Abstract
Intracellular nucleoside reverse transcriptase inhibitor (NRTI) concentrations are associated with plasma HIV-1 response. Coadministration of protease inhibitors with NRTIs can affect intra-cellular concentrations due to protease inhibitor inhibition of efflux transporters. Tenofovir-diphosphate (TFV-DP) concentrations within peripheral blood mononuclear cells were compared among individuals receiving either atazanavir or darunavir-based regimens. There was a trend towards higher TFV-DP concentrations among women and among participants receiving atazanavir. TFV-DP intracellular concentrations were positively associated with undetectable plasma HIV-1 RNA.
Combination antiretroviral therapy (cART) results in virologic suppression and robust immune reconstitution, leading to prolonged life expectancy in HIV-1 infection. Despite cART, eradication of HIV-1 has not been achievable, as the virus remains detectable in suspected reservoirs, including peripheral blood mononuclear cells (PBMCs), even with plasma virologic suppression [1].
Nucleoside reverse transcriptase inhibitors (NRTIs), the cART ‘backbone’, are prodrugs requiring intracellular phosphorylation to produce active metabolites. Clinical studies have shown significant associations between intracellular NRTI concentrations and virologic response [2–5]. Intracellular NRTI concentrations may be modulated by drug–drug interactions mediated by membrane transporter inhibition [6,7]. For example, PBMCs express efflux transporters, including p-glyco-protein (p-gp) and multidrug resistance associated proteins (MRPs), which can be inhibited by protease inhibitors. In particular, higher intracellular concentrations of tenofovir-diphosphate (TFV-DP), the active metabolite of tenofovir disoproxil fumarate (TDF), were achieved when coadministered with lopinavir/ritonavir (LPV/RTV) compared with a nonprotease inhibitor regimen [7].
Atazanavir (ATV) and darunavir (DRV), two protease inhibitors recommended as first line for ART-naive patients, are both p-glycoprotein (p-gp) inhibitors [8–10], although ATV is a more potent p-gp inhibitor than DRV [11]. To determine whether intracellular concentrations of TFV-DP differ in patients receiving ATV vs. DRV-based regimens, we compared TFV-DP concentrations in PBMCs of participants receiving RTV-boosted ATV vs. RTV-boosted DRV and assessed relationships with plasma HIV-1 RNA.
This was a substudy of a cross-sectional investigation of HIV-1 infected patients at the Ponce de Leon Center in Atlanta, Georgia, receiving regimens of daily TDF/emtricitabine (300 mg/200 mg) as well as RTV (100 mg)-boosted and either once-daily ATV (300 mg) or DRV (800 mg). Eligibility criteria included documented adherence to cART and undetectable plasma HIV RNA for at least 6 months before study entry. PBMCs were isolated from blood collected at trough times for 30 participants from May to December 2012 [12]. The Emory University Institutional Review Board and Grady Research Oversight Committee approved this study. All study participants gave written informed consent.
PBMCs were collected and prepared using a well documented method [13]. Two million PBMCs were suspended in 70% methanol; supernatants were dried and stored at −20°C until analysed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) as previously described [14], with minor modifications. Intracellular TFV-DP was separated using a Kinetex XB-C18 column (100 × 2.1 mm) with 2.6 μm particle size (Phenomenex, Torrance, California, USA) at a flow rate of 200 μl/min. About 2 mmol/l NH3H2PO4 with 3 mmol/l hexylamine as solvent A and acetonitrile as solvent B was used in a gradient elution programme as follows: 3–25% B from 0 to 18 min, 25–80% B from 18 to 22 min, 80% B from 22 to 25 min. Equilibration time between two injections was 10 min. An API5000 triple-quadrupole mass spectrometer in positive mode was used for detection, by multiple reaction monitoring (MRM): TFV-DP (448 → 176). Plasma HIV-1 RNA was measured with COBAS! Ampliprep/COBAS Taqman version 2.0 HIV-1 assay (Roche Molecular Systems, Inc) [15].
Intracellular drug concentrations were log transformed; geometric means and 95% confidence intervals (CIs) were compared for each arm using a two-sided, two-sample t-test. Relationships between intracellular TFV-DP and detection of plasma HIV-1 RNA were assessed using point biserial correlation tests for each arm.
Study population demographic and clinical characteristics were previously reported [12]. Thirty patients were enrolled (n = 15 on ATV and n = 15 on DRV): 23/30 (76.7%) male, 26/30 (86.7%) black and median age 46.9 years (interquartile range, IQR, 37.9–51.9). Five women were in the ATV group and two in the DRV group (P = 0.4).
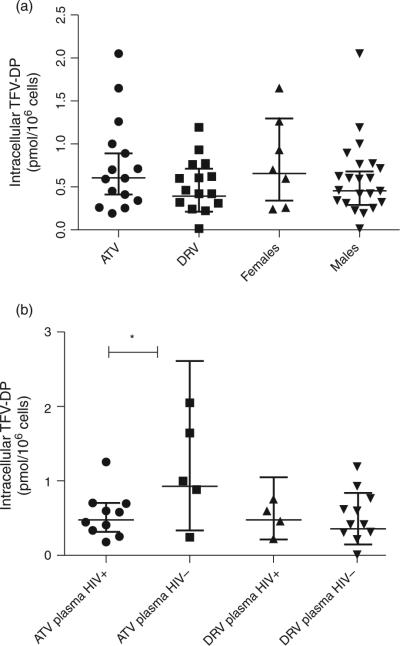
Higher geometric mean intracellular concentrations of TFV-DP were seen in the ATV arm than in the DRV arm (0.60 pmol/106 cells, 95% CI 0.41–0.89 vs. 0.39 pmol/106 cells, 95% CI 0.21–0.71), and in women than in men [0.65 pmol/106 cells (95% CI 0.33–1.29) vs. 0.44 pmol/106 cells (95% CI 0.29–0.68)], although neither reached statistical significance (Fig. 1a).
Fig. 1. Scatterplots of intracellular tenofovir-diphosphate concentrations in pmol/106 cells are shown with brackets indicating geometric mean and 95% confidence intervals.
(a) Intracellular concentrations are compared between atazanavir (ATV, circles) vs. darunavir (DRV, squares) arms, and between women (up-pointing triangles) vs. men (down-pointing triangles). (b) Intracellular TFV-DP concentrations are compared between participants in each treatment arm stratified by HIV viral load. ATV arm with plasma HIV viral load ≥40 copies/ml (plasma HIV RNA+, circles) vs. plasma HIV viral load <40 copies/ml (plasma HIV RNA-, squares); DRV arm with plasma HIV RNA+ (up-pointing triangle) vs. plasma HIV RNA- (down-pointing triangle). *P < 0.05.
Ten of 15 patients in the ATVarm had a detectable plasma viral load [median 225 copies/ml (IQR 140–310)] compared with four of 15 in the DRV arm [median 200 copies/ml (IQR 95–3520)], P = 0.03. A significant positive correlation was seen between intracellular TFV-DP concentration and undetectable plasma HIV RNA (<40 copies/ml) in the ATVarm (r = −0.56, P=0.03) but not in the DRV arm (r=−0.02, P = 0.94), Fig. 1b.
TFV-DP intracellular concentrations were slightly higher than prior published reports: Pruvost et al. [7] found TFV-DP Ctrough median of 0.222 pmol/106 cells (IQR 0.160–0.376 pmol/106 cells), while Dumond et al. [16] found TFV-DP mean concentration to be 0.112 pmol/ 106 cells (SD 0.077 pmol/106), although significant interindividual variability has been documented with intracellular TFV concentrations. Consistent with Pruvost et al. [7], women trended towards higher TFV-DP concentrations than men. This phenomenon has been described with other phosphorylated NRTIs, including zidovudine-triphosphate (TP) and lamivudine-TP [5]. Although biologic mechanisms for these sex differences remain unclear, women may phosphorylate NRTIs differently than men and hormonal differences may play a role. A significant positive correlation between intracellular TFV-DP and undetectable plasma HIV-1 RNA was seen with ATV but not DRV. One mechanistic explanation for this is that DRV has greater antiviral activity than ATV [17], making plasma HIV-1 RNA suppression less dependent upon intracellular TFV-DP concentrations for patients on DRV.
In conclusion, TFV-DP concentrations in PBMCs were similar in both treatment arms. The trend towards higher intracellular concentrations in the ATV arm could be explained by the fact that five of seven women were in that group: further studies should explore this sex effect. In addition, TFV-DP concentrations predicted detectable plasma HIV-1 RNA in the ATV arm, emphasizing the importance of adequate antiretroviral penetration into PBMCs for achieving virologic control in patients receiving this protease inhibitor.
Future prospective studies should evaluate intracellular ART concentrations throughout the dosing interval coupled with longitudinal HIV-1 RNA measurements. These data would elucidate the virologic impact of PBMC drug concentrations over time. Finally, studies must be adequately powered to investigate sex differences in antiretroviral pharmacology with a goal of informing clinical practice.
Acknowledgements
All listed authors met the criteria for authorship set forth by the International Committee for Medical Journal Editors. Author contributions were as follows: C.D.L and I.O. were lead study investigators, designed and wrote the study protocol, acquired/analysed/interpreted data and wrote and critically reviewed the article. S.T., Y.J. and R.F.S performed assays for intracellular TFV-DP quantification and wrote and critically reviewed the article. V.C.M., W.S.A. and S.S. were study investigators and wrote and critically reviewed the manuscript. A.V. conducted all processing of PBMCs, contributed to study design and wrote and critically reviewed the manuscript. E.P.A. assisted in study design and interpretation of pharmacologic data, and wrote and critically reviewed the manuscript.
C.D.L. is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and KL2TR000455 and the Bristol-Myers Squibb Virology Fellows Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
R.F.S. is supported in part by CFAR grant NIH 5P30-AI-050409, and the Department of Veterans Affairs. This work is also supported by the Emory CFAR (P30 AI050409).
We are grateful to the faculty, staff and research participants at the Ponce de Leon Center for their time and dedication to this project.
C.D.L. received funding from the Bristol-Myers Squibb Virology Fellowship Award and payment from the Massachusetts Medical Society for development of a board review product. I.O. and S.S. have received funding from Bristol-Myers Squibb. W.S.A. is a member of the HIVMA IDSA board and received payment from the Massachusetts Medical Society.
Footnotes
Conflicts of interest All other authors have no conflicts of interest to report.
References
- 1.Palmer S, Josefsson L, Coffin JM. HIV reservoirs and the possibility of a cure for HIV infection. J Intern Med. 2011;270:550–560. doi: 10.1111/j.1365-2796.2011.02457.x. [DOI] [PubMed] [Google Scholar]
- 2.Stretcher BN, Pesce AJ, Frame PT, Greenberg KA, Stein DS. Correlates of zidovudine phosphorylation with markers of HIV disease progression and drug toxicity. AIDS. 1994;8:763–769. doi: 10.1097/00002030-199406000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Kiser JJ, Aquilante CL, Anderson PL, King TM, Carten ML, Fletcher CV. Clinical and genetic determinants of intracellular tenofovir diphosphate concentrations in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;47:298–303. doi: 10.1097/qai.0b013e31815e7478. [DOI] [PubMed] [Google Scholar]
- 4.Fletcher CV, Kawle SP, Kakuda TN, Anderson PL, Weller D, Bushman LR, et al. Zidovudine triphosphate and lamivudine triphosphate concentration-response relationships in HIV-infected persons. AIDS. 2000;14:2137–2144. doi: 10.1097/00002030-200009290-00010. [DOI] [PubMed] [Google Scholar]
- 5.Anderson PL, Kakuda TN, Kawle S, Fletcher CV. Antiviral dynamics and sex differences of zidovudine and lamivudine triphosphate concentrations in HIV-infected individuals. AIDS. 2003;17:2159–2168. doi: 10.1097/00002030-200310170-00003. [DOI] [PubMed] [Google Scholar]
- 6.Jorajuria S, Dereuddre-Bosquet N, Becher F, Martin S, Porcheray F, Garrigues A, et al. ATP binding cassette multidrug transporters limit the anti-HIV activity of zidovudine and indinavir in infected human macrophages. Antivir Ther. 2004;9:519–528. [PubMed] [Google Scholar]
- 7.Pruvost A, Negredo E, Theodoro F, Puig J, Levi M, Ayen R, et al. Pilot pharmacokinetic study of human immunodeficiency virus-infected patients receiving tenofovir disoproxil fumarate (TDF): investigation of systemic and intracellular interactions between TDF and abacavir, lamivudine, or lopinavir-ritonavir. Antimicrob Agents Chemother. 2009;53:1937–1943. doi: 10.1128/AAC.01064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujimoto H, Higuchi M, Watanabe H, Koh Y, Ghosh AK, Mitsuya H, et al. P-glycoprotein mediates efflux transport of darunavir in human intestinal Caco-2 and ABCB1 gene-transfected renal LLC-PK1 cell lines. Biol Pharm Bull. 2009;32:1588–1593. doi: 10.1248/bpb.32.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janneh O, Anwar T, Jungbauer C, Kopp S, Khoo SH, Back DJ, et al. P-glycoprotein, multidrug resistance-associated proteins and human organic anion transporting polypeptide influence the intracellular accumulation of atazanavir. Antivir Ther. 2009;14:965–974. doi: 10.3851/IMP1399. [DOI] [PubMed] [Google Scholar]
- 10.Lee LS, Soon GH, Shen P, Yong EL, Flexner C, Pham P. Darunavir/ritonavir and efavirenz exert differential effects on MRP1 transporter expression and function in healthy volunteers. Antivir Ther. 2010;15:275–279. doi: 10.3851/IMP1505. [DOI] [PubMed] [Google Scholar]
- 11.Tong L, Phan TK, Robinson KL, Babusis D, Strab R, Bhoopathy S, et al. Effects of human immunodeficiency virus protease inhibitors on the intestinal absorption of tenofovir disoproxil fumarate in vitro. Antimicrob Agents Chemother. 2007;51:3498–3504. doi: 10.1128/AAC.00671-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delille CA, Pruett ST, Marconi VC, Lennox JL, Armstrong WS, Arrendale RF, et al. Effect of protein binding on unbound atazanavir and darunavir cerebrospinal fluid concentrations. J Clin Pharmacol. 2014;54:1063–1071. doi: 10.1002/jcph.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King T, Bushman L, Kiser J, Anderson PL, Ray M, Delahunty T, et al. Liquid chromatography-tandem mass spectrometric determination of tenofovir-diphosphate in human peripheral blood mononuclear cells. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;843:147–156. doi: 10.1016/j.jchromb.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 14.Fromentin E, Gavegnano C, Obikhod A, Schinazi RF. Simultaneous quantification of intracellular natural and antiretroviral nucleosides and nucleotides by liquid chromatography-tandem mass spectrometry. Anal Chem. 2010;82:1982–1989. doi: 10.1021/ac902737j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damond F, Avettand-Fenoel V, Collin G, Roquebert B, Plantier JC, Ganon A, et al. Evaluation of an upgraded version of the Roche Cobas AmpliPrep/Cobas TaqMan HIV-1 test for HIV-1 load quantification. J Clin Microbiol. 2010;48:1413–1416. doi: 10.1128/JCM.01409-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baheti G, Kiser JJ, Havens PL, Fletcher CV. Plasma and intra-cellular population pharmacokinetic analysis of tenofovir in HIV-1-infected patients. Antimicrob Agents Chemother. 2011;55:5294–5299. doi: 10.1128/AAC.05317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shen L, Peterson S, Sedaghat AR, McMahon MA, Callender M, Zhang H, et al. Dose-response curve slope sets class-specific limits on inhibitory potential of anti-HIV drugs. Nat Med. 2008;14:762–766. doi: 10.1038/nm1777. [DOI] [PMC free article] [PubMed] [Google Scholar]