Abstract
Parental support is a powerful regulator of stress and fear responses for infants and children, but recent evidence suggests it may be an ineffective stress buffer for adolescents. The mechanisms underlying this developmental shift are not well-understood. The goal of the present study was to examine the independent and joint contributions of pubertal status and chronological age in explaining this shift. A sample of 75 typically-developing youth (M age = 12.95 years, SD = 0.70, range = 11.7–14.6 years; 37 females) was recruited to complete a modified Trier Social Stress Test (TSST-M) in the laboratory. Participants were recruited in such a way as to disentangle pubertal stage and chronological age by phone screening for markers of pubertal stage and then recruiting roughly equal numbers of younger and older, pre/early and mid/late pubertal youth who were then randomly assigned within groups to condition. The TSST-M was used as the stressor and youth prepared either with their parent or stranger (parent condition: N = 39). Pubertal stage was confirmed by the Petersen Pubertal Development Scale at the time of testing and treated, along with chronological age, as a continuous variable in the analyses. The results revealed an interaction of pubertal stage and support condition for cortisol reactivity to the TSST-M such that preparing for the speech with the parent became a less potent buffer of the HPA axis as pubertal stage increased. Age did not interact with condition in predicting cortisol reactivity. In contrast, the parent’s presence during speech preparation decreased in its effectiveness to hasten recovery of the HPA axis as children got older, but pubertal stage was not predictive of recovery rate. These patterns were specific to cortisol and were not observed with salivary alpha-amylase levels or subjective stress ratings for the task. These analyses suggest that the switch away from using parents as social buffers may be the result of neurobiological mechanisms associated with puberty.
Keywords: social buffering, HPA axis, cortisol, parenting
1. Introduction
For the young of many mammalian species including our own, contact and proximity with the attachment figure is critical to survival. When threatened or stressed, young animals and children seek this proximity and the presence of the attachment figure provides a powerful buffer of the infant’s fear and stress systems (Hennessy et al., 2009). Termed parental social buffering, this regulating effect of the attachment figure’s presence on key stress mediating systems, such as the hypothalamic-pituitary-adrenocortical (HPA) system, has been demonstrated multiple times in infants and toddlers (Gunnar et al., 1996; Nachmias et al., 1996; Spangler and Schieche, 1998; Ahnert et al., 2004).
Stress buffering has been observed in adults as well. Among adults, romantic relationship partners (Kirschbaum et al., 1995) and close friends (Fontana et al., 1999; Uno et al., 2002) have been shown to reduce cortisol and autonomic responses to social stressors. Furthermore, the presence of another person is not sufficient for stress buffering; instead, it seems that a degree of intimacy with the person providing the buffering is required (Kirschbaum et al., 1995). Indeed, for adults, a period of self-disclosure that enhances intimacy is sufficient to increase the value of a stranger’s presence as a source of social buffering of the HPA axis (Smith et al., 2009).
Until recently, there had been little work examining parental social buffering beyond infancy. One research group did show that among 7- to 12-year old females, recovering from a social stressor task with their mother reduced cortisol to baseline faster than doing so without any maternal contact, and even talking to their mother on the phone provided some benefit (Seltzer et al., 2010). This study also showed that contact with the mother, in person or by phone, increased the production of oxytocin (Seltzer et al., 2010). In work with adults, nasal oxytocin has been shown to produce effects comparable to a partner’s presence in reducing cortisol responses to a social stressor task (Heinrichs et al., 2003). Evidence of oxytocin as a potential mediator or correlate of social buffering underscores the importance of the bond between social partners as critical to the buffering potency of the other person’s presence.
One question with regards to parental social buffering is how long in development the buffering potency of the parent lasts. If social buffering organized around parents is part of an immature mode of coping with danger and threat, then one would expect it to diminish in potency as the child approaches independence. Recently we examined the effectiveness of the parent as a social buffer of the HPA axis among children aged 9- and 10-years-old and adolescents aged 15- and 16-years-old (Hostinar et al., 2015). Among the children, the presence of the parent during the time the child prepared for the speech in the Trier Social Stress Test completely blocked elevations in cortisol despite the fact that the parent was not present during the speech and math section of the task. Among the adolescents the parent’s presence had no effect. In addition, as would be expected, basal levels of cortisol were higher among the adolescents than the children (Dahl and Gunnar, 2009). In other work, researchers have shown that with adolescence, the mother’s presence no longer buffers the amygdala responses to threat stimuli, allowing fear conditioning to occur even when the mother is present and not indicating fear of the conditioned stimulus (Gee et al., 2014).
Thus, there is evidence of a reduction of parental social buffering potency with the transition from childhood to adolescence. The question addressed in this study is the extent to which this reduction is associated with puberty or with age changes in processes that are unrelated to puberty. We hypothesized that the capacity of the parent’s presence to reduce reactivity of the HPA axis to a social stressor would decrease in relation to pubertal stage and not child age. To provide a more fine-grained analysis, we differentiated reactivity and recovery components of the HPA response to the social stressor task as suggested by Juster and colleagues (2012). To help determine whether the parent’s presence operated through reducing how frightened or anxious the children were, we obtained the children’s self-reports of stress at different points in the procedure. We also collected measures of alpha-amylase, an indirect indicator of autonomic arousal, to examine whether this phenomenon is specific to the HPA axis or applies to both stress-mediating systems.
2. Methods
2. 1. Participants
A total of 81 youth ages 11–14 were recruited from a department-maintained participant pool and were enrolled in the study. Exclusion criteria included the use of steroid or psychotropic medications, and diagnosis of Autism Spectrum Disorder, Fetal Alcohol Spectrum Disorder, or any other developmental disorder. Six adolescents were excluded from analysis for taking medications that likely affect cortisol levels (e.g., corticosteroids, diabetes injections, vasopressin analogues, antidepressant, testosterone injections, beta-blockers, immunosuppressants). Participant data below is reported without these 6 excluded participants. A total of 75 typically developing youth (M age = 12.95, SD = 0.70, range = 11.67–14.58 years; 37 females) were included in all analyses. Approximately half of each sex was assigned to prepare for the stressor task with their parent present (23 males/16 females) and the others prepared with the stranger present (15 males/21 females). To balance pubertal status between the sexes, the average age of the males (M age = 13.27 years, SD = 0.69, range: 11.75–14.58) was about a half-year older than that of the females (M age = 12.62 years, SD = 0.53, range: 11.67–13.67). Participants were randomly assigned within age and pubertal groups to prepare their speech with either a parent or stranger (parent condition: N = 39).
Annual household income ranged from less than $15,000 to more than $200,000 per year. The percent of families that had incomes greater than $150,000 was 25.6, 57.7% had incomes from $75,001–150,000, 14.1% had incomes from $35,001–75,000, and 2.6% had incomes less than $35,000. There were three individuals who refused to report income. The distribution for parental educational level (of the parent who attended the session) was 13.9% high school or GED graduate, 7.6% 2-year college or associate’s degree, 41.8% bachelor’s or 4-year college degree, and 36.7% postgraduate degree (two individuals did not report education level). Neither parent education nor family income differed as a function of sex, pubertal status or parent/stranger condition. There was a significant correlation between age and parent education, with older adolescents having a parent with lower educational attainment r(73) = −0.26, p = 0.03, but there was no relation between age and income level, p > 0.10. The procedures and purposes of the study were approved by the University of Minnesota Institutional Review Board. Parents were recruited by phone, and those who did not meet exclusion criteria and agreed to have their adolescents participate were scheduled.
2. 2. Procedures
2.2.1 Recruitment
Potential participants were stratified by age into younger and older groups. For males, younger was defined as being below 13.5 years old, and for females, younger was defined as being below 12.5 years old. A phone recruiter asked parents of males 5 questions from the Petersen Pubertal Development Scale (described below) in order to classify participants as pre-pubertal or pubertal. For females, the recruiter asked parents if their daughter had begun menstruating in order to classify her as pre-pubertal or pubertal. Youth were then recruited to obtain roughly equal numbers of pre/early and mid/late pubertal participants in the younger and older age group. This method allowed us to reduce the association between age and pubertal stage so that both factors could be examined in the same analysis without problems of co-linearity. The final numbers of male and female children in each puberty/age group are shown in Table 1.
Table 1.
Number of male and female participants in each puberty/age group.
| Group | Male | Female | Total |
|---|---|---|---|
| Pre-Early Puberty/Younger | 13 | 10 | 23 |
| Pre-Early Puberty/Older | 7 | 9 | 16 |
| Mid-Late Puberty/Younger | 9 | 9 | 18 |
| Mid-Late Puberty/Older | 9 | 9 | 18 |
| Total | 38 | 37 | 75 |
Note. Total N = 75. Numbers shown were for recruitment purposes only, to tease apart age and pubertal status. Analyses used continuous measures of pubertal status and age.
2.2.2 Pubertal status
Once at the laboratory, pubertal status was re-assessed using adolescent self-report. The Pubertal Development Scale (Petersen et al., 1988; Carskadon and Acebo, 1993) assessed the extent of participants’ sex-specific bodily changes associated with puberty onset: growth in height, body hair, skin changes, deepening of voice, and facial hair for males; growth in height, body hair, skin changes, breast development, and menstruation for females. Responses were 1 = not yet started, 2 = barely started, 3 = definitely started, and 4 = seems complete (Carskadon and Acebo, 1993). Menstruation was coded as 1 if it had not begun and 4 if it had begun. This measure yields a mean score from 1 (puberty has not begun) to 4 (puberty is complete), which was used for analysis of puberty as a continuous variable. A check on the effectiveness of the recruitment strategy revealed that, as planned, age and pubertal stage were not correlated, r(75) = 0.06, p = 0.64.
2.2.3 Session timeline
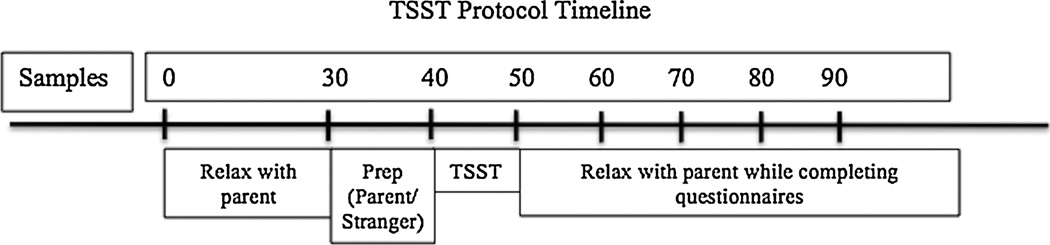
Participants were scheduled for one session in which all data were collected. All participants were accompanied by a parent and arrived to the laboratory between 15:30 and 16:30 in order to account for diurnal variation in cortisol. Parents were told over the phone that mothers were preferred to accompany the adolescent to the session but that fathers could attend if needed. Mothers (77%) were the parent in most of the sessions with no difference in sex of parent across age groups χ2(2, N = 75) = 2.19, p = 0.34, pubertal groups χ2(2, N = 75) = 2.36, p = 0.31, or conditions χ2(2, N = 75) = 1.45, p = 0.47. Each session included the following timeline (see Figure 1): (1) Parent and child worked independently on questionnaires in the laboratory after the consent process (30 min), (2) saliva sample #1 collection, then participant moved to a separate room (either with parent or with female experimenter) and received TSST-M instructions for the speech preparation period (5 min), (3) speech preparation with parent or stranger (5 min), (4) sample #2; participant moved to speech room and completed TSST alone regardless of condition (10 min), (5) samples #3–7; participant relaxed with parent regardless of condition while working on questionnaires (samples collected every 10 min), (6) debriefing of both participants and parents was conducted. The only part of the experiment that differed by condition was the 5-minute speech preparation period with the parent or experimenter; all participants were with their parent before speech preparation and after the completion of the TSST-M.
Figure 1.
N = 75. A timeline of the TSST-M protocol by minutes since the beginning of the session. The first saliva sample used to compute reactivity was collected at 30 minutes after the start of the session, and the following 6 samples were collected every 10 minutes after the first. Note that the only difference in parent presence for the two conditions is during speech preparation.
2.2.4. Stress paradigm
The stressor procedures were identical to those described in Hostinar et al. (2015) and will be summarized here. A modified version of the Trier Social Stress Test (TSST-M; Yim, et al., 2010) was used in which youths are asked to imagine they are introducing themselves to a new classroom of students and they should tell the class some good and bad characteristics about themselves. This was followed by the standard TSST-C mental arithmetic (Buske-Kirschbaum et al., 1997). In addition, rather than two live judges, the participant was told that there were two teachers behind a one-way mirror who would judge their performance. The teachers introduced themselves via pre-recorded audiotape. Prior to the TSST-M, participants were randomly assigned to condition. Participants in the parent condition received support from their parent, who was instructed to assist their child in any way deemed useful. In the stranger condition, the participant’s parent remained in the waiting room while the stranger sat in the room and supported the participant for the duration of their speech preparation. In both conditions, the participants were alone in the room during the TSST-M.
2.2.5. Cortisol and alpha-amylase
Eight saliva samples were collected throughout the research session to provide baseline, response, and recovery cortisol and alpha-amylase levels. Participants used the passive drool method to collect saliva through a straw into pre-labeled 1.5mL Eppendorf (Hamburg, Germany) tubes. Participants were instructed to refrain from consuming large, protein-filled meals, milk, caffeine, or energy drinks for two hours before arriving at the laboratory. After collection of all samples, saliva was stored in a −20°C laboratory freezer until being shipped to the University of Trier, Germany for assaying. A time-resolved fluorescence immunoassay (dissociation-enhanced lanthanide fluorescent immunoassay [DELFIA]) was used to detect cortisol levels. Alpha-amylase was measured using 2-chloro-4-nitrophenyl-alpha-D-maltotrioside as a substrate (Lorentz et al., 1999). Optical density was measured after 1 minute of incubation and then again after 2 minutes of further incubation, and the difference between these measurements was calculated. Using this measurement and other factors (e.g., sample volume, dilution factor), the activity of alpha-amylase was calculated. Intra-assay CVs for cortisol and alpha-amylase ranged from 4.0% to 6.7%, and inter-assay CVs ranged from 5.38% to 7.46%. All eight samples from each participant were assayed in duplicate and within the same batch to prevent between-batch variation. An average of duplicate samples was used in analyses.
2.2.6 Daily diary
The parent and child each completed a daily diary for which they reported information about the participant relevant to cortisol collection including time of wake-up, medication usage, caffeine consumption, physical activity, distressing events experienced that day (e.g. arguments with siblings or parents), and number of hours of sleep during the previous night. Adolescent reports were the primary source of information. However, for type of medication used by the adolescent, the parent’s report on this variable was used instead when the child’s information was missing, too vague or incomplete. After excluding participants taking medications with known effects on cortisol, a medication count variable was created using the Granger and colleagues (2009) method (M = 0.23, SD = 0.63, range 0–2). Time since wake was calculated by subtracting the adolescent’s reported time of wake from the time of first saliva sample collection.
2.2.7 Self-reported stress
Participants completed a questionnaire asking how stressed they felt at five different points in the assessment: arrival to the session, speech preparation, speech delivery, math assessment, and at the end of the session (e.g., “How stressful was giving the speech?”). They could respond as 1 = calm, 2 = low stress, 3 = medium stress, 4 = somewhat high stress, 5 = very high stress. On average, adolescents showed large increases in perceived stress levels during the speech and math portions of the TSST-M: arrival (M = 1.84, SD = 0.90), speech preparation (M = 2.76, SD = 0.99), speech (M = 3.86, SD = 0.85), math (M = 3.82, SD = 1.06), and end of session (M = 1.44, SD = 0.67).
2.3 Data Analytic Plan
A piecewise latent growth curve model was used to examine the cortisol and alpha-responses around a theoretically meaningful time point (the onset of the TSST). The reactivity and recovery slopes for both cortisol and alpha-amylase were extracted using estimates derived in Mplus in order to test hypotheses about reactivity and recovery with linear regression models. Although most individuals’ cortisol levels peaked 10 minutes post-TSST-M and alpha-amylase levels peaked immediately post-TSST, some individuals peaked 10 minutes later for either measure. As a result, landmark registration was used so that each person’s peak alpha-amylase and cortisol responses were utilized as the point of highest reactivity and the beginning of recovery. Typically peak cortisol levels were 20 to 30 minutes post-TSST onset, so reactivity was the slope of cortisol between the onset of speech preparation and their peak cortisol level. The peak in alpha-amylase levels was typically 10 to 20 minutes post-TSST onset, so reactivity was the slope between speech preparation onset and the peak alpha-amylase level. Recovery was measured from the peak to the final sample. Although subjects were recruited into pubertal and age groups, age and pubertal status were examined continuously in the analyses. Four linear regressions were conducted (2 cortisol/alpha-amylase by 2 reactivity/recovery) with age, pubertal stage and condition (parent vs. stranger) as the primary independent variables in Step 1 of the regressions. If sex, medication count, or time-since-wake significantly predicted cortisol or alpha-amylase reactivity or recovery, it was included in the analysis as a covariate. For cortisol reactivity, the Mplus-derived cortisol intercept before the start of the TSST-M was included as a covariate; for cortisol recovery, both the cortisol intercept and reactivity slope were included to control for their effects. For alpha-amylase reactivity, the Mplus-derived alpha-amylase intercept before the start of the TSST-M was used as a covariate; for alpha-amylase recovery, both alpha-amylase intercept and reactivity slope were used. Age x puberty, puberty x condition, and age x condition interactions were entered into step 2. The 3-way age x puberty x condition interaction was entered into the 3rd step of the regression. Finally, similar analyses were conducted with Mplus-derived self-reported stress reactivity (slope from arrival at lab to speech delivery) and recovery (speech delivery to end of session) as dependent variables and age, puberty, and condition (and their interactions) to determine whether stress buffering acts by decreasing subjective feelings of stress.
3. Results
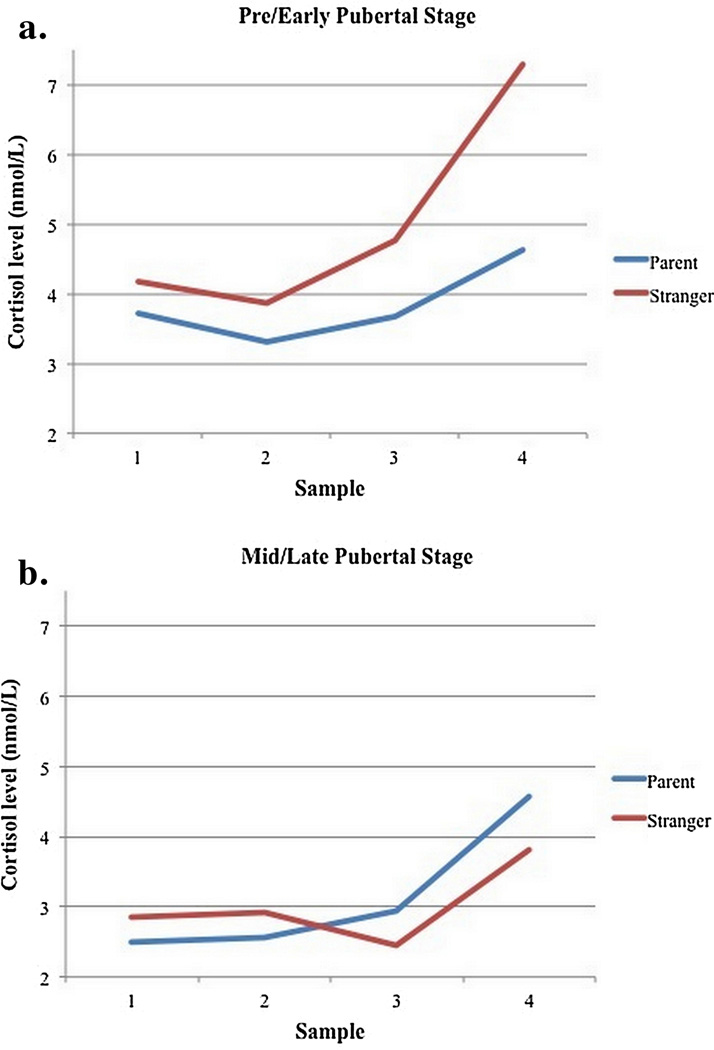
The results for the cortisol analyses are shown in Tables 1 and 2. There were significant effects of cortisol intercept on reactivity with higher intercepts associated with lower reactivity. Age was positively associated with reactivity. Sex and medication count did not predict cortisol reactivity, so these were excluded from the final equations. Finally, the puberty by condition association was not significant but provided an indication of potential effects on reactivity in the predicted direction (p = .08, Table 2). Specifically, preparing for the speech with the parent was associated with reduced reactivity for the youth at earlier but not later pubertal stages. To probe the effect of puberty by condition, the pre-pubertal vs. pubertal groups were used for analysis rather than the continuous variable. These follow-up analyses were conducted using pre/early pubertal versus mid/late pubertal stage categorically grouped female participants as mid/late pubertal if they had begun menstruating, and males as mid/late pubertal if their average scores were 2.5 or greater. In this analysis, all of the significant effects of the continuous analysis remained. In addition, the pubertal stage by condition interaction was significant, β = 0.15, t(74) = 2.38, p < 0.05 (see Figure 2). Simple slopes analysis revealed that, as predicted, the effect of condition is significant in the adolescents in early puberty (t = −2.08, p < 0.05) but not mid-to-late puberty, (t = 1.32, p = 0.19).
Table 2.
| Variable | B | SE(B) | β | t−value | p−value |
|---|---|---|---|---|---|
| Step 1 | |||||
| Cortisol Intercept | −0.40 | 0.03 | −0.82 | −12.60 | 0.00*** |
| Pubertal Status | −0.03 | 0.04 | −0.05 | −0.69 | 0.49 |
| Age | 0.10 | 0.04 | 0.17 | 2.63 | 0.01* |
| Condition | −0.02 | 0.04 | −0.04 | −0.56 | 0.58 |
| Step 2 | |||||
| Cortisol Intercept | −0.40 | 0.03 | −0.82 | −12.58 | 0.00*** |
| Pubertal Status | −0.02 | 0.04 | −0.04 | −0.57 | 0.57 |
| Age | 0.10 | 0.04 | 0.18 | 2.70 | 0.01** |
| Condition | −0.02 | 0.04 | −0.04 | −0.54 | 0.59 |
| Puberty x Age | 0.00 | 0.04 | 0.00 | 0.00 | 1.00 |
| Age x Condition | 0.03 | 0.04 | 0.04 | 0.652 | 0.52 |
| Puberty x Condition | 0.08 | 0.04 | 0.13 | 1.89 | 0.06† |
| Step 3 | |||||
| Cortisol Intercept | −0.40 | 0.03 | −0.82 | −12.51 | 0.00*** |
| Pubertal Status | −0.02 | 0.04 | −0.03 | −0.46 | 0.65 |
| Age | 0.10 | 0.04 | 0.17 | 2.49 | 0.02* |
| Condition | −0.02 | 0.04 | −0.03 | −0.48 | 0.63 |
| Puberty x Age | 0.00 | 0.04 | 0.00 | 0.00 | 1.00 |
| Age x Condition | 0.03 | 0.04 | 0.04 | 0.63 | 0.53 |
| Puberty x Condition | 0.07 | 0.04 | 0.12 | 1.81 | 0.08† |
| Age x Puberty x Condition | −0.02 | 0.04 | −0.03 | −0.41 | 0.68 |
Note. N = 75. Hierarchical linear regression results with cortisol reactivity (Mplusgenerated) as the dependent variable. < 0.10†, < 0.05*, < 0.01**, < 0.001***.
Figure 2.
N = 75. Cortisol Reactivity (nmol/L) for adolescents in pre/early pubertal stage vs. mid/late pubertal stage when preparing with a parent or with a stranger. Sample 1 was collected at the beginning of the speech preparation period, and samples 2–7 were collected every 10 minutes thereafter. Samples 4–5 represent the peak of cortisol production post-TSST that occurred 20–30 minutes after the onset of the speech. Means for each sample were calculated controlling for the effect of age and cortisol levels at arrival to the session. Simple slopes analysis revealed that the effect of condition is significant in the adolescents in early puberty (p < 0.05) but not mid-to-late puberty (p = 0.19).
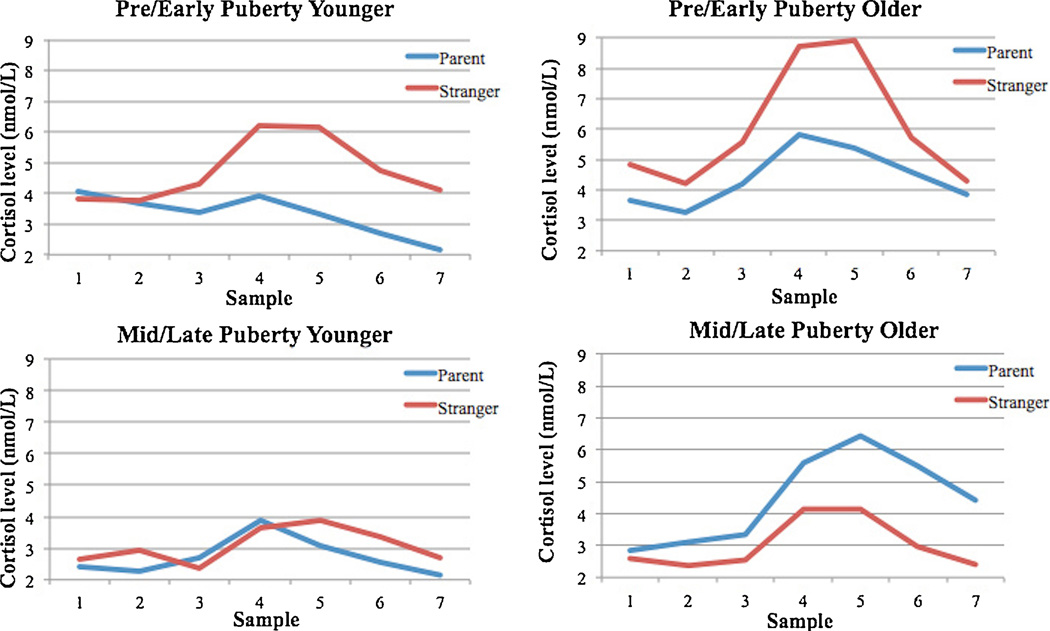
Considering the regression examining cortisol recovery (Table 3), as shown with cortisol reactivity in the model, cortisol intercept predicted a faster recovery. In addition, age but not puberty interacted with condition, β = 0.23, t(74) = 2.08, p < 0.05, suggesting that with age the parent’s presence had less of an impact on recovery of the HPA axis. However, simple slopes analysis was not statistically significant in the parent condition, t = 1.81, p = 0.08, or the stranger condition, t = − 1.13, p = 0.26. Sex and medication count did not predict cortisol recovery and were not included in the final analysis. Graphs of the mean cortisol levels by condition for each puberty x age group can be found in Figure 3.
Table 3.
| Variable | B | SE(B) | β | t-value | p-value |
|---|---|---|---|---|---|
| Step 1 | |||||
| Cortisol Intercept | −0.03 | 0.01 | −0.63 | −3.16 | 0.00** |
| Cortisol Reactivity | −0.03 | 0.02 | −0.26 | −1.29 | 0.2 |
| Time Since Wake | −0.01 | 0.01 | −0.25 | −2.25 | 0.03* |
| Pubertal Status | 0.00 | 0.01 | −0.01 | −0.11 | 0.91 |
| Age | 0.00 | 0.01 | 0.06 | 0.53 | 0.6 |
| Condition | 0.00 | 0.01 | −0.01 | −0.05 | 0.96 |
| Step 2 | |||||
| Cortisol Intercept | −0.04 | 0.01 | −0.70 | −3.54 | 0.00** |
| Cortisol Reactivity | −0.04 | 0.02 | −0.34 | −1.71 | 0.09† |
| Time Since Wake | −0.01 | 0.01 | −0.26 | −2.40 | 0.02* |
| Pubertal Status | 0.00 | 0.01 | 0.00 | 0.02 | 0.99 |
| Age | 0.01 | 0.01 | 0.09 | 0.80 | 0.43 |
| Condition | 0.00 | 0.01 | 0.00 | −0.02 | 0.99 |
| Puberty x Age | 0.01 | 0.01 | 0.10 | 0.88 | 0.38 |
| Age x Condition | 0.02 | 0.01 | 0.23 | 2.08 | 0.04* |
| Puberty x Condition | 0.01 | 0.01 | 0.09 | 0.79 | 0.43 |
| Step 3 | |||||
| Cortisol Intercept | −0.04 | 0.01 | −0.70 | −3.47 | 0.00** |
| Cortisol Reactivity | −0.04 | 0.02 | −0.34 | −1.66 | 0.1 |
| Time Since Wake | −0.01 | 0.01 | −0.26 | −2.35 | 0.02* |
| Pubertal Status | 0.00 | 0.01 | −0.01 | −0.09 | 0.93 |
| Age | 0.01 | 0.01 | 0.10 | 0.89 | 0.38 |
| Condition | 0.00 | 0.01 | −0.01 | −0.08 | 0.94 |
| Puberty x Age | 0.01 | 0.01 | 0.10 | 0.87 | 0.39 |
| Age x Condition | 0.02 | 0.01 | 0.23 | 2.08 | 0.04* |
| Puberty x Condition | 0.01 | 0.01 | 0.10 | 0.84 | 0.40 |
| Age x Puberty x Condition | 0.00 | 0.01 | 0.06 | 0.49 | 0.63 |
Note. N = 75. Hierarchical linear regression results with cortisol recovery (Mplusgenerated) as the dependent variable. < 0.10†, < 0.05*, < 0.01**, < 0.001***.
Figure 3.
N = 75. Mean cortisol levels (nmol/L) across the session for the 4 puberty x age groups. Samples 4–5 represent the peak of cortisol production post-TSST that occurred 20–30 minutes after the onset of the speech.
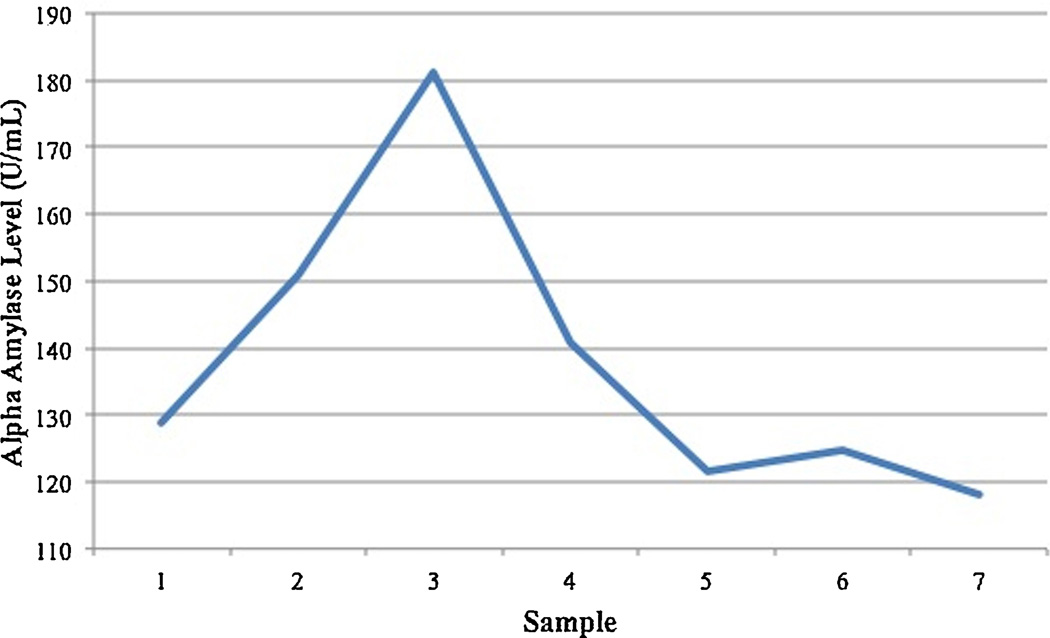
The analysis of alpha-amylase revealed a pattern of reactivity to the stressor and recovery (Figure 4). However, with the exception of higher alpha-amylase intercept predicting slower recovery, β = 0.57, t(74) = 5.71, p < 0.001, all of the other covariates, predictors and their interaction were non-significant (p’s > .10). [The analyses are available upon request].
Figure 4.
N = 75. Alpha-amylase level (U/mL) across the session. The peak at sample 3 represents the alpha-amylase level at the end of the TSST-M.
A regression with self-reported stress reactivity controlling for the Mplus-derived stress intercept suggested a potential effect of condition with lower reported stress in the parent condition, β = −0.21, t(74) = −1.95, p = 0.06, and no main effects of age or puberty (p’s > .10). There was a significant interaction between pubertal status and age, β = −0.26, t(74) = −2.43, p < 0.05, with the young pubertal participants having the greatest self-reported stress reactivity. Simple slopes analyses examining this interaction revealed a significant effect of puberty within the older adolescents (t = −3.17, p < 0.01), but not the younger adolescents (t = 0.51, p = 0.62). No other interactions were significant (p’s > .10). Self-reported stress recovery controlling for intercept and stress reactivity was predicted by pubertal status, β = −0.15, t(74) = −2.44, p < 0.05, with pubertal participants reporting faster recovery than pre-pubertal participants. No other main effects or interactions were significant (p’s > .10).
3. 1. Conclusions
Age was positively associated with cortisol reactivity to the TSST-M. There was an interaction between pubertal stage (early vs. mid-late puberty) and condition on reactivity such that preparing for the speech with the parent was associated with reduced reactivity for the youth in early puberty but not mid-late puberty. For cortisol recovery, the parent’s presence had less of an impact on recovery of the HPA axis with age. This pattern of buffering in cortisol levels was not observed in alpha-amylase levels or subjective stress.
4. Discussion
The current study is the first to examine the distinct roles of pubertal-versus age-related changes in parental social buffering of the HPA axis. This study not only attempts to disentangle the effects of age and puberty but considers two important parameters of HPA axis functioning separately: reactivity and recovery. Importantly, reactivity of the HPA axis represents activation of the system and mobilization of resources whereas recovery represents regulation of the HPA axis and return to baseline. Probing different components of HPA activity offers a more complete picture of overall functioning (e.g., Juster et al., 2012), and simultaneously considering the effects of age and puberty can shed light on specific mechanisms of operation at each level. Results of the current study suggest that parents have diminished effectiveness in buffering HPA axis reactivity as adolescents advance in pubertal development. This finding is in line with previous research demonstrating strong increases in cortisol reactivity as a function of puberty (e.g., Gunnar et al., 2009; Stroud et al., 2009; Blumenthal et al., 2014). Thus, it is possible that parental buffering of reactivity operates through a number of neurophysiological mechanisms that shift during puberty. Such pathways may include changes in neural activity, which enhance sensitivity to social-evaluative threat across puberty or alter neural connections between regions of the brain associated with social processing and evaluation of threat. It is possible that children need maternal support to demonstrate more mature affective processing while adolescents have more developed connectivity that allows them to process in a more independent manner. However, adolescents may not have fully developed the capabilities to appropriately deal with stressors independently of parents, which could result in psychiatric symptoms and disorders without significant support. This hypothesis should be tested in future research as PFC-amygdala connectivity may moderate HPA activity under conditions of psychosocial stress. Changes in oxytocin system functioning may also render parents ineffective at moderating reactivity to threat (Hostinar et al., 2014). Indeed, animal models have demonstrated that the oxytocin system undergoes extensive changes across puberty, including a substantial increase in oxytocin-containing neurons in the hypothalamus (van Eerdenburg et al., 1990) and upregulation of neuronal oxytocin mRNA (Chibbar et al., 1990), which is driven by both gonadal steroids and neural maturation. Studies have demonstrated increases in oxytocin and decreases in HPA activation when parental support is provided in childhood (Seltzer et al., 2010) and friend or partner support is provided in adulthood (Heinrichs et al., 2003; Grewen et al., 2005; Kirschbaum et al., 1995). Therefore, it seems that changes in oxytocin system functioning that accompany puberty may at least partially explain our finding that parents lose their buffering power as adolescents progress in pubertal development.
While parental buffering of HPA activation depends on puberty, conversely, the parental buffering of HPA axis recovery appears to depend more on age. The fact that parents were less able to help adolescents recover from the stressor with age suggests that parents lose the ability to assist in recovery from a stressor across adolescence, independent of pubertal development. It may be that parents are able to help younger but not older adolescents because older adolescents rely more on peers for social support following stress. Alternatively, perhaps no one can assist older adolescents in their recovery from stress. If no individual is able to assist in recovery from a stressor, this could explain the general increase in stress-related psychopathology (e.g., depression) observed in adolescence (see Costello et al., 2011, for review). Another possibility is that, with age, more developmentally salient individuals (i.e., peers or romantic partners) are more effective at helping adolescents return to baseline.
Theoretically, the idea that peers, specifically friends, better regulate the stress response for older adolescents is more likely. Adolescents report becoming more emotionally intimate with peers while becoming more distant from parents with age (Hunter and Youniss, 1982; Cauce, 1986; Harris, 1995; Hartup, 1996). As intimacy has been described as a key feature of the social buffering of stress (Smith et al., 2009), it is plausible that friends rather than parents may be better at helping older adolescents return to baseline after initiation of the stress response. Indeed, a study of older adolescents ages 12–16 revealed that recovering from a stressor with a friend who exhibited low negative qualities in the friendship was related to better HPA axis recovery (Calhoun et al., 2014). Our results suggest that the social changes associated with increasing age explain why parents were ineffective at helping to regulate the stress response for older adolescents in our sample. Future research must examine how parent-child interactions in the lab and the quality of the parent-child relationship as perceived by both the parent and adolescent predict cortisol reactivity in response to psychosocial stress.
The results also showed that the pubertal shift in parental buffering did not extend to self-perceptions or activity of the autonomic nervous system as indexed by alpha-amylase. Overall, regardless of age or pubertal status, the youth showed a trend-level effect of reporting lower stress-reactivity if they prepared for the TSST-M speech with their parent. Youth who were further along in the pubertal transition reported faster recovery, regardless of whether they prepared with their parent or not. Thus, self-report revealed a non-significant but suggestive parental buffering effect that did not diminish with age or puberty. Alpha amylase showed a marked stress response and rapid recovery regardless of condition, age or pubertal status. These findings indicate that the pubertal shift in parental buffering of the HPA axis must rely on mechanisms that are independent of self-perception and regulation of the autonomic system. The salivary alpha-amylase findings also suggest that increasing autonomic output was needed in order to stand, give a speech and respond to mental arithmetic problems, and thus regardless of the parent’s presence, the child needed to increase energy availability. The same was not true of the HPA axis. Elevations in cortisol were probably not essential for performing the motoric and cognitive functions required by the TSST-M task.
Findings of the current study should be interpreted in the context of its limitations. First, our assessment of puberty relied on self-report. Although self-reported pubertal status is known to correlate with physicians’ physical assessment of pubertal development (e.g., Shirtcliff et al., 2009), it is always a possibility that adolescents in our sample were inaccurate and/or biased reporters (Dorn, Susman, Nottelmann, Inoff-Germain, & Chrousos, 1990). Therefore, future studies attempting to replicate this finding may benefit from utilizing physical examinations to objectively determine pubertal status. Likewise, previous studies have found that increased cortisol reactivity accompanies pubertal development primarily in females (Stroud et al., 2009). We did not find sex effects in the current study, and it is possible that our small sample size limited our power to detect sex differences. It should also be noted that we allowed the sex of the parent to vary. While most of the time it was the mother who accompanied the youth, in 17 cases it was the father. Because the sex of parent was not confounded with condition, age or pubertal status, this cannot account for the results. Nonetheless, in the future it would be good to examine whether mothers and fathers provide similar or different stress buffering potency and also whether at this age the quality of the parent-youth relationship makes a difference in how buffering potency survives the transition from childhood to adolescence and from dependence to independence. Further, regardless of condition, the adolescent spent time both before the speech preparation period and after the TSST-M with the parent, which could have influenced our results. Particularly in the recovery phase, parental presence may result in individual differences in the rate at which all individuals return to baseline. However, it is also remarkable that there are still effects of social buffering on reactivity and recovery after just 5 minutes with the parent versus a stranger. Finally, there may be differences in responses between TSST protocols. For example, in this study there were no judges present in the room, and this may lead to differences in perceived stress or cortisol reactivity compared to a protocol where the panel of judges are in the room with the participant. This possibility should be further explored in future research.
In conclusion, results suggest that different aspects of development (i.e., puberty and age) distinctly affect the ability for parents to regulate different components of the HPA stress response (i.e., reactivity and recovery), and that parental buffering is specific to the HPA axis compared to the autonomic nervous system. Increased reliance on friends with age may contribute to the shift in HPA regulation (i.e., recovery from stress), while neurophysiological changes that accompany puberty may contribute to the shift in parental buffering of reactivity. It is also possible that parents do not fully lose effectiveness in social buffering of reactivity with puberty or assisting in recovery with age, but that the process of parental buffering may be stressor-specific. As such, at this point we can only conclude that parents lose their HPA axis buffering potency with the child’s puberty when children are confronted with social-evaluative stressors. It remains to be seen if this developmental change is also true for other types of stressors.
To further clarify mechanisms of operation, future research should employ longitudinal designs that capture the specific point in pubertal development when parents lose buffering over HPA axis reactivity to social stressors, which may shed light on the particular neural and/or hormonal systems involved. For example, localizing the loss of parental buffering at a certain pubertal stage may suggest that systems known to develop at that point in time contribute more to the shift. Furthermore, assessing the psychosocial events that may contribute to loss of parental regulation of HPA axis recovery with age will be particularly fruitful and may help inform intervention strategies. As a result, understanding potential mechanisms of the social buffering effect may inform treatments that decrease the deleterious effects of chronic or prolonged activation of the HPA stress system.
Parents can buffer children but not adolescents from increases in cortisol post-stressor.
We examine whether this switch across development tracks more with puberty or age.
Puberty is more closely associated with the inability of parents to lower cortisol reactivity.
The interaction of age and parent presence is more closely associated with cortisol recovery.
Salivary alpha-amylase levels or subjective stress could not explain cortisol results.
Acknowledgements
The authors were supported by the following grants: T32MH015755 from NIMH to JR Doom, NSF Graduate Research Fellowship to AA VanZomeren-Dohm, and F32HD078048 from NICHD to CE Hostinar. This research was supported by funds from the Canadian Institute for Advanced Research Experience-based Brain and Biological Development Program and NICHD HD075349. The authors would like to thank Bonny Donzella, Amanda Burkholder, Zamzam Ahmed, Hannah Meyers, and Michelle Brown for their assistance with the study.
Role of the Funding Source
Sponsors had no role in data collection, analysis, or interpretation, preparation of the manuscript, or the decision to submit the manuscript.
JRD collected, analyzed, and interpreted data and drafted/revised the article. CEH designed the study and revised the article. AAV collected data and drafted/revised the article. MRG designed the study and drafted/revised the article. All authors approved the final version of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All of the authors report no conflicts of interest.
References
- Ahnert L, Gunnar MR, Lamb ME, Barthel M. Transition to child care: Associations with infant-mother attachment, infant negative emotion, and cortisol elevations. Child Dev. 2004;75:639–650. doi: 10.1111/j.1467-8624.2004.00698.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal H, Leen-Feldner EW, Badour CL, Trainor CD, Babson KA. Pubertal maturation and cortisol level in response to a novel social environment among female adolescents. J. Adolescence. 2014;37:893–900. doi: 10.1016/j.adolescence.2014.06.005. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A, Jobst S, Wustmans A, Kirschbaum C, Rauth W, Hellhammer DH. Attenuated free cortisol response to psychosocial stress in children with atopic dermatitis. Psychosom. Med. 1997;59:419–426. doi: 10.1097/00006842-199707000-00012. [DOI] [PubMed] [Google Scholar]
- Calhoun CD, Helms SW, Heilbron N, Rudolph KD, Hastings PD, Prinstein MJ. Relational victimization, friendship, and adolescents’ hypothalamic–pituitary–adrenal axis responses to an in vivo social stressor. Dev. Psychopathol. 2014;26:605–618. doi: 10.1017/S0954579414000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA, Acebo CA. Self-administered rating scale for pubertal development. J. Adol. Health. 1993;14:190–195. doi: 10.1016/1054-139x(93)90004-9. [DOI] [PubMed] [Google Scholar]
- Cauce AM. Social networks and social competence: Exploring the effects of early adolescent friendships. Am. J. Commun. Psychol. 1986;14:607–628. doi: 10.1007/BF00931339. [DOI] [PubMed] [Google Scholar]
- Chibbar R, Toma J, Mitchell B, Miller F. Regulation of neural oxytocin gene expression by gonadal steroids in pubertal rats. Mol. Endocrinol. 1990;4:2030–2038. doi: 10.1210/mend-4-12-2030. [DOI] [PubMed] [Google Scholar]
- Costello EJ, Copeland W, Angold A. Trends in psychopathology across the adolescent years: What changes when children become adolescents, and when adolescents become adults? J. Child Psychol. Psyc. 2011;52:1015–1025. doi: 10.1111/j.1469-7610.2011.02446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Gunnar MR. Heightened stress responsiveness and emotional reactivity during pubertal maturation: Implications for psychopathology. Dev. Psychopathol. 2009;21:1–6. doi: 10.1017/S0954579409000017. [DOI] [PubMed] [Google Scholar]
- Dorn LD, Susman EJ, Nottelmann ED, Inoff-Germain G, Chrousos GP. Perceptions of puberty: Adolescent, parent, and health care personnel. Dev. Psychol. 1990;26:322–329. [Google Scholar]
- Fontana AM, Diegnan T, Villeneuve A, Lepore S. Nonevaluative social support reduces cardiovascular reactivity in young women during acutely stressful performance situations. J. Behav. Med. 1999;22:75–91. doi: 10.1023/a:1018751702934. [DOI] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam L, Telzer EH, Humphreys KL, Goff B, Shapiro M, Flannery J, Lumian DS, Fareri DS, Caldera C, Tottenham N. Maternal buffering of human amygdala-prefrontal circuitry during childhood but not during adolescence. Psychol. Sci. 25:2067–2078. doi: 10.1177/0956797614550878. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Hibel LC, Fortunato CK, Kapelewski CH. Medication effects on salivary cortisol: tactics and strategy to minimize impact in behavioral and developmental science. Psychoneuroendocrino. 2009;34:1437–1448. doi: 10.1016/j.psyneuen.2009.06.017. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom. Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Brodersen L, Nachmias M, Buss KA, Rigatuso J. Stress reactivity and attachment security. Dev. Psychobiol. 1996;29:191–204. doi: 10.1002/(SICI)1098-2302(199604)29:3<191::AID-DEV1>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Wewerka S, Frenn K, Long JD, Griggs C. Developmental changes in hypothalamus-pituitary-adrenal activity over the transition to adolescence: Normative changes and associations with puberty. Dev. Psychopathol. 2009;21:69–85. doi: 10.1017/S0954579409000054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JR. Where is the child’s environment? A group socialization theory of development. Psychol. Rev. 1995;102:458–489. [Google Scholar]
- Hartup WW. The company they keep: Friendships and their developmental significance. Child Dev. 1996;67:1–13. [PubMed] [Google Scholar]
- Heinrichs M, Baumgartner T, Kirschbaum C, Ehlert U. Social support and oxytocin interact to suppress cortisol and subjective responses to stress. Biol. Psychiat. 2003;54:1389–1398. doi: 10.1016/s0006-3223(03)00465-7. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front. Neuroendocrin. 2009;30:470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Hostinar CE, Johnson AE, Gunnar MR. Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Developmental Sci. 2015;18:281–297. doi: 10.1111/desc.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, Sullivan RM, Gunnar MR. Psychobiological mechanisms underlying the social buffering of the hypothalamic-pituitary-adrenocortical axis: A review of animal models and human studies across development. Psychol. Bull. 2014;140:256–282. doi: 10.1037/a0032671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter FT, Youniss J. Changing functions of three relationships during adolescence. Dev. Psychol. 1982;18:806–811. [Google Scholar]
- Juster RP, Perna A, Marin MF, Sindi S, Lupien SJ. Timing is everything: Anticipatory stress dynamics among cortisol and blood pressure reactivity and recovery in healthy adults. Stress. 2012;15:569–577. doi: 10.3109/10253890.2012.661494. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Klauer T, Filipp SH, Hellhammer DH. Sex-specific effects of social support on cortisol and subjective responses to acute psychological stress. Psychosom. Med. 1995;57:23–31. doi: 10.1097/00006842-199501000-00004. [DOI] [PubMed] [Google Scholar]
- Lorentz K, Gütschow B, Renner F. Evaluation of a direct α-amylase assay using 2-chloro-4-nitrophenyl-α-D-maltotrioside. Clin. Chem. Lab. Med. 1999;37:1053–1062. doi: 10.1515/CCLM.1999.154. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: The moderating role of attachment security. Child Dev. 1996;67:508–522. [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards MH, Boxer AM. Measuring pubertal status: Reliability and validity of a self-report measure. J. Youth Adolescence. 1988;7:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Seltzer LJ, Ziegler TE, Pollak SD. Social vocalizations can release oxytocin in humans. P. Roy. Soc. B-Biol. Sci. 2010;277:2661–2666. doi: 10.1098/rspb.2010.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AM, Loving TJ, Crockett EE, Campbell L. What’s closeness got to do with it? Men’s and women’s cortisol responses when providing and receiving support. Psychosom. Med. 2009;71:843–851. doi: 10.1097/PSY.0b013e3181b492e6. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, Pollak SD. Pubertal development: Correspondence between hormonal and physical development. Child Dev. 2009;80:327–337. doi: 10.1111/j.1467-8624.2009.01263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangler G, Schieche M. Emotional and adrenocortical responses of infants to the strange situation: the differential function of emotional expression. Int. J. Behav. Dev. 1998;22:681–706. [Google Scholar]
- Stroud LR, Foster E, Papandonatos GD, Handwerger K, Granger DA, Kivlighan KT, Niaura R. Stress response and the adolescent transition: Performance versus peer rejection stressors. Dev. Psychopathol. 2009;21:47–68. doi: 10.1017/S0954579409000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno D, Uchino BN, Smith TW. Relationship quality moderates the effect of social support given by close friends on cardiovascular reactivity in women. Int. J. Behav. Med. 2002;9:243–262. doi: 10.1207/s15327558ijbm0903_06. [DOI] [PubMed] [Google Scholar]
- van Eerdenburg FJ, Poot P, Molenaar GJ, van Leeuwen FW, Swaab DF. A vasopressin and oxytocin containing nucleus in the pig hypothalamus that shows neuronal changes during puberty. J. Comp. Neurol. 1990;301:138–146. doi: 10.1002/cne.903010113. [DOI] [PubMed] [Google Scholar]
- Yim IS, Quas JA, Cahill L, Hayawaka CM. Children’s and adults’ salivary cortisol responses to an identical psychosocial laboratory stressor. Psychoneuroendocrino. 2010;35:241–248. doi: 10.1016/j.psyneuen.2009.06.014. [DOI] [PubMed] [Google Scholar]