Abstract
The extracellular matrix (ECM) is a highly dynamic compartment that undergoes remodeling as a result of injury and repair. Over the past decade, mounting evidence in humans and rodents suggest that ECM remodeling is associated with diet-induced insulin resistance in several metabolic tissues. Additionally, integrin receptors for the ECM have also been implicated in the regulation of insulin action. This review will address what is currently known about the ECM, integrins and insulin action in the muscle, liver and adipose tissue. Understanding how ECM remodeling and integrin signaling regulates insulin action may aid in the development of new therapeutic targets for the treatment of insulin resistance and type 2 diabetes.
Keywords: Extracellular matrix, integrins, glucose homeostasis, insulin resistance, liver, muscle
Overview of the extracellular matrix and integrins
The extracellular matrix (ECM) (Glossary) is composed of a diverse network of proteins and proteoglycans [1]. It provides a scaffold for cells and modulates biological processes including differentiation, cell migration, repair and development [2, 3]. The interaction between cells and the ECM is important for all organs. The ECM communicates with cells through transmembrane cell surface receptors called integrins [4]. Integrins bind the ECM and transduce signals through the plasma membrane to activate intracellular signaling. Integrins themselves lack kinase activity. Thus, they are reliant on scaffolding proteins and downstream kinases for signal transduction. Integrins signal through various proteins including focal adhesion kinase (FAK) and integrin-linked kinase (ILK) (Box 1). The detailed structure and function of integrins have been reviewed elsewhere [1, 4, 5].
Box 1. Integrin signaling molecules.
Focal adhesion kinase
Focal adhesion kinase (FAK) is a cytoplasmic tyrosine kinase that localizes with integrin receptors at sites where cells attach to the ECM [89]. FAK undergoes rapid autophosphorylation at Tyr397 upon integrin-mediated cell adhesion [90], and this is associated with increased catalytic activity. Additionally, FAK can be regulated by the growth factor receptors epidermal growth factor receptor (EGFR), fibroblast growth factor receptor (FGFR) and the insulin receptor [63, 91]. This results in the activation of several downstream signaling cascades including the MAPK and PI3K signaling pathways [63, 91]. In addition to its signaling properties, FAK is important for cytoskeletal stabilization and focal adhesion turnover [92].
Integrin-linked kinase and insulin action
Integrin-linked kinase (ILK) is a highly conserved intracellular scaffolding protein. It interacts with the β1, β2 and β3-integrin cytoplasmic domains and numerous cytoskeleton-associated proteins. It is composed of three distinct domains: an N-terminus that contains five ankyrin repeats, a pleckstrin homology-like domain and a pseudokinase domain at the C-terminus. Considering that it is a scaffolding protein, it has been proposed that ILK modulates intracellular signaling through its ability to recruit a kinase or multiple kinases into a multiprotein complex. This complex then facilitates the activation of downstream signaling molecules upon insulin stimulation. The pseudokinase domain of ILK is an essential domain for the recruitment of adaptor proteins and/or signaling molecules including several proteins involved in insulin action such as PKB/Akt, PDK1 and GSK-3β. Overexpression of ILK or insulin treatment results in increased GSK-3 and Akt phosphorylation [93]. Co-transfection of Akt with wild-type ILK in 293 cells resulted in an enhancement of phosphorylation of Akt Ser473 [93]. Several studies have shown that the ablation of ILK results in decreased Akt Ser473 phosphorylation [94–96]. Moreover, ILK is connected to growth factor receptors through the adaptor protein Nck2 [97]. Therefore, although ILK lacks intrinsic kinase activity, it has been shown to regulate the activation of numerous intracellular growth factor signaling cascades [98–100].
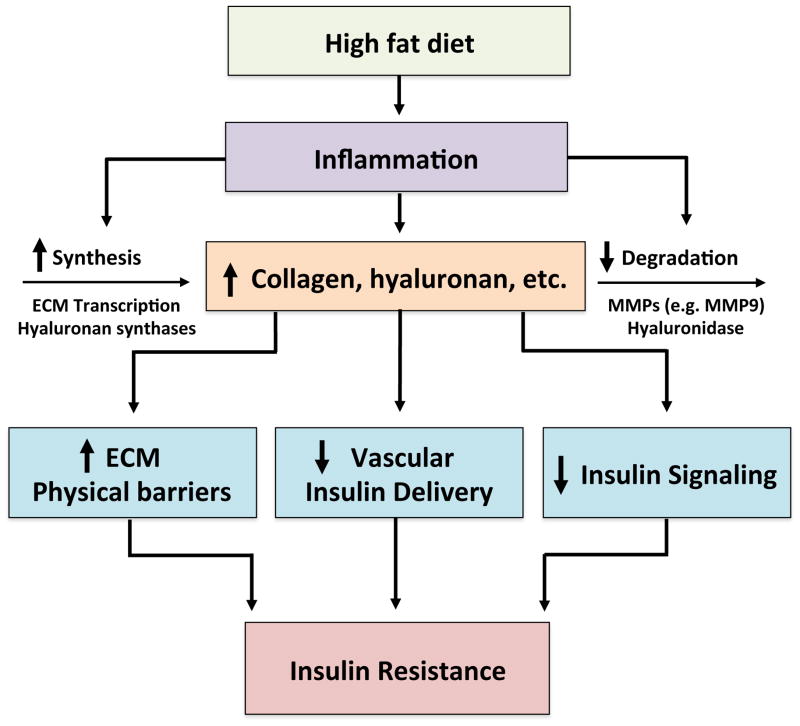
The ECM is a dynamic structure that remodels during times of injury and repair [6]. Pathological states are associated with ECM remodeling and alterations in integrin expression. In obese conditions, the expression of ECM proteins increases several-fold, while a shift appears to occur from low-density ECM proteins to more fibril-forming proteins. Several recent lines of evidence suggest that ECM remodeling and changes in integrin signaling in the diet-induced obese (DIO) state are associated with insulin resistance [7–16]. The potential mechanisms whereby this occurs are represented in Figure 1. Herein we discuss recent findings related to the emerging link between ECM remodeling, integrin signaling and insulin resistance in the skeletal muscle, liver, and adipose tissue.
Figure 1.
A link between extracellular matrix remodeling and insulin resistance.
A diet high in fat generates a state of chronic inflammation. This inflammatory response leads to increased ECM synthesis and decreased ECM degradation, resulting in increased deposition and remodeling of ECM. Increased levels of ECM lead to increased physical barriers for insulin and glucose transport, decreased vascular insulin delivery and decreased insulin signaling. The combination of all of these factors then culminates in insulin resistance.
The Skeletal Muscle
Mechanisms of High Fat Diet-induced ECM Remodeling in the Skeletal Muscle
Inflammation and elevated transforming growth factor (TGF) β signaling are associated with muscle ECM remodeling, in obese mice and humans [17]. Mice fed a high fat diet (HFD) exhibit increased infiltration of pro-inflammatory M1-activated (CD11c+) macrophages in muscle [18]. Additionally, CD68+ macrophages are elevated in obese individuals [19]. The association between ECM remodeling and inflammation was further shown in a study by Kang et al. [9]. In this study, 20 weeks of HF feeding in mice led to increased muscle collagen content associated with increased gene expression of the pro-inflammatory marker tumor necrosis factor (TNFα) and the macrophage marker F4/80. Importantly, gene expression for these inflammatory markers was diminished in mouse models of improved insulin sensitivity and decreased muscle collagen deposition. It is possible that increased recruitment of pro-inflammatory macrophages may lead to ECM remodeling via TGFβ-mediated Smad activation [20]. Smad3 activation is elevated in skeletal muscle biopsies of obese individuals compared to lean controls [17]. Collectively, this suggests that ECM remodeling in obese skeletal muscle occurs as a result of increased inflammation.
The Skeletal Muscle ECM and Glucose Metabolism
Insulin resistant muscle in obese and type 2 diabetic (T2D) humans is characterized by increased collagen deposition [7, 8]. Rapid weight gain in healthy young males resulted in impaired insulin sensitivity and the up-regulation of several muscle ECM genes [21]. There was no evidence of local adipose tissue or systemic inflammation despite weight gain, suggesting a key role for muscle ECM in the regulation of glucose homeostasis rather than secondary effects due to adipose tissue inflammation.
Muscle collagen content is also increased in DIO, insulin resistant mice [9]. Studies by Kang et al. showed that increased collagen deposition in the DIO state is due to in part to decreased muscle matrix metallopeptidase 9 (MMP9) activity [9], and that the genetic deletion of MMP9 in mice increases collagen deposition in the muscle and exacerbates muscle insulin resistance in HF-fed mice [15].
Hyaluronan is an anionic, nonsulfated glycosaminoglycan. As a major component of the ECM, hyaluronan has multiple functions, including creating space between cells [22]. Serum hyaluronan is increased in T2D [23]. Insulin resistant animals have increased hyaluronan in muscles [16], aorta [24], and kidneys [25]. Elevated muscle hyaluronan levels are associated with muscle insulin resistance in the obese state. A reduction of muscle hyaluronan by intravenous injection of pegylated human recombinant hyaluronidase PH-20 (PEGPH20) results in a dose-dependent increase in glucose infusion rate and muscle glucose uptake during a hyperinsulinemic-euglycemic clamp [16]. This study showed for the first time that whole-body depletion of an ECM polysaccharide rescues insulin sensitivity in C57BL/6J HF-fed mice.
There are several hypotheses as to how increased muscle ECM in the HF-fed state contributes to insulin resistance, which may co-exist. A first hypothesis, the ECM is a physical barrier to both glucose and insulin diffusion. Proteins buildup in the interstitial space and this impedes substrate delivery to the muscle by increasing diffusion distance. A second hypothesis is that increases in muscle ECM impair neo-vascular growth and vascular function. The ECM is in close contact with the endothelium. Blood flow and capillary recruitment are critical for proper glucose and insulin delivery to the muscle. Vascular dysfunction and capillary rarefaction (reduced capillary density) have long been implicated in the development of muscle insulin resistance and T2D [26]. Reduced blood flow to the muscle is correlated with insulin resistance, and conversely, the number of muscle capillaries is positively related to peripheral insulin action [27, 28]. Additionally, three weeks of treatment with the hormone relaxin, improved muscle insulin action through effects on the vasculature [29]. Kang and colleagues have provided consistent evidence that increased muscle capillaries are associated with improved muscle insulin action in HF-fed mice [9, 15]. This was evident in several mouse models, including the muscle-specific mitochondrial targeted catalase transgenic mice [9], chronic sildenafil-treated mice [9] and hyaluronidase-treated mice [16]. In contrast, decreased muscle capillaries are associated with exacerbated muscle insulin resistance in the global MMP9 knockout mouse [15]. It is important to consider that the first and second hypotheses are inextricably linked, as a decrease in capillarity will increase spatial barriers and diffusion distance for hormones and nutrients. Collectively, these data strongly suggest endothelial dysfunction and muscle capillary rarefaction are potential mechanisms by which ECM remodeling mediates muscle insulin resistance. Finally, the ECM may signal directly through muscle integrins to modulate insulin action (Figure 2). This is discussed in detail below.
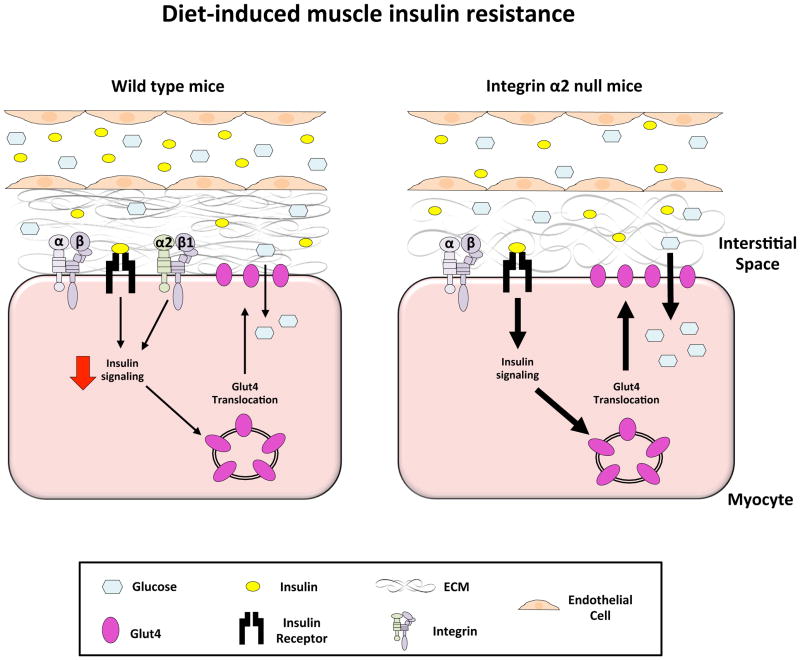
Figure 2.
The role of integrin α2β1 diet-induced muscle insulin resistance.
In the HF-fed state, capillary density and endothelial function are impaired. This results in decreased potential for glucose and insulin transport into the interstitial space despite hyperglycemia and hyperinsulinemia. Moreover, increased ECM deposition in the interstitial space also provides a physical barrier to glucose and insulin transport to the myocyte. Insulin signaling within the myocyte is impaired and this may be attributed to increased integrin α2β1 signaling as a consequence of increased deposition of the ECM. This results in impaired Glut4 translocation and decreased glucose transport into the myocyte. In contrast, the genetic deletion of the integrin α2 subunit results in improved insulin-stimulated muscle glucose uptake.
Integrins and Skeletal Muscle Insulin Resistance
Skeletal muscle expresses seven integrin α subunits (α1, α3, α4, α5, α6, α7, and αv), and are all associated with the β1 integrin subunit [30]. Remarkably, few studies have addressed the role of integrin signaling in the muscle with respect to muscle insulin resistance in vivo. The muscle-specific deletion of integrin β1 in chow-fed mice results in decreased whole-body insulin sensitivity, and decreased insulin-stimulated muscle glucose uptake during a hyperinsulinemic-euglycemic clamp [31]. Notably, the loss of skeletal muscle β1 has no effect on liver or adipose tissue glucose metabolism. The decrease in insulin-stimulated muscle glucose uptake was associated with decreased muscle glycogen synthesis and decreased Akt S473 phosphorylation. Moreover, the whole-body deletion of integrin α2 in obese, HF-fed mice, partially reverses diet-induced muscle insulin resistance, as evidenced by increased insulin-stimulated muscle glucose uptake during a hyperinsulinemic-euglycemic clamp, and increased insulin signaling [9] (Figure 3). These data suggest that integrin signaling might be a mechanistic link between the muscle ECM and insulin resistance.
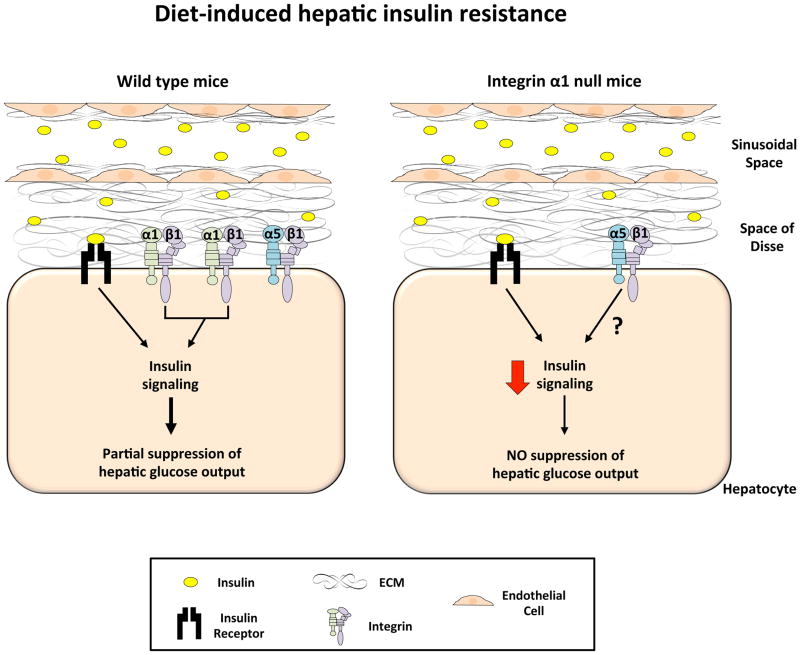
Figure 3.
The role of integrin α1β1 in diet-induced hepatic insulin resistance.
In the HF-fed state, sinusoidal capillarization occurs and this, in addition to increased ECM buildup in the space of Disse, results in decreased insulin transport to the hepatocyte despite hyperinsulinemia. Protein expression of the integrin α1 subunit is increased and this leads to increased α1β1 cell signaling. Upon insulin stimulation, the combination of both insulin and integrin α1β1 signaling results in some insulin signaling and the partial suppression of hepatic glucose output. In contrast, the genetic deletion of the integrin α1 subunit results in severe hepatic insulin resistance and no insulin-mediated suppression of hepatic glucose output. This is attributed to decreased insulin signaling. It is possible that this effect is mediated by integrin α5β1, the only other known integrin expressed on the hepatocyte, however this is currently unknown.
The downstream integrin signaling molecule, FAK, has been implicated in the regulation of insulin action in the muscle [32–34]. FAK tyrosine phosphorylation is decreased in muscle from HF-fed rats [32]. The in vivo siRNA-mediated knockdown of FAK results in hyperglycemia, hyperinsulinemia, impaired glucose tolerance and decreased insulin action in chow-fed mice [34]. The overexpression of FAK in C2C12 mouse myoblasts increases insulin-stimulated glucose uptake [35]. Conversely, C2C12 cells transfected with siRNA against FAK exhibit decreased insulin-stimulated glucose uptake [32]. L6 myocytes transfected with antisense FAK display decreased insulin signaling associated with decreased insulin-stimulated glucose uptake, decreased glycogen synthesis and impaired Glut4 translocation [33]. Collectively, these studies suggest that integrins mediate muscle glucose metabolism via their effects on both vascularization and glucose transport through Glut4.
No information exists about the role of ILK in the regulation of muscle glucose homeostasis. Mice with a muscle-specific deletion of ILK have been generated and are viable [36]. Considering the interaction of ILK with several known insulin signaling molecules such as Akt and GSK-3β, it is highly possible that ILK modulates muscle insulin action. Future studies should be designed to determine whether ILK regulates muscle insulin action in the DIO mouse model.
ECM remodeling and mechano-signal transduction
ECM remodeling is also reflected by alterations in mechano-signal transduction to the nucleus and mitochondria [37, 38]. This may be a consequence of disturbances in actin and intermediate filament organization and/or the sarcoglycan complex, a junction whereby the myofiber interacts with the ECM. The sarcoglycan complex is critical for both force and mechano-signal transduction to the nucleus and mitochondrion. Disturbances in this complex produce metabolic effects [39]. Mice lacking the sarcoglycan complex in the muscle and adipose tissue demonstrate whole body insulin resistance attributed to impaired insulin-stimulated muscle glucose uptake [39]. Additionally, changes in the ECM of insulin resistant human muscle are accompanied by decreased abundance of the key filament organizational proteins, actinin 2 and desmin. It is plausible that these alterations may impair the ability of the muscle to adapt to exercise via compromised mechano-signal transduction to the nucleus or mitochondria that, under normal conditions, would induce gene transcription in response to exercise. In support of the ECM modulating mitochondrial function in the skeletal muscle, there is evidence that alterations in the collagen VI composition of the matrix affect mitochondrial function [37, 38]. Insulin resistant muscle is characterized by alterations in exercise tolerance and mitochondrial function, thus this provides another route whereby the ECM may regulate muscle insulin action.
The Liver
Mechanisms of HFD-induced ECM Remodeling in the Liver
The liver ECM expands with over-nutrition. Mice fed a HFD display increased hepatic staining for α-smooth muscle actin (SMA, a marker of stellate cell activation) and collagen, as well as increased collagen type I α1 gene expression [11]. Mice fed a HFD with high fructose water exhibit increased hepatic collagen type I α1 gene expression [10]. Moreover, Williams et al. recently demonstrated that mice fed a 60% HFD exhibit increased gene expression for collagen types I and III [12].
The specific process whereby ECM remodeling in the liver occurs in the presence of over-nutrition is undefined. However, one prevailing hypothesis is a “two hit” hypothesis [40]. The “first hit” is the accumulation of lipid metabolites. This leads to a series of events including lipotoxicity, oxidative stress, and inflammation that produce a “second hit”. The “second hit” promotes tissue injury and the activation of stellate cells. This process is initiated by autocrine and paracrine stimuli including inflammatory cytokines and growth factors such as TGFβ [41–43]. Increased TGFβ signaling is associated with hepatic collagen synthesis [44]. Once activated, stellate cells deposit ECM proteins in the space of Disse as part of a wound healing response, resulting in changes in the ECM and fibrosis [45]. Although it has been widely proposed that stellate cells are the main contributor to ECM deposition in the liver, it is possible that other cell types are involved [46]. The notion that stellate cells are the main contributor was based on in vitro studies performed in cell culture [47, 48]. However, several cell types in the intact liver are capable of ECM synthesis, including hepatocytes, endothelial cells, as well as stellate cells [2]. Considering that hepatocytes comprise approximately 80% of the liver [49], it is feasible that they contribute to the hepatic ECM.
The Liver ECM and Glucose Metabolism
T2D in humans is associated with hepatic ECM remodeling [50, 51]. Patients with T2D exhibit increased staining for collagen type IV, α-SMA and a tendency for increased laminin staining [50]. In a separate study, liver biopsies from diabetic patients showed increased perisinusoidal fibrosis, characterized by immunostaining for laminin in sinusoidal spaces, as well as collagen type IV and α-SMA in the space of Disse [51]. It is important to note that early markers of ECM remodeling occur in diabetic patients prior to more advanced fibrosis and cirrhosis.
It is evident that a diet high in fat is associated with insulin resistance and ECM remodeling in the liver. Bonner et al. [29] showed that three weeks of relaxin treatment in HF-fed mice, results in decreased hepatic collagen type III, and a subsequent improvement in hepatic insulin action. In light of this, it is important to note that only one study to date has demonstrated a causal link between ECM remodeling and insulin resistance [16]. Kang et al. showed that depletion of systemic hyaluronan via tail vein injection of a long-acting hyaluronidase reverses HFD-induced liver insulin resistance [16].
CD44, the main hyaluronan cell surface receptor, is associated with T2D, as shown by expression-based genome-wide association studies (GWAS) [52]. CD44 is ubiquitously expressed, and its expression level in liver is positively correlated with hepatic steatosis and insulin resistance, in obese humans and DIO mice [52, 53]. Kodama et al. reported that anti-CD44 antibody treatment lowers glycemia, improves insulin sensitivity and hepatic steatosis in DIO mice [54]. It is important to note that CD44 can also interact with other ligands, such as osteopontin, collagens and MMPs. Therefore, it is unclear whether the phenotype of mice lacking functional CD44 is due to prevention of hyaluronan or osteopontin or both. Hence, the role of CD44 signaling in diet-induced insulin resistance remains unclear, and warrants future investigation. Collectively, these studies highlight the role of the liver ECM in the regulation of glucose homeostasis.
There are two existing hypotheses as to how the hepatic ECM contributes to changes in insulin action. The first is through cellular and microcirculatory changes as a result of diet-induced ECM remodeling. In the liver, it is reasonable to speculate that diet-induced ECM remodeling (i.e. sinusoidal capillarization) sensitizes the liver to further damage and may facilitate maladaptive changes in hepatic insulin action [55]. The liver is a major site of insulin clearance. It is estimated that 50% of insulin is extracted by the liver during the first pass, via a receptor-mediated process [56–58]. Hepatic insulin extraction from the circulation reflects the ability of the liver to adequately respond to an insulin stimulus. Patients with cirrhosis and chronic hepatitis display decreased hepatic insulin extraction, compared to normal subjects [59]. This decrease in insulin clearance can be attributed to either liver damage or shunting of the portal-systemic circulation [59]. An extension of this is impaired insulin action and ultimately insulin resistance. A second hypothesis is that, as in muscle, the ECM signals through integrins and this regulates insulin action.
Integrins and Liver Insulin Resistance
Six α integrin subunits (α1, α2, α3, α4, α5 and α6) are expressed in the liver, all of which are associated with the β1 integrin [60]. Of these six α subunits, only two integrins have been shown to be expressed on the hepatocyte: integrin α1β1 and α5β1. Integrin α5β1 is a fibronectin receptor, and integrin α1β1 is a collagen binding integrin. The genetic whole body deletion of the integrin α1 subunit in mice exists and is viable [61]. Studies show that integrin α1β1 protects against the development of hepatic insulin resistance [9, 12] (Figure 4). Williams et al. demonstrated that integrin α1 protein expression is upregulated in hepatocytes isolated from HF-fed mice, compared to chow-fed controls [12]. Thus, to determine whether this response protected against hepatic metabolic impairments in DIO mice, insulin sensitivity was determined in integrin α1-null mice and their wild-type littermates. This study showed that deletion of the integrin α1 subunit results in severe hepatic insulin resistance in HF-fed mice and decreased hepatic insulin signaling. It is currently unknown whether some unidentified integrin α1 subunit binding protein is modulating the observed protective effect. Moreover, the role of integrin α5β1 in hepatic insulin action in vivo has not been investigated. It is possible, in the integrin α1 subunit null mice, that the severe hepatic insulin resistance is attributable to enhanced integrin α5β1 signaling. Future studies should be conducted to identify novel integrin α1 subunit binding partners and/or to determine whether integrin α5β1 contributes to or protects against diet-induced hepatic insulin resistance.
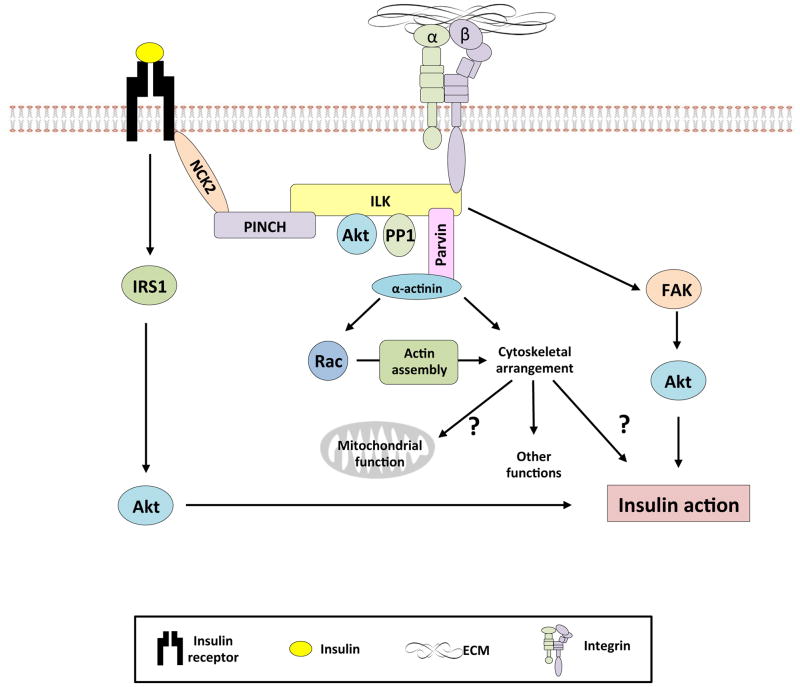
Figure 4.
Proposed model whereby integrins regulate insulin action.
In the presence of insulin, integrin signaling through both integrin linked kinase (ILK) and focal adhesion kinase (FAK) promotes insulin action. Canonical insulin signaling occurs, however it is possible that other mechanisms exist whereby insulin exerts its actions within the cell. Several studies show that FAK is an important regulator of insulin action in both the muscle and liver. Less is known about ILK. However, Nck2 is an adaptor protein shared by both the insulin receptor and ILK. This suggests that there may be a physical link between the insulin receptor and integrins through Nck2 and ILK, allowing the centralization of signaling through this complex. Akt, a critical insulin signaling molecule, is a known binding partner of ILK. Additionally, integrin signaling has been shown to modulate the assembly of the cytoskeleton and this may have effects on both mitochondrial function and insulin action.
FAK has been heavily implicated in the regulation of glucose homeostasis and insulin action in the liver [33, 34, 62]. FAK undergoes rapid tyrosine phosphorylation in livers from healthy rats upon insulin stimulation under euglycemic conditions in vivo [62]. This is consistent with a separate study showing that HepG2 cells transfected with mutant FAK constructs display decreased Akt Ser473 and GSK-3 Ser9 phosphorylation [63]. There was no difference in the insulin receptor phosphorylation or PI3K activity, upon insulin stimulation suggesting that FAK exerts its actions on insulin signaling downstream of the insulin receptor [64]. FAK tyrosine phosphorylation is decreased in HF-fed mice [12]. The in vivo siRNA-mediated knockdown of FAK results in hyperglycemia, hyperinsulinemia, and impaired glucose tolerance in chow-fed mice [34]. Finally, fa/fa rats treated with a TNF-α neutralizing agent exhibited increased hepatic FAK phosphorylation associated with decreased hepatic glucose output during an insulin clamp [62, 65]. These studies suggest that decreased integrin signaling through FAK may facilitate the development of hepatic insulin resistance.
Little is known about the role of integrin-linked kinase (ILK) in the regulation of hepatic insulin action. However, several studies suggest that ILK modulates the activation of several key insulin signaling proteins, including Akt and GSK-3β [66–68]. The stimulation of hepatic stellate cells in rats using carbon tetrachloride (CCl4) resulted in increased ILK protein expression, associated with enhanced phosphorylation of Akt, while the inhibition of ILK by siRNA prevented this [66]. In contrast, the phosphorylation of Akt Ser473 is not affected in mice with a hepatocyte-specific deletion of ILK [67], and ILK-deficient cells are capable of phosphorylating Akt at both Thr308 and Ser473 upon insulin stimulation, similar to control cells [68]. Thus, the role of ILK in the regulation of Akt and GSK-3 phosphorylation is currently unresolved and more studies are necessary to address whether ILK mediates insulin signaling in vivo. The regulation of hepatic insulin action by integrins is multifaceted, and more studies are necessary to determine the actions of each integrin and integrin signaling molecule on hepatic insulin action, to aid in the determination of future therapeutics.
The Adipose Tissue
Mechanisms of High Fat Diet-induced ECM Remodeling in Adipose Tissue
The adipose tissue responds dynamically to nutrient excess through adipocyte hypertrophy and hyperplasia [69]. This is followed by increased production of pro-inflammatory adipokines, immune cell infiltration and ECM remodeling. Excessive collagen deposition has been observed in the adipose tissue of various models of overnutrition [13, 70, 71]. Collagen VI is a highly enriched ECM protein in adipose tissue and its expression is increased in obese humans [70]. Additionally, collagen gene expression (types I, III, V, and VI) is increased in adipose tissue from obese leptin receptor deficient db/db mice, and this is further exacerbated when the mice are fed a HFD [13]. Collectively, this suggests that ECM remodeling is a characteristic of obese adipose tissue.
Hypoxia and inflammation are stimuli for ECM remodeling during adipose tissue expansion. HF feeding in rodents leads to the doubling of fat cell area accompanied by local hypoxia [72]. Hypoxia in obese adipose tissue occurs as the local vasculature fails to expand appropriately to meet the demands of increased fat mass. In support of this, gene expression of vascular endothelial growth factor (VEGF)-A and vessel density is decreased in the adipose tissue of ob/ob mice [72]. Hypoxia then leads to HIF1α activation and the secretion of pro-inflammatory cytokines from adipocytes [72, 73]. The combination of hypoxia and inflammation culminates in the pathological expansion of adipose ECM as adipocytes and recruited macrophages express and secrete collagens [70, 71, 74]. The mechanisms underlying ECM remodeling in adipose tissue have been reviewed in detail elsewhere [69, 75].
The Adipose Tissue ECM and Glucose Metabolism
One hallmark of metabolically dysfunctional adipose tissue is the pathological accumulation of ECM proteins. Increased collagen deposition is a physical barrier for adipocyte expansion during the development of obesity and this promotes the shunting of lipids into other tissues. A role for collagen in the obese, metabolically impaired adipose tissue was recently established [71]. The deletion of collagen VI in ob/ob mice results in un-impeded adipocyte expansion, improved glucose tolerance and insulin signaling. Additionally, the overexpression of the α3 chain of collagen VI (endotrophin) in mice stimulates deposition of other collagen types, mainly collagen I, III and VI, and insulin resistance, in the presence of a HFD [76].
Thrombospondin 1 (THBS1) is a large adhesive ECM glycoprotein expressed predominantly in visceral adipose tissue and its expression is elevated in insulin-resistant, obese humans [19]. In mice, HF feeding acutely induces adipose tissue Thbs1 expression and increases circulating THBS1 levels [14]. Genetic deletion of Thbs1 in mice protects against HFD induced adipose tissue inflammation and insulin resistance [14]. Circulating THBS1 may also induce fibrosis in skeletal muscle and induce insulin resistance [14]. This is evident as Thbs1-null skeletal muscle is protected from HFD-induced collagen deposition and insulin resistance. Moreover, expression of the pro-inflammatory ECM glycoprotein, tenacin C, is also upregulated in the adipose tissue of obese mice and humans [77] and may contribute to insulin resistance.
The composition of ECM reflects a balance between matrix synthesis and degradation. Adipose tissue remodeling is altered in the obese state by ECM proteolysis via the fibrinolytic systems and matrix metalloproteinases (MMPs) [69]. MMPs are a family of zinc dependent proteinases responsible for the degradation of ECM proteins [78]. MMP dysregulation has been implicated in the pathophysiology of obesity and diabetes. Plasma MMP2 and MMP9 concentrations are increased in obese [79] and diabetic [80, 81] individuals. Additionally, gene expression of adipose MMP9 correlates positively with altered HOMA-IR index in morbidly obese individuals [82].
MMPs are regulated by tissue inhibitors of metalloproteinases (TIMPs), which comprise a family of four protease inhibitors: TIMP1, TIMP2, TIMP3 and TIMP4 [83]. Circulating TIMP-1 and TIMP-2 are increased in patients with metabolic syndrome and diabetes [81]. Overexpression of TIMP1 in pancreatic β-cells protects mice from streptozotocin-induced β-cell death and diabetes [84]. Likewise, genetic deletion of TIMP2 results in obesity and glucose intolerance in chow-fed mice that is further exacerbated in HF-fed mice [85]. TIMP3 is reduced in the adipose tissue of experimental models of obesity and insulin resistance [86]. Genetic deletion of TIMP3 in mice promotes adipose tissue inflammation [87] and TIMP3 overexpression in macrophages protects against adipose tissue inflammation and insulin resistance [88]. These data suggest that increased tissue TIMPs are protective from insulin resistance. This is paradoxical as TIMPs inhibit MMP activities. Thus in-depth investigations into the role of MMPs and TIMPs in obesity and insulin resistance should be a fruitful future direction for research.
Concluding remarks and future perspectives
The regulation of insulin action in the DIO state is complex. Many questions remain as to how metabolic disease develops and persists over time. The ECM and integrins are emerging as critical regulators of insulin action in the muscle, liver and adipose tissue. Until recently, few studies had addressed the contribution of the extracellular compartment to the regulation of glucose metabolism. The observation that ECM remodeling occurs in both human and rodent models of insulin resistance and T2D was a great step forward in understanding this previously uncharacterized portion of insulin sensitive tissues. ECM remodeling in the obese state has been attributed to increased inflammation and the subsequent up regulation of pro-fibrotic signaling molecules including TGFβ. It is currently unknown how the ECM regulates insulin action; however several hypotheses exist to explain this phenomenon. First, ECM remodeling generates a mechanical barrier for (a) glucose and insulin transport in the muscle and liver and (b) adipocyte hypertrophy in the adipose tissue under conditions of overnutrition. Second, changes in the composition of the ECM results in downstream alterations in integrin signaling that culminate in impaired insulin action. In light of these hypotheses, several important outstanding questions endure (Box 2). Future studies that seek to determine the mechanisms underlying diet-induced ECM remodeling and the mechanistic link between ECM remodeling, integrin signaling and insulin action in metabolic tissues are vital to advancing this great new line of investigation. In conclusion, the ECM and integrins are important regulators of insulin action and represent novel therapeutic targets to treat the underlying insulin resistance associated with T2D.
Box 2. Outstanding questions.
What role does inflammation have in diet-induced ECM remodeling? Which components of the inflammatory process are involved? Are the inflammatory stimuli acting locally or are they systemic?
When does ECM remodeling occur during a time-course of HF feeding in rodent models? How does this relate to insulin action?
Does ECM remodeling lead to insulin resistance by generating a physical barrier for glucose and insulin transport?
How do integrins regulate insulin action in vivo? What components of the integrin signaling cascade are important for insulin action?
Highlights.
Extracellular matrix remodeling occurs in response to diet-induced obesity.
Extracellular matrix remodeling is associated with insulin resistance.
ECM-integrin interactions regulate insulin action in vivo.
Acknowledgments
This work was supported by National Institutes of Health Grants DK54902 (DHW), DK050277 (DHW) and DK059637 (DHW).
Glossary
- Collagen
the most abundant structural protein consisting of three α polypeptide chains folded into a triple helix formation. Collagen proteins are divided into subgroups depending on their organization and/or molecular size that include the fibril forming collagens type I and III, the basement membrane associated collagen type IV and collagen type V, a minor ECM component
- Cirrhosis
late stage fibrosis of the liver as a result of different liver diseases and conditions such as hepatitis and chronic ethanol ingestion
- Endothelial dysfunction
deleterious alterations in endothelial physiology characterized by impaired endothelium-dependent vasodilation due to decreased availability of vasodilators such as NO and/or an increase in endothelium-derived contracting factors
- Extracellular matrix (ECM)
the space outside the cell composed of a complex meshwork of different proteins, proteoglycans, glycoproteins, polysaccharides and other structural proteins
- Glycosaminoglycans
large linear polysaccharides containing repeating disaccharide units with an amino sugar (either GlcNAc or GalNAc) and an uronic acid. Five identified glycosaminoglycan chains exist: hyaluronan, dermatan, keratin, chondroitin and keratan
- Homeostatic model assessment of insulin resistance (HOMA-IR)
method to assess insulin resistance and β-cell function, from basal (fasting) glucose and insulin or C-peptide concentrations
- Hyaluronan
an anionic, nonsulfated glycosaminoglycan. It is a major component of the ECM and has multiple functions, including creating space between cells and facilitating cell migration
- Hyperinsulinemic-euglycemic clamp (insulin clamp)
the gold standard for assessing insulin action in vivo. During the insulin clamp, insulin is infused at a constant rate and glucose is infused at a variable rate to maintain euglycemia. The amount of glucose that is infused reflects the insulin sensitivity. The insulin clamp can be combined with tracer techniques to determine sites of insulin resistance
- Interstitial space
the narrow, fluid filled areas that surround the cells of a tissue
- Myofibroblasts
cells in a state between a fibroblast and a smooth muscle cell. Fibrogenic cells are not part of the normal tissue and are only present following cellular injury. Often characterized by the presence of ruffled membranes and a highly active endoplasmic reticulum
- Nonalcoholic fatty liver disease (NAFLD)
also known as fatty liver disease, refers to the accumulation of excess lipids in liver cells that can induce inflammation and fibrosis
- Oxidative stress
the imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses that may result in tissue damage
- Relaxin
a protein hormone that acts through two G-protein coupled receptors RXFP1 and RXFP2 and has effects on the cardiovascular system. The vascular effects of relaxin include vasodilation and a decrease in systemic vascular resistance
- Space of Disse
The sinusoidal endothelium is separated from hepatocytes by the space of Disse where all metabolites from the bloodstream must pass through to reach the hepatocytes. The surface area of hepatocytes exposed to the space of Disse is greatly enhanced by the presence of microvilli. Under normal conditions, the space of Disse is filled with loosely assembled, low-density extracellular matrix (ECM) proteins
- Stellate cells
previously known as Ito cells, are quiescent vitamin A rich cells. Following liver injury, they transforms into activated proliferative and fibrogenic myofibroblasts. This process is initiated by autocrine and paracrine stimuli including inflammatory cytokines and growth factors
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Hernandez A, et al. The extracellular matrix in hepatic regeneration. FASEB J. 1995;9:1401–1410. doi: 10.1096/fasebj.9.14.7589981. [DOI] [PubMed] [Google Scholar]
- 3.Schuppan D. Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis. 1990;10:1–10. doi: 10.1055/s-2008-1040452. [DOI] [PubMed] [Google Scholar]
- 4.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 5.Moser M, et al. The tail of integrins, talin, and kindlins. Science. 2009;324:895–899. doi: 10.1126/science.1163865. [DOI] [PubMed] [Google Scholar]
- 6.Pozzi A, et al. Integrins: sensors of extracellular matrix and modulators of cell function. Nephron Experimental nephrology. 2003;94:e77–84. doi: 10.1159/000072025. [DOI] [PubMed] [Google Scholar]
- 7.Richardson DK, et al. Lipid infusion decreases the expression of nuclear encoded mitochondrial genes and increases the expression of extracellular matrix genes in human skeletal muscle. J Biol Chem. 2005;280:10290–10297. doi: 10.1074/jbc.M408985200. [DOI] [PubMed] [Google Scholar]
- 8.Berria R, et al. Increased collagen content in insulin-resistant skeletal muscle. American journal of physiology Endocrinology and metabolism. 2006;290:E560–565. doi: 10.1152/ajpendo.00202.2005. [DOI] [PubMed] [Google Scholar]
- 9.Kang L, et al. Diet-induced muscle insulin resistance is associated with extracellular matrix remodeling and interaction with integrin alpha2beta1 in mice. Diabetes. 2011;60:416–426. doi: 10.2337/db10-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wada T, et al. Eplerenone ameliorates the phenotypes of metabolic syndrome with NASH in liver-specific SREBP-1c Tg mice fed high-fat and high-fructose diet. American journal of physiology Endocrinology and metabolism. 2013;305:E1415–1425. doi: 10.1152/ajpendo.00419.2013. [DOI] [PubMed] [Google Scholar]
- 11.Dixon LJ, et al. Caspase-1 as a central regulator of high fat diet-induced non-alcoholic steatohepatitis. PloS one. 2013;8:e56100. doi: 10.1371/journal.pone.0056100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Williams AS, et al. Integrin alpha1-null Mice Exhibit Improved Fatty Liver When Fed a High Fat Diet Despite Severe Hepatic Insulin Resistance. J Biol Chem. 2015 doi: 10.1074/jbc.M114.615716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber J, et al. Prevention of high-fat diet-induced adipose tissue remodeling in obese diabetic mice by n-3 polyunsaturated fatty acids. Int J Obes (Lond) 2007;31:1004–1013. doi: 10.1038/sj.ijo.0803511. [DOI] [PubMed] [Google Scholar]
- 14.Inoue M, et al. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology. 2013;154:4548–4559. doi: 10.1210/en.2013-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang L, et al. Matrix metalloproteinase 9 opposes diet-induced muscle insulin resistance in mice. Diab tologia. 2014;57:603–613. doi: 10.1007/s00125-013-3128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang L, et al. Hyaluronan accumulates with high-fat feeding and contributes to insulin resistance. Diabetes. 2013;62:1888–1896. doi: 10.2337/db12-1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watts R, et al. Increased Smad signaling and reduced MRF expression in skeletal muscle from obese subjects. Obesity (Silver Spring) 2013;21:525–528. doi: 10.1002/oby.20070. [DOI] [PubMed] [Google Scholar]
- 18.Hong EG, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58:2525–2535. doi: 10.2337/db08-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varma V, et al. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. American journal of physiology Endocrinology and metabolism. 2009;296:E1300–1310. doi: 10.1152/ajpendo.90885.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav H, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell metabolism. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tam CS, et al. Weight gain reveals dramatic increases in skeletal muscle extracellular matrix remodeling. J Clin Endocrinol Metab. 2014;99:1749–1757. doi: 10.1210/jc.2013-4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nature reviews Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- 23.Dasu MR, et al. Increased toll-like receptor (TLR) activation and TLR ligands in recently diagnosed type 2 diabetic subjects. Diabetes Care. 2010;33:861–868. doi: 10.2337/dc09-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chajara A, et al. Increased hyaluronan and hyaluronidase production and hyaluronan degradation in injured aorta of insulin-resistant rats. Arterioscler Thromb Vasc Biol. 2000;20:1480–1487. doi: 10.1161/01.atv.20.6.1480. [DOI] [PubMed] [Google Scholar]
- 25.Lewis A, et al. Diabetic nephropathy, inflammation, hyaluronan and interstitial fibrosis. Histol Histopathol. 2008;23:731–739. doi: 10.14670/HH-23.731. [DOI] [PubMed] [Google Scholar]
- 26.Jansson PA. Endothelial dysfunction in insulin resistance and type 2 diabetes. J Intern Med. 2007;262:173–183. doi: 10.1111/j.1365-2796.2007.01830.x. [DOI] [PubMed] [Google Scholar]
- 27.Solomon TP, et al. Progressive hyperglycemia across the glucose tolerance continuum in older obese adults is related to skeletal muscle capillarization and nitric oxide bioavailability. J Clin Endocrinol Metab. 2011;96:1377–1384. doi: 10.1210/jc.2010-2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonner JS, et al. Muscle-specific vascular endothelial growth factor deletion induces muscle capillary rarefaction creating muscle insulin resistance. Diabetes. 2013;62:572–580. doi: 10.2337/db12-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonner JS, et al. Relaxin treatment reverses insulin resistance in mice fed a high-fat diet. Diabetes. 2013;62:3251–3260. doi: 10.2337/db13-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gullberg D, et al. Integrins during muscle development and in muscular dystrophies. Front Biosci. 1998;3:D1039–1050. doi: 10.2741/a344. [DOI] [PubMed] [Google Scholar]
- 31.Zong H, et al. Insulin resistance in striated muscle-specific integrin receptor beta1-deficient mice. J Biol Chem. 2009;284:4679–4688. doi: 10.1074/jbc.M807408200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bisht B, et al. Focal adhesion kinase regulates insulin resistance in skeletal muscle. Diab tologia. 2007;50:1058–1069. doi: 10.1007/s00125-007-0591-6. [DOI] [PubMed] [Google Scholar]
- 33.Huang D, et al. Reduced expression of focal adhesion kinase disrupts insulin action in skeletal muscle cells. Endocrinology. 2006;147:3333–3343. doi: 10.1210/en.2005-0382. [DOI] [PubMed] [Google Scholar]
- 34.Bisht B, et al. In vivo inhibition of focal adhesion kinase causes insulin resistance. The Journal of physiology. 2008;586:3825–3837. doi: 10.1113/jphysiol.2008.157107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bisht B, et al. Focal Adhesion Kinase contributes to insulin-induced actin reorganization into a mesh harboring Glucose transporter-4 in insulin resistant skeletal muscle cells. BMC cell biology. 2008;9:48. doi: 10.1186/1471-2121-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gheyara AL, et al. Deletion of integrin-linked kinase from skeletal muscles of mice resembles muscular dystrophy due to alpha 7 beta 1-integrin deficiency. Am J Pathol. 2007;171:1966–1977. doi: 10.2353/ajpath.2007.070555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zamurs LK, et al. Aberrant mitochondria in a Bethlem myopathy patient with a homozygous amino acid substitution that destabilizes the collagen VI alpha2(VI) chain. J Biol Chem. 2015;290:4272–4281. doi: 10.1074/jbc.M114.632208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Irwin WA, et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat Genet. 2003;35:367–371. doi: 10.1038/ng1270. [DOI] [PubMed] [Google Scholar]
- 39.Groh S, et al. Sarcoglycan complex: implications for metabolic defects in muscular dystrophies. J Biol Chem. 2009;284:19178–19182. doi: 10.1074/jbc.C109.010728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Day CP, et al. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 41.Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000;275:2247–2250. doi: 10.1074/jbc.275.4.2247. [DOI] [PubMed] [Google Scholar]
- 42.Gressner OA, et al. Differential effects of TGF-beta on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytes. J Hepatol. 2007;47:699–710. doi: 10.1016/j.jhep.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 43.Eng FJ, et al. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. American journal of physiology Gastrointestinal and liver physiology. 2000;279:G7–G11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- 44.Carmiel-Haggai M, et al. A high-fat diet leads to the progression of non-alcoholic fatty liver disease in obese rats. FASEB J. 2005;19:136–138. doi: 10.1096/fj.04-2291fje. [DOI] [PubMed] [Google Scholar]
- 45.McCuskey RS, et al. Hepatic microvascular dysfunction during evolution of dietary steatohepatitis in mice. Hepatology. 2004;40:386–393. doi: 10.1002/hep.20302. [DOI] [PubMed] [Google Scholar]
- 46.Bataller R, et al. Hepatic stellate cells as a target for the treatment of liver fibrosis. Semin Liver Dis. 2001;21:437–451. doi: 10.1055/s-2001-17558. [DOI] [PubMed] [Google Scholar]
- 47.Friedman SL, et al. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985;82:8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maher JJ, et al. Collagen measured in primary cultures of normal rat hepatocytes derives from lipocytes within the monolayer. J Clin Invest. 1988;82:450–459. doi: 10.1172/JCI113618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Postic C, et al. DNA excision in liver by an albumin-Cre transgene occurs progressively with age. Genesis. 2000;26:149–150. doi: 10.1002/(sici)1526-968x(200002)26:2<149::aid-gene16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 50.Jaskiewicz K, et al. Fibrogenesis in fatty liver associated with obesity and diabetes mellitus type 2. Dig Dis Sci. 2008;53:785–788. doi: 10.1007/s10620-007-9942-x. [DOI] [PubMed] [Google Scholar]
- 51.Harrison SA. Liver disease in patients with diabetes mellitus. J Clin Gastroenterol. 2006;40:68–76. doi: 10.1097/01.mcg.0000190774.91875.d2. [DOI] [PubMed] [Google Scholar]
- 52.Kodama K, et al. Expression-based genome-wide association study links the receptor CD44 in adipose tissue with type 2 diabetes. Proc Natl Acad Sci U S A. 2012;109:7049–7054. doi: 10.1073/pnas.1114513109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bertola A, et al. Elevated expression of osteopontin may be related to adipose tissue macrophage accumulation and liver steatosis in morbid obesity. Diabetes. 2009;58:125–133. doi: 10.2337/db08-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kodama K, et al. Anti-CD44 Antibody Treatment Lowers Hyperglycemia and Improves Insulin Resistance, Adipose Inflammation, and Hepatic Steatosis in Diet-Induced Obese Mice. Diabetes. 2015;64:867–875. doi: 10.2337/db14-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farrell GC, et al. Hepatic microcirculation in fatty liver disease. Anat Rec (Hoboken) 2008;291:684–692. doi: 10.1002/ar.20715. [DOI] [PubMed] [Google Scholar]
- 56.Madison LL, et al. Evidence for a direct effect of insulin on hepatic glucose output. Metabolism. 1959;8:469–471. [PubMed] [Google Scholar]
- 57.Duckworth WC, et al. Insulin degradation: progress and potential. Endocr Rev. 1998;19:608–624. doi: 10.1210/edrv.19.5.0349. [DOI] [PubMed] [Google Scholar]
- 58.Field JB. Extraction of insulin by liver. Annu Rev Med. 1973;24:309–314. doi: 10.1146/annurev.me.24.020173.001521. [DOI] [PubMed] [Google Scholar]
- 59.Duckworth WC, et al. Insulin degradation by hepatocytes in primary culture. Endocrinology. 1981;108:1142–1147. doi: 10.1210/endo-108-4-1142. [DOI] [PubMed] [Google Scholar]
- 60.Volpes R, et al. Distribution of the VLA family of integrins in normal and pathological human liver tissue. Gastroenterology. 1991;101:200–206. doi: 10.1016/0016-5085(91)90478-4. [DOI] [PubMed] [Google Scholar]
- 61.Gardner H, et al. Deletion of integrin alpha 1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- 62.Cheung AT, et al. Tumor necrosis factor-alpha induces hepatic insulin resistance in obese Zucker (fa/fa) rats via interaction of leukocyte antigen-related tyrosine phosphatase with focal adhesion kinase. Diabetes. 2000;49:810–819. doi: 10.2337/diabetes.49.5.810. [DOI] [PubMed] [Google Scholar]
- 63.Huang D, et al. Focal adhesion kinase (FAK) regulates insulin-stimulated glycogen synthesis in hepatocytes. J Biol Chem. 2002;277:18151–18160. doi: 10.1074/jbc.M104252200. [DOI] [PubMed] [Google Scholar]
- 64.El Annabi S, et al. Focal adhesion kinase and Src mediate integrin regulation of insulin receptor phosphorylation. FEBS Lett. 2001;507:247–252. doi: 10.1016/s0014-5793(01)02981-7. [DOI] [PubMed] [Google Scholar]
- 65.Cheung AT, et al. An in vivo model for elucidation of the mechanism of tumor necrosis factor-alpha (TNF-alpha)-induced insulin resistance: evidence for differential regulation of insulin signaling by TNF-alpha. Endocrinology. 1998;139:4928–4935. doi: 10.1210/endo.139.12.6336. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, et al. Involvement of integrin-linked kinase in carbon tetrachloride-induced hepatic fibrosis in rats. Hepatology. 2006;44:612–622. doi: 10.1002/hep.21315. [DOI] [PubMed] [Google Scholar]
- 67.Gkretsi V, et al. Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology. 2007;45:1025–1034. doi: 10.1002/hep.21540. [DOI] [PubMed] [Google Scholar]
- 68.Sakai T, et al. Integrin-linked kinase (ILK) is required for polarizing the epiblast, cell adhesion, and controlling actin accumulation. Genes Dev. 2003;17:926–940. doi: 10.1101/gad.255603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Catalan V, et al. Role of extracellular matrix remodelling in adipose tissue pathophysiology: relevance in the development of obesity. Histol Histopathol. 2012;27:1515–1528. doi: 10.14670/HH-27.1515. [DOI] [PubMed] [Google Scholar]
- 70.Pasarica M, et al. Adipose tissue collagen VI in obesity. J Clin Endocrinol Metab. 2009;94:5155–5162. doi: 10.1210/jc.2009-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Khan T, et al. Metabolic dysregulation and adipose tissue fibrosis: role of collagen VI. Mol Cell Biol. 2009;29:1575–1591. doi: 10.1128/MCB.01300-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Halberg N, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang B, et al. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Keophiphath M, et al. Macrophage-secreted factors promote a profibrotic phenotype in human preadipocytes. Mol Endocrinol. 2009;23:11–24. doi: 10.1210/me.2008-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun K, et al. Fibrosis and adipose tissue dysfunction. Cell metabolism. 2013;18:470–477. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun K, et al. Endotrophin triggers adipose tissue fibrosis and metabolic dysfunction. Nature communications. 2014;5:3485. doi: 10.1038/ncomms4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Catalan V, et al. Increased tenascin C and Toll-like receptor 4 levels in visceral adipose tissue as a link between inflammation and extracellular matrix remodeling in obesity. J Clin Endocrinol Metab. 2012;97:E1880–1889. doi: 10.1210/jc.2012-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thrailkill KM, et al. Matrix metalloproteinases: their potential role in the pathogenesis of diabetic nephropathy. Endocrine. 2009;35:1–10. doi: 10.1007/s12020-008-9114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Derosa G, et al. Matrix metalloproteinase-2 and -9 levels in obese patients. Endothelium. 2008;15:219–224. doi: 10.1080/10623320802228815. [DOI] [PubMed] [Google Scholar]
- 80.Signorelli SS, et al. Plasma levels and zymographic activities of matrix metalloproteinases 2 and 9 in type II diabetics with peripheral arterial disease. Vasc Med. 2005;10:1–6. doi: 10.1191/1358863x05vm582oa. [DOI] [PubMed] [Google Scholar]
- 81.Hopps E, et al. Gelatinases and their tissue inhibitors in a group of subjects with metabolic syndrome. J Investig Med. 2013;61:978–983. doi: 10.2310/JIM.0b013e318294e9da. [DOI] [PubMed] [Google Scholar]
- 82.Tinahones FJ, et al. Obesity-associated insulin resistance is correlated to adipose tissue vascular endothelial growth factors and metalloproteinase levels. BMC physiology. 2012;12:4. doi: 10.1186/1472-6793-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brew K, et al. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiang H, et al. TIMP-1 transgenic mice recover from diabetes induced by multiple low-dose streptozotocin. Diabetes. 2007;56:49–56. doi: 10.2337/db06-0710. [DOI] [PubMed] [Google Scholar]
- 85.Jaworski DM, et al. Sexually dimorphic diet-induced insulin resistance in obese tissue inhibitor of metalloproteinase-2 (TIMP-2)-deficient mice. Endocrinology. 2011;152:1300–1313. doi: 10.1210/en.2010-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Demeulemeester D, et al. Overexpression of tissue inhibitor of matrix metalloproteinases-1 (TIMP-1) in mice does not affect adipogenesis or adipose tissue development. Thromb Haemost. 2006;95:1019–1024. doi: 10.1160/TH05-11-0742. [DOI] [PubMed] [Google Scholar]
- 87.Menghini R, et al. Tissue inhibitor of metalloproteinase 3 deficiency causes hepatic steatosis and adipose tissue inflammation in mice. Gastroenterology. 2009;136:663–672. e664. doi: 10.1053/j.gastro.2008.10.079. [DOI] [PubMed] [Google Scholar]
- 88.Menghini R, et al. TIMP3 overexpression in macrophages protects from insulin resistance, adipose inflammation, and nonalcoholic fatty liver disease in mice. Diabetes. 2012;61:454–462. doi: 10.2337/db11-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hanks SK, et al. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Plows LD, et al. Integrin engagement modulates the phosphorylation of focal adhesion kinase, phagocytosis, and cell spreading in molluscan defence cells. Biochim Biophys Acta. 2006;1763:779–786. doi: 10.1016/j.bbamcr.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 91.Schlaepfer DD, et al. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- 92.Ilic D, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 93.Delcommenne M, et al. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci U S A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Troussard AA, et al. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J Biol Chem. 2003;278:22374–22378. doi: 10.1074/jbc.M303083200. [DOI] [PubMed] [Google Scholar]
- 95.Wang HV, et al. Integrin-linked kinase stabilizes myotendinous junctions and protects muscle from stress-induced damage. J Cell Biol. 2008;180:1037–1049. doi: 10.1083/jcb.200707175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.White DE, et al. Targeted ablation of ILK from the murine heart results in dilated cardiomyopathy and spontaneous heart failure. Genes Dev. 2006;20:2355–2360. doi: 10.1101/gad.1458906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vaynberg J, et al. Weak protein-protein interactions as probed by NMR spectroscopy. Trends Biotechnol. 2006;24:22–27. doi: 10.1016/j.tibtech.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Qian F, et al. Interaction between integrin alpha(5) and fibronectin is required for metastasis of B16F10 melanoma cells. Biochem Biophys Res Commun. 2005;333:1269–1275. doi: 10.1016/j.bbrc.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 99.Hill MM, et al. Identification of a plasma membrane Raft-associated PKB Ser473 kinase activity that is distinct from ILK and PDK1. Curr Biol. 2002;12:1251–1255. doi: 10.1016/s0960-9822(02)00973-9. [DOI] [PubMed] [Google Scholar]
- 100.Persad S, et al. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase activity and amino acids arginine 211 and serine 343. J Biol Chem. 2001;276:27462–27469. doi: 10.1074/jbc.M102940200. [DOI] [PubMed] [Google Scholar]