover a coupling beteen vascualr osteoclasts with vascualr calcification in atherolscelrosis.
- 39.Li X, Giachelli CM. Sodium-dependent phosphate cotransporters and vascular calcification. Curr Opin Nephrol Hypertens. 2007;16:325–358. doi: 10.1097/MNH.0b013e3281c55ef1. [DOI] [PubMed] [Google Scholar]
- 40.Moe SM, Duan D, Doehle BP, O’Neill KD, Chen NX. Uremia induces the osteoblast differentiation factor Cbfa1 in human blood vessels. Kidney Int. 2003;63:1003–1011. doi: 10.1046/j.1523-1755.2003.00820.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen NX, Duan D, O’Neill KD, Wolisi GO, Koczman JJ, Laclair R, et al. The mechanisms of uremic serum-induced expression of bone matrix proteins in bovine vascular smooth muscle cells. Kidney international. 2006:1046–1053. doi: 10.1038/sj.ki.5001663. 2006/07/14. [DOI] [PubMed] [Google Scholar]
- 42.Larsson TE, Olauson H, Hagstrom E, Ingelsson E, Arnlov J, Lind L, et al. Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arteriosclerosis, thrombosis, and vascular biology. 2010:333–339. doi: 10.1161/ATVBAHA.109.196675. 2009/12/02. [DOI] [PubMed] [Google Scholar]
- 43.Tonelli M, Sacks F, Pfeffer M, Gao Z, Curhan G. Relation between serum phosphate level and cardiovascular event rate in people with coronary disease. Circulation. 2005:2627–2633. doi: 10.1161/CIRCULATIONAHA.105.553198. 2005/10/26. [DOI] [PubMed] [Google Scholar]
- 44.Turtle KR, Short RA. Longitudinal relationships among coronary artery calcification, serum phosphorus, and kidney function. Clinical journal of the American Society of Nephrology : CJASN. 2009:1968–1973. doi: 10.2215/CJN.01250209. 2009/12/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.West SL, Swan VJ, Jamal SA. Effects of calcium on cardiovascular events in patients with kidney disease and in a healthy population. Clinical journal of the American Society of Nephrology : CJASN. 2010:S41–S47. doi: 10.2215/CJN.05860809. 2010/02/03. [DOI] [PubMed] [Google Scholar]
- 46. Pai A, Leaf EM, El-Abbadi M, Giachelli CM. Elastin degradation and vascular smooth muscle cell phenotype change precede cell loss and arterial medial calcification in a uremic mouse model of chronic kidney disease. Am J Pathol. 2011;178:764–773. doi: 10.1016/j.ajpath.2010.10.006.. Determine smooth muscle phenotype change in medial vascualr clacificaiton in mouse CKD model.
- 47.Lomashvili K, Garg P, O’Neill WC. Chemical and hormonal determinants of vascular calcification in vitro. Kidney international. 2006:1464–1470. doi: 10.1038/sj.ki.5000297. 2006/03/15. [DOI] [PubMed] [Google Scholar]
- 48.Jono S, McKee MD, Murry CE, Shioi A, Nishizawa Y, Mori K, et al. Phosphate regulation of vascular smooth muscle cell calcification. Circulation research. 2000:E10–E17. doi: 10.1161/01.res.87.7.e10. 2000/09/29. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Yang HY, Giachelli CM. Role of the sodium-dependent phosphate cotransporter, Pit-1, in vascular smooth muscle cell calcification. Circulation research. 2006:905–912. doi: 10.1161/01.RES.0000216409.20863.e7. 2006/03/11. [DOI] [PubMed] [Google Scholar]
- 50.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, et al. Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: a potential mechanism for accelerated vascular calcification in ESRD. Journal of the American Society of Nephrology : JASN. 2004:2857–2867. doi: 10.1097/01.ASN.0000141960.01035.28. 2004/10/27. [DOI] [PubMed] [Google Scholar]
- 51.Yang H, Curinga G, Giachelli CM. Elevated extracellular calcium levels induce smooth muscle cell matrix mineralization in vitro. Kidney international. 2004:2293–2299. doi: 10.1111/j.1523-1755.2004.66015.x. 2004/12/01. [DOI] [PubMed] [Google Scholar]
- 52. Yamada S, Taniguchi M, Tokumoto M, Toyonaga J, Fujisaki K, Suehiro T, et al. The antioxidant tempol ameliorates arterial medial calcification in uremic rats: important role of oxidative stress in the pathogenesis of vascular calcification in chronic kidney disease. J Bone Miner Res. 2012;27:474–485. doi: 10.1002/jbmr.539.. Demonstrate that uremia-induced oxidative stress in rats with chronic kidney disease results in the development of aortic calcification, which can be ameliorated by use of tempol, an antioxidant.
- 53.Yin H, Shi ZG, Yu YS, Hu J, Wang R, Luan ZP, et al. Protection against osteoporosis by statins is linked to a reduction of oxidative stress and restoration of nitric oxide formation in aged and ovariectomized rats. European journal of pharmacology. 2012:200–206. doi: 10.1016/j.ejphar.2011.11.024. 2011/12/02. [DOI] [PubMed] [Google Scholar]
- 54.Goettsch C, Rauner M, Hamann C, Sinningen K, Hempel U, Bornstein SR, et al. Nuclear factor of activated T cells mediates oxidised LDL-induced calcification of vascular smooth muscle cells. Diabetologia. 2011:2690–2701. doi: 10.1007/s00125-011-2219-0. 2011/06/28. [DOI] [PubMed] [Google Scholar]
- 55.Deng L, Huang L, Sun Y, Heath JM, Wu H, Chen Y. Inhibition of FOXO1/3 promotes vascular calcification. Arterioscler Thromb Vase Biol. 2014 doi: 10.1161/ATVBAHA.114.304786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Heiss A, DuChesne A, Denecke B, Grotzinger J, Yamamoto K, Renne T, et al. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. The Journal of biological chemistry. 2003:13333–13311. doi: 10.1074/jbc.M210868200. 2003/01/31. [DOI] [PubMed] [Google Scholar]
- 57.Schafer C, Heiss A, Schwarz A, Westenfeld R, Ketteler M, Floege J, et al. The serum protein alpha 2-Heremans-Schmid glycoprotein/fetuin-A is a systemically acting inhibitor of ectopic calcification. The Journal of clinical investigation. 2003:357–366. doi: 10.1172/JCI17202. 2003/08/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sage AP, Lu J, Tintut Y, Demer LL. Hyperphosphatemia-induced nanocrystals upregulate the expression of bone morphogenetic protein-2 and osteopontin genes in mouse smooth muscle cells in vitro. Kidney international. 2011:414–422. doi: 10.1038/ki.2010.390. 2010/10/15.. Determine the effects and potential mecahnisms of Fetuin-A in inhibiting hyperphosphatemia-induced calcification of vascular smooth muscle cells.
- 59.veKemik POF. The relationship between fetuin-a and bone mineral density in postmenopausal. Osteoporosis. 2013;28:195–201. [Google Scholar]
- 60.Terkeltaub RA. Inorganic pyrophosphate generation and disposition in pathophysiology. American journal of physiology Cell physiology. 2001:C1–c11. doi: 10.1152/ajpcell.2001.281.1.C1. 2001/06/13. [DOI] [PubMed] [Google Scholar]
- 61.Lomashvili KA, Narisawa S, Millan JL, O’Neill WC. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney international. 2014:1351–1356. doi: 10.1038/ki.2013.521. 2014/04/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osorio A, Ortega E, Torres JM, Sanchez P, Ruiz-Requena E. Biochemical markers of vascular calcification in elderly hemodialysis patients. Mol Cell Biochem. 2013;374:21–27. doi: 10.1007/s11010-012-1500-y. [DOI] [PubMed] [Google Scholar]
- 63.Montanez-Barragan A, Gomez-Barrera I, Sanchez-Nino MD, Ucero AC, Gonzalez-Espinoza L, Ortiz A. Osteoprotegerin and kidney disease. J Nephrol. 2014 doi: 10.1007/s40620-014-0092-x. [DOI] [PubMed] [Google Scholar]
- 64.Siomou E, Challa A, Printza N, Giapros V, Petropoulou F, Mitsioni A, et al. Serum osteoprotegerin, RANKL and fibroblast growth factor-23 in children with chronic kidney disease. Pediatr Nephrol. 2011;26:1105–1114. doi: 10.1007/s00467-011-1870-5. [DOI] [PubMed] [Google Scholar]
- 65.Walsh MC, Choi Y. Biology of the TRANCE axis. Cytokine Growth Factor Rev. 2003;14:251–263. doi: 10.1016/s1359-6101(03)00027-3. [DOI] [PubMed] [Google Scholar]
- 66.Bucay N, Sarosi I, Dunstan CR, Morony S, Tarpley J, Capparelli C, et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Orita Y, Yamamoto H, Kohno N, Sugihara M, Honda H, Kawamata S, et al. Role of osteoprotegerin in arterial calcification: development of new animal model. Arteriosclerosis, thrombosis, and vascular biology. 2007:2058–2064. doi: 10.1161/ATVBAHA.107.147868. 2007/07/07. [DOI] [PubMed] [Google Scholar]
- 68.Osako MK, Nakagami H, Koibuchi N, Shimizu H, Nakagami F, Koriyama H, et al. Estrogen inhibits vascular calcification via vascular RANKL system: common mechanism of osteoporosis and vascular calcification. Circ Res. 2010;107:466–475. doi: 10.1161/CIRCRESAHA.110.216846. [DOI] [PubMed] [Google Scholar]
- 69.Mohammadpour AH, Shamsara J, Nazemi S, Ghadirzadeh S, Shahsavand S, Ramezani M. Evaluation of RANKL/OPG serum concentration ratio as a new biomarker for coronary artery calcification: a pilot study. Thrombosis. 2012;2012:306263. doi: 10.1155/2012/306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Morony S, Tintut Y, Zhang Z, Cattley RC, Van G, Dwyer D, et al. Osteoprotegerin inhibits vascular calcification without affecting atherosclerosis in ldlr(−/−) mice. Circulation. 2008;117:411–420. doi: 10.1161/CIRCULATIONAHA.107.707380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shimizu H, Nakagami H, Osako MK, Hanayama R, Kunugiza Y, Kizawa T, et al. Angiotensin II accelerates osteoporosis by activating osteoclasts. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2008:2465–2475. doi: 10.1096/fj.07-098954. 2008/02/08. [DOI] [PubMed] [Google Scholar]
- 72.Osako MK, Nakagami H, Shimamura M, Koriyama H, Nakagami F, Shimizu H, et al. Cross-talk of receptor activator of nuclear factor-kappaB ligand signaling with renin-angiotensin system in vascular calcification. Arteriosclerosis, thrombosis, and vascular biology. 2013:1287–1296. doi: 10.1161/ATVBAHA.112.301099. 2013/04/13. [DOI] [PubMed] [Google Scholar]
- 73. Rinotas V, Niti A, Dacquin R, Bonnet N, Stolina M, Han CY, et al. Novel genetic models of osteoporosis by overexpression of human RANKL in transgenic mice. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2014:1158–1169. doi: 10.1002/jbmr.2112. 2013/10/16.. Develop a mouse model harboring human RANKL and demonstrates a correlation of the levels of transgenic RANKL with the severity of osteoporotic phenotypes.
- 74.Cummings SR, San Martin J, McClung MR, Siris ES, Eastell R, Reid IR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765. doi: 10.1056/NEJMoa0809493. [DOI] [PubMed] [Google Scholar]
- 75. Samelson EJ, Miller PD, Christiansen C, Daizadeh NS, Grazette L, Anthony MS, et al. RANKL inhibition with denosumab does not influence 3-year progression of aortic calcification or incidence of adverse cardiovascular events in postmenopausal women with osteoporosis and high cardiovascular risk. J Bone Miner Res. 2014;29:450–457. doi: 10.1002/jbmr.2043.. Demonstrate the effects of RANKL antibody on osteoporosis and aortic calcification in postmenopausal women with advnced age. Although it shows RANKL antibody has no overall effects on 3-year progression of aortic calficiation, it appears to inhibit calcification in patients with less calcification and increase calcificaiton in patients with severe calcification, suggesting different.
- 76.Panizo S, Cardus A, Encinas M, Parisi E, Valcheva P, Lopez-Ongil S, et al. RANKL increases vascular smooth muscle cell calcification through a RANK-BMP4-dependent pathway. Circ Res. 2009;104:1041–1048. doi: 10.1161/CIRCRESAHA.108.189001. [DOI] [PubMed] [Google Scholar]
- 77. Deuell KA, Callegari A, Giachelli CM, Rosenfeld ME, Scatena M. RANKL enhances macrophage paracrine pro-calcific activity in high phosphate-treated smooth muscle cells: dependence on IL-6 and TNF-alpha. Journal of vascular research. 2012:510–521. doi: 10.1159/000341216. 2012/09/06.. Determine a role of pro-calcific cytokines produced by macrophages via RANKL signaling in promoting calcification of smooth muscle cells.
- 78.Lin HH, Liou HH, Wu MS, Lin CY, Huang CC. Long-term sevelamer treatment lowers serum fibroblast growth factor 23 accompanied with increasing serum Klotho levels in chronic haemodialysis patients. Nephrology (Carlton, Vic) 2014:672–678. doi: 10.1111/nep.12319. 2014/08/13. [DOI] [PubMed] [Google Scholar]
- 79.Tokumoto M, Mizobuchi M, Finch JL, Nakamura H, Martin DR, Slatopolsky E. Blockage of the renin-angiotensin system attenuates mortality but not vascular calcification in uremic rats: sevelamer carbonate prevents vascular calcification. American journal of nephrology. 2009:582–591. doi: 10.1159/000192844. 2009/01/16. [DOI] [PubMed] [Google Scholar]
- 80.Phan O, Ivanovski O, Nguyen-Khoa T, Mothu N, Angulo J, Westenfeld R, et al. Sevelamer prevents uremia-enhanced atherosclerosis progression in apolipoprotein E-deficient mice. Circulation. 2005;112:2875–2882. doi: 10.1161/CIRCULATIONAHA105.541854. [DOI] [PubMed] [Google Scholar]
- 81. Nikolov IG, Joki N, Nguyen-Khoa T, Guerrera IC, Maizel J, Benchitrit J, et al. Lanthanum carbonate, like sevelamer-HCl, retards the progression of vascular calcification and atherosclerosis in uremic apolipoprotein E-deficient mice. Nephrol Dial Transplant. 2012;27:505–513. doi: 10.1093/ndt/gfr254.. Demonstrate the effects of a phosphate binder on vascular calcification in CKD mouse model.
- 82.Ciceri P, Elli F, Brenna I, Volpi E, Romagnoli S, Tosi D, et al. Lanthanum prevents high phosphate-induced vascular calcification by preserving vascular smooth muscle lineage markers. Calcified tissue international. 2013:521–530. doi: 10.1007/s00223-013-9709-7. 2013/02/19. [DOI] [PubMed] [Google Scholar]
- 83.Ohtake T, Kobayashi S, Oka M, Furuya R, Iwagami M, Tsutsumi D, et al. Lanthanum carbonate delays progression of coronary artery calcification compared with calcium-based phosphate binders in patients on hemodialysis: a pilot study. Journal of cardiovascular pharmacology and therapeutics. 2013:439–416. doi: 10.1177/1074248413486355. 2013/04/26. [DOI] [PubMed] [Google Scholar]
- 84.Alam S, Hussain A, Daiwajna R, Tan J. Clinical efficacy of sevelamer hydrochloride in patients with end-stage renal disease: a retrospective study. Singapore medical journal. 2013:263–266. doi: 10.11622/smedj.2013105. 2013/05/30. [DOI] [PubMed] [Google Scholar]
- 85.Raggi P, James G, Burke SK, Bommer J, Chasan-Taber S, Holzer H, et al. Decrease in thoracic vertebral bone attenuation with calcium-based phosphate binders in hemodialysis. J Bone Miner Res. 2005;20:764–772. doi: 10.1359/JBMR.041221. [DOI] [PubMed] [Google Scholar]
- 86.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM. Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney international. 2007:438–441. doi: 10.1038/sj.ki.5002059. 2007/01/04. [DOI] [PubMed] [Google Scholar]
- 87.McCullough PA, Chinnaiyan KM. Annual progression of coronary calcification in trials of preventive therapies: a systematic review. Arch Intern Med. 2009;169:2064–2070. doi: 10.1001/archinternmed.2009.382. [DOI] [PubMed] [Google Scholar]
- 88.Alam MU, Kirton JP, Wilkinson FL, Towers E, Sinha S, Rouhi M, et al. Calcification is associated with loss of functional calcium-sensing receptor in vascular smooth muscle cells. Cardiovascular research. 2009:260–268. doi: 10.1093/cvr/cvn279. 2008/10/15. [DOI] [PubMed] [Google Scholar]
- 89.Henaut L, Boudot C, Massy ZA, Lopez-Fernandez I, Dupont S, Mary A, et al. Calcimimetics increase CaSR expression and reduce mineralization in vascular smooth muscle cells: mechanisms of action. Cardiovascular research. 2014:256–265. doi: 10.1093/cvr/cvt249. 2013/11/13. [DOI] [PubMed] [Google Scholar]
- 90.Ciceri P, Volpi E, Brenna I, Elli F, Borghi E, Brancaccio D, et al. The combination of lanthanum chloride and the calcimimetic calindol delays the progression of vascular smooth muscle cells calcification. Biochemical and biophysical research communications. 2012:770–773. doi: 10.1016/j.bbrc.2012.01.097. 2012/02/09. [DOI] [PubMed] [Google Scholar]
- 91.Ivanovski O, Nikolov IG, Joki N, Caudrillier A, Phan O, Mentaverri R, et al. The calcimimetic R-568 retards uremia-enhanced vascular calcification and atherosclerosis in apolipoprotein E deficient (apoE−/−) mice. Atherosclerosis. 2009:55–62. doi: 10.1016/j.atherosclerosis.2008.10.043. 2009/01/03. [DOI] [PubMed] [Google Scholar]
- 92.Henley C, Davis J, Miller G, Shatzen E, Cattley R, Li X, et al. The calcimimetic AMG 641 abrogates parathyroid hyperplasia, bone and vascular calcification abnormalities in uremic rats. European journal of pharmacology. 2009:306–313. doi: 10.1016/j.ejphar.2009.05.013. 2009/05/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nakayama K, Nakao K, Takatori Y, Inoue J, Kojo S, Akagi S, et al. Long-term effect of cinacalcet hydrochloride on abdominal aortic calcification in patients on hemodialysis with secondary hyperparathyroidism. International journal of nephrology and renovascular disease. 2013:25–33. doi: 10.2147/IJNRD.S54731. 2014/01/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2011:1327–1339. doi: 10.1093/ndt/gfq725. 2010/12/15. [DOI] [PubMed] [Google Scholar]
- 95.Mori K, Shioi A, Jono S, Nishizawa Y, Morii H. Expression of matrix Gla protein (MGP) in an in vitro model of vascular calcification. FEBS Lett. 1998;433:19–22. doi: 10.1016/s0014-5793(98)00870-9. [DOI] [PubMed] [Google Scholar]
- 96.Zhou S, Fang X, Xin H, Guan S. Effects of alendronate on the Notch1RBPJkappa signaling pathway in the osteogenic differentiation and mineralization of vascular smooth muscle cells. Molecular medicine reports. 2013:89–94. doi: 10.3892/mmr.2013.1489. 2013/05/28. [DOI] [PubMed] [Google Scholar]
- 97.Hashiba H, Aizawa S, Tamura K, Kogo H. Inhibition of the progression of aortic calcification by etidronate treatment in hemodialysis patients: long-term effects. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2006:59–64. doi: 10.1111/j.1744-9987.2006.00345.x. 2006/03/25. [DOI] [PubMed] [Google Scholar]
- 98.Hartle JE, Tang X, Kirchner HL, Bucaloiu ID, Sartorius JA, Pogrebnaya ZV, et al. Bisphosphonate therapy, death, and cardiovascular events among female patients with CKD: a retrospective cohort study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012:636–644. doi: 10.1053/j.ajkd.2011.11.037. 2012/01/17. [DOI] [PubMed] [Google Scholar]
- 99.Tanko LB, Qin G, Alexandersen P, Bagger YZ, Christiansen C. Effective doses of ibandronate do not influence the 3-year progression of aortic calcification in elderly osteoporotic women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2005:184–190. doi: 10.1007/s00198-004-1662-x. 2004/06/16. [DOI] [PubMed] [Google Scholar]
- 100.Toussaint ND, Lau KK, Strauss BJ, Polkinghorne KR, Kerr PG. Effect of alendronate on vascular calcification in CKD stages 3 and 4: a pilot randomized controlled trial. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2010:57–68. doi: 10.1053/j.ajkd.2009.12.039. 2010/03/30. [DOI] [PubMed] [Google Scholar]