Abstract
Background
Hypertension is a common complication of chronic kidney disease (CKD) and persists among most patients with end-stage renal disease (ESRD) despite the provision of conventional thrice weekly hemodialysis.
Methods
We analysed the effects of frequent hemodialysis on blood pressure in the randomized controlled Frequent Hemodialysis Network Trials. The Daily Trial randomized 245 patients to 12 months of 6× (“frequent”) versus 3× (“conventional”) weekly in-center hemodialysis; the Nocturnal Trial randomized 87 patients to 12 months of 6× weekly nocturnal hemodialysis versus 3× weekly predominantly home-based hemodialysis.
Results
In the Daily Trial, compared to 3× weekly hemodialysis, two months of frequent hemodialysis lowered pre-dialysis systolic blood pressure by −7.7 mmHg [95%CI: −11.9 to −3.5] and diastolic blood pressure by −3.9 mmHg [95%CI: −6.5 to −1.3]. In the Nocturnal Trial, compared to 3× weekly hemodialysis, two months of frequent hemodialysis lowered systolic blood pressure by −7.3 mmHg [95%CI: −14.2 to −0.3] and diastolic blood pressure by −4.2 mmHg [95%CI: −8.3 to −0.1]). In both trials blood pressure treatment effects were sustained until month 12. Frequent hemodialysis resulted in significantly fewer antihypertensive medications (Daily: −0.36 medications [95%CI: −0.65 to −0.08]; Nocturnal: −0.44 mediations [95%CI: −0.89 to −0.03]). In the Daily Trial, the relative risk per dialysis session for intradialytic hypotension was lower with 6×/week HD but given the higher number of sessions per week, there was a higher relative risk for IDH requiring saline administration.
Conclusions
Frequent hemodialysis reduces blood pressure and the number of prescribed antihypertensive medications.
Keywords: blood pressure, hypertension, hemodialysis, frequent hemodialysis, nocturnal hemodialysis
Introduction
Hypertension is diagnosed in over 80% of patients on maintenance hemodialysis1. While there is no agreement on optimal blood pressure (BP) targets or the therapeutic means to achieve BP goals2, recent meta-analyses indicate that antihypertensive therapy in patients on maintenance HD is associated with lower mortality3,4. Previous studies suggest that 6× weekly HD compared to the conventional 3× weekly regimen reduces BP5–15. Two small randomized 6-months-long trials16,17 reported 7 – 23 mmHg reductions in pre-HD systolic BP (SBP) and 4–12 mmHg in diastolic BP (DBP). However, there is little information about how frequent HD affects BP over time. To our knowledge, no published study has described the effects of frequent HD on episodes of intradialytic hypotension, a common problem associated with poor short-term and long-term outcomes18. We previously reported the primary results of two parallel year-long randomized controlled trials on the effects of frequent HD19,20. Here we provide a description of BP dynamics in both FHN trials, the influence of baseline factors on treatment effects, the association between changes in pre-HD SBP and fluid status, and the effects of frequent HD on antihypertensive therapy and intradialytic hypotensive events.
Subjects and Methods
FHN Trials
The FHN Daily and Nocturnal Trials (ClinicalTrials.gov#NCT00264758) are multicenter, randomized, prospective trials of in-center short daily HD and predominantly home-based nocturnal HD. The designs, inclusion and exclusion criteria of both trials were described previously21. Pertinent to this report was the less stringent exclusion criterion for residual renal function in the Nocturnal Trial (average of the urea and creatinine clearances >10 mL/min/1.73m2) compared to the Daily Trial (>3 mL/min per 35 L urea distribution volume)22. Post-HD target weight was prescribed by the patient’s attending nephrologist. Patients were enrolled between 3/2006 and 5/2009 and the trials concluded in 5/2010. Both trials were conducted in accordance with the Declaration of Helsinki and were approved by the Institutional Review Board at each participating site. An independent Data Safety Monitoring Board provided oversight of both trials.
Measurements
In the daily trial blood pressure was measured according to facility protocol by an automated device before and after HD. Pre- and post-HD BP were averaged over one week each month. Patients in the nocturnal study kept flow sheets for all of their home treatments and brought them to the clinic once a month. Patients on home hemodialysis monitored their blood pressure initially every 30 minutes per treatment for a week and then every hour for two to three weeks. Nocturnal patients were advised to monitor their BP every hour during the night for the first month after they went home. The reason we did this was to detect if the patient had any hypotensive episodes during the night. Once the investigators were comfortable that hypotensive episodes did not occur, patients were advised to stop measuring blood pressure every hour and just measure it before and after dialysis treatment. Caregivers were trained to measure BP and look for symptoms when a patient was dialyzed at home or at night.
Pre-HD and post-HD weights were recorded during the same week each month that the BP measurements were taken. Interdialytic weight gain (IDWG) was estimated from the intradialytic weight loss, calculated as pre-HD weight minus post-HD weight per dialysis session; the average IDWG over the recorded week was used for estimating associations with changes in BP. Brain natriuretic peptide (B-type natriuretic peptide; BNP), was measured in F1 and F12.
Antihypertensive Therapy
Category and dose of prescribed antihypertensive medications were recorded at baseline and every 4 months during the course of the trials. The quantities of medications belonging to relevant drug classes were summed over four week periods. Adjustment of antihypertensive therapy was at the discretion of the attending nephrologist.
Intradialytic Hypotensive Episodes (IDHE)
We defined sessions associated with IDHE as those HD treatments during which hypotensive symptoms led to either lowering the ultrafiltration rate or to saline administration. In the nocturnal trial patients were taught to give themselves 250 to 500 mL of normal saline whenever they experienced hypotensive symptoms during the dialysis treatment. We analyzed relative rates as well as the absolute counts of sessions associated with IDHE. Incidents of sessions associated with IDHE during one week periods were recorded monthly. A maximum of one IDHE was counted per HD session.
Subgroup Analyses
We defined a priori three baseline factors that might modify the effect of frequent HD on the change in average pre-HD SBP during follow-up months 3 to 5 (F3–5) and 10 to 12 (F10–12). The primary assessment of treatment interactions with quantitative subgroup factors was based on a test for linear interaction which treated the subgroup factor as a continuous variable; estimated treatment effects are also provided for the subgroups defined by the indicated cut-offs for descriptive purposes. These three factors were a) baseline pre-HD SBP ≤ 145 or > 145 mmHg, b) baseline IDWG ≤ 3 or > 3 kg, and c) baseline urine volume (Daily Trial: ≤ 100 or >100 mL per day; Nocturnal Trial: ≤ 500 or > 500 mL per day). We also conducted a posteriori subgroup analyses in patients with baseline urinary volumes of 0, 1 – 400, and >400 mL/day. Exploratory analyses were conducted in patients whose SBP declined 30 mmHg or more from baseline to F10–12.
Statistical Methods
We summarized categorical variables using proportions and continuous variables using mean ± SD or median with 10th and 90th percentiles where data were skewed. Descriptive summaries of changes in treatment-related variables are provided for the patients with non-missing values at four time points: baseline, F2, F3–5, and F10–12.
We estimated the effects of the randomized treatment assignment on pre-HD SBP, DBP and other continuous outcomes with a mixed effects analysis that included a time interaction with the baseline value of each outcome for both trials and with clinical centers for the Daily Trial. The mixed effects analyses incorporated baseline and monthly measurements; we used a combined compound-symmetry first order autoregressive covariance matrix to account for correlations in measurements over time23. This analytic approach incorporated baseline BP measurements in cases where patients died or dropped out of the study during the follow-up period. We estimated treatment effects for the mean changes from baseline to the average values during F2, F3–5, and F10–12 with change to F10–12 representing the pre-specified measure of most interest. While not pre-specified, we conducted an additional analysis with adjustment for prescribed dialysate sodium concentration.
We obtained treatment group comparisons for the number of prescribed antihypertensive medications at F3–5 and F10–12 using exact permutation tests stratified by the baseline amounts.
For each of the three pre-specified subgroup factors, we used linear regression analyses to relate the change in pre-HD SBP to treatment assignment, the pre-specified baseline covariates, and to corresponding interaction terms. The primary assessment of treatment interactions with quantitative subgroup factors was based on a test for linear interaction which treated the subgroup factor as a continuous variable. In the Daily Trial, we present P-values for the subgroup interactions without adjustment for multiple comparisons. Due to its limited sample size, we treated all subgroup analyses in the Nocturnal Trial as exploratory without significance testing.
To assess the relationship between changes in SBP and changes in indicators of fluid status we depicted the association of changes in pre-HD SBP with changes in other factors for individual patients using scatter plots with separate nonparametric local regression curves for each treatment group24 and we also computed Spearman correlations between the changes for each group.
We report both the absolute numbers and relative rates of IDHE. Treatment-based differences in both outcomes were tested using generalized estimating equations. Analyses were not adjusted for baseline values of IDHEs or other covariates
We performed all analyses using SAS version 9.2. Two-tailed P-values <0.05 were considered statistically significant unless otherwise indicated.
Results
In the Daily Trial 245 patients were randomized to 12 months of 6× weekly HD or 3× weekly in-center HD. In the Nocturnal Trial 87 patients were randomized to 12 months of 6× weekly nocturnal HD or 3× weekly conventional predominantly home-based HD, four patients went through all of follow-up being dialyzed in-center (Table 1).
Table 1.
Baseline Characteristics
| Variables | Daily Trial | Nocturnal Trial | |||
|---|---|---|---|---|---|
|
3× weekly (N=120) |
6× weekly (N=125) |
3× weekly (N=42) |
6× weekly (N=45) |
||
| Age (years) | 52.0 ± 14.1 | 48.9 ± 13.6 | 54.0 ± 12.9 | 51.7 ± 14.4 | |
| Male | 73 (60.8%) | 78 (62.4) | 28 (66.7) | 29 (64.4) | |
| Race | |||||
| Black | 53 (44.2%) | 49 (39.2%) | 11 (26.2%) | 12 (26.7% | |
| White | 46 (38.3%) | 43 (34.4%) | 21 (50.0%) | 27 (60.0%) | |
| Native American, Aboriginal Canadian, Alaskan Native | 4 (3.3%) | 4 (3.2%) | 2 (4.8%) | 1 (2.2% | |
| Asian | 5 (4.2%) | 11 (8.8%) | 7 (16.7%) | 5 (11.1%) | |
| Native Hawaiian or other Pacific Islander | 3 (2.5%) | 1 (0.8%) | 0 (0%) | 0 (0%) | |
| Other/Mixed/Unknown | 9 (7.5%) | 17 (13.6%) | 1 (2.4%) | 0 (0%) | |
| Hispanic/Latino Ethnicity | 31 (26%) | 38 (30%) | 0 (0%) | 0 (0%) | |
| Diabetes | 50 (41.7%) | 50 (40.0%) | 18 (42.9%) | 19 (42.2%) | |
| Hypertension1 | 111 (92.5%) | 117 (93.6%) | 39 (92.9%) | 41 (91.1%) | |
| Coronary artery disease | 16 (13.3%) | 11 (8.8%) | 4 (9.5%) | 5 (11.1%) | |
| Congestive heart failure | 24 (20.0%) | 25 (20.0%) | 7 (16.7%) | 5 (11.1%) | |
| Atrial fibrillation | 9 (7.5%) | 5 (4.0%) | 0 (0.0%) | 6 (13.3%) | |
| Peripheral arterial disease | 10 (8.33%) | 15 (12.0%) | 7 (16.7%) | 8 (17.8%) | |
| Stroke | 9 (7.5%) | 9 (7.2%) | 1 (2.4%) | 1 (2.2%) | |
| COPD | 5 (4.2%) | 6 (4.8%) | 2 (4.8%) | 2 (4.4%) | |
| Body Mass Index (kg/m2) | 27.6 ± 6.8 | 27.5 ± 6.6 | 28.4 ± 7.6 | 29.8 ± 8.3 | |
| ESRD vintage (years) | |||||
| <2 (%) | 38.2 | 29.6 | 71.4 | 62.2 | |
| 2–5 (%) | 35.0 | 27.2 | 11.9 | 17.8 | |
| >5 (%) | 35.8 | 43.2 | 16.7 | 20.0 | |
| Residual urinary volume (L/day) | 0 (0, 0.54) | 0 (0, 0.60) | 0.54 (0, 1.25) | 0.40 (0, 1.33) | |
| Interdialytic weight gain (kg) | |||||
| Per session | 3.12 ± 0.93 | 3.12 ± 0.99 | 2.44 ± 1.47 | 2.27 ± 1.35 | |
| Per week | 9.22 ± 2.96 | 9.17 ± 3.00 | 7.16 ± 4.54 | 6.63 ± 3.60 | |
| Haemoglobin (g/dL) | 12.0 ± 1.2 | 11.9 ± 1.3 | 11.9 ± 1.1 | 11.6 ± 1.1 | |
| Serum sodium (mmol/L) | 138 ± 3 | 138 ± 3 | 139 ± 3 | 138 ± 3 | |
| Dialysate sodium (mmol/L) | 141 ± 3 | 139 ± 2 | 140 ± 2 | 140 ± 2 | |
| Medications use | |||||
| ESA | 111 (92.5%) | 117 (93.6%) | 37 (88.1%) | 38 (84.4%) | |
| Antihypertensives | 105 (87.5%) | 109 (87.2%) | 35 (83.3%) | 38 (84.4%) | |
| ACEI | 38 (31.7%) | 42 (33.6%) | 12 (28.6%) | 7 (15.6%) | |
| ARB | 25 (20.8%) | 30 (24.0%) | 3 (7.1%) | 9 (20.0%) | |
| Dihydropyridine CCB | 53 (44.2%) | 62 (49.6%) | 15 (35.7%) | 18 (40.0%) | |
| Non-Dihydropyridine CCB | 6 (5.0%) | 6 (4.8%) | 3 (7.1%) | 2 (4.4%) | |
| Beta blockers | 77 (64.2%) | 70 (56.0%) | 21 (50.0%) | 30 (66.7%) | |
| Peripheral alpha blockers | 4 (3.3%) | 1 (0.8%) | 4 (9.2%) | 2 (4.4%) | |
| Centrally acting agents | 24 (20.0%) | 22 (17.6%) | 3 (7.1%) | 5 (11.1%) | |
| Non-specific vasodilators | 13 (10.8%) | 22 (17.6%) | 0 (0%) | 2 (4.4%) | |
| Diuretics | 16 (13.3%) | 17 (13.6%) | 6 (14.3%) | 11 (24.4%) | |
Results are shown as mean ±standard deviation, median (10th& 90th percentiles), or frequency (%), as indicated. ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; COPD, chronic obstructive pulmonary disease; ESA, erythropoiesis stimulating agent.
Hypertension defined as average pre-dialysis systolic BP > 140/90, patient taking antihypertensive medication(s), or the diagnosis of hypertension from clinical records
Fourteen patients died in the Daily Trial (5 in 6× weekly and 9 in 3× weekly cohorts), and 3 in the Nocturnal Trial (2 in the nocturnal and 1 in the 3× weekly cohorts).
Treatment Effects on Blood Pressure Dynamics and Antihypertensive Therapy
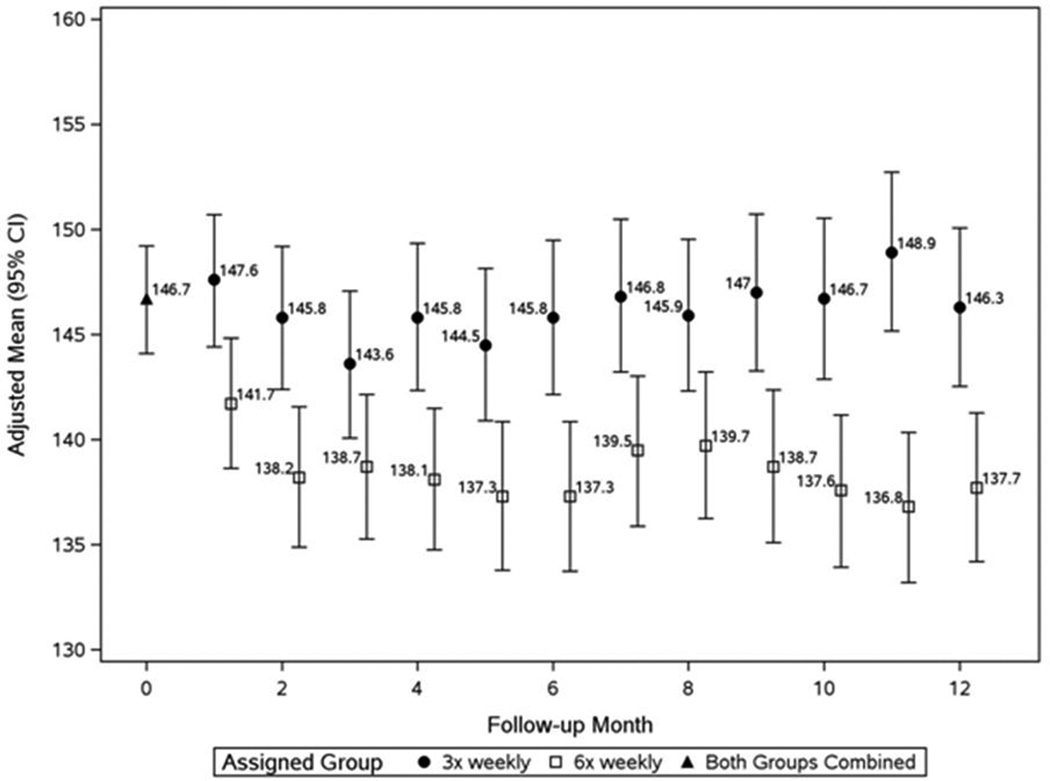
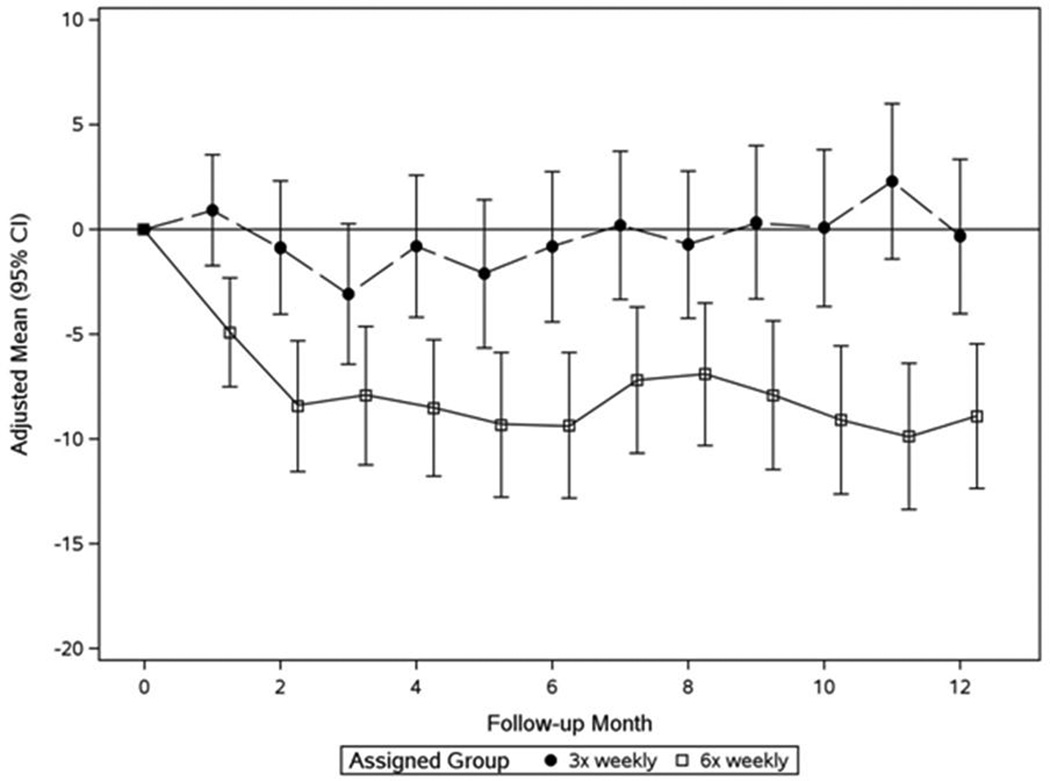
In the Daily Trial, compared to 3× weekly HD, 6× weekly HD resulted in a significant reduction of pre-HD SBP by 7.7 mmHg and DBP by 3.9 mmHg at month 2 of the trial (F2) (Figures 1a and 1b, Table 2a). The SBP reduction remained relatively stable for the subsequent 10 months; the mean reduction of pre-HD SBP at F10–12 was 10 mmHg (Figures 1a and 1b, Table 2a). Comparable dynamics were observed for pre-HD DBP, post-HD SBP, and post-HD DBP (Table 2a). Sixteen patients (7%) experienced a decline in pre-HD SBP of ≥30 mmHg between baseline and F10–12 (13 in the 6× weekly group; 3 in the 3× weekly arm, Table 3). The most prominent clinical characteristic among these patients was a relatively high baseline SBP (164±17.8 mmHg).
Figure 1.
a: Monthly pre-dialysis systolic blood pressure in the Daily Trial
Patients in the 6× weekly group (open squares) had significantly lower values than the 3× weekly group (closed circles) at F1 (P<0.001) and thereafter (mixed effects analysis adjusting for baseline value of outcome and clinical center).
b: Monthly mean changes of pre-dialysis SBP from baseline in the Daily Trial
Patients in the 6× weekly group (open squares) had significantly lower values than the 3× weekly group (closed circles) at F1 (P<0.001) and thereafter (mixed effects analysis adjusting for baseline value of outcome and clinical center).
Table 2.
| a. Daily Trial - Treatment Effects on Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Treatment | Observed Data Mean (SD)1. |
6× vs. 3× Treatment Comparison: Mean Changes from Baseline (95% CI)2. |
|||||
| Baseline | F2 | F3–5 | F10–12 | F2 | F3–5 | F10–12 | ||
| Pre-HD SBP [mmHg] | 3× weekly | 146.6 (17.8) | 145.5 (22.3) | 144.8 (17.5) | 147.3 (18.5) | −7.7 (−11.9, −3.5)3 | −6.6 (−10.3,−2.9)3 | −10.0 (−13.9, −6.0)3 |
| 6× weekly | 147.0 (18.9) | 138.7 (20.7) | 137.7 (19.7) | 137.4 (20.5) | ||||
| Pre-HD DBP [mmHg] | 3× weekly | 78.2 (11.6) | 79.4 (13.6) | 78.2 (12.6) | 79.6 (12.0) | −3.9 (−6.5, −1.3)2 | −3.4 (−5.6, −1.2)2 | −5.1 (−7.4, −2.8)3 |
| 6× weekly | 81.5 (11.7) | 76.7 (11.8) | 76.5 (11.7) | 76.0 (11.5) | ||||
| Post-HD SBP [mmHg] | 3× weekly | 134.6 (18.3) | 135.8 (22.7) | 133.6 (19.4) | 134.2 (18.9) | −8.7 (−12.9, −4.6)3 | −8.4 (−12.0, −4.8)3 | −7.9 (−11.8, −3.9)3 |
| 6× weekly | 132.6 (19.0) | 125.7 (17.2) | 124.4 (15.9) | 125.2 (16.0) | ||||
| Post-HD DBP [mmHg] | 3× weekly | 71.9 (11.1) | 73.4 (12.4) | 76.5 (10.4) | 74.1 (12.1) | −3.4 (−5.9, −1.0)2 | −3.1 (−5.2, −1.1)2 | −3.4 (−5.6, −1.2)2 |
| 6× weekly | 72.9 (11.7) | 70.2 (10.0) | 73.9 (13.1) | 72.4 (13.4) | ||||
| Pre-HD Weight [kg] | 3× weekly | 81.8 (20.3) | 82.1 (20.7) | 81.9 (20.5) | 82.0 (20.4) | −1.6 (−2.2, −1.1)3 | −1.3 (−2.0,−0.6)3 | −0.2 (−1.1, 0.7) |
| 6× weekly | 80.2 (21.3) | 78.9 (21.4) | 79.3 (21.5) | 80.3 (21.5) | ||||
| Post-HD Weight [kg] | 3× weekly | 78.9 (19.8) | 79.5 (20.3) | 79.1 (19.9) | 79.2 (19.9) | −0.6 (−1.1, −0.1)1 | −0.36 (−1.07, 0.34) | 0.79 (−0.1, 1.68) |
| 6× weekly | 77.0 (20.8) | 78.0 (20.5) | 77.1 (21.2) | 78.2 (21.2) | ||||
| Interdialytic Weight Gain [kg] | 3× weekly | 3.14 (0.96) | 3.15 (1.11) | 3.11 (1.08) | 3.10 (1.04) | −1.1 (−1.2, −0.9)3 | −0.92 (−1.08, −.76)3 | −1.0 (−1.1, −0.8)3 |
| 6× weekly | 3.16 (0.99) | 2.08 (0.89) | 2.15 (0.77) | 2.11 (0.86) | ||||
| Ultrafiltration Rate (mL/min) | 3× weekly | 14.8 (4.1) | 14.6 (4.6) | 14.6 (4.7) | 14.5 (4.3) | −1.1 (−2.1, −0.1)1 | −0.4 (−1.3, 0.4) | −0.6 (−1.5, 0.3) |
| 6× weekly | 15.0 (5.5) | 13.7 (4.6) | 14.3 (4.1) | 13.9 (4.7) | ||||
| Treatment Time (min / session) | 3× weekly | 214 (27.8) | 213 (29.9) | 215 (31.5) | 217 (30.3) | −64.6 (−69.1, −60.2)3 | −63.2 (−67.9, −58.6)3 | −63.8 (−68.7, −58.9)3 |
| 6× weekly | 219 (27.2) | 150 (24.5) | 153 (26.6) | 154 (29.5) | ||||
| Number of Prescribed Anti-hypertensive Drugs [per patient] | 3× weekly | 2.3 (1.4) | -- | 2.1 (1.4) | 2.0 (1.4) | -- | −0.38 (−0.70 to −0. 08)1 | −0.36 (−0.65 to −0.08)1 |
| 6× weekly | 2.2 (1.6) | -- | 1.6 (1.5) | 1.4 (1.3) | ||||
| b. Nocturnal Trial - Treatment Effects on Outcome Variables | ||||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Treatment | Observed Data (Mean ± SD)1. |
6× vs. 3× Treatment Comparison: Mean Changes from Baseline (95% CI)2. |
|||||
| Baseline | F2 | F3–5 | F10–12 | F2 | F3–5 | F10–12 | ||
| Pre-HD SBP [mmHg] | 3× weekly | 152.6 (22.2) | 155.7 (23.4) | 155.0 (20.6) | 150.7 (18.6) | −7.3 (−14.2,−0.3)1 | −9.4 (−15.5, −3.2)2 | −8.0 (−14.5, −1.6)1 |
| 6× weekly | 144.9 (13.7) | 141.7 (22.8) | 140.1 (18.9) | 137.0 (20.5) | ||||
| Pre-HD DBP [mmHg] | 3× weekly | 82.9 (13.9) | 84.4 (13.1) | 83.8 (11.8) | 83.0 (12.7) | −4.2 (−8.3, −0.1)1 | −4.9 (−8.5, −1.3)2 | −4.5 (−8.3, −0.7)1 |
| 6× weekly | 79.5 (11.0) | 77.8 (13.6) | 76.7 (11.5) | 76.0 (13.9) | ||||
| Post-HD SBP [mmHg] | 3× weekly | 139.2 (21.2) | 140.8 (22.2) | 140.4 (21.6) | 132.3 (2.8) | −1.2 (−9.0, 6.5) | −6.3 (−13.0, 0.5) | −2.0 (−9.2, 5.2) |
| 6× weekly | 136.1 (18.5) | 137.6 (25.5) | 133.0 (22.6) | 129.3 (20.3) | ||||
| Post-HD DBP [mmHg] | 3× weekly | 76.3 (14.9) | 78.4 (13.3) | 76.5 (10.4) | 74.1 (12.1) | −1.4 (−5.7, 2.9) | −2.4 (−6.1, 1.3) | −1.3 (−5.2, 2.6) |
| 6× weekly | 75.1 (11.9) | 77.0 (14.4) | 73.9 (13.1) | 72.4 (13.4) | ||||
| Pre-HD Weight [kg] | 3× weekly | 85.7 (25.1) | 85.3 (24.9) | 85.7 (25.7) | 86.6 (26.3) | −0.5 (−1.5, 0.5) | −0.4 (−2.0, 1.1) | 0.2 (−2.4, 2.9) |
| 6× weekly | 90.8 (29.0) | 89.0 (29.2) | 89.7 (28.8) | 91.1 (28.9) | ||||
| Post-HD Weight [kg] | 3× weekly | 83.5 (24.1) | 82.9 (24.4) | 83.3 (25.0) | 84.1 (25.6) | 0.1 (−0.9, 1.0) | 0.0 (−1.4, 1.4) | 0.6 (−1.9, 3.1) |
| 6× weekly | 88.6 (28.2) | 88.6 (29.0) | 87.8 (28.6) | 89.1 (28.6) | ||||
| Interdialytic Weight Gain [kg] | 3× weekly | 2.42 (1.24) | 2.36 (1.24) | 2.43 (1.14) | 2.55 (1.02) | −0.6 (−1.0, −0.3)3 | −0.44 (−0.76, −0.17)2 | −0.46 (−0.77, −0.15)2 |
| 6× weekly | 1.72 (0.77) | 1.69 (0.82) | 1.91 (0.65) | 2.04 (0.87) | ||||
| Ultrafiltration Rate (mL/min) | 3× weekly | 10.9 (6.2) | 9.2 (4.1) | 9.6 (4.02) | 10.4 (3.8) | −4.2 (−5.7, −2.7)3 | −3.9 (−5.2, −2.7)3 | −4.1 (−5.4, −2.8)3 |
| 6× weekly | 10.1 (6.4) | 4.7 (2.8) | 5.6 (2.9) | 6.0 (3.63) | ||||
| Treatment Time (min / session) | 3× weekly | 229 (32.9) | 261 (78.2) | 260 (79.7) | 258 (74.8) | 120 (96.4, 143.6)3 | 128 (106, 149)3 | 112 (89, 134)3 |
| 6× weekly | 230 (31.9) | 381 (69.4) | 382 (60.7) | 372 (85.1) | ||||
| Number of Prescribed Anti-hypertensive Drugs [per patient] | 3× weekly | 1.6 (1.1) | -- | 1.6 (1.2) | 1.6 (1.2) | -- | −0.46 (−0.98 to −0.004)1 | −0.44 (−0.89 to −0.03)1 |
| 6× weekly | 1.9 (1.3) | -- | 1.3 (1.6) | 1.1 (1.6) | ||||
For patients with non-missing baseline, 4 month and 12 month values (3× weekly N=92–93, 6× weekly N=103–104)
Results controlling for baseline value and clinical center
P < 0.05,
P < 0.01,
P < 0.001
For patients with non-missing baseline, 4 month and 12 month values (3× weekly N=39, 6× weekly N=41)
Results controlling for baseline value.
P < 0.05,
P < 0.01,
P < 0.001
Table 3.
Daily Trial – Demographic and clinical characteristics for subjects with 12-month SBP declines of at least 30 mmHg. Changes (Δ) are calculated as F10–12 minus baseline (BL).
| Pat # | Arm | Pre-HD SBP [mm Hg] |
# Antihyper- tensive drugs |
Age (yr) |
Vintage (yr) |
IDWG [kg] |
Pre-HD Weight [kg] |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | Δ | BL | Δ | BL | Δ | BL | Δ | ||||
| 1 | 6× weekly | 191 | −63.9 | 3 | 0 | 45.8 | 3.2 | 2.4 | −0.94 | 73.5 | 2.0 |
| 2 | 3× weekly | 196 | −49.8 | 1 | 1 | 53 | 4.9 | 5.2 | −1.76 | 103.4 | −9.1 |
| 3 | 6× weekly | 177 | −49.6 | 4 | −4 | 55.2 | 6.7 | 4.4 | −1.86 | 95.9 | 2.3 |
| 4 | 6× weekly | 160 | −42.8 | 4 | 0 | 41.9 | 6.0 | 5.2 | −1.97 | 79.3 | −2.2 |
| 5 | 6× weekly | 155 | −41.4 | 1 | −1 | 34.3 | 8.1 | 4.4 | −3.77 | 76.2 | −8.8 |
| 6 | 6× weekly | 171 | −40.4 | 1 | 0 | 79.6 | 1.7 | 3.0 | −1.99 | 70.1 | 1.6 |
| 7 | 6× weekly | 164 | −37.0 | 3 | −2 | 41.5 | 7.2 | 3.6 | −1.31 | 75.7 | 2.5 |
| 8 | 6× weekly | 157 | −34.6 | 4 | −2 | 35.6 | 4.0 | 1.4 | −0.82 | 103.9 | 2.5 |
| 9 | 6× weekly | 130 | −33.7 | 2 | −2 | 59.7 | 6.6 | 2.2 | −0.76 | 45.1 | −0.1 |
| 10 | 6× weekly | 190 | −32.9 | 0 | 0 | 64.2 | 3.8 | 3.2 | −0.81 | 96.1 | −2.6 |
| 11 | 6× weekly | 168 | −32.5 | 2 | −1 | 58.8 | 1.0 | 3.4 | −1.20 | 79.2 | 1.5 |
| 12 | 3× weekly | 160 | −30.9 | 0 | 1 | 37 | 3.0 | 4.8 | −0.05 | 101.5 | −2.3 |
| 13 | 6× weekly | 159 | −30.7 | 3 | −2 | 42.4 | 5.2 | 4.2 | −1.71 | 106.9 | −0.9 |
| 14 | 6× weekly | 152 | −30.7 | 0 | 0 | 53.9 | 3.6 | 1.8 | 0.79 | 71.1 | 8.2 |
| 15 | 6× weekly | 148 | −30.2 | 6 | −4 | 51.1 | 19.3 | 2.4 | −0.77 | 92.9 | −4.3 |
| 16 | 6× weekly | 146 | −30.1 | 1 | −1 | 77.4 | 0.6 | 2.8 | −1.46 | 75.6 | −2.6 |
| Remainder 3× weekly 1 | 145 [17.1] | 1.6 [15.3] | 2.2 [1.4] | −0.3 [1.1] | 52.2 [14.1] | 5.1 [5.3] | 3.1 [0.91] | −0.02 [0.76] | 81.5 [20.9] | 0.37 [3.21] | |
| Remainder 6× weekly 1 | 145 [17.8] | −5.3 [15.0] | 2.2 [1.6] | −0.7 [1.6] | 48.3 [13.5] | 6.4 [6.9] | 3.1 [0.99] | −1.01 [0.81] | 80.7 [21.6] | 0.14 [4.00] | |
Statistics for subjects with values at both baseline and F10–12.
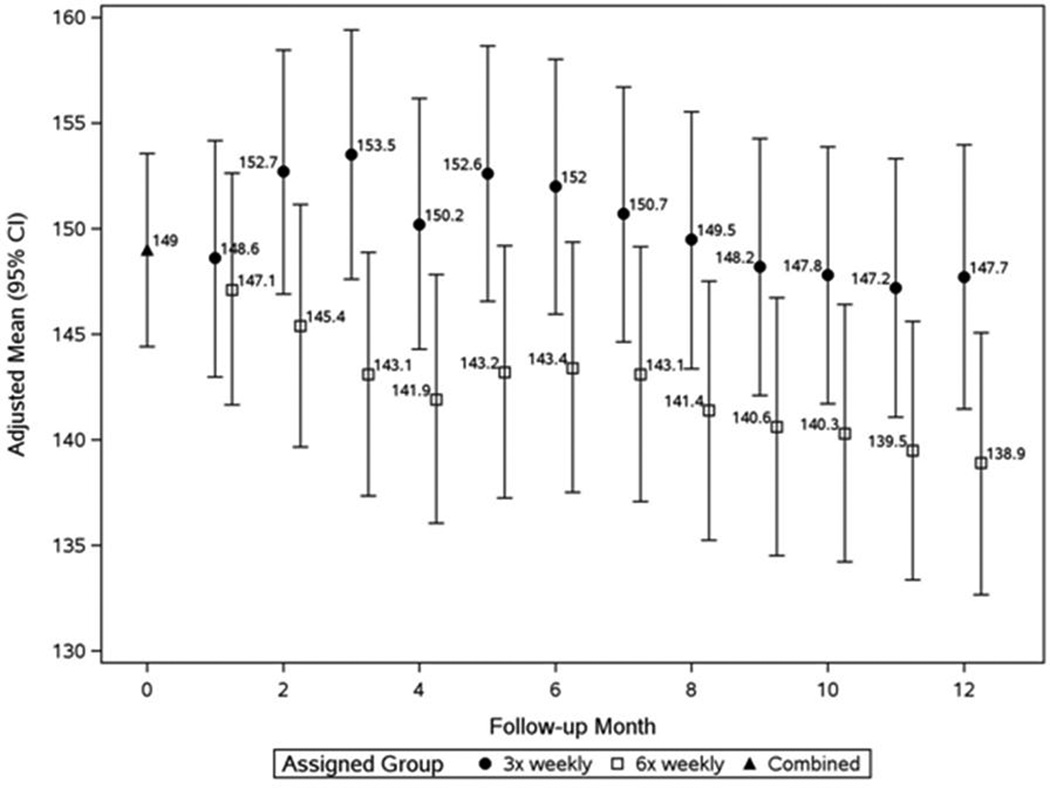
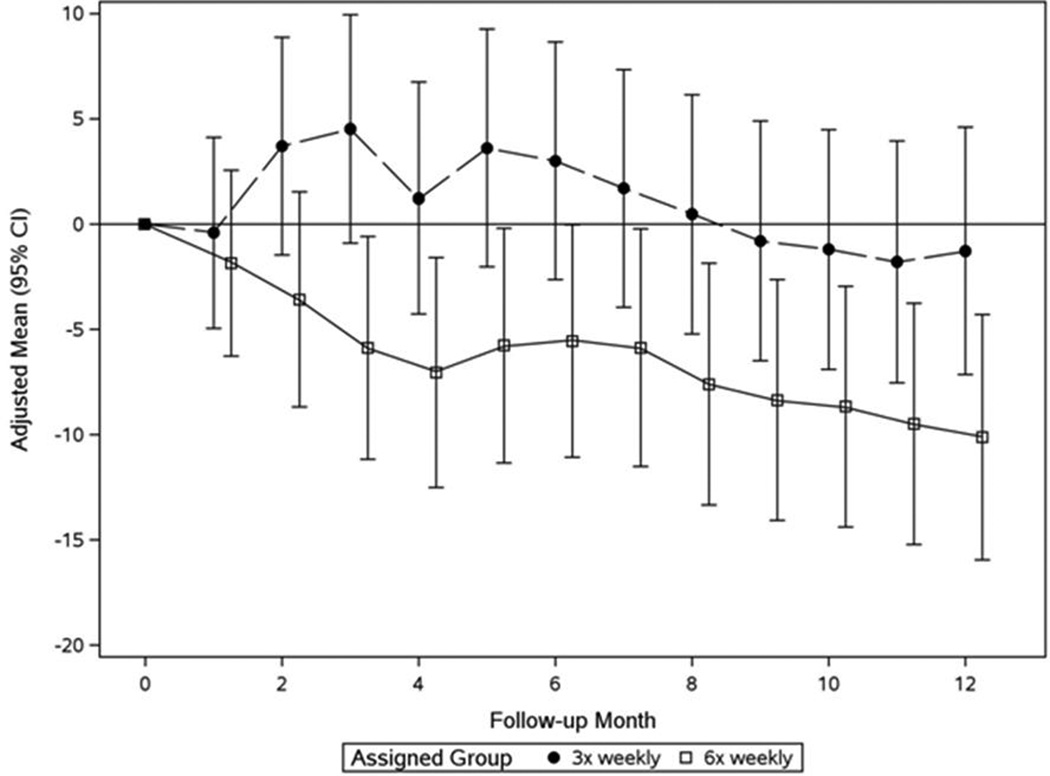
In the Nocturnal Trial at F2, the pre-HD SBP treatment effect was 7.3 mmHg and 4.2 mmHg for pre-HD DBP. These relative BP changes remained stable thereafter (Figures 2a and 2b, Table 2b). No significant treatment effects were observed on post-HD SBP and DBP (Table 2b). In 7 subjects (8%) pre-HD SBP fell by ≥30 mmHg between baseline and F10–12 (4 in the 6× weekly nocturnal arm; 3 in the conventional arm, Table 4). Older age (60.6±13.3 years) and a higher pre-HD SBP (166±14.7 mmHg) at baseline distinguished these patients.
Figure 2.
a: Monthly pre-dialysis SBP in the Nocturnal Trial
Patients in the 6× weekly group (open squares) had significantly lower values than the 3× weekly group (closed circles) at F3–5 (P=0.003) and thereafter (mixed effects analysis adjusting for baseline value of outcome).
b: Monthly mean changes of pre-dialysis SBP from baseline in the Nocturnal Trial
Patients in the 6× weekly group (open squares) had significantly lower values than the 3× weekly group (closed circles) at F3–5 (P=0.003) and thereafter (mixed effects analysis adjusting for baseline value of outcome).
Table 4.
Nocturnal Trial - Demographic and clinical characteristics for subjects with 12-month SBP declines of at least 30 mmHg. Changes (Δ) are calculated as F10–12 minus baseline (BL).
| Pat # | Arm | Pre-HD SBP [mm Hg] |
# Antihyper- tensive drugs |
Age (yr) |
Vintage (yr) |
IDWG [kg] |
Pre-HD Weight [kg] |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | Δ | BL | Δ | BL | Δ | BL | Δ | ||||
| 1 | 6× weekly | 155 | −43.1 | 2 | −2 | 66.7 | 19.0 | 1.8 | −0.55 | 89.5 | −0.3 |
| 2 | 6× weekly | 160 | −41.9 | 2 | −2 | 77.9 | 2.4 | 2.6 | −1.27 | 90.6 | −6.7 |
| 3 | 6× weekly | 156 | −40.7 | 1 | 0 | 48.4 | 0.9 | 2.8 | −1.89 | 94.0 | −0.6 |
| 4 | 3× weekly | 194 | −37.7 | 1 | 2 | 76.4 | 1.3 | 2.2 | 0.49 | 87.5 | −1.3 |
| 5 | 3× weekly | 177 | −34.2 | 2 | 2 | 51.3 | 0.6 | 3.0 | −1.06 | 56.2 | 1.1 |
| 6 | 6× weekly | 154 | −33.8 | 3 | −3 | 58.4 | 0.6 | 3.0 | −0.76 | 103.0 | −12.4 |
| 7 | 3× weekly | 166 | −33.4 | 3 | −3 | 45.1 | 3.6 | 2.0 | 0.13 | 97.3 | −2.7 |
| Remainder 3× weekly 1 | 151 [21.4] | 0.9 [13.2] | 1.6 [1.1] | 0.0 [1.0] | 53.8 [12.8] | 3.4 [7.5] | 2.4 [1.5] | 0.2 [1.1] | 86.1 [25.1] | 0.9 [7.2] | |
| Remainder 6× weekly 1 | 144 [13.5] | −4.1 [15.5] | 1.9 [1.3] | −0.8 [1.7] | 50.6 [14.2] | 3.3 [4.9] | 2.2 [1.4] | −0.1 [1.5] | 89.5 [29.0] | 0.9 [6.4] | |
Statistics for subjects with values at both baseline and F10–12.
The number of prescribed antihypertensive drugs was significantly lower among 6× weekly subjects by F3–5 both in the Daily Trial (−0.38 [95%CI: −0.70 to −0.008]; Table 2a) and the Nocturnal Trial (−0.46 [95%CI: −0.98 to −0.004]; Table 2b).
We also conducted a not pre-specified analysis with adjustment for prescribed dialysate sodium concentrations. This additional analysis indicated a significant treatment effect for pre-HD SBP in the Daily Trial, but not in the Nocturnal Trial (Supplemental Table 1).
Treatment Effects on Indicators of Fluid Volume
In the Daily Trial, in the 6× weekly group post HD weight at F2 was relatively lower compared to the 3× weekly control group. The difference was not significant later in the trial (Table 2a). In the Nocturnal Trial, there was no treatment effect on post HD weight at any time point (Table 2b). In contrast, pre-HD weight decreased by 1.3 to 1.6 kg more among Daily 6× weekly subjects in the first 2–5 months compared to conventional HD (P<0.001). However, by F10–12, this difference was no longer significant (Table 2a). In the Nocturnal Trial, there were no significant changes in either the pre- or the post-HD weights by treatment group (Table 2b).
In the Daily Trial, in both treatment groups changes in IDWG from baseline remained remarkably constant throughout the study (3× weekly: ~3.1 kg; 6× weekly: ~1.0 kg) (Table 2a). In the Nocturnal Trial the overall IDWG was ~2.4 kg in the 3× weekly group and ~ 1.9 kg in the 6× weekly group with a significant difference of ~ 0.5 kg between the two treatment arms from F2 onwards (Table 2b).
Intradialytic Hypotensive Episodes (IDHE) (Table 5)
Table 5.
Frequency of sessions associated with intradialytic hypotensive episodes (IDHE)
| Trial | Type of IDHE | Count of Sampled Dialysis Sessions 1 with and without IDHE |
Relative Risk (95% CI)** |
P-value 2 | |
|---|---|---|---|---|---|
| 3× weekly Group |
6× weekly Group |
||||
| Daily | No symptoms of hypotension | 2973 | 5945 | ||
| 1) Symptoms of hypotension led to lowering of UF rate or reduced blood flow | 218 | 293 | 1.26 (0.89, 1.77) |
0.18 | |
| 2a) Symptoms of hypotension led to administration of saline | 129 | 209 | 1.53 (1.11, 2.09) | 0.0086 | |
| 2b) Symptoms of hypotension led to lowering of UF rate and administration of saline. | 120 | 220 | |||
| Nocturnal | No symptoms of hypotension | 1261 | 2159 | ||
| 1) Symptoms of hypotension led to lowering of UF rate or reduced blood flow | 43 | 38 | 0.85 (0.33, 2.17) | 0.74 | |
| 2a) Symptoms of hypotension led to administration of saline | 51 | 11 | 0.35 (0.18, 0.69) |
0.0024 | |
| 2b) Symptoms of hypotension led to lowering of UF rate and administration of saline. | 37 | 19 | |||
Dialysis session data recorded over periods of one week each month.
Top relative risk statistics and P-values reflect tests for whether patients in one treatment group were more likely to experience sessions associated with IDHEs which were treated by reducing the UF rate only (IDHE category 1). Bottom statistics reflect tests for whether patients in one treatment group were more likely to experience sessions associated with IDHEs which were treated with saline (IDHE categories 2a & 2b).
In the Daily Trial there was a trend for a lower percentage of sessions associated with IDHEs relative to the number of sampled HD sessions in the 6× weekly group compared to the 3× weekly group (P=0.056). However, with more HD sessions in the 6× weekly group, the absolute number of sessions associated with IDHE episodes requiring saline administration was also greater resulting in a relative risk of 1.53 (95%CI: 1.11 to 2.09) for 6× weekly patients in the Daily Trial.
In the Nocturnal Trial the relative frequency of sessions associated with IDHE was significantly lower in the 6× weekly group (P<0.001). The relative risk for sessions associated with IDHE requiring saline administration for 6× weekly subjects was 0.35 (95%CI: 0.18 to 0.69) compared to 3× weekly subjects.
Baseline Predictors of pre-HD SBP Response to Frequent Dialysis
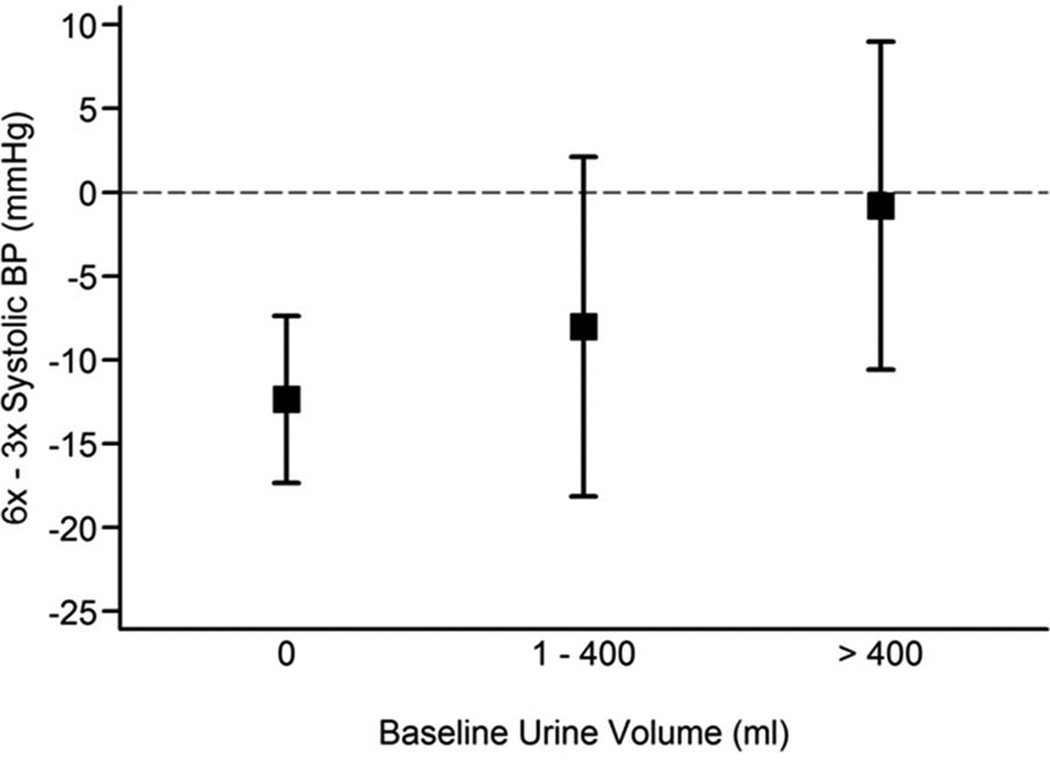
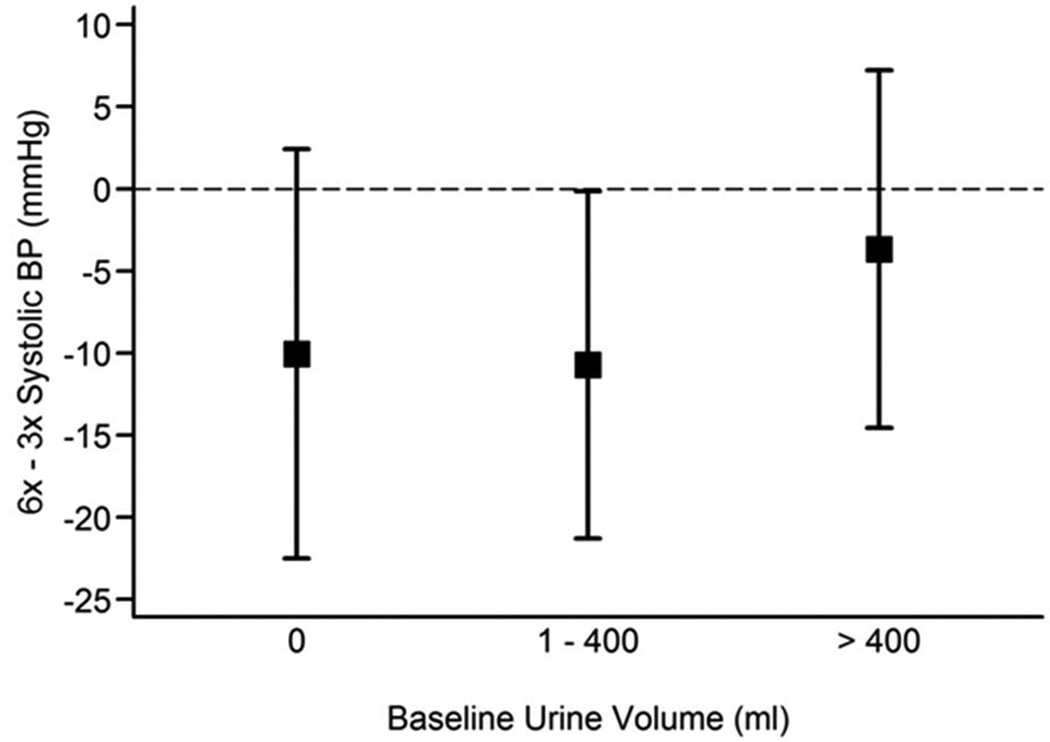
Neither baseline pre-HD SBP nor the baseline interdialytic weight gain (IDWG) significantly influenced the treatment effect on BP in both trials (data not shown). However, the effect of frequent HD on pre-HD SBP was significantly greater among Daily Trial patients who were anuric at baseline, when compared to those who produced any urine (interaction P = 0.05) (Figure 3). A comparable modification of urine volume on the treatment effect was not seen in the Nocturnal Trial (interaction P = 0.73) (Figure 4).
Figure 3. Estimated treatment effect on pre-dialysis SBP by magnitude of daily urine volume at baseline in the Daily Trial.
Tests were performed on the continuous form of urine volume but results were represented using urine volume categories, simply for illustrative purposes. Patients who had no urine output at baseline had a greater reduction in SBP in the frequent dialysis group compared to the conventional 3× weekly group (P for interaction = 0.05).
Figure 4. Estimated treatment effect on pre-dialysis systolic BP by magnitude of daily urine volume at baseline in the Nocturnal Trial.
Tests were performed on the continuous form of urine volume but results were represented using urine volume categories, simply for illustrative purposes. Urine output at baseline had no modifying effect on the reduction in SBP in the frequent dialysis group compared to the conventional 3× weekly group.
Correlational Analyses
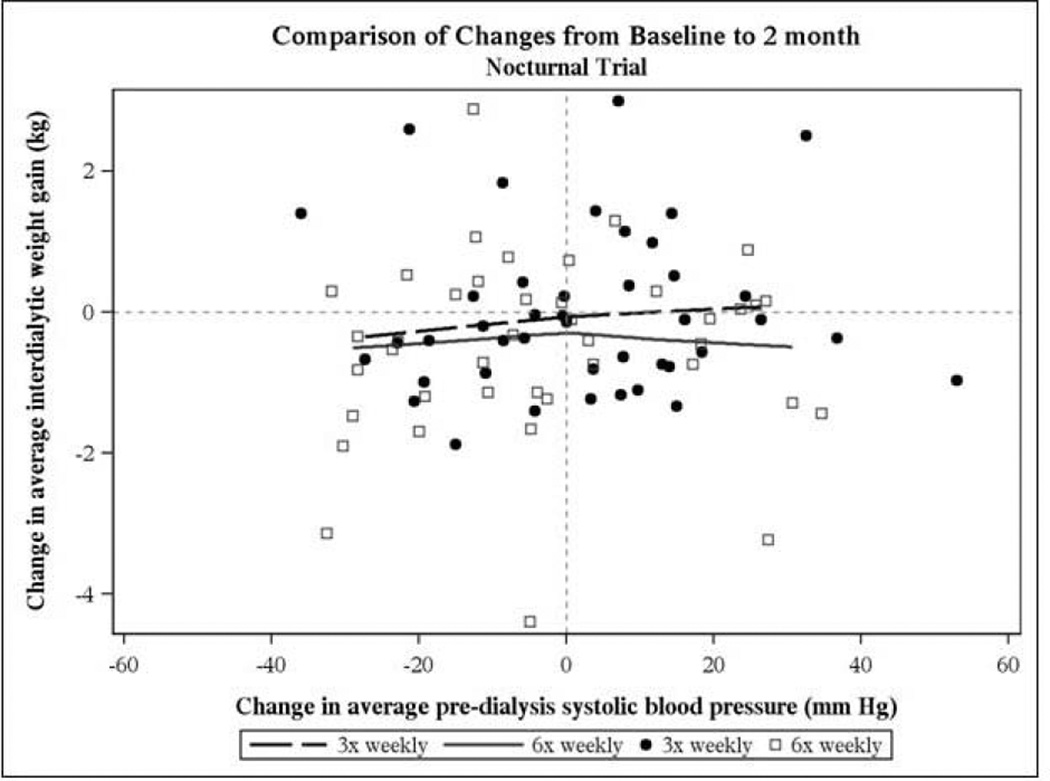
In the Daily Trial changes in pre-HD SBP between baseline and F2 were modestly correlated with changes in IDWG in the 3× weekly group (Spearman rho = 0.21, P=0.035), but not in the 6× weekly groups (Figure 5). This lack of correlation was also true at F10–12. In the Nocturnal Trial, there was no correlation between the change in pre-HD SBP and the change in IDWG in F2 (Figure 6).There was also no correlation between the change in post-HD weight and the change in pre-HD BP in either trial (data not shown). In both trials changes in BNP between F1 and F12 and changes in pre-HD SBP were not correlated (data not shown).
Figure 5. Daily Trial.
Association between change in pre-dialysis systolic BP and change in interdialytic weight gain between baseline and F2. There was a modest correlation in the 3× weekly group (Spearman rho = 0.21, P=0.035), but not in the 6× weekly group
Figure 6. Nocturnal Trial.
Association between change in pre-HD systolic BP and change in interdialytic weight gain between baseline and F2. There was no statistically significant correlation in either the 3× weekly (closed circles) and 6× weekly (open squares) groups.
Discussion
Frequent HD reduces BP and the number of prescribed antihypertensive medications whether it is given in shorter duration daily or in longer nocturnal sessions. Our results are comparable to those seen in observational studies and randomized trials of daily in-center HD, in which reductions in pre-HD SBP of 7 to 23 mmHg and in DBP of 4 to 12 mmHg have been reported5–13,16. In addition to greater declines in BP, patients who received frequent HD were also prescribed significantly fewer antihypertensive medications, a finding in keeping with previous studies5–8,11,17.
BP dynamics appeared to differ slightly between daily and nocturnal 6× weekly HD (Table 2a, 2b). We speculate that shorter dialysis vintage and larger urine volumes in the Nocturnal Trial may have attenuated the impact of frequent dialysis compared to the Daily trial. Subgroup analysis in the Daily Trial indicated a significant interaction between daily urine volume at baseline and BP response, so that anuric patients had greatest relative decline in BP with frequent HD. These findings suggest that the larger residual urinary volume in the Nocturnal Trial at baseline may have tempered BP lowering in this group.
Intradialytic hypotensive episodes (IDHE) are a major concern for HD patients. While in patients in the frequent HD arms of both FHN trials pre-HD SBP and DBP were lower than at baseline, there was a lower percentage of sessions associated with IDHE relative to the total number of sampled HD sessions in the 6× weekly groups compared to the 3× weekly groups in both trials. The reasons for the lower rates of sessions associated with IDHE are not completely clear, but the lower ultrafiltration rate observed with more frequent HD is a reasonable possibility (Table 2a, 2b). However, while the relative frequency of sessions associated with IDHE decreased in the 6× weekly group in the Daily Trial, the absolute number of IDHE per patient over a given time increased, resulting in a significantly higher relative risk for sessions associated with IDHE requiring the administration of saline (Table 5). In the Nocturnal Trial the relative risk of sessions associated with IDHE was decreased in the 6× weekly arm. The smaller number of sessions associated with IDHE among 6× weekly patients in the Nocturnal Trial may be related to differences in monitoring; it is conceivable that in a sleeping patient it would be less likely that ultrafiltration rate would be adjusted or saline be given.
While these trials were not designed to study the mechanisms responsible for BP reductions with more frequent HD, we speculate that several pathways may be responsible. A reduction in interdialytic fluid gain with 6× weekly dialysis may lead to a reduction in BP. Our data appear to support prior studies that frequent dialysis reduced predialysis BP and the number of antihypertensive agents. Blood pressure rises in the interdialytic period at a rate that depends on the interdialytic weight gain. This has been confirmed using ambulatory and home BP measurements. Patients who had daily dialysis had weight excursions in a narrower range than those in conventional dialysis group, and in both trials the weekly average IDWG was lower in the frequent HD arms. Thus, one could hypothesize that reduction in BP may simply reflect lower weight excursions, lower interdialytic weight gain, and therefore lower predialysis blood pressure. In order to further explore the association between changes in IDWG and pre-HD SBP, we analyzed this in individual patients in each group. We focused on the changes 2 months after randomization, reasoning that these earlier changes would be less confounded by other factors such as physiological adjustments to a lower BP, changing medications, provider adjustments to the prescribed target weight, differences in diet, and changes in body composition that might occur later in the trials. When the relationship between change in IDWG and change in pre-HD SBP was examined at the individual patient level, the association was modest in the 3× weekly arm in the Daily Trial (Spearman rho = 0.21, P=0.035) and not significant in the 6× weekly groups in both trials (Figures 5 and 6). In addition, correlational analysis of changes in pre-HD SBP and changes in BNP, an indicator of volume status, showed no relationship in both trials. These results call into question a direct effect of interdialytic fluid intake on pre-HD SBP. While not excluding the role of extracellular volume differences, other factors, including reduced sympathetic tone25, improved endothelial function, and more efficient removal of pressor substances26 may play a role in the decline in BP with frequent HD. In a not pre-specified analysis we adjusted for the effect of prescribed dialysate levels on pre-HD SBP. In this analysis the treatment effect of frequent HD on pre-HD SBP remained significant in the Daily but not in the Nocturnal Trial. Notwithstanding the fact that no data on actually delivered dialysate sodium concentrations is available, we believe that this observation deserves future research into the relationship between actually delivered dialysate sodium and blood pressure.
The Daily and Nocturnal Trials are the largest published randomized trials of frequent HD, permitting robust evaluations of these interventions on BP. However, there are important limitations to the data. First, actual antihypertensive drug dose was not recorded; only the prescribed antihypertensive drug dose could be relied on as an indicator of antihypertensive therapy. Secondly, despite randomization in the Nocturnal Trial, average pre-HD SBP at baseline was 7.7 mmHg higher in the 3× weekly arm (152.6±22.2 vs. 144.9±13.7 mmHg; Table 2b). This baseline difference would be expected to favor a more pronounced BP decline in the 3× weekly arm. However, BP reduction was actually more pronounced in the 6× weekly nocturnal arm. Thirdly, we did not rigorously standardize methods for measuring BP and IDHE. However this limitation should have been mitigated by our focus on changes over time since the center-specific modes of BP measurement probably remained fixed during the study; in addition, any differences would have been expected to be balanced by randomization. Fourth, ambulatory BP monitoring was not performed in either trial and home blood pressure measurements were required only at baseline but not at the end of study. Ambulatory BP monitoring would have facilitated to investigate in greater detail the time course and mechanistic concepts related to the BP dynamics. Fifth, there is a potential for ascertainment bias, because of the unmasked design. However, it is almost inconceivable that an intervention such as frequent hemodialysis can be delivered in a masked fashion. Finally, additional measures of extracellular (e.g. bioimpedance spectroscopy) and / or intravascular volume (e.g. diameter of inferior vena cava) would be very useful. Unfortunately, measurements of fluid status by means of bioimpedance spectroscopy are available only in certain selected patient subsets. In conclusion, compared to 3× weekly HD, 6× weekly HD produced a comparable reduction of BP in both the Daily and Nocturnal Trials, indicating that frequent HD reduces BP in both frequent in-center HD and with frequent nocturnal HD sessions at home. The BP reduction was accompanied by fewer prescribed antihypertensive drugs. Favorable reductions in BP should be considered when balancing the relative risks and benefits of frequent HD in selected patients with ESRD.
Supplementary Material
Acknowledgment
The authors wish to dedicate this manuscript to Dr. John Stokes, our colleague and collaborator who untimely left us. John’s insight, focus and scholarship served as a guiding light for us all. The whole FHN family is forever indebted to Dr. Stokes, and we will miss his keen observations, his dedication and his friendship.
Source of Funding
Supported by the National Institutes of Health (NIH), National Institutes of Diabetes and Digestive and Kidney Diseases, the Center for Medicare and Medical Services, and the NIH Research Foundation. Contributors to the NIH Foundation in support of the FHN trials included Amgen, Baxter, and Dialysis Clinics. Additional support was provided by DaVita, Dialysis Clinics, Fresenius Medical Care, Renal Advantage, Renal Research Institute, and Satellite Healthcare.
Footnotes
Conflict of Interest
Drs. Peter Kotanko and Nathan W. Levin are full time employees of the Renal Research Institute, a wholly owned subsidiary of Fresenius Medical Care Holdings, Inc. which may be considered an entity with a financial interest in providing dialysis services and devices; Drs. Peter Kotanko and Nathan W. Levin hold stock in Fresenius Medical Care.
The other authors declare no conflict of interest.
References
- 1.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG. Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med. 2003;115(4):291–297. doi: 10.1016/s0002-9343(03)00366-8. [DOI] [PubMed] [Google Scholar]
- 2.Levin NW, Kotanko P, Eckardt KU, Kasiske BL, Chazot C, Cheung AK, et al. Blood pressure in chronic kidney disease stage 5D-report from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2010;77(4):273–284. doi: 10.1038/ki.2009.469. [DOI] [PubMed] [Google Scholar]
- 3.Heerspink HJ, Ninomiya T, Zoungas S, de Zeeuw D, Grobbee DE, Jardine MJ, et al. Effect of lowering blood pressure on cardiovascular events and mortality in patients on dialysis: a systematic review and meta-analysis of randomised controlled trials. Lancet. 2009;373:1009–1015. doi: 10.1016/S0140-6736(09)60212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agarwal R, Sinha AD. Cardiovascular protection with antihypertensive drugs in dialysis patients: systematic review and meta-analysis. Hypertension. 2009;53(5):860–866. doi: 10.1161/HYPERTENSIONAHA.108.128116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woods JD, Port FK, Orzol S, Buoncristiani U, Young E, Wolfe RA, et al. Clinical and biochemical correlates of starting "daily" hemodialysis. Kidney Int. 1999;55(6):2467–2476. doi: 10.1046/j.1523-1755.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- 6.Nesrallah G, Suri R, Moist L, Kortas C, Lindsay RM. Volume control and blood pressure management in patients undergoing quotidian hemodialysis. Am J Kidney Dis. 2003;42(1 Suppl):13–17. doi: 10.1016/s0272-6386(03)00532-8. [DOI] [PubMed] [Google Scholar]
- 7.Ting GO, Kjellstrand C, Freitas T, Carrie BJ, Zarghamee S. Long-term study of high-comorbidity ESRD patients converted from conventional to short daily hemodialysis. Am J Kidney Dis. 2003;42(5):1020–1035. doi: 10.1016/j.ajkd.2003.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Traeger J, Galland R, Delawari E, Arkouche W, Hadden R. Six years' experience with short daily hemodialysis: do the early improvements persist in the mid and long term? Hemodial Int. 2004;8(2):151–158. doi: 10.1111/j.1492-7535.2004.01089.x. [DOI] [PubMed] [Google Scholar]
- 9.Fagugli RM, Buoncristiani U, Ciao G. Anemia and blood pressure correction obtained by daily hemodialysis induce a reduction of left ventricular hypertrophy in dialysed patients. Int J Artif Organs. 1998;21(7):429–431. [PubMed] [Google Scholar]
- 10.Piccoli GB, Mezza E, Quaglia M, Bermond F, Bechis F, Burdese M, et al. Flexibility as an implementation strategy for a daily dialysis program. J Nephrol. 2003;16(3):365–372. [PubMed] [Google Scholar]
- 11.Kooistra MP, Vos J, Koomans HA, Vos PF. Daily home haemodialysis in The Netherlands: effects on metabolic control, haemodynamics, and quality of life. Nephrol Dial Transplant. 1998;13(11):2853–2860. doi: 10.1093/ndt/13.11.2853. [DOI] [PubMed] [Google Scholar]
- 12.Andre MB, Rembold SM, Pereira CM, Lugon JR. Prospective evaluation of an in-center daily hemodialysis program: results of two years of treatment. Am J Nephrol. 2002;22(5–6):473–479. doi: 10.1159/000065280. [DOI] [PubMed] [Google Scholar]
- 13.Koshikawa S, Akizawa T, Saito A, Kurokawa K. Clinical effect of short daily in-center hemodialysis. Nephron Clin Pract. 2003;95(1):c23–c30. doi: 10.1159/000073015. [DOI] [PubMed] [Google Scholar]
- 14.Walsh M, Culleton B, Tonelli M, Manns B. A systematic review of the effect of nocturnal hemodialysis on blood pressure, left ventricular hypertrophy, anemia, mineral metabolism, and health-related quality of life. Kidney Int. 2005;67(4):1500–1508. doi: 10.1111/j.1523-1755.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 15.Suri RS, Nesrallah GE, Mainra R, Garg AX, Lindsay RM, Greene T, et al. Daily hemodialysis: a systematic review. Clin J Am Soc Nephrol. 2006;1(1):33–42. doi: 10.2215/CJN.00340705. [DOI] [PubMed] [Google Scholar]
- 16.Fagugli RM, Reboldi G, Quintaliani G, Pasini P, Ciao G, Cicconi B, et al. Short daily hemodialysis: blood pressure control and left ventricular mass reduction in hypertensive hemodialysis patients. Am J Kidney Dis. 2001;38(2):371–376. doi: 10.1053/ajkd.2001.26103. [DOI] [PubMed] [Google Scholar]
- 17.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298(11):1291–1299. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 18.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66(3):1212–1220. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 19.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363(24):2287–2300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rocco MV, Lockridge RS, Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80(10):12. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suri RS, Garg AX, Chertow GM, Levin NW, Rocco MV, Greene T, et al. Frequent Hemodialysis Network (FHN) randomized trials: study design. Kidney Int. 2007;71(4):349–359. doi: 10.1038/sj.ki.5002032. [DOI] [PubMed] [Google Scholar]
- 22.Rocco MV, Larive B, Eggers PW, Beck GJ, Chertow GM, Levin NW, et al. Baseline characteristics of participants in the Frequent Hemodialysis Network (FHN) daily and nocturnal trials. Am J Kidney Dis. 2010;57(1):90–100. doi: 10.1053/j.ajkd.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fitzmaurice GM, Laird NM, Ware JH, editors. Covariance Pattern Models. Philadelphia, PA: John Wiley & Sons; 2004. Applied Longitudinal Analysis. [Google Scholar]
- 24.Cleveland ER, Johnson RK, Cunningham PJ. Correlated responses of carcass and reproductive traits to selection for rate of lean growth in swine. J Anim Sci. 1988;66(6):1371–1377. doi: 10.2527/jas1988.6661371x. [DOI] [PubMed] [Google Scholar]
- 25.Blankestijn PJ, Ligtenberg G. Volume-independent mechanisms of hypertension in hemodialysis patients: clinical implications. Semin Dial. 2004;17(4):265–269. doi: 10.1111/j.0894-0959.2004.17324.x. [DOI] [PubMed] [Google Scholar]
- 26.Saad E, Charra B, Raj DS. Hypertension control with daily dialysis. Semin Dial. 2004;17(4):295–298. doi: 10.1111/j.0894-0959.2004.17330.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.