Abstract
Objectives
Ureter injury is a serious complication of laparoscopic surgery. Current strategies to identify the ureters, such as placement of a ureteral stent, carry additional risks for patients. To non-invasively reduce ureteral injury, we hypothesize that the systemically-injected near-infrared (NIR) dye IRDye800CW-CA can be used to visualize the ureter intraoperatively.
Methods
Adult female mixed breed pigs weighing 24–41 kg (n=2 per dose) were given a 30, 60, or 120 μg/kg systemic injection of IRDye800CW-CA. Using an FDA-cleared laparoscopic NIR system (PINPOINT), images of the ureter and bladder were captured every 10 minutes for 60 minutes after injection. To determine the biodistribution of the dye, tissues were collected for ex vivo analysis with the Pearl Impulse system. Image J software was used to quantify fluorescence signal and signal-to-background ratio (SBR) for the intraoperative images.
Results
The ureter was identified in all pigs at each dose with peak intensity being reached by 30 minutes and remaining elevated throughout the imaging (60 minutes). The 60μg/kg dose was determined to be optimal for differentiating ureter according to absolute fluorescence (>60 counts/pixel) and SBR (3.1). Urine fluorescence was inversely related to plasma fluorescence (R2=−0.82). Ex vivo imaging of kidney, ureter, bladder, and abdominal wall tissues revealed low fluorescence.
Conclusions
Systemic administration of IRDye800CW-CA shows promise in providing ureteral identification with high specificity during laparoscopic surgery. The low dose required, rapid time to visualization, and absence of invasive ureteral instrumentation inherent to this technique may reduce complications related to pelvic surgery.
Introduction
Laparoscopic surgery is a rapidly growing practice in the fields of gynecologic and colorectal surgery. Recent studies report 42% of all colorectal resections and greater than 30% of gynecologic procedures are attempted in this manner.1–3 A laparoscopic approach offers several advantages over traditional open abdominal surgery including decreased postoperative pain, shorter hospital length of stay, fewer surgical site infections.4–9 In gastrointestinal surgery, laparoscopic procedures have clearly shown decreased overall hospital cost when compared to open surgery.6,9 In gynecologic surgery, laparoscopic hysterectomies (LH) can take longer than vaginal and abdominal hysterectomies; however, LH carry similar benefits to the vaginal approach like shorter hospital stays and faster return to normal activities while maintaining low overall costs.10,11
One major drawback to this approach is iatrogenic ureteral injury. Although infrequent, laparoscopic ureteral injury remains a serious complication with significant associated morbidity. Studies have reported an incidence of 0.1 – 7.6% for colorectal and gynecologic surgery with more than 80% going unrecognized intraoperatively.2,12,13 The Cochrane Review found patients almost 2.5 (OR 2.41, 95% CI 1.21–4.82) times more likely to have a ureteral or bladder injury during a laparoscopic hysterectomy.10 Decreased tactile sensation, two-dimensional imaging, and patient-specific factors disrupting the normal anatomy all contribute to the increased incidence observed with a laparoscopic approach. It is important to note that even with high-definition, three-dimensional images viewed during robotic surgery, ureteral injury occurs in 1.7% of patients undergoing hysterectomy.14 Current techniques for intraoperative ureter identification include ureteral stent placement, direct visualization after intraoperative dissection, x-ray fluoroscopy, and visible dyes. However, both stents and fluoroscopy carry additional risk to the patient, and visible dyes lack adequate sensitivity.15–18
Near-infrared (NIR) fluorescence imaging is a rapidly emerging field for real-time illumination of anatomic structures. With emissions in the 700 – 900 nm range, NIR avoids interference with auto-fluorescence of other tissues and can penetrate approximately 1 cm of tissue.19,20 The NIR dye, IRDye800CW-CA, (LI-COR Biosciences) is a modified version of the hepatically-cleared IRDye800CW NHS-Ester that has been studied in animal models and is currently being used in multiple clinical trials21–23 (clinicaltrials.gov, identifiers NCT01987375, NCT0197273, NCT01508572, NCT02113202, NCT02129933). To facilitate urinary tract imaging, however, IRDye800CW-CA contains a carboxylate group that is excreted renally. An advantage of using IRDye800CW-CA is the absorption and emission spectra overlap with indocyanine green (ICG), which can be imaged with the PINPOINT device (Novadaq Technologies, Inc.). The PINPOINT, an FDA-cleared imaging system designed to image perfusion and bile pathways using ICG, combines traditional laparoscopic imaging with NIR fluorescence imaging. Since 2013, over 80 PINPOINT systems have been installed in primarily major academic institutions but also private hospitals in both metropolitan and rural based settings.
Although previous studies have demonstrated feasibility of ureter imaging using open field cameras, an increasing proportion of pelvic surgery is being performed via a minimally invasive approach.17 To this end, we investigated the potential of IRDye800CW-CA in combination with the PINPOINT device to selectively detect the anatomic location of the ureter during laparoscopic surgery. This is the first study to evaluate this dye in combination with an FDA-cleared laparoscopic imaging system. A secondary objective was to determine the optimal dose of IRDye800CW-CA for intraoperative ureter visualization.
Materials and Methods
Reagents
The NIR imaging probe used was IRDye800CW-CA, a carboxylic acid derivative of IRDye800CW (LI-COR Biosciences, Lincoln, NE), excitation 774 nm and emission 789 nm. The dye was reconstituted as a 10mM solution with dimethyl sulfoxide and stored at −80°C in the dark until use. Prior to injection, the dye was brought to room temperature and a weight-based dose of IRDye800CW-CA was calculated based on the desired dose (30 μg, 60 μg, or 120 μg) and the weight of the animal (kg). The μg/kg dose was then diluted to a total volume of 5 mL in phosphate-buffered saline (PBS).
Laparoscopic NIR Device
Image collection was accomplished with the PINPOINT system (Novadaq Technologies Inc., Toronto, Ontario, Canada) using a 10mm, 0-degree scope. Images were captured in traditional bright field view, NIR fluorescence view, and overlay view. Videos were captured in NIR fluorescence (Supplemental Video 1) and overlay (Supplemental Video 2) views.
Animal Models
Adult female mixed breed pigs weighing 24–41 kg were purchased from Snyder Farms (Gadsden, AL). Pigs were housed in accordance with the Institutional Animal Care and Use Committee guidelines and all IRDye800CW-CA dye studies were conducted according to protocols approved by the Animal Resources Program and IACUC. Telazol 4.4 mg/kg and Xylazine 2.2 mg/kg were given for induction of anesthesia and inhalant anesthesia (Isofluorane 2.5%) was used during imaging procedures. Each pig was intubated and underwent bilateral posterior auricular venous catheter placement. Lactated ringer’s solution was infused throughout the study to ensure adequate hydration status and urine production. Pigs were placed supine and secured to operating tables. A 12mm trocar was introduced in the midline superior to the umbilicus and pneumoperitoneum was established with carbon dioxide. Two 5mm trocars were placed under laparoscopic guidance on either side of the abdomen inferior to the umbilicus. Heart rate, blood pressure, respiratory rate, and oxygen saturation were monitored throughout the experiment. All pigs were euthanized according to approved protocols at the conclusion of imaging.
Ureteral Fluorescence Imaging and Analysis
Operating room tables were placed in steep Trendelenberg position and pigs were injected intravenously with 30 μg/kg, 60 μg/kg, or 120 μg/kg dose (n=2 per dose) of IRDye800CW-CA while maintaining NIR laparoscopic visualization of the pelvis. Bright field, NIR, and overlay images were captured for each animal starting at the time of injection (time 0) and proceeding every 10 minutes for a total of 60 minutes post injection. Included in the field of view for each image were the bladder, one or both ureters, the uterus, the colon, and the abdominal wall. No ureter dissection and exposure was performed for any animal. Both ureters were ultimately visualized in all animals; however, bladder distention and uterine and colon position prevented simultaneous visualization of both ureters in the same field of view at some time points. Complete visualization of both ureters was then made possible with atraumatic manipulation of the bladder, uterus, or colon.
Images were exported as TIFF files and analyzed using Image J software. All images were set as 32-bit in Image J. Regions of interest (ROI) were created for each animal at each time point: 2 for bladder, 2 for ureter, 2 for colon, 2 for uterus, and 2 for abdominal wall. All ROI’s were the same area. Mean fluorescence counts from size-matched ROI’s were recorded and averaged across animals for each tissue at every time point and reported as fluorescence signal.
Ex Vivo Tissue Imaging and Analysis
Blood samples were collected prior to IRDye800CW-CA infusion, and after infusion at 10 minutes, 30 minutes, and 60 minutes in K2 EDTA 3 mL collection tubes (BD Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ) (n=2 per dose). Samples were placed on ice until further processing. Once all samples were collected, tubes were centrifuged at 3×103 revolutions per minute for 10 minutes at 4° Celsius.
Urine samples were collected from the bladder via a percutaneous approach before IRDye800CW-CA infusion, and after infusion at 30 and 60 minutes (n=1 per dose for baseline samples and n=2 per dose for 30 and 60 minute samples).
For fluorescence quantification, urine and plasma samples were aliquotted into 0.2 mL centrifuge tubes and imaged with the Pearl Impulse System (LI-COR Biosciences, Lincoln, NE), which is designed to image IRDye800. Image Studio software (LI-COR Biosciences, Lincoln, NE), which is designed for the quantification of Pearl images, was used for analysis. Mean fluorescence counts from size-matched ROI’s were recorded and averaged across animals for the plasma and urine at every time point and reported as fluorescence signal. To correlate plasma and urine fluorescence, a scatter plot was generated. Regression analysis was performed using average mean fluorescence signal in plasma and average mean fluorescence signal in urine.
At the conclusion of in vivo imaging, kidney, ureter, bladder, and abdominal wall tissues were collected for background comparison (n=2 per dose for each tissue) via a midline laparotomy. Prior to imaging, tissues were washed thoroughly with PBS. Because the amount of tissue being imaged can affect fluorescence signal, weight was standardized across all tissue samples at ~55 mg. Tissues were imaged with the Pearl Impulse System, and images were analyzed with Image Studio software. Mean fluorescence counts from size-matched ROI’s were recorded and averaged across animals for each tissue at every time point and reported as fluorescence signal.
Results
Ureteral Fluorescence Imaging
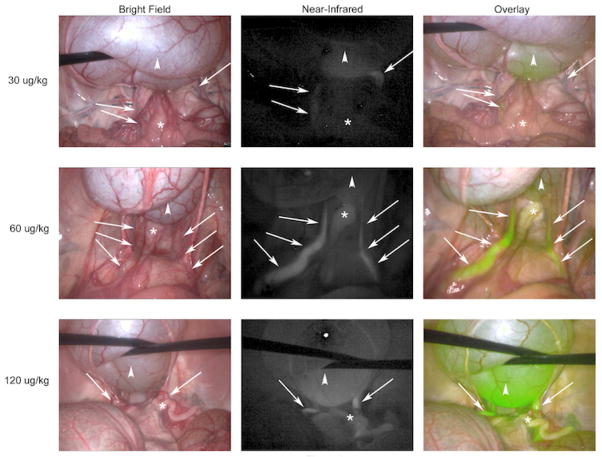
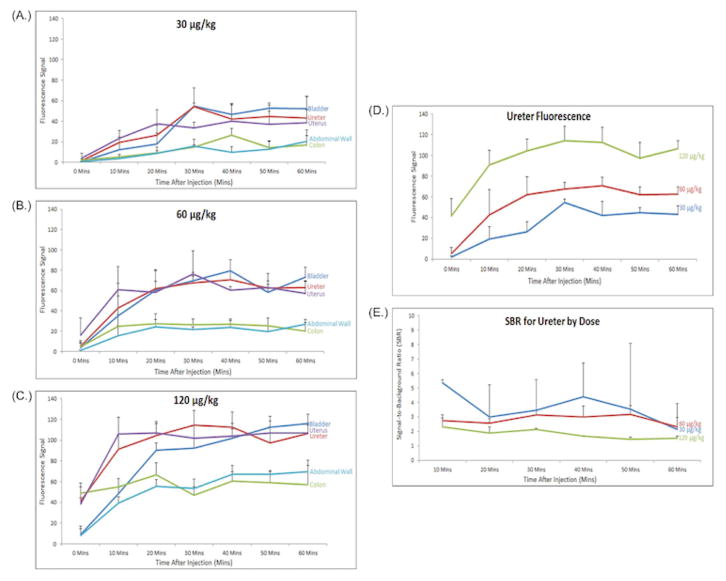
A dose escalation of IRDye800CW-CA was performed using doses that were chosen based on preliminary experiments with 3.0 μg/kg, 7.5 μg/kg, and 15 μg/kg, all of which provided suboptimal ureter visualization (data not shown). Subsequently, doses of 30 μg/kg, 60 μg/kg, and 120 μg/kg were used (n=2 per dose). Laparoscopic imaging with the PINPOINT system yielded bright field, NIR, and overlay images of the pelvis (Figure 1). Ureters are difficult to identify in the bright field image; however, use of the NIR filter and overlay features of the imaging system allow for clear delineation of the path of the ureters bilaterally. The benefit of the overlay view is maintaining traditional bright field views of the surgical field with simultaneous fluorescent illumination of the ureters. An increase in fluorescence signal correlated with IRDye800CW-CA dose, as demonstrated in Figure 1 and quantified in Figure 2. Peak ureter fluorescence was observed at 30 minutes post-injection for the 30 μg (Figure 2A) dose (fluorescence signal = 54) and 30 – 40 minutes post-injection for the 60 μg (Figure 2B) and 120 μg (Figure 2C) doses (fluorescence signal = 67 – 70 and 112 – 114, respectively). The fluorescence signal of the ureter at the 120 μg dose was significantly different (p-value < 0.05) than the 30 μg and 60 μg doses at all time points (Figure 2D). The fluorescence signal for the ureter at the 60 μg dose was significantly different (p-value < 0.05) than the 30 μg dose at 20 minutes and 40 – 60 minutes. There was an increase in fluorescence signal of the bladder throughout the 60-minute study for all 3 doses. The uterus also produced high fluorescence signals, which were higher than that of the ureter at several time points for each dose, especially at time points closer to the infusion of IRDye800CW-CA. It is unclear exactly why uterine fluorescence signals were higher at times than those of the ureters. Ureteral peristalsis produced alternating periods of intense and absent fluorescence, so uterine fluorescence is higher than ureteral fluorescence when there is no urine in the ureter to produce a signal. Additionally, continuous vascular supply to the bicornuate porcine uterus that has a thin myometrium allows the PINPOINT device to easily detect an intense fluorescence signal. In all doses, fluorescence signals in the colon and abdominal walls peaked shortly after the systemic injection of the dye as it coursed through vessels of the abdominal wall and viscera. As the dye was cleared from the blood by the kidneys, the fluorescence signals of the colon and abdominal wall remained relatively constant but less intense than the signals from the ureters and bladder.
Figure 1. Ureter Visualization With PINPOINT Device.
Representative images from the 30 minute time point are shown for each dose of IRDye800CW-CA. (Arrows denote the course of the ureter; Arrowheads denote the bladder; * denotes the uterus)
Figure 2. Fluorescence Signals and Signal-to-Background Ratio (SBR) Over Time.
Overall fluorescence of each tissue over time for the (A.) 30 μg, (B.) 60 μg, and (C.) 120 μg doses. (D.) Comparison of ureter fluorescence over time by dose. (E.) Comparison of SBR for the ureter over time by dose. Values shown are mean fluorescence signal, error bars represent standard error.
Signal-to-background ratios (SBR) were calculated for the ureters versus the abdominal wall for 10 minutes after infusion until 60 minutes (Figure 2E). SBR for Time 0 was not calculated as IRDye800CW-CA had not fully circulated and been allowed reach the urine at this time point. SBR’s were highest for the 30 μg dose with a peak SBR of 5.4 at 10 minutes after infusion and lowest for the 120 μg dose with a peak SBR of 2.1 at 30 minutes. Finally, the 60 μg dose had SBR’s that were relatively constant for the duration of the study (2.5 – 3.1) with peak SBR of 3.1 at 30 minutes.
Clearance of IRDye800CW-CA
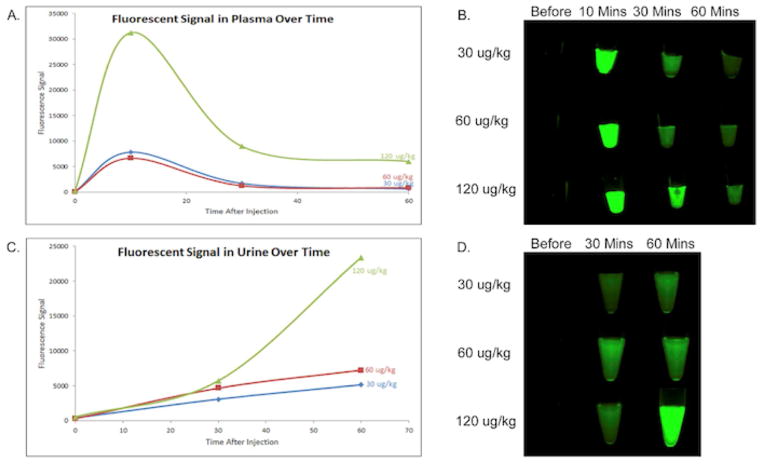
Fluorescence signals from plasma and urine samples collected at several time points over the course of the study confirmed a strong inverse relationship, with an average R2=−0.82 for all 3 doses (Figure 3). As expected, baseline samples before infusion for both urine and plasma had very low fluorescence signals. A peak in plasma fluorescence signal was seen at 10 minutes after infusion, and signal continued to decrease at 30 and 60 minutes for each dose (Figures 3A and 3B). Urine fluorescence signal, however, continued to increase at 30 and 60 minutes for each dose (Figures 3C and 3D).
Figure 3. Fluorescence Signals of the Plasma and Urine Over Time.
(A. and B.) Fluorescence signals over time in the plasma for all doses. (C. and D.) Fluorescence signals over time in the urine for all doses. Values shown are mean fluorescence signal, error bars represent standard error. Images were captured with the 800 nm filter on the Pearl.
Ex Vivo Tissue Imaging
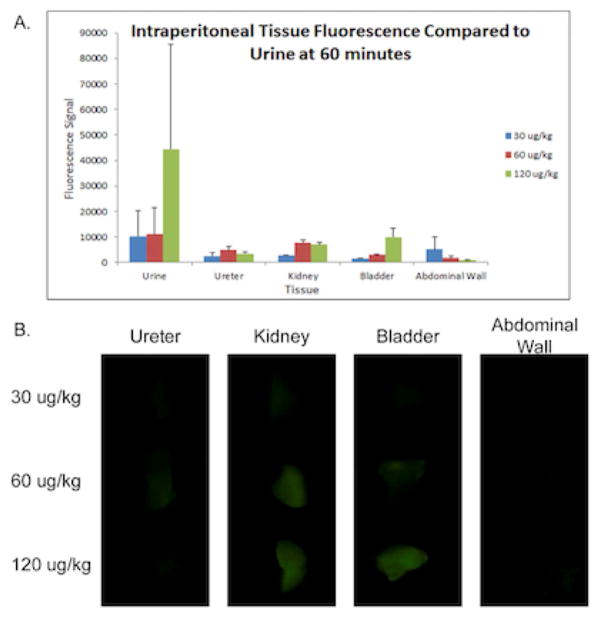
Tissue samples were also collected post-mortem for ex vivo imaging to confirm that the IRDye800CW-CA was present in the urine but not in the urinary tract tissues. Abdominal wall tissue was examined for background. Ex vivo ureter and abdominal wall tissues had the lowest fluorescence. Kidney and bladder had higher fluorescence than ureter and abdominal wall; however, the fluorescence signals were much lower than that of the urine at 60 minutes (Figure 4).
Figure 4. Fluorescence of Ex Vivo Tissue Samples.
(A.) Comparison of the 60-minute urine fluorescence and all ex-vivo tissues. Values shown are mean fluorescence signal, error bars represent standard error. (B.) Pearl images of the tissues using the 800 nm and white filters.
Discussion
Overlay imaging combining traditional laparoscopic and NIR fluorescence imaging is ideal for the operative setting and several platforms are currently available for use including the SPY Elite System (Manufactured by Novadaq, Toronto, Canada and distributed by Lifecell Corporation, Branchburg, NJ), Firefly (Intuitive Surgical Inc., Sunnyvale, CA), and the PINPOINT System. Although all of these devices are designed to image ICG, IRDye800CW-CA was chosen for this study because it is renally excreted, has spectral overlap with ICG, and animal studies in rats have shown it to be nontoxic in large doses.24 This study is the first to demonstrate the ability of IRDye800CW-CA to visualize the ureter using a commercially-available laparoscopic system. These data demonstrate that the ureter is fluorescently visualized within 10 minutes after dye injection and remains distinguishable from surrounding tissue for up to 60 minutes using the PINPOINT system. Additionally, an inverse relationship was demonstrated between the fluorescence of the plasma and that of the urine, indicating rapid renal clearance. Finally, fluorescence examination of the tissues revealed that the IRDye800CW-CA specifically accumulated in the blood and urine.
Other studies evaluating IRDye800CW-CA for ureter identification have used both open and laparoscopic imaging platforms in pigs; however, several factors differentiate this study from previous work. Tanaka et al employed an imaging device able to capture video, NIR, and overlay images, but the device was designed for open procedures and partial exposure of the ureter was required prior to imaging as thicker surrounding tissue limited complete ureter visualization with NIR images.17 This study fails to recapitulate an intraoperative scenario where ureters could potentially be identified without dissection of any kind. Additionally, while this study characterized the excretion of the dye in the urine, no other tissues were examined. In contrast, Schols et al recently utilized a laparoscopic imaging system similar to this study; however, the device is not FDA-approved for use in the United States and is unable to provide an overlay image.25 Furthermore, the study examined only 2 doses of IRDye800CW-CA, one of which was deemed suboptimal for ureter visualization, and did not assess any tissue samples.
The current study evaluated the combination of IRDye800CW-CA with a laparoscopic system capable of reproducing bright field, NIR, and overlay images and is commercially-available and FDA-cleared. The ureter did not require any surgical exposure for identification, and was therefore not placed at additional risk of injury. A true dose escalation (3.0 μg/kg – 120 μg/kg) was employed, and SBR values similar to previous reports were identified. Finally, in contrast to other studies, multiple tissues were surveyed for the presence of IRDye800CW-CA, and trends in fluorescence were identified.
Identification of the optimal dose will depend significantly on the imaging hardware and the ambient light environment. In this study, a discrepancy exists between the overall fluorescence values and the SBR’s for the ureter. The 30 μg/kg dose had the lowest overall fluorescence signal, but had the highest SBR’s. This was due to the extremely low background signals at this dose. During the procedure, the ureter was subjectively more difficult to identify at this dose; however, it was more easily differentiated from the uterus, which had high fluorescence at all doses secondary to its increased vascularity. The opposite effect occurred for the 120 μg/kg dose, where higher background signals led to the lowest SBR’s despite the highest overall fluorescence signal for the ureter. Subjectively, the ureter was highly visible at this dose, particularly in the overlay mode; however, it was often difficult to distinguish next to the overwhelming background signal from the uterus, which was dose-dependent. In light of these competing values and based on subjective experience during the study, the authors observed that the 60 μg/kg dose was, therefore, optimal for this study. Overall fluorescence signal remained high for the ureter, which was consistent with SBR’s between 2.5 and 3.1 throughout the course of imaging. Furthermore, subjective experience proved that intraoperative identification of the ureter was easily accomplished in both the NIR and overlay modes, and there was less interference from the background signal produced by the uterus at this dose (Supplemental Videos 1–2).
Moving forward, several factors must be considered. While the safety and toxicity of IRDye800CW-CA has been documented in rat models, safety has yet to be determined in large animals or humans.24 Additionally, only one hour of imaging was captured after administration of the dye, and it is currently unknown how long a fluorescent signal will persist. Such considerations may be important when undertaking pelvic procedures that last several hours. Furthermore, due to anatomic aspects of the porcine urethra, bladders were unable to be catheterized. As urine was produced and allowed to accumulate during our experiment, a large amount of fluorescent signal was associated with the bladder. This resulted in a “shining” effect onto surrounding structures, particularly at the higher doses of IRDye800CW-CA. Variance in urine fluorescence was also seen between animals secondary to the excreted dye being diluted by the initial volume of urine present in each bladder. These artifacts would be eliminated in most surgical situations where a catheter is employed. Furthermore, in addition to the bladder, the background organ with the highest fluorescence was the uterus, often complicating the distinction between itself and the ureter at the 120 μg/kg dose. However, alternate dosing may yield a higher SBR in patients with a prior hysterectomy or in a male. Finally, in the clinical setting, intraoperative ureter identification is frequently complicated by abnormal conditions or findings in the pelvis like adhesions, increased body mass index, and bleeding. No abnormal disease was present in the models examined in this study, and future investigations are needed to determine the efficacy of this technique under such circumstances.
An additional limitation to this technique of ureteral identification is the availability of the imaging system. The PINPOINT system costs approximately $170,000, and it is currently available at over 80 hospitals across the country. While pricing may prevent smaller hospitals from offering this service, larger centers that already use this device to assist laparoscopic surgeons in identifying vascular blood flow and tissue perfusion have the potential to systemically inject IRDye800CW-CA and expand the use of the PINPOINT system to improve the efficiency of ureteral identification.
In conclusion, this animal study demonstrates that NIR fluorescence imaging is a promising utility for the non-invasive intraoperative identification of the ureter. The combination of IRDye800CW-CA with an FDA-cleared laparoscopic device has the potential to substantially decrease the incidence of a serious complication related to laparoscopic pelvic surgery.
Supplementary Material
Left ureteral peristalsis at 50 minutes after injection of 60 μg/kg of IRDye800CW-CA.
Left ureteral peristalsis at 50 minutes after injection of 60 μg/kg of IRDye800CW-CA.
Synopsis.
Near-infrared fluorescence imaging using IRDye® 800CW-CA in conjunction with the PINPOINT device can specifically detect ureter location showing promising utility for non-invasive ureter imaging.
Acknowledgments
The authors would like to thank Cheryl Killingsworth DVM, Deidra Isbell LVT, Laura Cornett LVT, and Amanda Moon LVT of the Animal Resources Program (University of Alabama at Birmingham, Birmingham, Alabama) for their help and support of this project.
Footnotes
There are no conflicts of interest for the acknowledged contributors.
Disclosures:
IRDye800CW-CA was donated by LI-COR. The PINPOINT System was supplied on an equipment loan from Novadaq Technologies Inc. and the Pearl Impulse was supplied on an equipment loan from LI-COR. Warner Huh received honorarium as a member of Novadaq’s scientific advisory board independent from the work presented in this manuscript. For the remaining authors, no conflicts of interest were declared. This work is supported by grants from the NIH (T32-CA091078).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wright JD, Ananth CV, Lewin SN, et al. Robotically assisted vs laparoscopic hysterectomy among women with benign gynecologic disease. Jama. 2013;309(7):689–698. doi: 10.1001/jama.2013.186. [DOI] [PubMed] [Google Scholar]
- 2.Park JH, Park JW, Song K, Jo MK. Ureteral injury in gynecologic surgery: a 5-year review in a community hospital. Korean journal of urology. 2012;53(2):120–125. doi: 10.4111/kju.2012.53.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simorov A, Shaligram A, Shostrom V, Boilesen E, Thompson J, Oleynikov D. Laparoscopic colon resection trends in utilization and rate of conversion to open procedure: a national database review of academic medical centers. Annals of surgery. 2012;256(3):462–468. doi: 10.1097/SLA.0b013e3182657ec5. [DOI] [PubMed] [Google Scholar]
- 4.Makinen J, Brummer T, Jalkanen J, et al. Ten years of progress--improved hysterectomy outcomes in Finland 1996–2006: a longitudinal observation study. BMJ open. 2013;3(10):e003169. doi: 10.1136/bmjopen-2013-003169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobayashi E, Nagase T, Fujiwara K, et al. Total laparoscopic hysterectomy in 1253 patients using an early ureteral identification technique. The journal of obstetrics and gynaecology research. 2012;38(9):1194–1200. doi: 10.1111/j.1447-0756.2012.01849.x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MZ, Hollenbeak CS, Stewart DB. Laparoscopic colectomy is associated with a lower incidence of postoperative complications than open colectomy: a propensity score-matched cohort analysis. Colorectal disease : the official journal of the Association of Coloproctology of Great Britain and Ireland. 2014;16(5):382–389. doi: 10.1111/codi.12537. [DOI] [PubMed] [Google Scholar]
- 7.Kiran RP, El-Gazzaz GH, Vogel JD, Remzi FH. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: data from national surgical quality improvement program. Journal of the American College of Surgeons. 2010;211(2):232–238. doi: 10.1016/j.jamcollsurg.2010.03.028. [DOI] [PubMed] [Google Scholar]
- 8.Bilimoria KY, Bentrem DJ, Merkow RP, et al. Laparoscopic-assisted vs. open colectomy for cancer: comparison of short-term outcomes from 121 hospitals. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2008;12(11):2001– 2009. doi: 10.1007/s11605-008-0568-x. [DOI] [PubMed] [Google Scholar]
- 9.Juo YY, Hyder O, Haider AH, Camp M, Lidor A, Ahuja N. Is minimally invasive colon resection better than traditional approaches?: First comprehensive national examination with propensity score matching. JAMA surgery. 2014;149(2):177–184. doi: 10.1001/jamasurg.2013.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. The Cochrane database of systematic reviews. 2009;(3):CD003677. doi: 10.1002/14651858.CD003677.pub4. [DOI] [PubMed] [Google Scholar]
- 11.Lee T, Shepherd JP, Kantartzis KL, Ahn KH, Bonidie MJ. Cost analysis when open surgeons perform minimally invasive hysterectomy. JSLS : Journal of the Society of Laparoendoscopic Surgeons/Society of Laparoendoscopic Surgeons. 2014;18(4) doi: 10.4293/JSLS.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palaniappa NC, Telem DA, Ranasinghe NE, Divino CM. Incidence of iatrogenic ureteral injury after laparoscopic colectomy. Archives of surgery. 2012;147(3):267–271. doi: 10.1001/archsurg.2011.2029. [DOI] [PubMed] [Google Scholar]
- 13.da Silva G, Boutros M, Wexner SD. Role of prophylactic ureteric stents in colorectal surgery. Asian journal of endoscopic surgery. 2012;5(3):105–110. doi: 10.1111/j.1758-5910.2012.00134.x. [DOI] [PubMed] [Google Scholar]
- 14.Propst K, Chura J. Is routine cystoscopy warranted after robotic hysterectomy? Obstetrics and gynecology. 2014;123 (Suppl 1):127S. [Google Scholar]
- 15.Brandes S, Coburn M, Armenakas N, McAninch J. Diagnosis and management of ureteric injury: an evidence-based analysis. BJU international. 2004;94(3):277–289. doi: 10.1111/j.1464-410X.2004.04978.x. [DOI] [PubMed] [Google Scholar]
- 16.Bothwell WN, Bleicher RJ, Dent TL. Prophylactic ureteral catheterization in colon surgery. A five-year review. Diseases of the colon and rectum. 1994;37(4):330–334. doi: 10.1007/BF02053592. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka E, Ohnishi S, Laurence RG, Choi HS, Humblet V, Frangioni JV. Real-time intraoperative ureteral guidance using invisible near-infrared fluorescence. The Journal of urology. 2007;178(5):2197–2202. doi: 10.1016/j.juro.2007.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wood EC, Maher P, Pelosi MA. Routine use of ureteric catheters at laparoscopic hysterectomy may cause unnecessary complications. The Journal of the American Association of Gynecologic Laparoscopists. 1996;3(3):393–397. doi: 10.1016/s1074-3804(96)80070-3. [DOI] [PubMed] [Google Scholar]
- 19.Adams KE, Ke S, Kwon S, et al. Comparison of visible and near-infrared wavelength-excitable fluorescent dyes for molecular imaging of cancer. Journal of biomedical optics. 2007;12(2):024017. doi: 10.1117/1.2717137. [DOI] [PubMed] [Google Scholar]
- 20.Keereweer S, Van Driel PB, Snoeks TJ, et al. Optical image-guided cancer surgery: challenges and limitations. Clinical cancer research : an official journal of the American Association for Cancer Research. 2013;19(14):3745–3754. doi: 10.1158/1078-0432.CCR-12-3598. [DOI] [PubMed] [Google Scholar]
- 21.Day KE, Beck LN, Heath CH, Huang CC, Zinn KR, Rosenthal EL. Identification of the optimal therapeutic antibody for fluorescent imaging of cutaneous squamous cell carcinoma. Cancer biology & therapy. 2013;14(3):271–277. doi: 10.4161/cbt.23300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korb ML, Hartman YE, Kovar J, Zinn KR, Bland KI, Rosenthal EL. Use of monoclonal antibody-IRDye800CW bioconjugates in the resection of breast cancer. The Journal of surgical research. 2014;188(1):119–128. doi: 10.1016/j.jss.2013.11.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day KE, Sweeny L, Kulbersh B, Zinn KR, Rosenthal EL. Preclinical comparison of near-infrared-labeled cetuximab and panitumumab for optical imaging of head and neck squamous cell carcinoma. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2013;15(6):722–729. doi: 10.1007/s11307-013-0652-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marshall MV, Draney D, Sevick-Muraca EM, Olive DM. Single-dose intravenous toxicity study of IRDye 800CW in Sprague-Dawley rats. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2010;12(6):583–594. doi: 10.1007/s11307-010-0317-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schols RM, Lodewick TM, Bouvy ND, van Dam GM, Dejong CH, Stassen LP. Application of a new dye for near-infrared fluorescence laparoscopy of the ureters: demonstration in a pig model. Diseases of the colon and rectum. 2014;57(3):407–411. doi: 10.1097/DCR.0000000000000055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left ureteral peristalsis at 50 minutes after injection of 60 μg/kg of IRDye800CW-CA.
Left ureteral peristalsis at 50 minutes after injection of 60 μg/kg of IRDye800CW-CA.