Abstract
The epithelial-mesenchymal transition (EMT) has been postulated as a mechanism by which cancer cells acquire the invasive and stem-like traits necessary for distant metastasis. However, direct in vivo evidence for the role of EMT in the formation of cancer stem-like cells (CSC) and the metastatic cascade remains lacking. Here we report the first isolation and characterization of mesenchymal and EMT tumor cells, which harbor both epithelial and mesenchymal characteristics, in an autochthonous murine model of prostate cancer. By crossing the established Pb-Cre+/−;PtenL/L;KrasG12D/+ prostate cancer model with a vimentin-GFP reporter strain, generating CPKV mice, we were able to isolate epithelial, EMT and mesenchymal cancer cells based on expression of vimentin and EpCAM. CPKV mice (but not mice with Pten deletion alone) exhibited expansion of cells with EMT (EpCAM+/Vim-GFP+) and mesenchymal (EpCAM−/Vim-GFP+) characteristics at the primary tumor site and in circulation. These EMT and mesenchymal tumor cells displayed enhanced stemness and invasive character compared to epithelial tumor cells. Moreover, they displayed an enriched tumor-initiating capacity and could regenerate epithelial glandular structures in vivo, indicative of epithelia-mesenchyme plasticity. Interestingly, while mesenchymal tumor cells could persist in circulation and survive in the lung following intravenous injection, only epithelial and EMT tumor cells could form macrometastases. Our work extends the evidence that mesenchymal and epithelial states in cancer cells contribute differentially to their capacities for tumor initiation and metastatic seeding, respectively, and that EMT tumor cells exist with plasticity that can contribute to multiple stages of the metastatic cascade.
Keywords: EMT, prostate cancer, mouse models, cancer stem cells, metastasis
Introduction
Prostate cancer is the most commonly diagnosed male malignancy and the second leading cause of cancer-related death in Western men (1). Although localized prostate cancer is treatable, metastatic, late stage castration-resistant prostate cancer (mCRPC) is currently incurable and represents the major cause of prostate cancer-related death (2). Recent studies have focused on the processes and pathway alterations that cause prostate tumor cells to disseminate and metastasize. We and others have shown that activation of the PI3K/AKT and RAS/MAPK pathways is associated with metastatic prostate cancer, and that activation of both pathways is sufficient to induce distant metastasis and an epithelial-mesenchymal transition (EMT) at the primary tumor site in genetically engineered mouse models (3–5). The acquisition of an EMT phenotype within localized cancer has been demonstrated to be sufficient to trigger lethal metastatic disease (4, 6, 7) and promote CRPC (8–10) in multiple model systems.
EMT, in the context of cancer, allows epithelial cancer cells to acquire migratory and invasive characteristics, as well as overcome senescence, apoptosis, and anoikis, properties that are essential for tumor cell dissemination and distant metastasis (11). Moreover, recent studies have also implicated EMT in the acquisition of stem-like qualities (12–14) and drug resistance properties (15). However, evidence for the role of EMT in cancer stem cell formation and metastasis is mostly based on either in vitro manipulation of cultured cell lines to induce EMT or the expression of EMT signature markers in human cancer samples (10). Therefore, a direct role for EMT in prostate tumor progression, dissemination of circulating tumor cells (CTCs) into the blood stream, and seeding of metastases at distant sites remains unclear due to the lack of in vivo models that recapitulate the metastatic process.
We previously reported that deletion of the Pten tumor suppressor gene and conditional activation of the KrasG12D oncogene in the murine prostate epithelium (Pb-Cre+/−;PtenL/L;KrasG12D/+) leads to 100% penetrable macrometastasis to the lungs and liver, and an EMT phenotype at the primary tumor site (4). Although the Pb-Cre+/−;PtenL/L;KrasG12D/+ (CPK) prostate cancer mouse model recapitulates late-stage, metastatic human prostate cancer and associates EMT with prostate cancer metastasis, whether tumor cells that have undergone an EMT contribute directly to tumor progression, dissemination, and distant macrometastasis has yet to be established. In this study, we develop an in vivo system that allows tracking of the dynamic EMT program and isolation of cells from the CPK prostate cancer model that have either completed (mesenchymal) or are transitioning through an EMT (EMT) for characterization and functional testing. Our in vivo analysis suggests that mesenchymal and epithelial states contribute to different stages of the prostate cancer disease, and that EMT tumor cells, which have the plasticity to readily transition between epithelial and mesenchymal lineages, are able to contribute to multiple stages of the metastatic cascade.
Materials and Methods
Mouse strains
Vim-GFP reporter mice were purchased from GENSAT (16). After crossing Vim-GFP mice with the Cre+/−;PtenL/L;KrasG/+ model (4), Pb-Cre+/−;PtenL/L;Kras+/+;Vim-GFP male mice were crossed with Pb-Cre−;PtenL/L;KrasG12D/+ female mice to generate the Cre−;PtenL/L;KrasG/+;Vim-GFP (V), Cre+/−;PtenL/L;Kras+/+;Vim-GFP (CPV), and Cre+/−;PtenL/L;KrasG/+;Vim-GFP (CPKV) mouse models. These strains have been maintained on a mixed strain background. All studies with animals were performed under the regulation of the division of Laboratory Animal Medicine at the University of California at Los Angeles (UCLA).
Histology and immunohistochemistry
Immunohistochemistry (IHC) was performed on formalin-fixed, paraffin-embedded tissues. Antigen retrieval was performed by boiling sections in 10mM citrate buffer (pH6) for 30 minutes. The following primary antibodies were used: Vimentin (Cell Signaling; 5741), GFP (Cell Signaling; 2955), E-cadherin (BD Biosciences; 610181), P-S6 (Cell Signaling; 2215), PTEN (Cell Signaling; 9559), Ki67 (Vector Laboratories; VP-RM04), CK5 (Covance; PRB-160P), CK8 (Covance; MMS-162P), Synaptophysin (Dako; A0010), and Pan-Cytokeratin (Sigma; C1801).
Matrigel invasion assay
8μm transwell inserts (BD Biosciences) were coated with Matrigel (300 μg/ml) (BD Biosciences) and placed into 24-well culture plates. 5 ×104 sorted cells per population were resuspended in serum free media in the top chamber, while full serum media (Dulbecco’s modified eagle medium (DMEM) with 10% Fetal Bovine Serum (FBS)) was used in the bottom chamber. 24 hours later, invaded cells were fixed with methanol, stained with 0.2% crystal violet, and counted using a light microscope at 10X magnification.
Matrigel sphere assay
The Matrigel sphere assay was carried out as previously described (18). 5 × 103 sorted cells from each cell population were plated in triplicate.
BrdU pulse labeling
BrdU pulse labeling was carried out as previously described (19). 2 × 104 FACS sorted cells per population were cytospun onto coated cytoslides (Thermo Scientific) at 500 rpm for 5 minutes using Cytospin4 (Thermo Scientific). Cells were fixed with 4% paraformaldehyde for 15 minutes, and stained with a BrdU (BD Biosciences; 563445) primary antibody. 10 fields per slide were counted at 20x magnification.
Subcutaneous tumor regeneration assay
Prostate lobes/regions from CPKV mice were separated as described (Fig. 3B), serrated, mixed with Matrigel, and transplanted subcutaneously into NOD/SCID/IL2Rγ-null (NSG) mice. Once tumors reached 2 cm in size, mice were euthanized, and tumors were again serrated, mixed with Matrigel, and passaged into new recipient hosts.
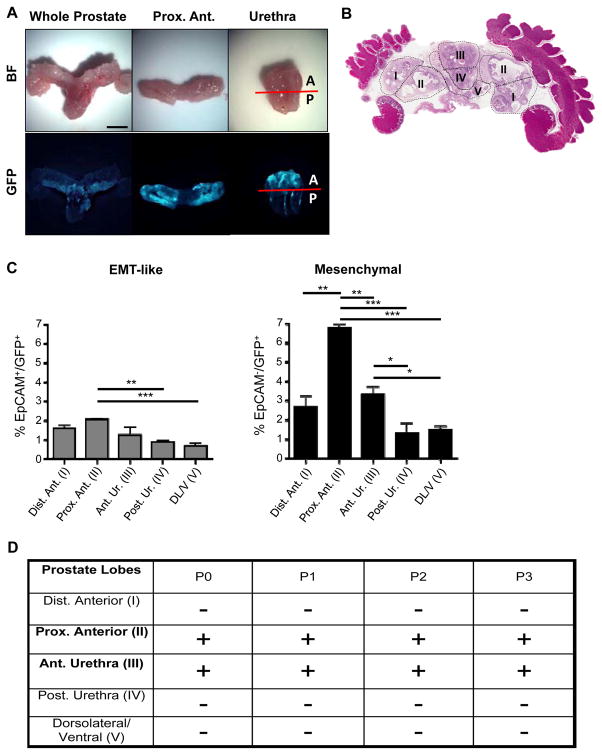
Figure 3.
Prostate regions enriched in Vim-GFP+ cells are able to regenerate transplantable tumors in vivo. A, Bright-field (BF) and fluorescent images of a whole-mount CPKV prostate (10 weeks). GFP expression in CPKV prostates is most prominent in the proximal region of the anterior lobes and in the anterior portion of the urethra. B, Diagram depicting how prostate regions/lobes were separated. I, distal region of the anterior lobe. II, proximal region of the anterior lobe. III, anterior portion of the urethra. IV, posterior portion of the urethra. V, dorsolateral/ventral lobes. C, FACS analysis demonstrates that the anterior portion of the urethra and proximal region of the anterior lobes express the highest percentage of mesenchymal cells compared to other regions/lobes from the prostates of CPKV mice (10–12 weeks). The percentage of EMT tumor cells was also slightly higher in the proximal region of the anterior lobes compared to other lobes/regions. D, The only CPKV prostate regions/lobes that produced transplantable tumors in NSG mice were the proximal region of the anterior lobe and anterior portion of the urethra. Data in C are represented as mean ± SEM. Bar, 4mm. A, anterior. P, posterior. Dist. Ant., distal region of the anterior lobe. Prox. Ant., proximal region of the anterior lobe. Ant. Ur., anterior portion of the urethra. Post. Ur., posterior portion of the urethra. DL/V, dorsolateral/ventral lobes. P0, passage 0. P1, passage 1. P2, passage 2. P3, passage 3. +, tumor. −, no tumor. *, P < 0.05, **, P < 0.01. ***, P < 0.001.
Orthotopic tumor regeneration assay
5 × 103 sorted cells per population were mixed in 50% Matrigel/media, loaded into a 10 μl Hamilton syringe (Microliter), and 2.5 × 103 cells were injected into each anterior lobe of the prostates of recipient NSG mice.
Tail vein injections
2.5 × 104 or 1 × 105 sorted cells from each population were resuspended in 200 μl of PBS and injected intravenously into NSG hosts. The presence of lung macrometastases was assessed by gross examination of formalin-fixed lung samples under a dissecting microscope.
Statistical analysis
Graphpad Prism software was used to calculate mean and standard deviation. Student’s t-test was used to calculate the statistical significance between the two groups of data. P < 0.05 is considered significant.
Results
Tracking EMT and mesenchymal tumor cells in an endogenous prostate cancer model using a Vimentin-GFP reporter line
In order to generate an in vivo tracking system to study the role of EMT in prostate cancer progression and metastasis, we crossed Vimentin-GFP mice (16) with the Pb-Cre+/−;PtenL/L;KrasG12D/+ (CPK) murine model of prostate cancer (4) we recently developed to create the Pb-Cre+/−;PtenL/L;KrasG12D/+;Vim-GFP model (CPKV). Vimentin-GFP reporter mice, in which GFP expression is driven from the endogenous Vimentin promoter on a bacterial artificial chromosome (BAC) (16), were chosen because Vimentin is one of the earliest upregulated genes during the EMT process (21), and its expression is associated with high Gleason scores, disease recurrence, and bone metastasis in human prostate cancers (22, 23).
In 10–12 week old CPKV prostates, GFP staining overlaps with endogenous Vimentin expression, which marks EMT regions within the stromal compartment surrounding GFP-negative epithelial glandular structures (Fig. 1A). These EMT regions also contain cells that are PTEN− and P-S6+, a surrogate marker for PTEN loss and activation of the PI3K pathway, confirming that these cells were originally derived from Ptenloxp/loxp prostate epithelial cells that underwent Cre recombination (Fig. 1A). As it is possible that endogenous stromal cells in the prostate, including CD45+ leukocytes, also express P-S6+, we stained prostate sections from 4 week old CPKV mice, a time-point when these mice have not yet developed invasive prostate tumors or an EMT phenotype, to identify if Vimentin+/GFP+ stromal cells in the prostate normally express P-S6+. Indeed, Vimentin+/GFP+ stromal cells in these prostates were PTEN+ and P-S6− (Supplementary Fig. S1A). Moreover, in WT Vim-GFP (V) prostates, all Vimentin+/GFP+ stromal cells are also P-S6− (Supplementary Fig. S1B). These results verify that P-S6 can be used as a marker in our CPKV model to distinguish Vimentin+/GFP+/PTEN+ stromal cells from Vimentin+/GFP+/PTEN− EMT cells derived from the Pten−/ − prostate epithelium.
Figure 1.
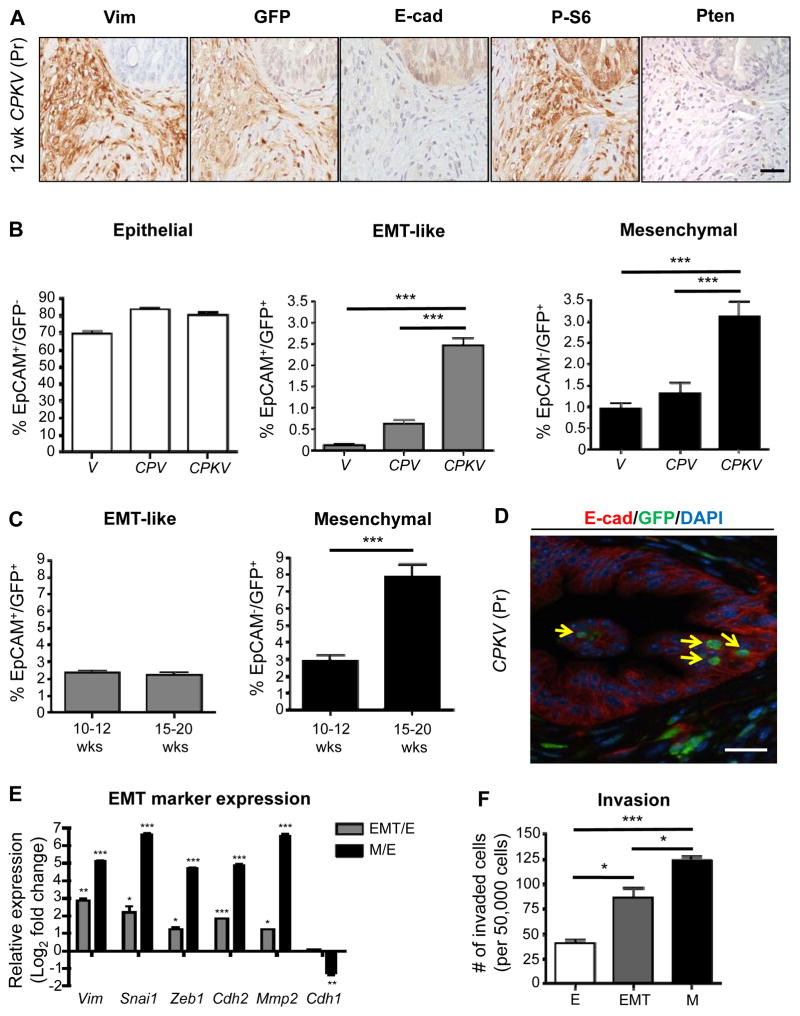
Tracking EMT and mesenchymal tumor cells in an endogenous prostate cancer model using a Vimentin-GFP reporter line. A, Prostates from CPKV mice (12 weeks) display EMT regions that are Vimentin (Vim)/GFP-positive surrounding E-cadherin (E-cad)-positive epithelial glandular structures. P-S6 marks cells that have undergone Cre recombination. B, Vimentin-GFP (GFP) and EpCAM were used to characterize epithelial (EpCAM+/GFP−), EMT (EpCAM+/GFP+), and mesenchymal (EpCAM−/GFP+) cell populations from the prostates of various Vim-GFP reporter mice (10–12 weeks) by FACS. CPKV mice have significant expansion of EMT and mesenchymal tumor cell populations. C, Expansion of mesenchymal tumor cell populations in CPKV prostates during late stage tumor progression (15–20 weeks). D, EMT cells (yellow arrow) that are GFP+ (green) and E-cadherin (E-cad)+ (red) are found within epithelial glandular structures in CPKV prostates (12 weeks). E, qPCR analysis confirms EMT signature gene expression in EMT and mesenchymal tumor cells isolated from CPKV prostates (10–12 weeks). F, Matrigel invasion assay reveals that EMT and mesenchymal tumor cells isolated from CPKV prostates (10–12 weeks) are significantly more invasive than epithelial tumor cells. Data in B, C, E, and F are represented as mean ± SEM. Bar, 50 μm. Pr, prostate. Lin−,CD45−/CD31−/Ter119−. *, P < 0.05, **, P < 0.01. ***, P < 0.001.
In order to isolate and characterize tumor cells with epithelial and mesenchymal characteristics from the prostates of CPKV mice, a FACS gating strategy was designed in which the epithelial cell adhesion molecule (EpCAM) was used as an epithelial marker and GFP as a mesenchymal marker (Supplementary Fig. S1A). Cells from CPKV prostates were first negatively selected from CD45+, CD31+, and Ter119+ fractions (referred to as Lin−) in order to avoid contamination from leukocyte, endothelial, and erythrocyte populations, respectively, as these cell types are known to express Vimentin (21). While age-matched WT Vim-GFP (V) (n=8) and Pb-Cre+/−;PtenL/L;Vim-GFP (CPV) (n=8) mutant prostates have a very rare population of cells that are EpCAM−/GFP+, which we will refer to as mesenchymal tumor cells, CPKV mutants (n=13) have a significant induction of the mesenchymal tumor cell population by 10 weeks of age (Fig. 1B and Supplementary Fig. S1C). Interestingly, a significant population of cells that co-express both epithelial and mesenchymal markers (EpCAM+GFP+), hereafter referred to as EMT cells, could also be isolated from 10–12 week old CPKV, and, to a small extent, CPV prostates, but not V prostates (Fig. 1B and Supplementary Fig. S1C). While there was a marginal increase in the percentage of EpCAM+GFP− epithelial cells within CPV and CPKV prostates compared to V prostates, this change was not significant (Fig. 1B and Supplementary Fig. S1A). Genomic PCR analysis confirmed that all three FACS isolated tumor cell populations were indeed derived from epithelial cells that initially underwent Cre recombination, as they exhibit Pten deletion and Kras activation (Supplementary Fig. S1D).
To assess the association of the EMT and mesenchymal tumor cell populations with prostate cancer progression, we compared 10–12 week old CPKV mutants (n=11), which have begun to develop poorly differentiated EMT morphology, to late stage 15–20 week old mutants (n=9) with sarcomatoid morphology (4). While there was a significant expansion of mesenchymal tumor cells during tumor progression, there was no change in the percentage of EMT tumor cells in the 15–20 week old mutants (Fig. 1C), indicating that EMT tumor cells may indeed represent a transition stage. Histologically, rare EMT tumor cells that co-expressed both epithelial (E-cadherin; red) and mesenchymal markers (Vim-GFP; Green) were found within epithelial acinar structures, indicating that the early steps of the EMT process do not occur exclusively along the leading edge of the tumor (Fig. 1D, yellow arrows).
Finally, we confirmed that EpCAM+/GFP+ and EpCAM−/GFP+ cell populations isolated from CPKV prostates indeed display EMT signature gene expression by qPCR analysis. While both EMT and mesenchymal tumor cells had induced expression of EMT signature genes compared to epithelial tumor cells, mesenchymal prostate tumor cells displayed more dramatically increased expression of the EMT transcription factors Snail and Zeb1, as well as a switch from E-cadherin (Cdh1) to N-cadherin (Cdh2) expression (Fig. 1E). Mesenchymal tumor cells were also significantly more invasive ex vivo compared to epithelial tumor cells, with EMT tumor cells displaying an intermediate invasive capacity (Fig. 1F). Taken together, we have developed a novel system by using a Vim-GFP reporter to faithfully track the dynamics of the EMT program and to isolate and characterize cells undergoing or having undergone an EMT in an endogenous prostate cancer model.
EMT and mesenchymal tumor cells have enhanced stemness properties
To explore whether EMT and mesenchymal tumor cells from an endogenous prostate cancer model have enhanced stemness qualities, tumor cell populations from primary CPKV prostates were isolated by FACS and grown in Matrigel cultures to assess their ability to form spheres, an in vitro assay used to assess stemness characteristics (18). Indeed, EMT tumor cells could generate significantly more spheres than both epithelial and mesenchymal tumor cells (Fig. 2A). The EMT tumor cell subpopulation also contained a higher proportion of Lin−Sca1+CD49fhigh (LSChi) stem/progenitor cells (Fig. 2B), which have been previously shown to have a basal cell phenotype, high sphere-forming activity, and be sufficient to initiate tumorigenesis and metastasis (4, 24). These findings suggest that EMT tumor cells may represent a subpopulation of tumor cells with enhanced stemness qualities.
Figure 2.
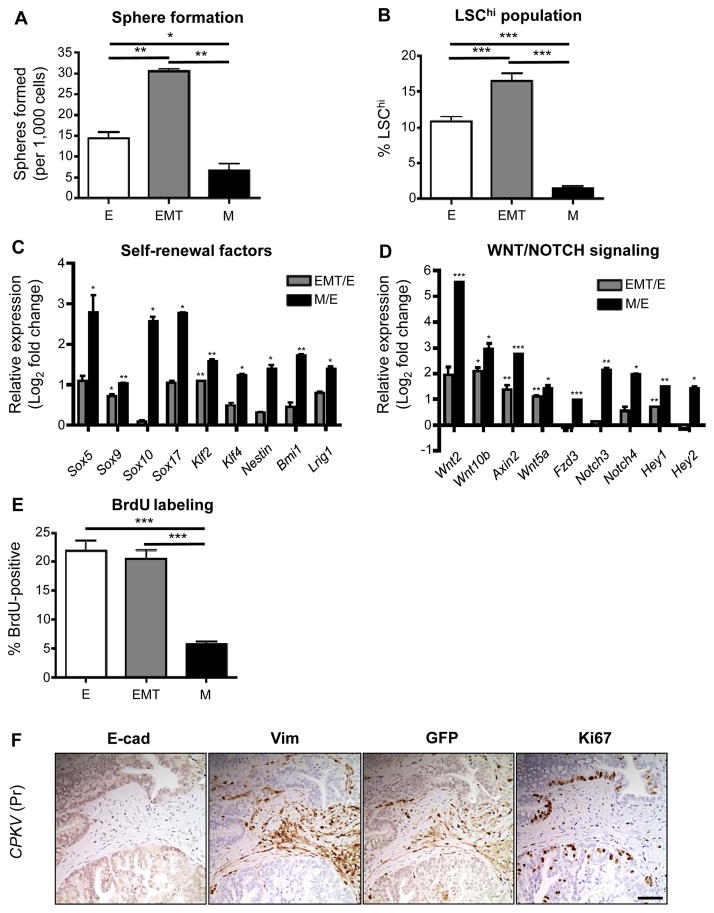
EMT and mesenchymal tumor cells have enhanced stemness properties. A, Matrigel sphere assay reveals that EMT tumor cells sorted from CPKV prostates (10–12 weeks) form more spheres than epithelial and mesenchymal tumor cells after 7 days in culture. B, EMT tumor cells have a higher LSChi content compared to epithelial and mesenchymal tumor cells from CPKV prostates (10–12 weeks), as quantified by FACS. C, EMT and mesenchymal tumor cells from CPKV prostates (10–12 weeks) have enhanced expression of self-renewal and stemness factors compared to epithelial cells. D, EMT and mesenchymal tumor cells from CPKV prostates (10–12 weeks) have enhanced expression of genes involved in WNT and NOTCH signaling compared to epithelial cells. E, Epithelial and EMT tumor cells from CPKV prostates (10–12 weeks) have a higher percentage of cells in S-phase compared to mesenchymal tumor cells, as measured by the percentage of BrdU+ cells. F, Ki67-positive cells are found preferentially in E-cadherin (E-cad)-positive glandular structures compared to Vimentin (Vim)/GFP-positive EMT regions in the stroma of CPKV prostates (12 weeks). Data in A through E are represented as mean ± SEM. Bar, 100 μm. Pr, prostate. *, P < 0.05, **, P < 0.01. ***, P < 0.001.
Compared to both epithelial and EMT tumor cell populations, mesenchymal tumor cells had reduced in vitro sphere-forming capacity (Fig. 2A). However, mesenchymal tumor cells had significantly increased expression of genes involved in maintenance of the stem cell state, including the Sox family members Sox5, Sox9, Sox10, and Sox17, the reprogramming factors Klf2 and Klf4, the self-renewal maintenance factors Bmi1, Nestin, and Lrig1, and members of the WNT and NOTCH developmental pathways, suggesting that mesenchymal tumor cells may also harbor stemness qualities (Fig. 2C and 2D). One possible explanation for their poor sphere-forming capacity may be that mesenchymal tumor cells exist in a quiescent state and therefore have less capacity to proliferate and form sphere structures (25, 26). To test this hypothesis, we pulse labeled CPKV mice with 5-bromo-2′-deoxyuridine (BrdU) for 24 hours prior to FACS sorting to assess the percentage of cycling cells in each population. While the epithelial and EMT tumor cell populations had a high percentage of cells in S-phase, the mesenchymal tumor cell population had a much lower percentage of cycling cells (Fig. 2E). In support of this finding, our Ki67 immunohistochemical analysis revealed that E-cadherin-positive glandular structures are more proliferative than GFP-positive mesenchymal regions in the stroma (Fig. 2F). In summary, both EMT and mesenchymal tumor cells from CPKV mutants have enhanced stemness characteristics, with EMT tumor cells displaying a proliferative, stem/progenitor cell phenotype, and mesenchymal tumor cells exhibiting a quiescent stem cell phenotype.
Prostate regions enriched in Vim-GFP+ cells are able to regenerate transplantable tumors in vivo
As prostate stem cells have been previously shown to reside in the proximal region of the prostate attached to the urethra (25), we next wanted to ascertain if EMT and mesenchymal tumor cells were also enriched in this stem cell niche. Gross fluorescent imaging of whole CPKV prostates revealed that Vim-GFP expression was most prominent in the proximal anterior lobes, and, interestingly, in the anterior portion of the urethra itself (Fig. 3A). When distinct anatomical regions of the prostate were separated from 10-week old CPKV mice with established tumors (Fig. 3B) and subjected to quantitative FACS analysis, the percentage of mesenchymal tumor cells was significantly higher in the proximal region of the anterior lobes and the anterior portion of the urethra, the regions immediately connected to the proximal region of the prostate, compared to other regions of CPKV prostates (Fig. 3C, right panel, n=3). Although the percentage of EMT tumor cells was slightly higher in the proximal region of anterior lobes, the percentage of EMT tumor cells did not change dramatically between the different regions of the prostate (Fig. 3C, left panel, n=3). This data suggests that mesenchymal tumor cells may indeed have properties of quiescent stem cells, which are localized to specific stem cell niches.
To test whether the regions of CPKV prostates with the highest percentage of EMT and mesenchymal tumor cells also have the highest tumor-initiating capacity, regions/lobes were separated from 10-week old CPKV prostates, mixed with matrigel, and implanted subcutaneously into NOD/SCID/IL2Rγ-null (NSG) mice. Remarkably, the only regions that were able to form subcutaneous tumors in vivo were from the proximal region of the anterior gland and anterior portion of the urethra, the same regions with the highest percentage of EMT and mesenchymal tumor cells (Fig. 3D, n=4). Tumors from both regions were able to be serially passaged, and these passaged tumors contained all 3 prostatic cell lineages (CK5+ basal, CK8+ luminal, and Synaptophysin+ neuroendocrine cells) (Supplementary Fig. S2), demonstrating that stem cells within these regions have the capacity to regenerate all prostate lineages.
EMT and mesenchymal tumor cells have enhanced tumor-initiating capacity and cellular plasticity in vivo
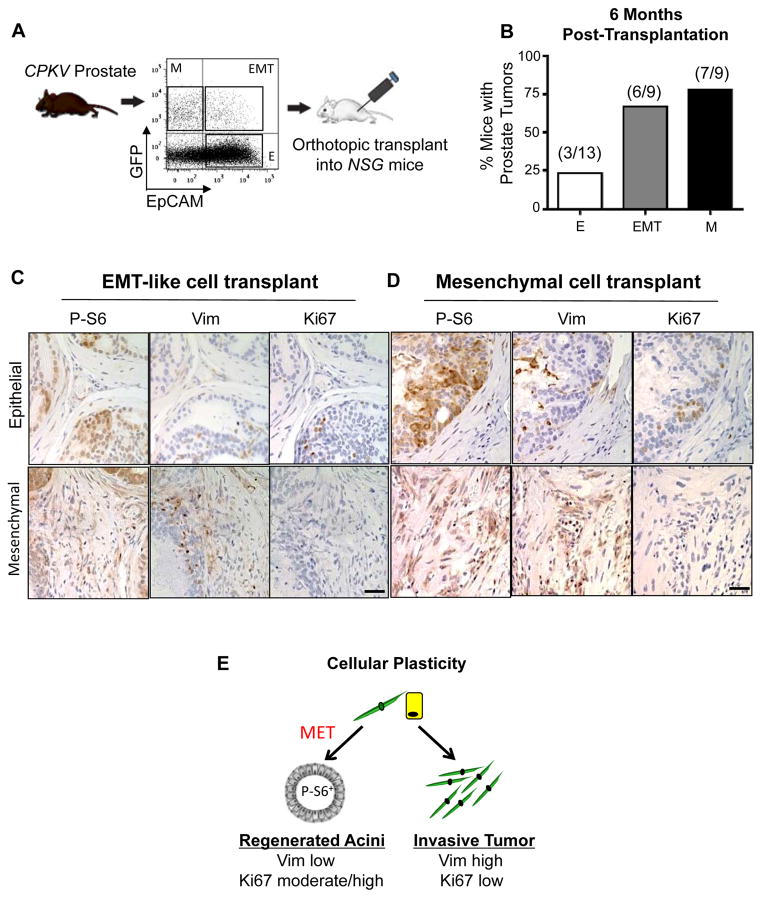
In order to directly assess the tumor-initiating capacity of epithelial, EMT and mesenchymal tumor cells isolated from CPKV mice, 5,000 sorted cells from each population were implanted orthotopically into the anterior lobes of NSG mice and allowed to incubate up to 6 months (Fig. 4A). Tumor pathology was defined histologically by the presence of abnormal glandular structures, stromal expansion, and positive staining for P-S6 and Ki67, which should not be present in normal prostate tissue (Fig. 4C and 4D and Supplementary Fig. S3A). While EMT and mesenchymal tumor cells have a high tumor-initiating capacity in vivo, with 6/9 and 7/9 mice injected with these tumor cells forming prostate tumors, respectively, only 3/13 mice injected with epithelial tumor cells formed tumor pathology in vivo (Fig. 4B and Supplementary Fig. S3A). EMT and mesenchymal tumor cells also formed tumors as early as 9 weeks and 6 weeks post-transplantation, respectively, whereas the epithelial tumor cells did not form tumors until 24 weeks post-transplantation (Supplementary Fig. S3B). Tumorigenic areas formed from transplanted epithelial tumor cells were also much less aggressive compared to those formed by EMT and mesenchymal tumor cells (T; Supplementary Fig. S3A). EMT and mesenchymal tumor cells generated tumors with vast regions of EMT morphology that were positive for both Vimentin and P-S6 (Fig. 4C and 4D, bottom panels). Despite initially being injected as GFP+ cells, EMT and mesenchymal tumor cells were also remarkably able to regenerate tumorous epithelial acinar structures with prostatic intraepithelial neoplasia (PIN) lesions that were Vimentin-negative and P-S6-positive (Fig. 4C and 4D, top panels), indicating that these cells have the plasticity to switch between mesenchymal and epithelial states. In general, while regenerated epithelial glandular structures were positive for proliferation markers (Ki67), invasive EMT regions were devoid of any proliferation markers in tumors generated from EMT and mesenchymal tumor cells (Fig. 4C and 4D), mimicking the phenotype seen in primary CPKV prostates (Fig. 2F). These results demonstrate that EMT and mesenchymal tumor cells have enhanced tumor-initiating capacity compared to epithelial tumor cells from the same CPKV mice, and that these cells have an inherent plasticity to switch between mesenchymal and epithelial states, as they are able to form invasive tumor regions that are P-S6+, Vimentin+, and Ki67lo, as well as regenerated glandular structures that are P-S6+, Ki67int/hi, and Vimentin− (Fig. 4E).
Figure 4.
EMT and mesenchymal tumor cells have enhanced tumor-initiating capacity and cellular plasticity in vivo. A, Schematic of experimental design for orthotopic transplantations. B, EMT and mesenchymal tumor cells form tumors more readily in vivo compared to epithelial tumor cells from CPKV prostates (10–12 weeks). C, IHC stains of anterior lobes from an NSG mouse transplanted with EMT tumor cells from CPKV prostates (10–12 weeks). P-S6 was used to trace transplanted cells, and Vim was used to mark mesenchymal cells. Top panel, transplanted EMT tumor cells form regenerated glandular structures. Bottom panel, transplanted EMT tumor cells form invasive mesenchymal tumor regions. D, Same as in C, except a prostate section from an NSG mouse transplanted with mesenchymal tumor cells from CPKV prostates (10–12 weeks). E, EMT (yellow) and mesenchymal (green) tumor cells from CPKV prostates have the plasticity to undergo an MET and regenerate epithelial glandular structures, or form invasive mesenchymal tumors in vivo. Bar, 50 μm.
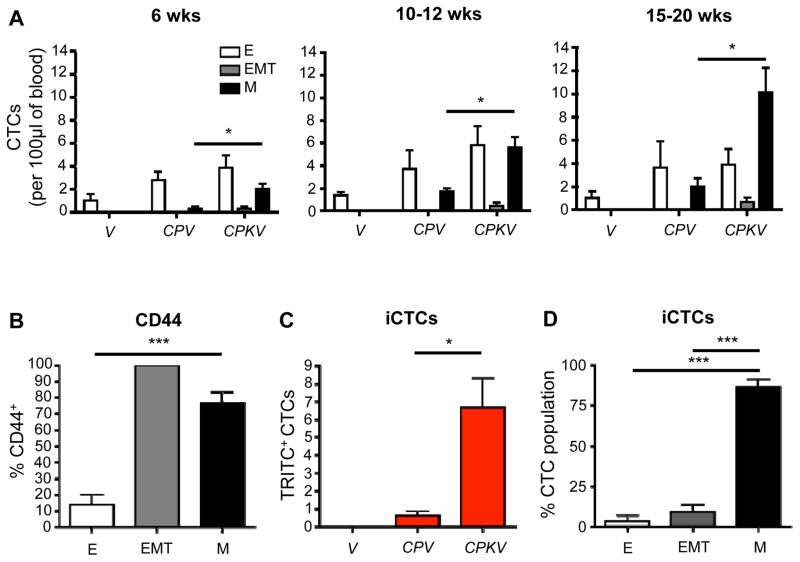
Increase in CTCs with mesenchymal and invasive characteristics during disease progression in CPKV mice
Circulating tumor cells (CTCs) represent a surrogate biomarker of metastatic disease and a predictive factor of overall survival in various malignancies, including prostate cancer (27, 28). The current FDA-approved CellSearch method for CTC enumeration uses antibodies against EpCAM to isolate CTCs with epithelial characteristics (29). However, recent studies in human breast and prostate cancer patients have revealed that a significant percentage of CTCs co-express both mesenchymal and epithelial markers or display fully mesenchymal characteristics (23, 30–32), through which they could pass undetected by the CellSearch system.
To determine if we could use the Vim-GFP reporter to isolate and characterize CTCs with EMT and mesenchymal characteristics at different disease stages, we collected peripheral blood from CPKV mutants. CTCs were isolated and characterized from the Lin− population by FACS analysis in a similar manner to how the primary tumor cell populations were isolated (Supplementary Fig. S4A). As early as 6 weeks of age and well before the detection of metastatic disease, epithelial, EMT and mesenchymal CTCs can be detected in the blood of CPKV mice (Fig. 5A, left panel, n=7). During the intermediate (10–12 weeks) and late stages (15–20 weeks) of tumor progression, there is a significant expansion of mesenchymal CTCs and rare EMT CTCs but not epithelial CTCs in CPKV mice, suggesting that only mesenchymal and EMT CTC counts correlate with progression towards metastatic disease (Fig. 5A, middle and right panels, n=10). Supporting this finding, CPV mice, which develop micrometastases in the lymph nodes but not distant macrometastasis to the lung or liver (33), show similar epithelial CTC numbers to age-matched CPKV mice but have very few CTCs with mesenchymal characteristics and no CTCs with EMT traits (Fig. 5A, n=5). This data suggests that 1) dissemination of metastatic tumor cells occurs early on during tumor initiation, 2) mesenchymal and EMT CTC counts correlate with metastatic disease, and 3) EMT tumor cells may quickly polarize to a mesenchymal or epithelial phenotype upon entering blood circulation.
Figure 5.
Increase in CTCs with mesenchymal and invasive characteristics during disease progression in CPKV mice. A, CPKV mice have a significant increase in mesenchymal and EMT CTCs compared to CPV or V mice during disease progression. B, Mesenchymal and EMT CTCs from CPKV mice (15–20 weeks) have significantly higher CD44 expression compared to epithelial CTCs. C, CPKV mice have significantly more invasive CTCs (iCTCs) compared to CPV and V mice (15–20 weeks). D, The majority of iCTCs have a mesenchymal phenotype. Data in A through D are represented as mean ± SEM. *, P < 0.05, ***, P < 0.001.
As it is thought that disseminated cells with metastatic seeding potential must possess stem cell characteristics in order to colonize distant tissues, we looked at expression of CD44, a putative cancer stem cell (CSC) marker (34), in our different CTC subpopulations. Throughout the various stages of disease progression in CPKV mice, the majority of CTCs with mesenchymal characteristics also expressed CD44, while only a small percentage of epithelial CTCs expressed this CSC marker (Fig. 5B, n=10). Interestingly, 100% of rare EMT CTCs that could be detected were CD44+ (Fig. 5B, n=10).
To further assess metastatic seeding potential of the CTC subpopulations, blood from CPKV mutants, as well as CPV and V mutants, was incubated on Vitatex culture plates, which are coated with a cell-adhesion matrix (CAM) that is used to assess the ability of tumor cells to invade collagen matrices (20, 35). Utilizing a Vitatex plate coated with a fluorescent red CAM, we are able to identify invasive CTCs (iCTCs), which represent CTCs with metastatic potential, by their ability to invade through the matrix and ingest TRITC-labeled CAM (Supplementary Fig. S4B). After 10 days in culture, blood isolated from CPKV mice (n=10) had a significantly larger number of TRITC+ iCTCs compared to blood from CPV (n=5) or V mice (n=3), supporting the notion that CTCs in CPKV mice contain more metastatic seeding potential (Fig. 5C). Moreover, when exploring the phenotype of these iCTCs from CPKV mice by FACS analysis, the majority of iCTCs had mesenchymal characteristics (Fig. 5D). The Vim-GFP reporter can therefore also be utilized for the isolation and characterization of CTC populations during endogenous prostate tumor cell dissemination and metastatic spreading. Our analysis reveals that CPKV mice have a significant increase in EMT and mesenchymal CTCs, but not epithelial CTCs, during disease progression.
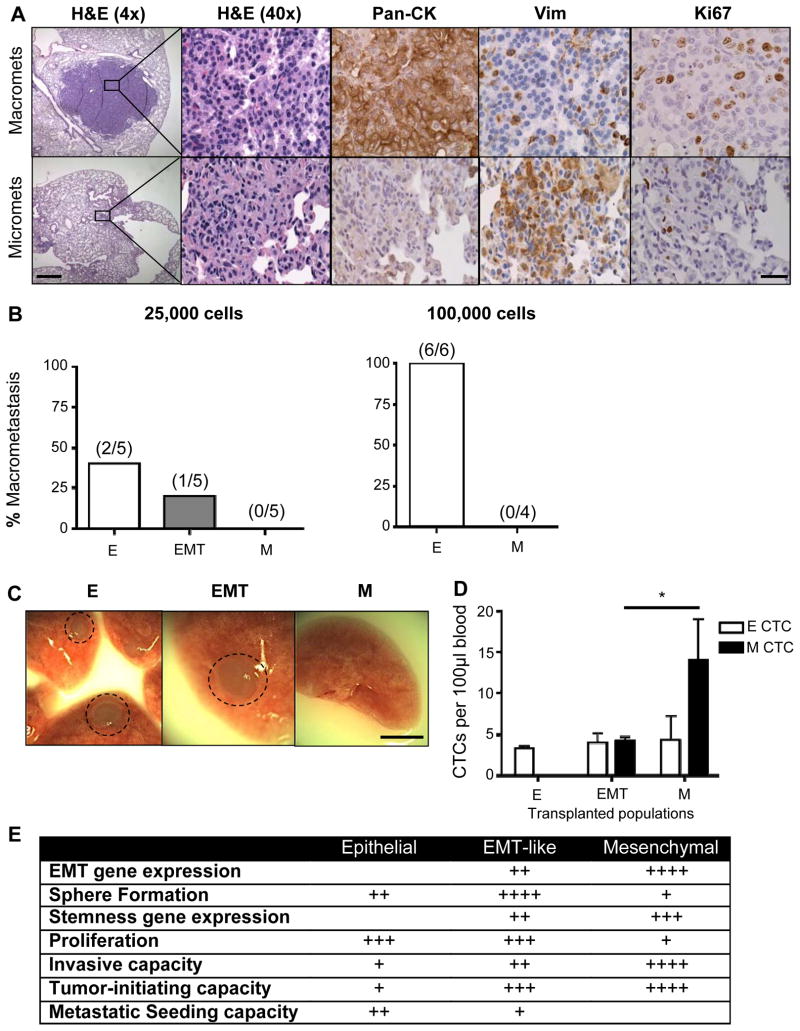
Epithelial tumor cells have enhanced metastatic seeding potential
The observation that metastatic lesions in humans often display an epithelial morphology suggests that tumor cells that have disseminated through an EMT may revert to an epithelial phenotype through a mesenchymal-epithelial transition (MET) in order to form macrometastases (36). To test whether an MET may be required for macroscopic metastasis in CPKV mice, we compared epithelial and mesenchymal marker expression in both micrometastases, which remain small, dormant lesions, and actively proliferating macrometastases in the lungs. While micrometastases express high levels of Vimentin and low levels of Pan-Cytokeratin (CK) and Ki67, macrometastases express low levels of Vimentin and high levels of CK and Ki67 (Fig. 6A). This data suggests that a reversion to an epithelial phenotype, marked by high levels of CK, may be required for dormant micrometastases with mesenchymal features to proliferate to form macrometastases.
Figure 6.
Epithelial tumor cells have enhanced metastatic seeding potential. A, IHC analysis of epithelial (Pan-CK), mesenchymal (Vim), and proliferation (Ki67) markers in micrometastases (micromets) and macrometastases (macromets) in primary CPKV lungs (18 weeks). Low magnification bar, 500 μm; high magnification bar, 50 μm. B, Percent of macrometastatic lesions in the lungs of NSG mice 16 weeks after intravenous transplantation of either 25,000 or 100,000 epithelial, EMT, or mesenchymal tumor cells from CPKV prostates (10–12 weeks). C, Whole-mount images of lungs isolated from NSG mice transplanted with 25,000 epithelial, EMT, or mesenchymal tumor cells from CPKV prostates (10–12 weeks). Circle, macrometastases. Bar, 4 mm. D, NSG mice transplanted intravenously with mesenchymal tumor cells (25,000) from CPKV prostates (10–12 weeks) contained a significantly higher number of CTCs persisting in the bloodstream compared to mice transplanted with either epithelial or EMT tumor cells 16 weeks post-transplantation. E, Table summarizing the characteristics of epithelial, EMT, and mesenchymal tumor cells isolated from CPKV prostates. Data in D are represented as mean ± SEM. *, P < 0.05
We next wanted to directly test the metastatic seeding capacity of epithelial, EMT and mesenchymal tumor cell populations by injecting these primary tumor cell populations isolated from CPKV mice intravenously into NSG mice. Remarkably, while mesenchymal tumor cells were unable to form macrometastases up to 16 weeks post-transplantation, epithelial tumor cells readily formed macrometases when either 25,000 (2/5) or 100,000 (6/6) tumor cells were injected (Fig. 6B and 6C). EMT tumor cells were also able to form metastases (1/5), albeit at a lower efficiency compared to epithelial tumor cells (Fig. 6B and 6C). Upon histological examination of the lungs post-transplantation, the macrometastases formed by both epithelial and EMT tumor cells were devoid of GFP+ cells and expressed high levels of CK (Supplementary Fig. S5), similar to lung macrometastases found in CPKV mice (Fig. 6A), confirming that macrometastatic spread requires reversion to an epithelial phenotype. While mesenchymal tumor cells did not form macrometastases or even small micrometastases in the lungs, solitary GFP+ cells were found in a quiescent, non-proliferative state (Ki67−) throughout the lungs (Supplementary Fig. S5). Interestingly, while few tumor cells remained in the circulation of mice transplanted with epithelial and EMT tumor cells 16 weeks post-intravenous injection, those transplanted with mesenchymal tumor cells had a significantly high number of CTCs with mesenchymal characteristics, suggesting that mesenchymal cells can persist and survive in the blood stream for long periods of time (Fig. 6D, n=4). These findings propose that while mesenchymal tumor cells are able to survive in the blood, extravasate, and persist as solitary, dormant cells in the lungs over the course of 16 weeks, they are unable to proliferate to generate frank metastases. EMT tumor cells, on the other hand, reside in a transitionary state, and have the capacity to transition to an epithelial state in order to proliferate and form macrometastases. Overall, while both EMT and mesenchymal prostate tumor cells have enhanced stemness characteristics and tumor-initiating capacity compared to epithelial tumor cells, only EMT tumor cells have the capacity to revert to an epithelial state and proliferate in the lungs to form macrometastases (Fig. 6E).
Discussion
As EMT is a plastic and dynamic process, the study of the EMT process through the in vitro manipulation of established cell lines, which often polarizes cells into a fixed mesenchymal state, may overlook much of the biology involved within the transition. Here, we demonstrate for the first time the isolation and characterization of both mesenchymal tumor cells that have fully completed an EMT, as well as EMT tumor cells that are in a transitory state between epithelial and mesenchymal programs from an endogenous murine cancer model. While previous studies have suggested that partial but not complete passage of cells into a mesenchymal state is associated with stemness and tumorigenicity (17, 37, 38), our study reveals that both EMT and mesenchymal tumors cells harbor stemness characteristics and tumor-initiating capacity. A striking distinction between these populations is their proliferative capacity, with mesenchymal tumor cells exhibiting characteristics of quiescent stem cells, and EMT tumor cells exhibiting characteristics of proliferating progenitor cells. Given that mesenchymal tumor cells localize to the stem cell niche in the proximal region of the prostate, it is likely that they are maintained in a quiescent state by factors in the surrounding microenvironment (26).
While both EMT and mesenchymal tumor cells demonstrate the plasticity to initiate proliferative epithelial tumor structures in the prostate microenvironment, only EMT cells are able to quickly revert to an epithelial phenotype to form macrometastatic colonies in the lungs. This suggests that mesenchymal tumor cells may exist in a more fixed state compared to EMT tumor cells, and may require additional stimuli, possibly mediated through paracrine factors secreted from the surrounding microenvironment, in order to acquire an epithelial phenotype and proliferate to form distant metastases. The modeling of dissemination in immunodeficient mice in our study, as well as by others (17, 39), presents a number of caveats that may affect the rate of successful metastatic colonization. First, NSG mice lack mature T cells, B cells, and NK cells, and have documented defects in macrophage activation. This is problematic, as the outgrowth of macrometastases has been shown to rely on the successful recruitment of myeloid and other inflammatory cell types to the metastatic site (40). Second, primary tumors release systemic factors that can educate distant sites in preparation for metastasizing cancer cells and mobilize bone marrow-derived cells to these sites to orchestrate what is termed the “premetastatic niche” (41–43). The lack of both a primary tumor and various immune cell subtypes in our dissemination model may indeed impact the metastatic niche required for colonization. Future studies exploring the contribution of distinct tumor cell populations to metastatic colonization will need to be carried out using lineage-tracing techniques in endogenous mouse models or through transplantation of tumor cells into immunocompetent syngeneic mice to fully recapitulate the role of the tumor microenvironment in metastatic colonization. Finally, as human prostate cancer metastasizes most frequently to the bone, it is plausible that the mesenchymal state, as opposed to the epithelial state, may be favored for metastatic colonization in the bone microenvironment. Indeed, a number of studies have demonstrated that human prostate cancer bone metastases have downregulation of E-cadherin and increased mesenchymal marker expression (44–47).
Given that mesenchymal CTCs increase in number significantly during disease progression in CPKV mice and harbor stemness properties, it is likely that mesenchymal tumor cells can settle in the distant organ sites and eventually form macrometastases, albeit over long latency periods. It has been well documented in the clinic that disseminated tumor cells (DTCs) can persist in a quiescent state at metastatic sites for decades after surgical removal of the primary tumor and eventually give rise to macrometastases (48, 49). It is possible that disseminated mesenchymal tumor cells in our CPKV mice model the clinical phenomenon of metastatic dormancy, and that the observation window of our metastasis assay (16 weeks) was not long enough to observe reactivation of these dormant, solitary DTCs to produce growing macrometastases. Moreover, while epithelial tumor cells can readily form metastases in the lung after entering the blood stream, it is unlikely that they can actively disseminate from the primary tumor in the endogenous setting. However, we cannot rule out the possibility that epithelial and mesenchymal tumor cells dynamically interact at different stages of the invasion-metastasis cascade to produce macrometastatic disease (17, 39). On the other hand, EMT tumor cells, by harboring the plasticity to readily transition between epithelial and mesenchymal states, have the capacity to complete the entirety of the invasion-metastasis cascade on their own. A preliminary transcriptional profiling study of each cell population revealed significant alterations in a number of key epigenetic regulators in the EMT and mesenchymal tumor cell populations, including HMGA2, which we found to be 1) significantly upregulated in human mCRPC, 2) required for epithelial-mesenchymal plasticity, and 3) successfully targeted by the histone deacteylase inhibitor (HDACi) LBH589 for therapeutic benefit in vivo (data in preparation). While more detailed analyses are warranted and still ongoing, these findings provide strong support for the use of therapeutic agents that target prostate tumor cell plasticity, as opposed to therapies that specifically target the epithelial or mesenchymal state. Although therapeutic targeting of mesenchymal tumor cells and the mesenchymal state could be useful in preventing tumor progression and dissemination, such therapeutic strategies could be counterproductive in patients that have DTCs or dormant micrometastases, since promoting reversion to an epithelial state could in turn promote metastatic growth. Further understanding of the mechanisms regulating epithelial-mesenchymal plasticity will help to unveil novel therapies that can be used to target tumor cell plasticity and hence inhibit integral steps of the metastastic cascade that ultimately leads to prostate cancer mortality.
Supplementary Material
Acknowledgments
Grant Support:
MR was supported by NIH T32 CA009056. DJM was supported by NIH F32 CA112988-01, CIRM TG2-01169 and Prostate Cancer Foundation Young Investigator Award. This work has been supported in part by awards from the Prostate Cancer Foundation, and grants from the NIH (R01 CA107166, RO1 CA121110, P50 CA092131 and U01 CA164188 to HW).
The authors thank members of the Hong Wu lab for their critical comments and suggestions. We also thank Naoko Kobayashi, Antreas Hindoyan, and Diana Moughon for their technical assistance.
Footnotes
Disclosure of Potential Conflicts of Interest: The authors do not have potential conflicts of interest.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA: a cancer journal for clinicians. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich A, Pfister D, Merseburger A, Bartsch G. Castration-resistant prostate cancer: where we stand in 2013 and what urologists should know. European urology. 2013;64:260–5. doi: 10.1016/j.eururo.2013.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, et al. Integrative genomic profiling of human prostate cancer. Cancer cell. 2010;18:11–22. doi: 10.1016/j.ccr.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, et al. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer research. 2012;72:1878–89. doi: 10.1158/0008-5472.CAN-11-3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aytes A, Mitrofanova A, Kinkade CW, Lefebvre C, Lei M, Phelan V, et al. ETV4 promotes metastasis in response to activation of PI3-kinase and Ras signaling in a mouse model of advanced prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3506–15. doi: 10.1073/pnas.1303558110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lue HW, Yang X, Wang R, Qian W, Xu RZ, Lyles R, et al. LIV-1 promotes prostate cancer epithelial-to-mesenchymal transition and metastasis through HB-EGF shedding and EGFR-mediated ERK signaling. PloS one. 2011;6:e27720. doi: 10.1371/journal.pone.0027720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josson S, Nomura T, Lin JT, Huang WC, Wu D, Zhau HE, et al. beta2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer research. 2011;71:2600–10. doi: 10.1158/0008-5472.CAN-10-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanaka H, Kono E, Tran CP, Miyazaki H, Yamashiro J, Shimomura T, et al. Monoclonal antibody targeting of N-cadherin inhibits prostate cancer growth, metastasis and castration resistance. Nature medicine. 2010;16:1414–20. doi: 10.1038/nm.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer research. 2012;72:527–36. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 10.Nauseef JT, Henry MD. Epithelial-to-mesenchymal transition in prostate cancer: paradigm or puzzle? Nature reviews Urology. 2011;8:428–39. doi: 10.1038/nrurol.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Craene B, Berx G. Regulatory networks defining EMT during cancer initiation and progression. Nature reviews Cancer. 2013;13:97–110. doi: 10.1038/nrc3447. [DOI] [PubMed] [Google Scholar]
- 12.Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704–15. doi: 10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PloS one. 2008;3:e2888. doi: 10.1371/journal.pone.0002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, et al. Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness. Cancer research. 2010;70:6945–56. doi: 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- 15.Marin-Aguilera M, Codony-Servat J, Reig O, Lozano JJ, Fernandez PL, Pereira MV, et al. Epithelial-to-mesenchymal transition mediates docetaxel resistance and high risk of relapse in prostate cancer. Molecular cancer therapeutics. 2014;13:1270–84. doi: 10.1158/1535-7163.MCT-13-0775. [DOI] [PubMed] [Google Scholar]
- 16.Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–25. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- 17.Celia-Terrassa T, Meca-Cortes O, Mateo F, de Paz AM, Rubio N, Arnal-Estape A, et al. Epithelial-mesenchymal transition can suppress major attributes of human epithelial tumor-initiating cells. The Journal of clinical investigation. 2012;122:1849–68. doi: 10.1172/JCI59218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nature protocols. 2010;5:702–13. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia AJ, Ruscetti M, Arenzana TL, Tran LM, Bianci-Frias D, Sybert E, et al. Pten Null Prostate Epithelium Promotes Localized Myeloid-Derived Suppressor Cell Expansion and Immune Suppression during Tumor Initiation and Progression. Molecular and cellular biology. 2014;34:2017–28. doi: 10.1128/MCB.00090-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedlander TW, Ngo VT, Dong H, Premasekharan G, Weinberg V, Doty S, et al. Detection and characterization of invasive circulating tumor cells derived from men with metastatic castration-resistant prostate cancer. International journal of cancer Journal international du cancer. 2014;134:2284–93. doi: 10.1002/ijc.28561. [DOI] [PubMed] [Google Scholar]
- 21.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cellular and molecular life sciences : CMLS. 2011;68:3033–46. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q, Helfand BT, Jang TL, Zhu LJ, Chen L, Yang XJ, et al. Nuclear factor-kappaB-mediated transforming growth factor-beta-induced expression of vimentin is an independent predictor of biochemical recurrence after radical prostatectomy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:3557–67. doi: 10.1158/1078-0432.CCR-08-1656. [DOI] [PubMed] [Google Scholar]
- 23.Armstrong AJ, Marengo MS, Oltean S, Kemeny G, Bitting RL, Turnbull JD, et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Molecular cancer research : MCR. 2011;9:997–1007. doi: 10.1158/1541-7786.MCR-10-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulholland DJ, Xin L, Morim A, Lawson D, Witte O, Wu H. Lin-Sca-1+CD49fhigh stem/progenitors are tumor-initiating cells in the Pten-null prostate cancer model. Cancer research. 2009;69:8555–62. doi: 10.1158/0008-5472.CAN-08-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, et al. Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis. The Journal of cell biology. 2002;157:1257–65. doi: 10.1083/jcb.200202067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salm SN, Burger PE, Coetzee S, Goto K, Moscatelli D, Wilson EL. TGF-{beta} maintains dormancy of prostatic stem cells in the proximal region of ducts. The Journal of cell biology. 2005;170:81–90. doi: 10.1083/jcb.200412015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scher HI, Jia X, de Bono JS, Fleisher M, Pienta KJ, Raghavan D, et al. Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. The lancet oncology. 2009;10:233–9. doi: 10.1016/S1470-2045(08)70340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Bono JS, Scher HI, Montgomery RB, Parker C, Miller MC, Tissing H, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clinical cancer research : an official journal of the American Association for Cancer Research. 2008;14:6302–9. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 29.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:920–8. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 30.Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339:580–4. doi: 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen CL, Mahalingam D, Osmulski P, Jadhav RR, Wang CM, Leach RJ, et al. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. The Prostate. 2013;73:813–26. doi: 10.1002/pros.22625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kallergi G, Papadaki MA, Politaki E, Mavroudis D, Georgoulias V, Agelaki S. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast cancer research : BCR. 2011;13:R59. doi: 10.1186/bcr2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, et al. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer cell. 2003;4:209–21. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 34.Tang DG, Patrawala L, Calhoun T, Bhatia B, Choy G, Schneider-Broussard R, et al. Prostate cancer stem/progenitor cells: identification, characterization, and implications. Molecular carcinogenesis. 2007;46:1–14. doi: 10.1002/mc.20255. [DOI] [PubMed] [Google Scholar]
- 35.Lu J, Fan T, Zhao Q, Zeng W, Zaslavsky E, Chen JJ, et al. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. International journal of cancer Journal international du cancer. 2010;126:669–83. doi: 10.1002/ijc.24814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–64. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 37.Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28:1435–45. doi: 10.1002/stem.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shamir ER, Pappalardo E, Jorgens DM, Coutinho K, Tsai WT, Aziz K, et al. Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. The Journal of cell biology. 2014;204:839–56. doi: 10.1083/jcb.201306088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuji T, Ibaragi S, Shima K, Hu MG, Katsurano M, Sasaki A, et al. Epithelial-mesenchymal transition induced by growth suppressor p12CDK2-AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer research. 2008;68:10377–86. doi: 10.1158/0008-5472.CAN-08-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nature reviews Cancer. 2009;9:239–52. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Psaila B, Lyden D. The metastatic niche: adapting the foreign soil. Nature reviews Cancer. 2009;9:285–93. doi: 10.1038/nrc2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hiratsuka S, Watanabe A, Aburatani H, Maru Y. Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nature cell biology. 2006;8:1369–75. doi: 10.1038/ncb1507. [DOI] [PubMed] [Google Scholar]
- 44.Umbas R, Schalken JA, Aalders TW, Carter BS, Karthaus HF, Schaafsma HE, et al. Expression of the cellular adhesion molecule E-cadherin is reduced or absent in high-grade prostate cancer. Cancer research. 1992;52:5104–9. [PubMed] [Google Scholar]
- 45.Sethi S, Macoska J, Chen W, Sarkar FH. Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. American journal of translational research. 2010;3:90–9. [PMC free article] [PubMed] [Google Scholar]
- 46.Lang SH, Hyde C, Reid IN, Hitchcock IS, Hart CA, Bryden AA, et al. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate carcinoma. The Prostate. 2002;52:253–63. doi: 10.1002/pros.10088. [DOI] [PubMed] [Google Scholar]
- 47.Kwok WK, Ling MT, Lee TW, Lau TC, Zhou C, Zhang X, et al. Up-regulation of TWIST in prostate cancer and its implication as a therapeutic target. Cancer research. 2005;65:5153–62. doi: 10.1158/0008-5472.CAN-04-3785. [DOI] [PubMed] [Google Scholar]
- 48.Giancotti FG. Mechanisms governing metastatic dormancy and reactivation. Cell. 2013;155:750–64. doi: 10.1016/j.cell.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aguirre-Ghiso JA. Models, mechanisms and clinical evidence for cancer dormancy. Nature reviews Cancer. 2007;7:834–46. doi: 10.1038/nrc2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.