Abstract
Targeting HER2 for the treatment of HER2-positive breast cancers is now a validated treatment paradigm. However evidence suggests that this family of receptors may have important roles outside of the realm of HER2 amplification. There is considerable interest in the development of biomarkers to identify such breast cancers.
In this issue of Clinical Cancer Research, Leary and colleagues report the results of a biomarker study conducted on a window trial of short-course pre-surgical lapatinib therapy in patients with early stage breast cancer (1). The patient population, although not large, encompasses the entire spectrum of breast cancers and the biomarker results provide a thought-provoking data set that reinforces the notion that HER family tyrosine kinase receptor signaling plays a wider role in breast cancers than we currently appreciate, and reminds us of an untapped potential in targeting this pathway for the treatment of breast cancers.
The Human Epidermal Growth Factor Receptor (HER) family is comprised of four highly homologous members, EGFR, HER2, HER3, and HER4 that are stimulated by a large family of ligands with varying degrees of receptor selectivity. The physiologic function of these receptors is tightly controlled at baseline by conformational restraints and an expression ceiling, and their activation follows highly orchestrated ligand-induced conformational dynamics leading to dimerization, enzymatic activation, and signaling tail phosphorylations. The overexpression or mutational alteration of these receptors in cancers overcomes such self-restraints, creating a pathologic state of signaling favorable for tumorigenic growth and heavily selected during the evolution of many cancer types. Examples of this include the commonly seen amplification and overexpression of HER2 in about 20% of breast cancers, and rare mutational activations of HER2, or amplification or mutational activation of EGFR. In addition, there is ample evidence that in some cancers, signaling through this family of receptors plays a key functional role without genetic alterations such as mutation or amplification. Evidence for this comes from observations of clinical response to the EGFR-targeting antibody cetuximab in colon cancers (2), response to the HER family TKI erlotinib in pancreatic cancers (3), or the response to the HER2 dimerization inhibitor pertuzumab in some cases of ovarian cancer (4).
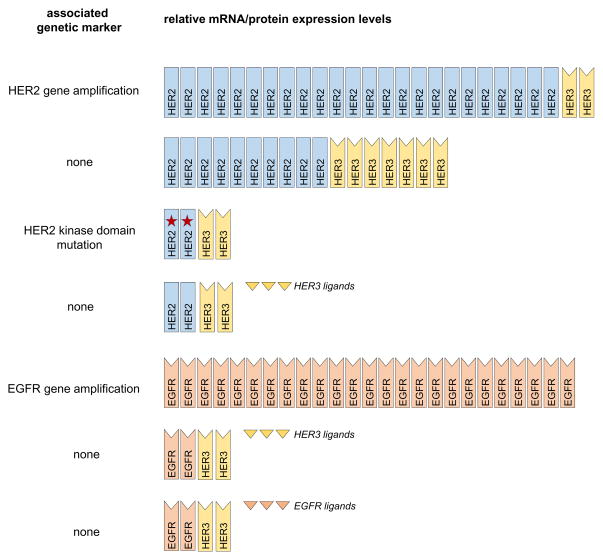
The identification of breast cancers wherein HER family members play key roles without mutation or amplification remains a difficult task and performing large clinical studies of all classes of HER family targeting agents in all subtypes of breast cancers in traditional clinical trial designs is not realistically feasible. This is where the current study by Leary et al plays in. In this study, the investigators used the pre-surgical window clinical trial design to conduct a short in-vivo experiment of lapatinib response in patients with all subtypes of breast cancer. Lapatinib is a highly selective tyrosine kinase inhibitor (TKI) with almost no activity outside of the HER family. As such, an antiproliferative response to lapatinib signifies a proliferative role for HER kinases in the biology of that individual cancer (summarized in Fig. 1). The anti-proliferative response was detected by Ki67 downregulation and correlated with a number of biomarkers of HER family expression and activity. The expected finding was the anti-proliferative response seen in HER2-positive breast cancers wherein overexpression of HER2 and constitutive HER2-HER3 signaling is known to be the disease driver. But the exploratory finding was the evidence of some anti-proliferative responses in HER2 negative breast cancers, with the biomarker analysis suggesting that these responses occurred in tumors with higher expression of HER3.
Figure 1.
A schematic summary of numerous genetic or expression scenarios of the HER family wherein breast cancer cells may be driven by the HER family of receptors or their ligands. The scenarios depicted are ones for which there is at least some evidence from clinical studies or cell lines to suggest a functional role. Many other hypothetical scenarios are plausible, but without any evidence. All these scenarios are expected to be sensitive to an EGFR/HER2 kinase inhibitor such as lapatinib and captured by a lapatinib screen. The first scenario represents the clinically diagnosed HER2-positive subtype of breast cancer. All other potential scenarios fall under the umbrella of clinical HER2-negative breast cancer and currently not assayed or identified in the course of clinical care.
While the role of HER3 as an obligate partner for amplified HER2 is well established, its role outside of this context has been speculated but no reliable markers have emerged to identify HER3-dependent tumors (5). The HER3 kinase domain is catalytically inactive and its signaling functions depend entirely on its HER family partners (6). As such, the attribution of a tumor promoting function to HER3 also implicates a role for one of its catalytically competent HER family partners. But how the HER3 signal is generated in such tumors without gene amplification or mutation events remains a matter of speculation. Some breast cancers may be driven by autocrine ligand loops as was discovered in a screen of cancer cell lines including breast cancer cell lines(7). Such tumors could be amenable to treatment with HER3 ligand-blocking antibodies, HER3 dimerization-blocking antibodies, possibly EGFR or HER2-targeting antibodies, or HER family TKIs. A similar paradigm invoking paracrine ligand stimulation may be present in other tumors, implying a much more interdependent tumor-stromal relationship. A potential for ligand-independent HER3 signaling can also be speculated. This hypothesis arises from in vitro experiments revealing that HER2 and HER3 are synergistic in transformation assays such that while the massive overexpression of HER2 is sufficient to transform cells, a concomitant increase in HER3 can reduce the threshold required for HER2 to transform cells (8). This leaves open the possibility that some breast cancers are driven by elevated expression of HER2 and HER3 in the absence of HER2 amplification, and that does not meet the criteria for HER2 overexpression. The concomitant elevation of HER2 and HER3 expression in the study by Leary is consistent with such a notion. Also potentially consistent with this is the evidence of trastuzumab responders in a subset of breast cancer patients without confirmed amplification and overexpression of HER2 (9).
A role for HER3 in partnership with EGFR has also been described in a multitude of cancers, most recently in triple negative breast cancers where a compensatory increase in EGFR-HER3 signaling appears to mitigate the efficacy of PI3K/Akt pathway inhibitors (10). This connects EGFR with a body of existing evidence that the PI3K/Akt pathway is frequently activated in triple negative breast cancers (11). Although amplification of EGFR is only rarely seen in triple negative breast cancers, its frequent expression and overexpression in this subtype of breast cancer has led to interest in EGFR as a target for the treatment of triple negative breast cancers. But its role has been difficult to understand and translate into therapy, and clinical studies with the EGFR-targeting antibody cetuximab have produced disappointing results. It remains possible that these triple negative breast cancers are driven by robust signaling from the EGFR-HER3 dimer through to downstream PI3K/Akt pathway signaling and effective treatment of these cancers will require targeting multiple components of this pathway.
A role for EGFR-HER3 signaling has been described in many other cancers including some lung cancers, melanomas, colon cancers, pancreatic cancers, ovarian cancers, and some cancers of the head & neck (5). In these cancers the hypothesis has been generated by observations that increased expression of HER3 in subsets of these cancers confers a worse prognosis, and strengthened by experimental models using knockdown techniques in cancer cell lines. In most of these cancers, the available evidence suggests that the tumor-promoting functions of HER3 are supported by the catalytic activity of EGFR, leading to numerous preclinical and clinical studies of EGFR targeting agents for the treatment of these cancers. Occasionally HER3 signaling can be mediated through promiscuous activity attributable to kinases from other families such as is seen in lung cancers with MET amplification (12). The extent and scope of such promiscuous activity among human cancers is not well known.
The reason why HER3 signaling is engaged in many different types of cancers is not entirely known, but it is easy to speculate that it is because of its competence at activation of PI3K/Akt pathway signaling. The HER3 signaling tail has six PI3K-binding motifs, more than most kinases, and activation of HER3 signaling may be engaged in many cancers as a means to PI3K pathway activation, analogous to genetic or epigenetic events in PI3K subunits, PTEN, or Akt that lead to the deregulation of this pathway.
Acknowledgments
M.M. Moasser is supported by the NIH under award numbers CA122216 and CA112970. M.R. Campbell is supported by a Susan G. Komen for the Cure postdoctoral fellowship.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Leary A, Evans A, Johnston SRD, A’Hern R, Bliss JM, Sahoo R, et al. Antiproliferative effect of lapatinib in HER2-positive and HER2-negative/HER3-high breast cancer: results of the presurgical randomized MAPLE trial (CRUK E/06/039) Clin Cancer Res. 2014 doi: 10.1158/1078-0432.CCR-14-1428. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–45. doi: 10.1056/NEJMoa033025. [DOI] [PubMed] [Google Scholar]
- 3.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 4.Makhija S, Amler LC, Glenn D, Ueland FR, Gold MA, Dizon DS, et al. Clinical activity of gemcitabine plus pertuzumab in platinum-resistant ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. J Clin Oncol. 2010;28:1215–23. doi: 10.1200/JCO.2009.22.3354. [DOI] [PubMed] [Google Scholar]
- 5.Campbell MR, Amin D, Moasser MM. HER3 comes of age: new insights into its functions and role in signaling, tumor biology, and cancer therapy. Clin Cancer Res. 2010;16:1373–83. doi: 10.1158/1078-0432.CCR-09-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jura N, Shan Y, Cao X, Shaw DE, Kuriyan J. Structural analysis of the catalytically inactive kinase domain of the human HER3 receptor. Proc Nat Acad Sci U S A. 2009;106:21608–13. doi: 10.1073/pnas.0912101106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson TR, Lee DY, Berry L, Shames DS, Settleman J. Neuregulin-1-mediated autocrine signaling underlies sensitivity to HER2 kinase inhibitors in a subset of human cancers. Cancer Cell. 2011;20:158–72. doi: 10.1016/j.ccr.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Alimandi M, Romano A, Curia MC, Muraro R, Fedi P, Aaronson SA, et al. Cooperative signaling of ErbB3 and ErbB2 in neoplastic transformation and human mammary carcinomas. Oncogene. 1995;10:1813–21. [PubMed] [Google Scholar]
- 9.Paik S, Kim C, Wolmark N. HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med. 2008;358:1409–11. doi: 10.1056/NEJMc0801440. [DOI] [PubMed] [Google Scholar]
- 10.Tao JJ, Castel P, Radosevic-Robin N, Elkabets M, Auricchio N, Aceto N, et al. Antagonism of EGFR and HER3 enhances the response to inhibitors of the PI3K-Akt pathway in triple-negative breast cancer. Sci Signal. 2014;7:ra29. doi: 10.1126/scisignal.2005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon V, Banerji S. Molecular pathways: PI3K pathway targets in triple-negative breast cancers. Clin Cancer Res. 2013;19:3738–44. doi: 10.1158/1078-0432.CCR-12-0274. [DOI] [PubMed] [Google Scholar]
- 12.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]