Abstract
We developed and tested a semi-automated algorithm to generate large data sets of ventilatory information (amplitude, premotor drive and timing) across a range of motor behaviors. Adult spontaneously breathing, anesthetized mice (n=27) underwent measurements of transdiaphragmatic pressure (Pdi) during eupnea, hypoxia-hypercapnia, and tracheal occlusion with values ranging from 8±1 to 9±2 to 44±3 cm H2O, respectively. Premotor drive to phrenic motor neurons (estimated by the rate of rise during initial 60 ms) was ~5-fold greater during tracheal occlusion compared to other behaviors. Variability in Pdi amplitude (normalized to spontaneously occurring sighs for each animal) displayed minimal evidence of complex temporal structure or dynamic clustering across the entire period of examination. Using a deterministic model to evaluate predictor variables for Pdi amplitude between successive inspiratory events, there was a large correlation for premotor drive and preceding Pdi amplitude vs. Pdi amplitude (r=0.52). These findings highlight substantial variability in Pdi amplitude that primarily reflects linear components rather than complex, dynamic effects over time.
Keywords: Automated software, Diaphragm muscle, Premotor drive, Respiratory system, Signal analysis
1. Introduction
Breathing is a life sustaining behavior accomplished by activation of ventilatory muscles such as the diaphragm (DIAm) in mammals. In fact, the DIAm is the primary muscle responsible for generating forces necessary for inspiration and contributes in coordinated fashion with other muscle groups to accomplish higher force non-ventilatory behaviors such as coughing or sneezing (Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck, 1989, 1991; Sieck, 1994). Clinically, transdiaphragmatic pressure (Pdi) measurements are commonly used in evaluating a patient’s ability to sustain ventilation for diagnostic purposes, c.f. the American Thoracic Society and the European Respiratory Society Statement on Respiratory Muscle Testing (2002). Across a range of species, Pdi approximates DIAm force, having been validated in sheep (Bazzy and Haddad, 1984), dogs (Hubmayr et al., 1990), cats (Sieck and Fournier, 1989), piglets (Watchko et al., 1986), hamsters (Sieck, 1991; Sieck, 1994), rats (Gill et al., 2015; Mantilla et al., 2010; Mantilla and Sieck, 2011) and mice (Greising et al., 2013b). Thus, data acquired from Pdi measurements is used to yield clinically and experimentally important information regarding the ability to generate DIAm force. Analyses of Pdi amplitude across motor behaviors may provide important information regarding premotor drive to respiratory muscles such as the DIAm.
Rhythmic activation of respiratory muscles, such as the DIAm, reflects timing characteristics that are likely imposed by central pattern generators (Feldman et al., 2013). The amplitude of a single breath, or inspiratory event, in turn relates to the specific premotor drive, and may be determined at the level of the motor unit pool for the different ventilatory muscles (Holstege, 2014; Mantilla et al., 2014). Analyses of variability in Pdi amplitude (i.e., variance and temporal structure of differences between successive inspiratory events) can provide useful insight into the neuromotor control of respiratory muscles, and may also inform conditions of injury, disease or aging. Recent studies using data acquired via DIAm electromyography (EMG) provide important insight into the timing of motor unit recruitment and the level of premotor drive associated with DIAm activation across a range of ventilatory and higher force non-ventilatory behaviors (Gill et al., 2015; Mantilla et al., 2014; Seven et al., 2014; Seven et al., 2013). By developing a semi-automated method to analyze Pdi it is possible to obtain and evaluate large data sets containing information about the amplitude and premotor drive of successive inspiratory events across motor behaviors. We hypothesize that the premotor drive to phrenic motor neurons is not related to the timing characteristics of each inspiratory event, indicating an independence of Pdi amplitude and timing. In the present study, deterministic models of variance in Pdi amplitude were generated to evaluate the relationship between the premotor drive, timing and Pdi amplitude characteristics for successive inspiratory events.
2. Methods
2.1. Animals
Adult mice (6 – 24 months of age, 36.3 ± 1.3 g body weight) of two different commonly used strains (C57BL/6J, n = 7; C57x129, n = 20) were studied. All experimental procedures were approved by the Institutional Animal Care and Use committee at the Mayo Clinic, in accordance with the National Institutes of Health Guidelines. Mice were anesthetized by an intramuscular injection of ketamine (90 mg/kg) and xylazine (10 mg/kg) prior to determining Pdi.
2.2. Pdi measurements
As previously described, Pdi measurements were conducted in spontaneously breathing, anesthetized animals (Gill et al., 2015; Greising et al., 2013b; Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck, 1991; Sieck, 1994; Sieck and Fournier, 1989). The abdomen was tightly constrained and the trachea was instrumented using a metal 19G cannula (Fishman #2512119). Following calibration as recommended by the manufacturer, a pair of Millar Mikro-Tip pressure transducer catheters (3.5 F, SPR-524; Millar Instrumentation, Houston TX) were inserted into the mid-esophagus and stomach. Data were digitized (2000 Hz) using a PowerLab 8/35 data acquisition system.
Measurements of Pdi were conducted during eupnea and following exposure to hypoxia (10% O2) and hypercapnia (5% CO2); each for 5 min. In addition, Pdi measurements were obtained during a 15 sec sustained tracheal occlusion. Measurements were also analyzed separately for any naturally-occurring deep breaths (“sighs”), as previously described (Gill et al., 2015; Greising et al., 2013b; Mantilla et al., 2010). Adequate recovery time was given between motor behaviors (usually ~5 min).
2.3. Analytical procedures
For the purposes of this study, each instance of DIAm activation measured as a peak in Pdi was considered an inspiratory event regardless of the motor behavior. Consistent with previous studies (Gill et al., 2015; Greising et al., 2013b; Mantilla et al., 2010), Pdi amplitude for a single inspiratory event was calculated manually as the difference between the maximum pressure and an observer-decided baseline in LabChart (Millar Instrumentation). Such measurements were conducted in inspiratory events from 4 mice (usually ~ 40 inspiratory events during eupnea and hypoxia-hypercapnia and the maximum 5 inspiratory events during tracheal occlusion). Respiratory frequency (min−1) was calculated by counting the number of inspiratory events over a 15 sec period during eupnea and hypoxia-hypercapnia.
2.3.1. Semi-automated Pdi measurements
In order to generate a large data set of Pdi measurements, a semi-automated technique was developed involving 3 steps: 1) Band-pass filtering: (0.3–30 Hz; in LabChart) to center the power of the signal around zero, remove any offset and high frequency noise components related to cardiac activity. The segment to be analyzed was selected and exported into a data file for further data analysis; 2) Threshold selection: Using MATLAB (The MathWorks Inc., Natick, MA, 2012) a threshold σ was arbitrarily set at 30% of the median value of all positive peaks (identified by the findpeaks function in MATLAB) in the segment of the Pdi recording being measured (usually ~3 min). In this fashion baseline cycles were below σ differentiating individual peak cycles above the threshold σ (Fig. 1). A single peak in each peak cycle was identified as the maximum (if in rare cases more than one peak was present); 3) Baseline definition: The baseline used in determining the amplitude of a peak was selected automatically by the average of all inflection points within the baseline cycle (i.e., upward and downward deflections were identified by a two-step application of the findpeaks function for both the original and inverted baseline cycle segment). The Pdi amplitude for a single inspiratory event (n) was calculated as the difference between the maximum peak and the preceding interpeak baseline. Average respiratory frequency was obtained from the instantaneous respiratory frequencies measured from the inverse of the peak-to-peak period. The executable analytical program is available upon request.
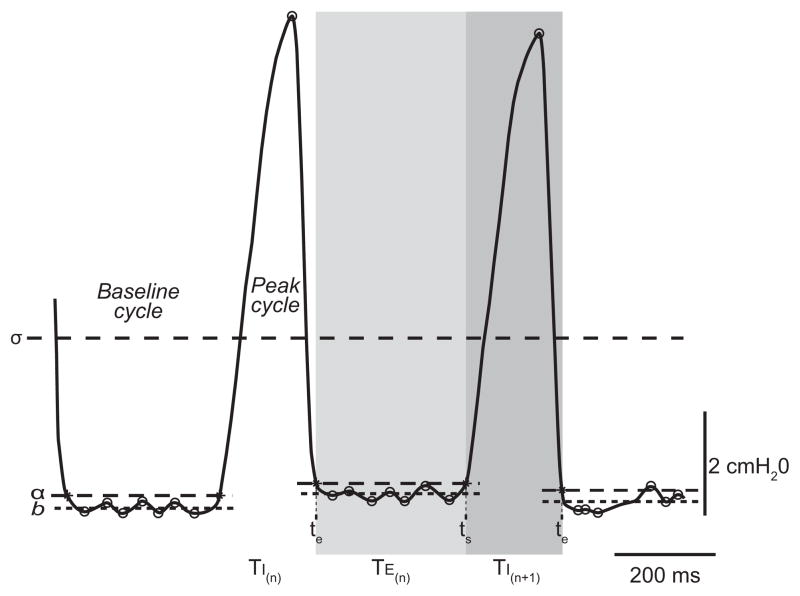
Figure 1.
Representative tracing of transdiaphragmatic pressure (Pdi) measurements in an adult spontaneously breathing mouse, highlighting the automated algorithm analysis of individual inspiratory events. The threshold σ differentiates baseline and peak cycles based on a running time average of the signal. Within the baseline cycle, the mean value of all inflexion points was used to determine the baseline value (b; dashed horizontal lines). Peak Pdi amplitude was calculated as the difference between the maximum point during the peak cycle and the baseline during the preceding baseline cycle. A threshold α was determined by adding 3% of the successive peak amplitude to the baseline within the preceding baseline cycle. This threshold α was used to determine the end-time (te) for the preceding peak and the start-time (ts) for the successive peak. These time points were used to determine the inspiratory time (TI; shaded dark gray region) and expiratory time (TE; shaded light gray region).
Statistical analyses comparing manual and semi-automated methods of Pdi analyses were conducted with JMP (JMP version 10.0; SAS Institute Inc.) using two main outcomes. Breath by breath comparisons were conducted for the same inspiratory events (20 during eupnea and 20 during hypoxia-hypercapnia for each spontaneously breathing mouse) in a total 4 mice. Intra-animal comparisons (using representative samples for each animal but without ensuring identical sampling for the two methods) were conducted in a total of 18 mice. Data for comparisons of both Pdi amplitude and respiratory frequency were examined by linear regression and Bland-Altman analysis (Bland and Altman, 1986) with a 95% confidence interval, as appropriate. Coefficient of variation (CV) for each parameter analyzed was determined for each animal and data are summarized across animals.
2.3.2. Analyses of Pdi amplitude
Seven mice (male, 12 months of age; 39.4 ± 2.8 g body weight) were used for semi-automated analysis of premotor drive, timing and Pdi amplitude characteristics for successive inspiratory events across various motor behaviors ranging from eupnea to higher force activation during tracheal occlusion. In recent studies, we showed that the period of phrenic motor unit recruitment spans the initial ~35–55% of the EMG peak duration across motor behaviors (Seven et al., 2013) and premotor drive to phrenic motor neurons is reflected by the rate of rise in DIAm EMG (Seven et al., 2014). Importantly, DIAm EMG measures such as the root mean squared EMG amplitude correlate with Pdi and both of these measures show a linear rate of rise during the period of motor unit recruitment (Mantilla et al., 2014; Mantilla et al., 2010). Accordingly, premotor drive (dPdi) was estimated for each inspiratory event by the change in Pdi amplitude within the initial ~30% of the inspiratory peak (i.e., 60 ms in mice). Additional timing characteristics for each inspiratory event were determined using the semi-automated analysis. A threshold α was obtained by adding 3% of the nth peak amplitude to the preceding interpeak baseline permitting automated determination of te(n−1) and ts(n) for each inspiratory event (Fig. 1). The start-time (ts) and end-time (te) for each peak were used to determine inspiratory time (TI (n); te(n) – ts(n)) across all motor behaviors. For ventilatory behaviors (eupnea and hypoxia-hypercapnia), expiratory time (TE (n); ts(n+1) – te(n)) and total time (TTOT(n); TI (n) + TE (n)) were also calculated. Data for premotor drive, timing and Pdi amplitude across animals were analyzed by a mixed linear model with individual animals as a random effect in order to compare Pdi or dPdi across motor behaviors using JMP.
2.3.3. Analyses of variability in Pdi amplitude
Additional analyses evaluating variability in Pdi amplitude (i.e., variance and temporal structure of differences between successive inspiratory events). Previous reports document differences in Pdi measurements across animals (Greising et al., 2013b; Mantilla et al., 2010). Accordingly, comparisons across animals were facilitated by normalizing Pdi amplitude in eupnea and hypoxia-hypercapnia to the average amplitude of spontaneous sighs measured during these ventilatory behaviors. Variance in normalized Pdi amplitude was analyzed by constructing standard Poincaré plots (Brennan et al., 2001, 2002) and using the methods developed by Dick and colleagues (Fishman et al., 2012) for evaluation of variability in long time series of physiological events. The publically-available, executable analytical program developed in MATLAB by these authors was used in such analyses. Specifically, the standard deviation of successive differences in normalized Pdi amplitudes (SD1) and the standard deviation in the variance of all normalized Pdi amplitudes (SD2) were calculated. In addition, temporal pattern variability was analyzed using time-delayed temporal Poincaré variability (TVPTD) and long-term temporal Poincaré variability (TVPA), as previously described (Fishman et al., 2012). TVPTD was analyzed with a time delay of up to 10 inspiratory events. SD1, SD2, and TVPA (moving window sizes of 1, 5, 10, 15, and 20 inspiratory events) were analyzed for the entire data set as well for 3 partitions to evaluate dynamic components (e.g., temporal clustering) of temporal variability in Pdi amplitude. Data on Pdi variability were analyzed in JMP by three-way ANOVA or one-way repeated measures ANOVA, as appropriate.
2.3.4. Predictor variables of Pdi amplitude
Linear regression models were also generated to examine the correlation between Pdi and timing characteristics and dPdi during eupnea and hypoxia-hypercapnia. Linear regression of Pdi(n) and Pdi(n+1) and all deterministic models were conducted with Statsmodels for Python (Python Language Reference version 2.7; Python Software Foundation). Variables included in linear models were the following: Pdi, dPdi, TI, TE, and TTOT for the index (n) and subsequent (n + 1) inspiratory event. The correlation coefficient (r) and number of inspiratory events analyzed (n) are presented for all pairwise regression models. Effects were classified based on the r values such that large (0.5–1.0), moderate (0.3 – 0.5), small (0.1 – 0.3) or no (0.0 – 0.1) correlation were considered (Cohen, 1988). Based on the results of pairwise correlation analyses, an additional linear regression model was developed for Pdi(n+1) vs. Pdi(n) and dPdi(n).
3. Results
3.1. Validation of semi-automated Pdi measurements
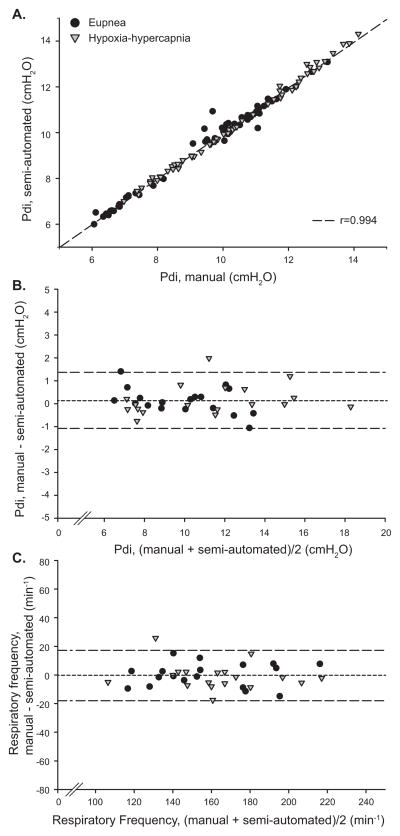
Adult mice from the ages of 6 through 24 months (~100 - 75% survival) were used to collect data on Pdi during spontaneous ventilation. During the ~5 min of either eupnea or hypoxia-hypercapnia, Pdi measurements were successfully obtained for all mice. A subset of inspiratory events was analyzed in duplicate by manual and semi-automated methods (n = 4 animals). In breath by breath comparisons, average Pdi generated during eupnea and hypoxia-hypercapnia was (mean ± SE) 9.8 ± 0.2 and 10.2 ± 0.2 cmH2O, respectively, with no differences across analytical methods (manual or semi-automated). Correlation and agreement between the manual and semi-automated methods were examined by linear regression and Bland-Altman analysis, respectively (Fig. 2). As expected, there was a large degree of correlation between the two methods (Fig. 2A) and there was excellent agreement (0.1 ± 0.5 cmH2O) between methods regardless of motor behavior. The manual and semi-automated methods of Pdi analysis were also compared in a set of 18 mice (6 month old: n = 14; 24 month old: n = 4) while using the larger sample size obtained with the semi-automated method (509 ± 36 inspiratory events per animal). Note that identical sampling was not ensured for each animal and behavior between methods. In this intra-animal comparison between analytical methods, there was again a large degree of correlation (r = 0.98; p < 0.001) and excellent agreement (0.1 ± 1.2 cmH2O) in determining Pdi amplitude (Fig. 2B). Of note, the difference between the methods was not statistically significant and within the equipment error according to the manufacturer (2 cmH2O). The extent of variability in Pdi amplitude during eupnea or hypoxia-hypercapnia was minor (CV = 1.5 ± 0.3% and 1.6 ± 0.4 % during eupnea and hypoxia-hypercapnia, respectively). Note that there were no significant differences in the extent of variability across age groups (CV = 9.5 ± 1.0 % and 8.1 ± 0.6 % for adult mice at 6 and 24 months of age, respectively; p = 0.216).
Figure 2.
Comparison between manual and semi-automated analyses of Pdi during eupnea and hypoxia-hypercapnia. A) Linear regression of Pdi amplitude data analyzed during identical inspiratory peaks (breath by breath comparison; n = 4 mice) determined across a range of motor behaviors demonstrates a large linear relationship (r = 0.99). Bland-Altman analyses for inter-animal comparisons (n = 18 mice) showed a significant agreement between analytical methods for B) Pdi amplitude and C) respiratory frequency. The dashed line represents the mean and dotted lines 2 SD from the mean.
During eupnea and hypoxia-hypercapnia, respiratory frequency was 158 ± 7 and 164 ± 6 min−1, respectively, with no differences across analytical methods (manual or semi-automated). In the intra-animal comparison, the correlation between analytical methods for respiratory frequency was large (r = 0.95; p < 0.001) and agreement was excellent, albeit within relatively wide limits (Fig. 2C; −1 ± 18 min−1). Using the semi-automated analytical method, the extent of variability in respiratory frequency was minor (CV = 7.9 ± 1.3 % and 8.7 ± 1.2 % during eupnea and hypoxia-hypercapnia, respectively). Note that there were no significant differences in the extent of variability across age groups (CV = 8.8 ± 1.1 % and 6.2 ± 0.5 % for 6 and 24 months of age, respectively; p = 0.216).
3.2. Pdi amplitude variability across a range of motor behaviors
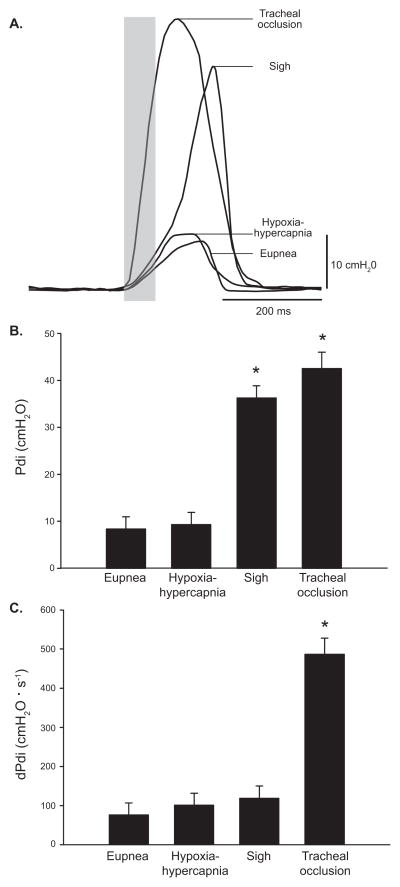
Measurements of Pdi were conducted in 7 mice (at 12 months of age) across a broad range of motor behaviors from eupnea, hypoxia-hypercapnia to spontaneous sighs and tracheal occlusion. Individual representative Pdi measurements obtained for inspiratory events in one mouse are shown for all motor behaviors examined (Fig. 3A). Average Pdi generated during eupnea and hypoxia-hypercapnia was significantly lower than that generated during spontaneous sighs and tracheal occlusion (Fig. 3B). As expected, Pdi increased rapidly during the initial phase of an inspiratory event, regardless of motor behavior. Thus, premotor drive to phrenic motor neurons was estimated from the rate of rise in Pdi, with dPdi calculated during the initial 60 ms (see the shaded area in Fig. 3A) for all motor behaviors. Average dPdi during tracheal occlusion was significantly greater (~5-fold) than during eupnea, hypoxia-hypercapnia or sighs (Fig. 3C), suggesting greater premotor drive during this higher force non-ventilatory behavior. There were statistically significant differences in TI across motor behaviors, with inspiratory events during sighs or tracheal occlusions being significantly longer than during eupnea or hypoxia-hypercapnia (Table 1). There were no statistically significant differences in TE or TTOT between eupnea and hypoxia-hypercapnia (Table 1). These parameters could not be analyzed during other non-rhythmic behaviors. The extent of variability (CV) in Pdi measurements, dPdi and timing characteristics is also presented in Table 1. There was relatively modest variability in Pdi amplitude (CV ~10%), dPdi (CV ~20%) or timing characteristics (CV ~10%) across motor behaviors.
Figure 3.
A) Representative tracings of Pdi across motor behaviors: eupnea, hypoxia-hypercapnia, spontaneously occurring deep breaths (i.e., sighs), and tracheal occlusion obtained in an adult mouse (12 month old). Premotor drive (dPdi) was estimated from the rate of rise in Pdi amplitude during the period of motor unit recruitment (~60 ms; shaded area). B) Pdi amplitude was significantly different across motor behaviors (n = 7 mice), as analyzed using a mixed linear model assigning animal as a random effect. The Pdi amplitude of inspiratory events increased from eupnea and hypoxia-hypercapnia to sighs and tracheal occlusion (p < 0.001). C) Estimated premotor drive is greater during tracheal occlusion compared to the other behaviors measured (p < 0.001). Data presented as least squared mean ± standard error; *significantly different than other motor behaviors.
Table 1.
Amplitude and timing characteristics of data collected from transdiaphragmatic pressure (Pdi) measurements
| Motor behavior | Inspiratory events | Peak amplitude (Pdi) | Premotor drive (dPdi) | Inspiratory time (TI) | Expiratory time (TE) | Total event time (TTOT) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n (per animal) | cmH2O | % CV | cmH2O/s | % CV | ms | % CV | ms | % CV | ms | % CV | |
| Eupnea | 218 ± 28 | 8.4 ± 1.0 | 9.7 ± 0.5 | 76.1 ± 11.2 | 23.4 ± 2.0 | 195.1 ± 6.5 | 8.9 ± 0.8 | 223.9 ± 18.5 | 12.4 ± 1.0 | 419.0 ± 23.7 | 6.5 ± 0.4 |
| Hypoxia-hypercapnia | 150 ± 33 | 9.3 ± 1.7 | 10.1 ± 0.5 | 101.0 ± 20.7 | 20.8 ± 3.9 | 201.9 ± 7.3 | 8.1 ± 1.1 | 201.2 ± 20.2 | 12.9 ± 1.2 | 412.0 ± 22.8 | 6.5 ± 0.4 |
| Sigh | 3 ± 0 | 36.3 ± 4.1 *† | 8.1 ± 2.8 | 118.6 ± 22.3 | 17.8 ± 4.6 | 245.8 ± 9.3*† | 1.7 ± 0.5 | - | - | - | - |
| Tracheal occlusion | 4 ± 0 | 43.6 ± 3.3 *† | 6.5 ± 2.6 | 492.8 ± 93.7 *†‡ | 20.4 ± 4.7 | 245.8 ± 19.7*† | 8.2 ± 4.0 | - | - | - | - |
| p-value | - | < 0.001 | - | < 0.001 | - | 0.002 | - | 0.407 | - | 0.677 | - |
mean ± SE, data analyzed by mixed linear model with animal as a random effect (n = 7 mice)
significantly different than eupnea;
significantly different from hypoxia-hypercapnia;
significantly different from sigh.
3.3. Variability in Pdi amplitude
To understand variability in Pdi amplitude (i.e., variance between inspiratory events and temporal structure) across ventilatory behaviors, Pdi was normalized to sighs for each animal. Differences in normalized Pdi amplitude between an inspiratory event (n) and its τh successor over time (n + τ) was analyzed up to 10 inspiratory events, representing TPVTD for τ = 1 to 10. There was no effect of the time delay (τ) or behavior (eupnea or hypoxia-hypercapnia) on the TPVTD (τ-repeated p = 0.766; behavior p = 0.566; interaction p = 0.786). These results suggest a mostly linear relationship in normalized Pdi amplitude between successive inspiratory events, but more complex temporal structure cannot be excluded. Accordingly, the distribution of the variance in normalized Pdi amplitude was also analyzed with SD1 and SD2 (Fig. 4) to compare the dynamics of each partition to the entire data set. In particular, SD2 would reflect epochs of different variability (e.g., high vs. low) within the time-series. Up to 3 partitions were considered. No effect of behavior or partition (p = 0.874) was found for either SD1 or SD2 (behavior: p ≥ 0.455; partition: p ≥ 0.874; interaction: p ≥ 0.965); indicating that normalized Pdi amplitude during ventilatory behaviors is uniform. For eupnea and hypoxia-hypercapnia, the standard deviation of successive differences (SD1) in normalized Pdi amplitude for the entire data set was 0.023 ± 0.004 and 0.026 ± 0.005, respectively. The standard deviation in the variance of all normalized Pdi amplitudes analyzed with the SD2, and SD2 for eupnea and hypoxia-hypercapnia was 0.023 ± 0.002 and 0.027 ± 0.004, respectively, for the entire data set.
Figure 4.
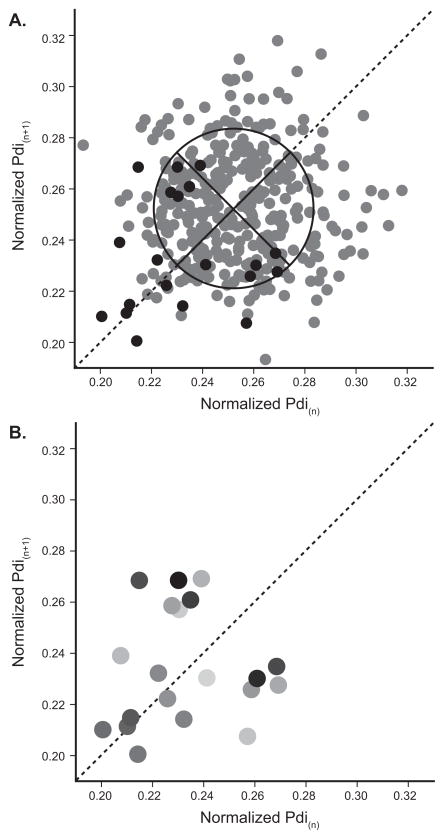
A) Poincaré plot of Pdi amplitudes (normalized to spontaneous sighs) for a representative mouse. All successive inspiratory events during eupnea are plotted in gray points. A subset of 20 consecutive inspiratory events is presented in black points. The line of symmetry is shown as dashed line and the oval with axis (solid lines) represent the SD1 (perpendicular to the line of symmetry) and SD2 (along the line of symmetry). See Methods for details of these measures. B) The subset of 20 consecutive inspiratory events is displayed in order, by the shading with the 1st inspiratory event in the lightest gray and the 20th inspiratory event in black.
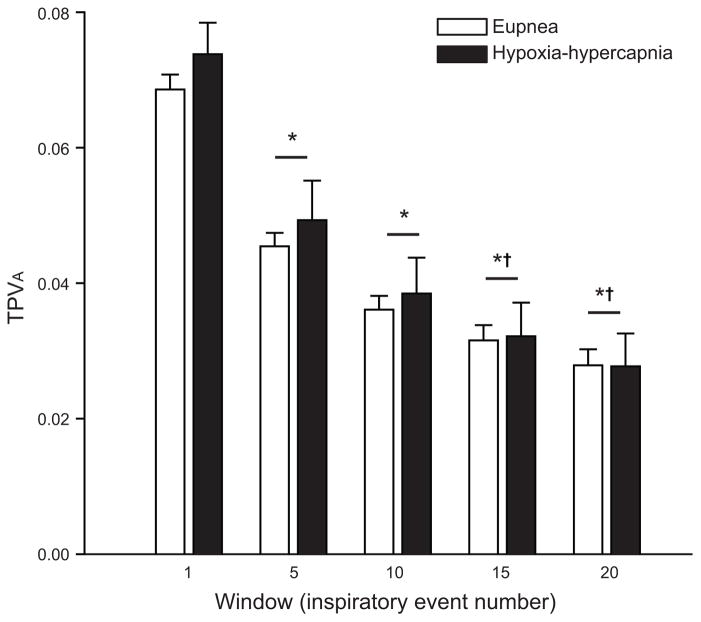
Greater information regarding the temporal structure of biological signals such as the Pdi amplitude can be ascertained by examining the variance in the centroid position of a discrete number of successive inspiratory events (window size) over time (TPVA; Fig. 5). Moving windows of up to 20 inspiratory events were considered. There was no difference between eupnea and hypoxia-hypercapnia (p = 0.354) or interaction between window size and behavior (p = 0.968) for the entire data set. As the moving window size increased, there was a decrease in the average TPVA (window p < 0.001). However, there was no effect on TPVA of partitioning the data set (up to 3 partitions were considered; behavior p = 0.393; partition p = 0.609; interaction p = 0.992). Collectively, there was minimal evidence of complex temporal structure in normalized Pdi amplitude. Thus, in anesthetized adult mice, dynamic processes do not contribute significantly to variability in Pdi amplitude during eupnea or exposure to hypoxia-hypercapnia.
Figure 5.
Long term temporal Poincaré variability (TPVA) in Pdi amplitude (normalized to spontaneous sighs) was determined by the variance of the centroid position of individual events over various window sizes from 1 to 20 during eupnea and hypoxia-hypercapnia. TPVA analysis allows for the changing dynamics of inspiratory event variability over time, specifically distributional variance and temporal correlations. Data analyzed by two-way ANOVA: interaction p = 0.969; main effect of window p < 0.001; main effect of behavior p = 0.354. *significantly different from window 1; †significantly different from window 5; ‡significantly different from window 15.
3.4. Predictors of Pdi amplitude
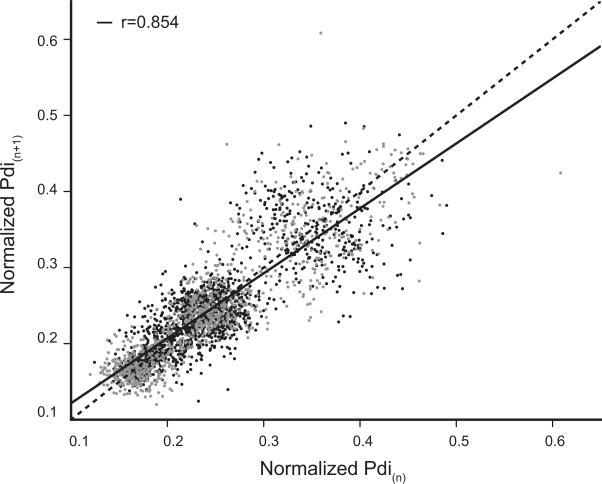
In a first analysis, the correlation between successive Pdi amplitudes during eupnea and hypoxia-hypercapnia was analyzed in a Poincaré plot (Fig. 6). Successive inspiratory events during eupnea and hypoxia-hypercapnia were analyzed independent of animal by using the normalized Pdi amplitude. The correlation between Pdi(n+1) and Pdi(n) (normalized to sigh) was large (r = 0.85; p < 0.001; Fig. 6). No differences were evident across ventilatory behaviors. The coefficient of determination (r2) for normalized Pdi amplitude was similar for eupnea (0.68) and hypoxia-hypercapnia (0.73).
Figure 6.
Poincaré plot for Pdi(n+1) vs. Pdi(n) (Pdi normalized to spontaneous sighs; n = 7 mice). Data was acquired across motor behaviors: eupnea – black dots, hypoxia-hypercapnia – gray dots. There was a large level of correlation between successive Pdi amplitudes across both eupnea and hypoxia-hypercapnia (r = 0.85; p < 0.001).
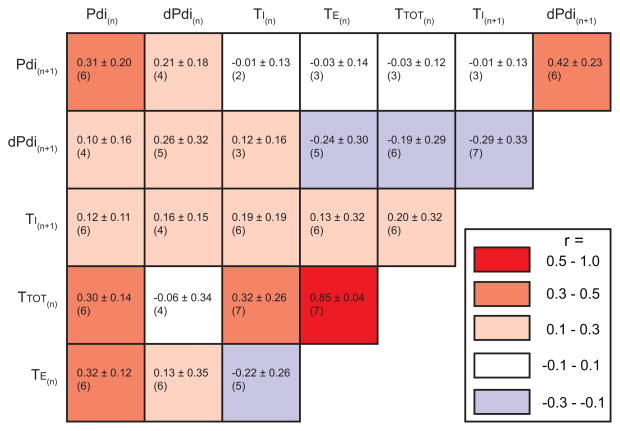
Parameters obtained from Pdi measurements were also analyzed in greater detail within each animal by conducting pairwise comparisons. The role of timing (TI, TE and TTOT, likely reflecting central pattern generator input), premotor drive (estimated by dPdi) and previous activation history (same characteristics for successive n and n+1 inspiratory events) as predictors of Pdi amplitude was analyzed in deterministic models. A correlation matrix was generated for pairwise comparisons in each animal (Fig. 7). In this matrix, the mean r value (± SD) and the number of animals displaying a significant correlation are presented. Only correlations for pairwise correlations that were consistently significant for at least 6 out of 7 animals were considered further, regardless of effect size based on the mean absolute r value (large: 0.5–1.0, moderate: 0.3 – 0.5, small: 0.1 – 0.3 or none: 0.0 – 0.1).
Figure 7.
Composite matrix of linear relationships between respiratory variables. Variables analyzed include Pdi amplitude, estimated premotor drive (dPdi), inspiratory time (TI), expiratory time (TE) and the total time (TTOT). Average (± SD) correlation coefficients (r) are reported in each interaction box along with the number of animals (out of 7) displaying a significant (p < 0.05) correlation. The color of each box indicates the average level of correlation according to the map (right) and ranges from positive (red) to negative (blue), with color intensity binned into 4 levels: none (0.0 – 0.1), small (0.1 – 0.3), moderate (0.3 – 0.5), and large (0.5–1.0).
Consistent with the results of normalized Pdi amplitude, Pdi amplitude showed moderate levels of correlation to preceding Pdi amplitude (positive; for Pdi(n+1) vs. Pdi(n)). Furthermore, Pdi amplitude showed moderate levels of correlation to dPdi (positive; for Pdi(n+1) vs. dPdi(n+1)), but no correlation to timing characteristics of the preceding inspiratory event (Pdi(n+1) vs. TI(n), TE(n), and TTOT(n)). Of note, pairwise comparisons between dPdi and timing characteristics support small levels of correlation to timing (both negative; for dPdi(n+1) vs. TI(n+1) and dPdi(n+1) vs. TTOT(n)). There results indicate that although lengthened TI and TTOT are associated with reduced dPdi for the successive inspiratory event during ventilatory behaviors, the effect is small. Taken together, these results support a main effect of premotor drive on Pdi amplitude with limited effects of timing characteristics.
In agreement with the small levels of correlation of premotor drive evident across successive inspiratory events (dPdi(n+1) vs. dPdi(n)), TI showed small levels of correlation with other timing parameters (positive; for TI(n+1) vs. TI(n), TE(n), and TTOT(n). Of note, TTOT showed large levels of correlation with TE (positive; for TTOT(n) vs. TE(n) and a moderate level of correlation with TI (positive; for TTOT(n) vs. TI(n).
In order to evaluate the combined effect of Pdi(n) and dPdi(n+1) on Pdi(n+1), a deterministic model was generated with animal as a random effect. There was a large, significant relationship between Pdi(n+1) vs. dPdi(n+1) and Pdi(n) in the model (p < 0.001), consistent with minimal differences in Pdi amplitude between two successive inspiratory events despite substantial variability across the entire data set. In 6 (out of 7) animals, the model was significant with r values between 0.46 and 0.88 indicative of a large level of correlation, and only one animal displayed no correlation (r = 0.06).
4. Discussion
This study substantiates that premotor drive to phrenic motor neurons determined using Pdi measurements does not reflect timing characteristics for successive inspiratory events. Variability in Pdi amplitude (normalized to spontaneously occurring sighs for each animal) displayed minimal evidence of complex temporal structure or dynamic clustering across the entire period of examination. Using a deterministic model to evaluate predictor variables for Pdi amplitude between successive inspiratory events, there was a large correlation for premotor drive and preceding Pdi amplitude vs. Pdi amplitude (r = 0.52). The findings also highlight the fact that the variability in Pdi amplitude evident over time in anesthetized mice primarily reflects linear components rather than complex, dynamic effects during rhythmic ventilatory behaviors (e.g., CV ~10%). These findings also support relative independence of Pdi amplitude (which can be modulated by respiratory system conditions, e.g., lung inflation, airway resistance) and timing characteristics (imposed by the central pattern generator).
4.1. Pdi measurements
There is substantial evidence in support of Pdi measurements as an estimate of DIAm force and as a reliable indicator of respiratory system performance (Bazzy and Haddad, 1984; Greising et al., 2013b; Hubmayr et al., 1990; Mantilla et al., 2010; Mantilla and Sieck, 2011; Sieck, 1991; Sieck, 1994; Sieck and Fournier, 1989; Watchko et al., 1986). As motor behaviors become more demanding there is an increase in the force generated by the DIAm that is measured by Pdi amplitude across multiple species (Gill et al., 2015; Greising et al., 2013b; Mantilla et al., 2010; Sieck and Fournier, 1989). Furthermore, measurements of Pdi amplitude correlate with DIAm root mean squared (RMS) EMG amplitude across a broad range of motor behaviors (Mantilla et al., 2010). In the current study, the Pdi generated ranged from 8 ± 1 cmH2O for eupnea to 44 ± 3 cmH2O for tracheal occlusion (in male and female mice with the age range of 6 – 24 months). These results are in general agreement with our previous work in 6 month old male mice in which Pdi during the two behaviors ranged from 10 ± 1 to 64 ± 7 cmH2O, respectively (Greising et al., 2013b).
In order to evaluate whether or not a relationship between the premotor drive, timing and Pdi amplitude characteristics exists across successive inspiratory events, a semi-automated algorithm was used to characterize variability across a large amount of Pdi data, increasing the statistical power and reliability of this type of study. In addition, comparisons across all animals were conducted after normalizing Pdi amplitude to spontaneously occurring sighs in each animal, which are higher force behaviors approaching levels necessary for maintenance of airway clearance such as tracheal occlusion. Indeed, during tracheal or airway occlusion, Pdi generated is ~70% of maximal Pdi in the mouse or rat (Gill et al., 2015; Greising et al., 2013b; Mantilla et al., 2010; Mantilla and Sieck, 2011).
4.2. Variability in Pdi amplitude
Variability in Pdi amplitude (i.e., variance between inspiratory events and temporal structure) was analyzed using normalized Pdi amplitude data across rhythmic ventilatory behaviors in adult anesthetized mice. During the entire period of examination, Pdi amplitude showed ~10% variance. However, there was minimal evidence of complex temporal structure or dynamic clustering. Differences in normalized Pdi amplitude for successive inspiratory events during eupnea and hypoxia-hypercapnia was evaluated comprehensively using methodology developed by Dick and colleagues for analyses of temporal patterns of variability (Fishman et al., 2012). There was no difference in the variance in Pdi amplitude between eupnea and exposure to hypoxia-hypercapnia in anesthetized mice, when examined either as the extent of variability (CV), the standard deviation of successive differences in normalized Pdi amplitudes (SD1) or the standard deviation in the variance of all normalized Pdi amplitudes (SD2). In addition, there was no evidence of periods of low vs. high variability as assessed by SD2 analyses across up to 3 partitions of the data set. Temporal pattern variability analyzed using time-delayed temporal Poincaré variability (TVPTD) with time delays of up to 10 inspiratory events supported analyses limited to consecutive inspiratory events (i.e., time delay = 1). Furthermore, long-term temporal Poincaré variability (TVPA) analyzed with moving window sizes of 1, 5, 10, 15, and 20 inspiratory events across 3 partitions did not reveal complex dynamic components (e.g., temporal clustering) in Pdi amplitude. Because this study utilized anesthetized mice, it is possible that anesthesia limited the extent of variability in respiratory parameters. For instance, when analyzing breath-to-breath variability in respiratory frequency it is evident that the variability across the entire data set (~8% CV), although present, is not large but is similar to previous reports of CV values in anesthetized mice (Gonsenhauser et al., 2004). Regardless, analyses of Pdi amplitude in this fashion reveal important characteristics of motor control of respiratory muscles.
4.3. Premotor drive to phrenic motor neurons
In the present study, Pdi amplitude was moderately and positively correlated to premotor drive (dPdi) and Pdi amplitude of the preceding inspiratory event. Premotor drive to the phrenic motor neurons was estimated by the change in rate of rise in Pdi amplitude within the initial 30% of the inspiratory peak, based on previous work which determined motor unit recruitment to occur within this time frame (Seven et al., 2013). In spontaneously breathing rats, premotor drive to the phrenic motor neurons was shown to be indexed by the RMS DIAm EMG amplitude during the initial 75 ms (i.e., RMS75) of the inspiratory event (Gill et al., 2015; Seven et al., 2014). Consistent with these measurements reflecting premotor drive to phrenic motoneurons, both dPdi (current study in mice) and RMS75 (previous work in rats) during tracheal occlusion are substantially greater than during other DIAm motor behaviors, consistent with greater drive being necessary for maintenance of airway patency and clearance (Mantilla et al., 2014). Indeed, drive during tracheal occlusion in mice dPdi was ~4-fold greater than during sigh and in rats during airway occlusion RMS75 was ~2 fold greater than sigh (Mantilla et al., 2014; Seven et al., 2014).
The premotor drive is imposed by central pattern generators but may be modulated at the level of the motor unit pool for the different ventilatory muscles, e.g., reflecting the effects of previous activation history on lung inflation (Holstege, 2014; Mantilla et al., 2014). In contrast, timing characteristics are predominantly determined by the central pattern generator (Feldman et al., 2013), with changes in motoneuron excitability exerting a minimal effect (e.g., as a result of changes in input resistance determined by intrinsic properties; c.f., (Jodkowski et al., 1987, 1988)). Accordingly, the lack of correlation between Pdi amplitude and timing characteristics is consistent with premotor drive to phrenic motor neurons as the primary predictor variable for successive inspiratory events rather than Pdi amplitude being imposed by the central pattern generator.
4.4. Predictors of Pdi amplitude
The mostly linear relationships during eupnea and hypoxia-hypercapnia allowed for variability in successive Pdi amplitudes to be examined by Poincaré plot. The Pdi amplitude (normalized to sigh) of successive inspiratory events showed a large correlation between inspiratory events across all animals. Further, Pdi amplitude showed moderate correlation to preceding inspiratory event Pdi amplitude in pairwise comparisons within each animal. Taken together, the results of pairwise comparisons examining predictors of Pdi amplitude including the minimal role of timing characteristics, importance of premotor drive and previous activation history support the large, significant relationship between Pdi(n+1) vs. dPdi(n+1) and Pdi(n). These findings indicate that in anesthetized adult mice exposed to eupnea or to hypoxia-hypercapnia, Pdi amplitude is consistent across successive inspiratory events.
4.5. Analytical methods to determine Pdi amplitude
The present study presents a straightforward global analysis of Pdi amplitude acquired from large sets of data. A strong linear relationship and degree of agreement was found between manual and semiautomated methods of Pdi analyses. However, similar analytical tools can be used for other respiratory measurements, e.g., EMG. Notably, the use of automated analytical tools such as that validated in the present study or other similar tools may prove important for the optimization of predictive algorithms in clinical care. For instance, information derived from deterministic models of Pdi amplitude could be used to determine the necessary amplitude of inspiratory events in mechanically ventilated patients. Indeed, ventilator-supported patients may benefit from such predictive algorithms in order to optimize the synchrony with patient efforts, e.g., during neurally-adjusted ventilatory assist (Cecchini et al., 2014). Future work should begin to examine how predictive models of Pdi amplitude such as that developed herein perform across a broader range of motor behaviors (e.g., exercise or sleep) and in humans (e.g., in intensive care units).
Historically, the analysis of respiratory timing variability has included highly complex models (i.e., noise titration techniques (Wysocki et al., 2006) or fractal dynamics and properties (Fadel et al., 2004)), selected predictor variables (i.e., interbreath interval (Goldman et al., 2008)), or within certain populations (i.e., preterm infants (Frey et al., 1998) or animal strains (Gonsenhauser et al., 2004)). For instance, the examination of respiratory timing variability before and after spontaneously occurring deep breaths has implicated sighs as a ‘reset’ of respiratory variability (Vlemincx et al., 2010; Wuyts et al., 2011). Based on the infrequent occurrence of sighs in anesthetized mice (present study; Greising et al., 2013b), it was not possible to evaluate the effects of sigh on variability in Pdi amplitude. However, future studies can directly address this point. The ability to analyze Pdi data using semi-automated analytical tools is thus an important addition to both clinical and experimental research repertoire.
It is worth noting when considering ventilatory behaviors such as eupnea vs. exposure to 10% O2 and 5% CO2 that mice exhibit relatively small changes in Pdi amplitude and respiratory frequency. Specifically for ventilatory behaviors, differences across species in the relative Pdi (as a fraction of maximal DIAm) may be related to variability in respiratory frequency, respiratory system compliance, and DIAm reserve capacity for force generation. Indeed, mice have reduced lung compliance and increased resistance in comparison to rats (Gomes et al., 2000). Studies examining variability in Pdi amplitude across species may thus illuminate differences in variability as they relate to respiratory system mechanics. For example, the higher respiratory frequency and higher proportion of fatigue-resistant fibers in the DIAm of mice compared to rats (Greising et al., 2013a; Prakash and Sieck, 1998; Sieck et al., 2012) is consistent with the relatively smaller fraction of maximal force generating capacity by the DIAm during ventilatory behaviors.
4.6. Conclusions
A semi-automated algorithm was developed to determine characteristics and predictors of Pdi amplitude and was used in the analysis of variability across large data sets. Analyses of large Pdi data sets allowed for investigation of variability in Pdi amplitude and the role of premotor drive to the phrenic motor neurons on respiratory muscles. Premotor drive to the phrenic motor neurons was largest during tracheal occlusion, a higher force non-ventilatory motor behavior associated with airway clearance. There was minimal evidence of complex temporal structure or dynamic clustering in Pdi amplitude over time. Using a deterministic model to evaluate predictor variables for Pdi amplitude between successive inspiratory events, evidence for a large correlation in premotor drive and preceding Pdi amplitude vs. Pdi amplitude was found. The results of the present study support the relative independence of premotor drive and timing of inspiratory events. Similar analyses of variability in Pdi amplitude can prove useful to studies of neuromotor control of respiratory muscles in health and disease.
Highlights.
Transdiaphragmatic pressure (Pdi) can be measured across multiple motor behaviors
Premotor drive (estimated from rate of rise) is greatest for near-maximal behaviors
Variability in Pdi amplitude is similar across ventilatory behaviors
Successive inspiratory events show moderate correlation in Pdi amplitude
Anesthetized mice show minimal temporal variability in Pdi amplitude
Acknowledgments
This research was supported by grants from National Institutes of Health grants R01-HL096750 and R01-AG044615 (CBM & GCS) and T32-HL105355 (SMG), and the Mayo Clinic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 166:518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 2.Bazzy AR, Haddad GG. Diaphragmatic fatigue in unanesthetized adult sheep. J Appl Physiol. 1984;57(1):182–190. doi: 10.1152/jappl.1984.57.1.182. [DOI] [PubMed] [Google Scholar]
- 3.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 4.Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincare plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng. 2001;48:1342–1347. doi: 10.1109/10.959330. [DOI] [PubMed] [Google Scholar]
- 5.Brennan M, Palaniswami M, Kamen P. Poincare plot interpretation using a physiological model of HRV based on a network of oscillators. Am J Physiol Heart Circ Physiol. 2002;283:H1873–1886. doi: 10.1152/ajpheart.00405.2000. [DOI] [PubMed] [Google Scholar]
- 6.Cecchini J, Schmidt M, Demoule A, Similowski T. Increased Diaphragmatic Contribution to Inspiratory Effort during Neurally Adjusted Ventilatory Assistance versus Pressure Support: An Electromyographic Study. Anesthesiology. 2014;121:1028–1036. doi: 10.1097/ALN.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- 8.Fadel PJ, Barman SM, Phillips SW, Gebber GL. Fractal fluctuations in human respiration. J Appl Physiol. 2004;97:2056–2064. doi: 10.1152/japplphysiol.00657.2004. [DOI] [PubMed] [Google Scholar]
- 9.Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu Rev Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fishman M, Jacono FJ, Park S, Jamasebi R, Thungtong A, Loparo KA, Dick TE. A method for analyzing temporal patterns of variability of a time series from Poincare plots. J Appl Physiol. 2012;113:297–306. doi: 10.1152/japplphysiol.01377.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frey U, Silverman M, Barabasi AL, Suki B. Irregularities and power law distributions in the breathing pattern in preterm and term infants. J Appl Physiol. 1998;85:789–797. doi: 10.1152/jappl.1998.85.3.789. [DOI] [PubMed] [Google Scholar]
- 12.Gill LC, Mantilla CB, Sieck GC. Impact of Unilateral Denervation on Transdiaphragmatic Pressure. Respir Physiol Neurobiol. 2015 doi: 10.1016/j.resp.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman LJ, Jarabo RM, Gomez RG. Airway pressure alters wavelet fractal dynamics and short-range dependence of respiratory variability. Respir Physiol Neurobiol. 2008;161:29–40. doi: 10.1016/j.resp.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol. 2000;89:908–916. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- 15.Gonsenhauser I, Wilson CG, Han F, Strohl KP, Dick TE. Strain differences in murine ventilatory behavior persist after urethane anesthesia. J Appl Physiol. 2004;97:888–894. doi: 10.1152/japplphysiol.01346.2003. [DOI] [PubMed] [Google Scholar]
- 16.Greising SM, Mantilla CB, Gorman BA, Ermilov LG, Sieck GC. Diaphragm muscle sarcopenia in aging mice. Experimental Gerontology. 2013a;48:881–887. doi: 10.1016/j.exger.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greising SM, Sieck DC, Sieck GC, Mantilla CB. Novel method for transdiaphragmatic pressure measurements in mice. Respir Physiol Neurobiol. 2013b;188:56–59. doi: 10.1016/j.resp.2013.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holstege G. The periaqueductal gray controls brainstem emotional motor systems including respiration. Prog Brain Res. 2014;209:379–405. doi: 10.1016/B978-0-444-63274-6.00020-5. [DOI] [PubMed] [Google Scholar]
- 19.Hubmayr RD, Sprung J, Nelson S. Determinants of transdiaphragmatic pressure in dogs. J Appl Physiol. 1990;69:2050–2056. doi: 10.1152/jappl.1990.69.6.2050. [DOI] [PubMed] [Google Scholar]
- 20.Jodkowski JS, Viana F, Dick TE, Berger AJ. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58:105–124. doi: 10.1152/jn.1987.58.1.105. [DOI] [PubMed] [Google Scholar]
- 21.Jodkowski JS, Viana F, Dick TE, Berger AJ. Repetitive firing properties of phrenic motoneurons in the cat. J Neurophysiol. 1988;60:687–702. doi: 10.1152/jn.1988.60.2.687. [DOI] [PubMed] [Google Scholar]
- 22.Mantilla CB, Seven YB, Sieck GC. Convergence of pattern generator outputs on a common mechanism of diaphragm motor unit recruitment. Prog Brain Res. 2014;209:309–329. doi: 10.1016/B978-0-444-63274-6.00016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mantilla CB, Seven YB, Zhan WZ, Sieck GC. Diaphragm motor unit recruitment in rats. Respir Physiol Neurobiol. 2010;173:101–106. doi: 10.1016/j.resp.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantilla CB, Sieck GC. Phrenic motor unit recruitment during ventilatory and non-ventilatory behaviors. Respir Physiol Neurobiol. 2011;179:57–63. doi: 10.1016/j.resp.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 26.Seven YB, Mantilla CB, Sieck GC. Recruitment of Rat Diaphragm Motor Units Across Motor Behaviors with Different Levels of Diaphragm Activation. J Appl Physiol. 2014;117:1308–1316. doi: 10.1152/japplphysiol.01395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seven YB, Mantilla CB, Zhan WZ, Sieck GC. Non-stationarity and power spectral shifts in EMG activity reflect motor unit recruitment in rat diaphragm muscle. Respir Physiol Neurobiol. 2013;185:400–409. doi: 10.1016/j.resp.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sieck DC, Zhan WZ, Fang YH, Ermilov LG, Sieck GC, Mantilla CB. Structure-activity relationships in rodent diaphragm muscle fibers vs. neuromuscular junctions. Respir Physiol Neurobiol. 2012;180:88–96. doi: 10.1016/j.resp.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieck GC. Recruitment and frequency coding of diaphragm motor units during ventilatory and non-ventilatory behaviors. In: Swanson GD, Grodins FS, Hughson RL, editors. Respiratory Control. Plenum Press; New York: 1989. pp. 441–450. [Google Scholar]
- 30.Sieck GC. Neural control of the inspiratory pump. NIPS. 1991;6:260–264. [Google Scholar]
- 31.Sieck GC. Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med. 1994;15:641–659. [PubMed] [Google Scholar]
- 32.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66:2539–2545. doi: 10.1152/jappl.1989.66.6.2539. [DOI] [PubMed] [Google Scholar]
- 33.Vlemincx E, Van Diest I, Lehrer PM, Aubert AE, Van den Bergh O. Respiratory variability preceding and following sighs: a resetter hypothesis. Biol Psychol. 2010;84:82–87. doi: 10.1016/j.biopsycho.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 34.Watchko JF, Mayock DE, Standaert A, Woodrum DE. Postnatal changes in transdiaphragmatic pressure in piglets. Pediatr Res. 1986;20:658–661. doi: 10.1203/00006450-198607000-00016. [DOI] [PubMed] [Google Scholar]
- 35.Wuyts R, Vlemincx E, Bogaerts K, Van Diest I, Van den Bergh O. Sigh rate and respiratory variability during normal breathing and the role of negative affectivity. Int J Psychophysiol. 2011;82:175–179. doi: 10.1016/j.ijpsycho.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Wysocki M, Fiamma MN, Straus C, Poon CS, Similowski T. Chaotic dynamics of resting ventilatory flow in humans assessed through noise titration. Respir Physiol Neurobiol. 2006;153:54–65. doi: 10.1016/j.resp.2005.09.008. [DOI] [PubMed] [Google Scholar]