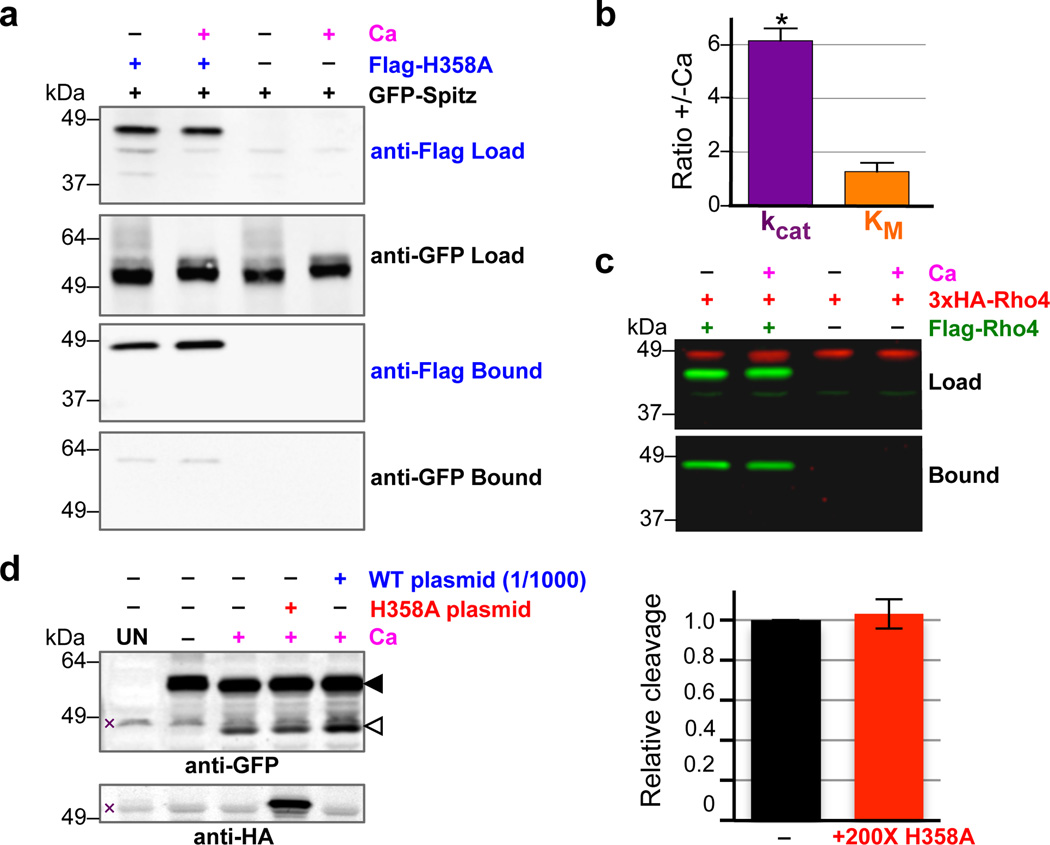
Figure 3. Intermolecular interactions do not mediate DmRho4 regulation by calcium.
a Anti-Flag coimmunoprecipitation analysis of catalytically inactive Flag-DmRho4-H358A and GFP-Spitz from S2R+ cells untreated or treated with calcium ionophore. b Effect of calcium on the steady-state kinetic parameters of intramembrane proteolysis by reconstituted DmRho4 (mean ± standard deviation of 3 independent experiments, compared using a paired t-test). c Anti-Flag coimmunoprecipitation of Flag-DmRho4 and HA-DmRho4 co-expressed in S2R+ cells untreated or treated with calcium ionophore. d Overexpressing catalytically-inactive DmRho4 (H358A) had no effect on the calcium-stimulated activity of endogenous DmRho4 in S2R+ cells (compare cleavage bands, denoted by open triangle, in lanes 3 versus 4, and quantified in graph on right). Expressing low levels of wildtype 3xHA-DmRho4 (1/1,000 amount of input plasmid) was used to quantify the level of endogenous DmRho4 (by comparing protease activity) relative to 3xHA-DmRho4-H358A expression (by comparing anti-HA signals). UN indicates S2R+ cells not transfected with GFP-Spitz (x denotes non-specific bands).