INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of death for both women and men, accounting for 1 in every 3 deaths in the United States (US) [1]. Since the mid-1980s, CVD has killed more women than men each year. In 2011 alone, CVD caused about 10,000 more deaths in women than men [1]. CVD in women is a disease of aging, rarely occurring before the 6th decade of life [2]. It has been proposed that deprivation of ovarian hormones, specifically estrogen, in menopause is causally related to increased CVD risk in aging women [3]. Observational and randomized controlled trials showed differential effects of menopausal hormone therapy (MHT), which included estrogen, on CVD risk: observational studies almost uniformly suggested benefit, while randomized trials showed harm, particularly in elderly women who were many years post-menopausal [4,5].
Multiple hypotheses have been proposed to explain the differences between the unfavorable effects of MHT in randomized studies and the body of observational evidence supporting the beneficial effects of MHT. Prominent among these is the “timing hypothesis” which proposes that MHT started in the perimenopausal or early postmenopausal period is cardioprotective, whereas MHT begun late after menopause increases the risk of CVD [6]. In this review we discuss observational studies and randomized controlled trials of MHT in women and examine the age-dependent effects of estrogen in animal models of acute vascular injury, as well as the effects of estrogen on cellular (macrophage and vascular smooth muscle cell (VSMC)) responses to inflammatory stimuli in vitro.
STUDIES OF MENOPAUSAL HORMONES IN WOMEN
Observational Studies
A meta-analysis of 25 observational studies showed a decreased relative risk of CVD and coronary heart disease (CHD) in postmenopausal women taking MHT compared to those who had never taken hormones (RR=0.70; CI, 0.65-0.75) [7]. The largest and most frequently cited of these, the Nurses’ Health Study (NHS), was a prospective observational study that enrolled 121,700 female nurses 30-55 years of age (Table 1) [8]. The 20 year follow up study of the 70,533 postmenopausal participants (accruing 808,825 person-years of follow up) demonstrated significantly fewer CVD events, non-fatal myocardial infarctions (MIs) or fatal CHD in women on MHT compared to MHT never-users after adjustment for age, body mass index (BMI), weight, diabetes history, hypertension, increased cholesterol, age of menopause, smoking, and family history (RR=0.61; 95% CI 0.52-0.71).
Table 1.
Studies of Menopausal Hormones
| Study | Type | Population | Type of MHT | Effect | Pros/Cons |
|---|---|---|---|---|---|
| Nurse's Health Study (NHS) [8] | Observational, follow up 20 years | Over 121,000 female nurses, 70,533 postmenopausal | 0.625 and 0.3 mg CEE varying doses chosen by participants | Decreases in CHD with both 0.625 and 0.3 mg of CEE | • Observational • Non-randomized • Non-placebo controlled |
| Heart and Estrogen/Progesterone Replacement Study (HERS) [13] | Randomized, placebo controlled, clinical trial | 2,763 postmenopausal women with coronary heart disease | 0.625 mg CEE + 2.5 mg of MPA | No benefit of MHT | • Mixture of estrogen and progestin was used. • Older women, average 66.7 years old |
| Women's Health Initiative Estrogen + Progesterone (WHI E + P) [16] | Randomized, placebo controlled, clinical trial | 16,608 postmenopausal women with intact uterus | 0.625 mg/d CEE and 2.5 mg/d MPA | Increased risk for CHD and invasive breast cancer | • Stopped early due to adverse effects • Older women, average 63 years old |
| Women's Health Initiative Estrogen Alone (WHI E) [17] | Randomized, placebo controlled, clinical trial | 10,739 postmenopausal women with hysterectomy | 0.625 mg/d CEE | No benefit to CHD Increased incidence of stroke | • Stopped early due to adverse effects • Older women, average 63 years old |
| Danish Osteoporosis Prevention Study (DOPS) [29] | Prospective, randomized study | 1006 healthy peri and early postmenopausal women ages 45-58 years old, 3-24 months after last menses | 2mg E2 | Decreased incidence of CVD outcomes and increased survival in MHT group | • Young, perimenopausal women. • Primarily Caucasian. • CVD not primary outcome |
| Kronos Early Estrogen Prevention Study (KEEPS) [32] | Randomized, double blind, placebo controlled study | 720 healthy postmenopausal women ages 42-56 years old, 6 months to 36 months from their last menses | 0.45 mg/d oral CEE plus 200mg oral progesterone 12 days monthly or 50 mg/d transdermal estrogen patch plus 200 mg oral progesterone 12 days monthly | No effect of MHT on CVD outcomes CIMT and CAC at 4 year follow up. Decreased menopausal vasomotor symptoms | • All healthy women, at low risk for CVD. • Does not support timing hypothesis • No adverse effects in MHT group |
| Early versus Late Intervention Trial with Estradiol (ELITE) [37] | 2 × 2 randomized, double blind, placebo controlled study | 643 early (<6 years) or late (>10 years) healthy post-menopausal women without CVD or diabetes | 1mg daily E2 | Decreases in atherosclerosis in early MHT group, not late. | • 90% compliance in MHT. • Supports timing hypothesis |
Abbreviations: CEE, conjugated equine estrogen, MPA medroxyprogesterone; CIMT, carotid intima-media thickness; CAC, coronary artery calcification E2, 17 β-estradiol
The major limitation of the NHS and other observational studies are its non-randomized design [9]. Observational studies are inherently unable to control for selection bias and for confounding differences between treatment groups. Although all of the participants in the NHS were female nurses, there may have been significant differences in unknown or unmeasured variables between the groups due to the non-randomized design of the study. Further studies demonstrated that women who chose to use MHT were more often Caucasian, healthier, wealthier and had more access to healthcare than non-users [10, 11, 12]. To control for these confounding factors, randomized placebo controlled trials were needed to determine the efficacy of MHT as a preventive strategy for CVD.
Randomized Controlled Trials
Heart and Estrogen/Progesterone Replacement Study
The Heart and Estrogen/Progesterone Replacement Study (HERS) was a randomized blinded placebo controlled study that tested the effects of MHT in postmenopausal women with pre-existing CHD (Table 1) [13]. HERS randomized 2,763 women with a mean age of 67 years to MHT with 0.625mg of conjugated equine estrogens (CEE) and 2.5mg of medroxyprogesterone acetate (MPA) daily or placebo and followed them for a mean of 4.1 years. There was no significant difference in the primary outcome (non-fatal MI or fatal CHD) between the 2 groups at the end of the study (RR=0.99; CI 95% 0.80-1.22) (Figure 1). However, there was a significant time trend with an early increase in risk associated with MHT use and subsequent decreased risk in years 4 and 5 (year 1 Relative Hazard (RH)=1.52 95% CI 1.01-2.29; years 4 and 5 RH= 0.67 95% CI 0.43-1.04, p=.009). The apparent benefit of long term (4-5 years) MHT on occurrence of CVD events reported in the primary outcome paper from the study was not confirmed in the extended (mean 6.8 year) unblinded follow up report (RR,0.97; 95% CI 0.82-1.14) [14]. HERS also showed that women in the MHT group were significantly more likely to have venous thromboembolic events and gallbladder disease than those on placebo [13, 14]. Following the demonstration in HERS that MHT did not reduce CVD events in postmenopausal women with established CHD, MHT was no longer recommended as a preventive treatment for CVD progression.
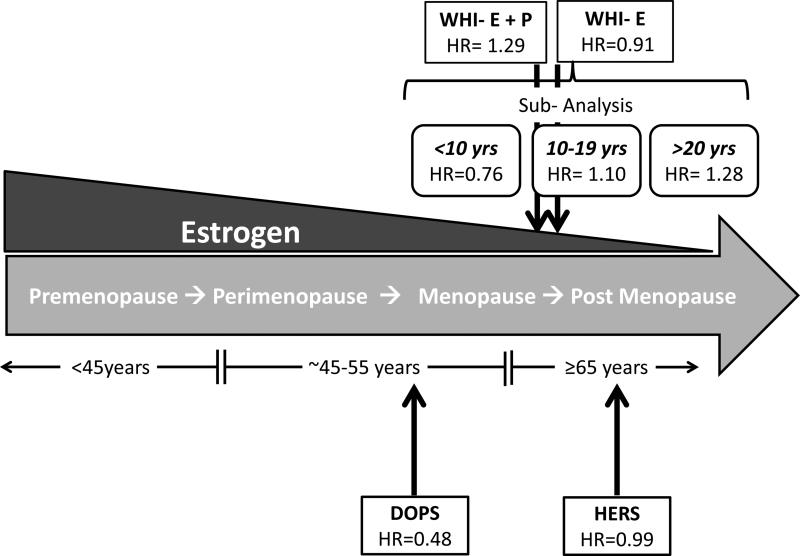
Figure 1. Studies of Menopausal Hormone Therapy (MHT) and the Timing Hypothesis.
Estrogen (E2) levels decrease with age and menopause status while cardiovascular disease (CVD) events increase with age in women. The Heart and Estrogen/Progesterone Replacement Study (HERS) showed no significant CHD benefit of MHT in postmenopausal women with previous diagnosis of CHD. The Women's Health Initiative (WHI) Estrogen + Progestin (WHI-E + P) study showed a significant increase in CHD events in the MHT group while the WHI Estrogen (WHI-E) alone study showed no significant benefit of MHT therapy. Further, when MHT treatment was stratified by time since menopause, the cumulative results from the WHI-E + P and WHI-E show significant decreases in CHD events in early menopausal women (<10yrs since menopause) compared to postmenopausal women (10-19 yrs and >20 yrs since menopause) using MHT. Results from the Danish Osteoporosis Prevention Study (DOPS) indicated a 50% reduction in CHD risk if MHT was begun immediately after menopause, in perimenopause. Arrows indicate average age of study participants. Hazard Ratio, HR for CHD events.
The negative results of the HERS trial, which conflicted with findings from the previous observational studies outlined above, could partially be explained by the recruitment of women with established CHD [13, 15]. Use of the synthetic progestin, MPA, and the formulation of estrogen, CEE, was also proposed to play a role in the negative results of HERS. Importantly, the unexpected negative findings of HERS reinforced the need for further clinical trials and mechanistic studies to determine the effects of estrogen on CVD prevention.
Women's Health Initiative
The Women's Health Initiative Estrogen + Progestin Study (WHI-E + P) was a randomized placebo controlled clinical trial that tested the effects of MHT with estrogen plus progestin (0.625 mg/d CEE and 2.5 mg/d MPA) on CVD events in 16,608 postmenopausal women who had an intact uterus (Table 1) [16]. WHI E + P was stopped after 5 years, 3 years earlier than expected, by the data safety monitoring board due to increased incidence of adverse events and no evidence of CVD benefit in the MHT arm. After an average follow up of 5.2 years the primary endpoint (non-fatal MI and fatal CHD) was significantly increased in women randomized to receive MHT compared to placebo (HR, 1.29; 95%CI 1.02-1.63) (Figure 1). Further, women in the MHT group had increased incidence of stroke (HR, 1.41; 95% CI 1.07-1.85), venous thromboembolism (HR, 2.11; 95%CI 1.58-2.82) and invasive breast cancer (HR, 1.26; 95%CI 1.00-1.59).
The randomized placebo controlled WHI Estrogen Study (WHI-E) enrolled 10,739 postmenopausal women who had undergone a hysterectomy and randomized them to receive estrogen (0.625 CEE) or placebo (Table 1) [17]. The WHI-E was stopped early by the NIH/NHLBI review board due to an increased incidence of stroke and no evidence of CVD benefit, although these outcomes did not cross pre-defined levels for trial termination by the data safety monitoring board [18]. After a mean follow up of almost 7 years, the primary outcome (non-fatal MI or CHD death) was not different between the 2 treatment groups (HR, 0.91; 95%CI 0.75-1.12) (Figure 1). The MHT group had an increased incidence of stroke (HR, 1.39; 95%CI 1.10-1.77) and a trend toward increased venous thromboembolism (HR, 1.33; 95%CI 0.99-1.79). Unlike the combination of CEE plus MPA, CEE alone did not increase invasive breast cancer incidence (HR, 0.77; 95%CI 0.59-1.01). The unblinded 10 year follow up of WHI-E showed no significant benefit of 6 years of MHT use on CVD events and no harmful effects of MHT in the overall population of postmenopausal women [19].
WHI and the Timing Hypothesis
MHT prescriptions almost doubled between 1995 and 1999 but decreased dramatically after 2002 as a result of WHI [20]. In 2004 an expert panel concluded that MHT should not be used as an intervention for CVD prevention [21]. The differential results between observational studies, such as NHS, and WHI studies were attributed to differences in study designs and study populations. The most prominent of these include: age and time since menopause, pre-existing CVD, the composition of the estrogen and progestin used, and their route of administration [22]. In 2005, Phillips and Langer proposed a unified hypothesis, the “timing hypothesis”, which stated that the time since menopause determined the effect of MHT on the cardiovascular system: MHT was cardioprotective in women who were perimenopausal or early postmenopausal, while MHT administered late in menopause had neutral or detrimental cardiovascular effects.
Subsequent follow up analyses of the WHI-E and WHI E + P studies that stratified data by age demonstrated a decreased HR for the primary outcomes of CHD death and non-fatal MI in women who began MHT within the first 10 years of menopause (HR, 0.76; 95%CI 0.50-1.16) and an increased HR in women who began therapy 10-19 years (HR, 1.10; 95%CI 0.84-1.45) or 20+ years after menopause (HR,1.28; 95%CI 1.03-1.58) (Figure 1) [23]. Although the p-value for trend was 0.02, this did not meet the predefined criterion for statistical significance of 0.01 which accounted for multiple testing. The trend was driven primarily by the WHI-E data, where a greater benefit was seen in women who were enrolled within 10 years of menopause (HR, 0.48; 95%CI 0.20-1.17).
A post hoc analysis of the WHI E + P study suggested increased CHD risk in the first 2 years of MHT use (HR,1.29; 95%CI 0.52-3.18) with subsequent decreased risk (HR, 0.64; 95%CI 0.21-1.99 in the first 8 years) in women randomized within 10 years of menopause [24]. The CHD-free survival curves crossed at around 6 years (95% CI 2-10 years), suggesting possible benefit with prolonged treatment of younger women. Similar analyses that included all women recruited in the WHI-E showed a significant decrease in CHD risk after 7 years of MHT compared to placebo (RR=0.46; 95% CI 0.28-0.78) [25].
A recent publication synthesized data from WHI-E (intervention average 7.2 years) and WHI-E+P (intervention average 5.6 years) on primary, secondary and quality of life outcomes after 13 years’ cumulative follow up [26]. The results confirmed previous findings that MHT usage in the intervention phase of WHI-E + P resulted in increased incidence of CHD events and invasive breast cancer and showed that the risks had dissipated in the 13 year cumulative follow up. In contrast, MHT with unopposed CEE was associated with nonsignificant trends toward reductions in CHD (HR, 0.94; 95%CI 0.78-1.14) and invasive breast cancer (HR, 0.79; 95%CI, 0.61-1.02) in the WHI-E population as a whole. Younger women, aged 50-59, had a decreased risk for all-cause-mortality, MI and major CVD outcomes. However, post intervention outcomes after 13 years showed no protective effects of MHT on primary or secondary CVD outcomes in either WHI study. Because the hazards of MHT use appeared to outweigh the benefits, the authors concluded that their findings do not support the use of CEE alone or CEE + MPA as treatment for chronic disease prevention.
The WHI Observational Study (WHI-OS) was a multicenter prospective study that compared the effect of oral and transdermal MHT (which contained E2) use on CVD outcomes in 93,676 postmenopausal women [27]. The 10.4 year mean follow up excluded from the analysis 38,024 women who had never used MHT and 13,931 who had previously used MHT but discontinued use. The remaining women were stratified by the formulation of estrogen used: 2,149 used oral CEE at a low dose (<0.625mg); 24,399 used oral CEE at the conventional dose (0.625mg); 3,396 used oral CEE at a high dose (>0.625mg); 3,024 used oral E2 and 2,187 used transdermal E2. However, there was a non-statistically significant trend toward decreased major CHD outcomes in women using transdermal E2 compared to conventional dose CEE (HR, 0.63; 95%CI 0.37-1.06). Limitations of this study include its observational nature, the small sample size in 4 of the 5 treatment groups, and the small number of CVD and CHD events observed.
An analysis that projected the findings of the WHI-E in women aged 50 to 59 years to the entire population of comparable women in the US estimated that failure to treat hysterectomized women with estrogen therapy was associated with 18,500-90,000 premature deaths over a 10 year period [28]. This analysis suggests the declining use of MHT in younger hysterectomized women may be associated with a significant number of deaths. Further research is needed in younger women to allow patients and physicians to make educated and informed decisions on MHT use in the perimenopausal period.
Danish Osteoporosis Prevention Study
The Danish Osteoporosis Prevention Study (DOPS), a prospective randomized study of 1006 healthy peri- and early postmenopausal women 45-58 years old, evaluated the CVD outcomes of MHT started 3-24 months after last menses (Table 1) [29]. Women were randomly assigned to receive MHT (2mg synthetic 17-β estradiol [E2] in those who had undergone hysterectomy and E2 with 1 mg norethisterone acetate added for 10 days every month in those with an intact uterus) or no treatment starting in 1990-1993. After a mean follow up of 10 years, and following the publication of the WHI findings, women were encouraged to stop MHT due to concerns for adverse effects of MHT. After termination of randomization, the women were followed up for an additional 6 years for a total follow up of 16 years. Both at the end of randomization (HR, 0.48; 95%CI 0.26-0.87) and at the 16 years of follow up (HR, 0.61; 95%CI 0.39-0.94) there was a decrease in the primary endpoint of death, non-fatal MI or admission to hospital for heart failure in women who used MHT (Figure 1). There was a trend towards reduction in MI (HR, 0.45; 95%CI 0.16-1.31) and all-cause mortality (HR, 0.66; 95%CI 0.41-1.08) with MHT and no difference in breast cancer incidence (HR, 0.90; 95%CI 0.52-1.57) at the end of follow up. These data provide additional evidence that early estrogen treatment is protective against CVD in menopausal women. Further support for the timing hypothesis comes from a significant interaction between age and randomization on outcomes, whereby younger women (<50 years) derived greater benefit.
Kronos Early Estrogen Prevention Study
The Kronos Early Estrogen Prevention Study (KEEPS) was a randomized double blind placebo controlled study that enrolled 720 healthy postmenopausal women 42-56 years old who were 6 to 36 months from their last menses (Table 1) [30]. Participants were randomized to receive oral CEE 0.45 mg/d plus 200mg oral progesterone 12 das monthly, 50 mg/d transdermal E2 patch plus 200 mg oral progesterone 12 das monthly or placebo. The primary outcome of the study was the change in carotid intima-media thickness (CIMT) by high-resolution ultrasonography. Secondary outcomes included changes in coronary artery calcification (CAC) and serum levels of C-reactive protein (CRP), IL-6, HDL cholesterol, LDL cholesterol, triglycerides, sex-hormone binding globulin, glucose and insulin.
During 4-year follow up, CIMT increased by 0.0076mm per year on average, and there was no difference between groups in the progression of CIMT. Similarly, there was no difference between the groups in the rate of progression of CAC (21% in the placebo group, 17% in the oral CEE group, and 19% in the transdermal E2 group) [31, 32]. Compared to placebo, oral CEE reduced LDL and increased HDL cholesterol, and reduced triglycerides, CRP and sex hormone binding globulin levels. Transdermal E2 decreased total cholesterol, HDL and non-HDL cholesterol, and increased sex hormone binding globulin levels. Both oral CEE and transdermal E2 were associated with lower levels of fasting insulin and decreased Homeostasis Model Assessment of Insulin Resistance (HOMA) score compared to placebo.
Both estrogen treatment groups had significantly lower incidence of vasomotor symptoms than placebo. The improvement in symptom burden was associated with improvement in some markers of CVD, but no effect on atherosclerosis progression as outlined above. Thus, data from KEEPs support use of MHT for vasomotor symptoms in early menopause. Since the population recruited in KEEPs was small, relatively healthy and at low CVD risk, generalizations of these findings to perimenopausal and early menopausal women with established CVD or multiple CVD risk factors is limited.
Genetic polymorphisms may alter susceptibility to CVD and other diseases. DNA was isolated from leukocytes of 610 KEEPS participants and screened for 13,229 single nucleotide polymorphisms (SNPs) [33]. There were no correlations between genetic polymorphisms for estrogen receptor α (ERα), estrogen receptor β (ERβ), CIMT and CAC at baseline. Four SNPs were significantly associated with CIMT: SNPs of MAP4K4 and IL5 were positively associated with CIMT, whereas 2 CCL5 (RANTES) SNPS were negatively associated with CIMT. IL5 is a TNFα mediator and plays a role in B cell differentiation. CCL5 is an inflammatory and immune modulator, and increased levels of CCL5 have been shown to be associated with atherosclerosis in mouse models [34, 35]. Three SNPs were associated with CAC score; SNPS of IRAK2 and SERPINA1 were positively correlated with CAC, while an ABO SNP was negatively associated with CAC [33]. IRAK2 is a transcriptional protein that affects transcription mRNA stability and IL-1 induced NFκB signaling, and SERPINA1 is a protein that is associated with lung fibrosis. O blood type has been identified as a risk factor for venous thrombosis in a GWAS study in women and men [36]. Functional studies, both preclinical and clinical, are needed to determine the effects of these SNPs on cellular function, CVD progression and response to MHT.
Early Versus Late Intervention Trial with Estradiol
The Early Versus Late Intervention Trial with Estradiol (ELITE) (NCT00114517) is a randomized double blind placebo controlled study that tested the effects of 1mg/day oral E2 (combined with transvaginal micronized progesterone in those with an intact uterus) in 643 healthy women without CVD or diabetes who were either early (<6 years) or late (>10 years) after the onset of menopause (Table 1) [37]. The primary outcome of the study was the rate of change in CIMT. Preliminary data from ELITE, showed that over a period of 6 years, E2 treatment of women in early, but not late menopause was associated with slower progression of CIMT. The interaction by age group was statistically significant. Further, MHT adherence was >90% among the women in the trial, suggesting that the decreased CIMT progression is due to MHT treatment. These exciting preliminary data lend further support to the timing hypothesis.
The above studies in women have resulted in inconclusive and conflicting results on the efficacy of MHT in CVD prevention. To understand these conflicting results we must further elucidate the role of estrogen in the cardiovascular system. Cell and animal model systems facilitate this process by allowing us to answer specific mechanistic questions with respect to estrogen's cardioprotective effects. Work determining the role of estrogen in acute vascular injury will be described below.
PRE-CLINICAL STUDIES
E2 inhibits the vascular injury response in young rodents
E2 (17 β-estradiol) administration delays CVD progression and attenuates the inflammatory response to acute vascular injury in animal models, including mice, rats, and monkeys [38]. We have demonstrated that the vasoprotective effects of E2 are sexually dimorphic (female > male) and age dependent (young > aged) (Figure 2). Using the highly reproducible rat carotid balloon injury model, we initially demonstrated that, compared to intact female rats, intact male rats had increased neointima formation [38, 39]. Further, E2 decreased neointima formation in both orchiectomized males and ovariectomized (OVX) females but testosterone had no effect on neointima formation [39]. Administration of MPA alone did not alter the neointimal response to balloon injury, but combining MPA with E2 blunted the effects of E2 on neointima formation in female OVX rats [40, 41]. Our observation that MPA blocks the vasoprotective effects of E2 in the setting of vascular injury predicted the findings of the WHI-E + P clinical trial, in which MHT containing the progestin MPA was found to increase CVD events in postmenopausal women. A subsequent time course study in the OVX rat model showed that E2 treatment begun immediately prior to balloon injury and continued for 3 das was as effective as long term treatment in preventing neointima formation [42]. Further, delay of E2 treatment until 7 days post injury abolished its inhibitory effect on neointima formation. These observations indicate that the E2 effect on the vascular injury response is mediated by molecular events that occur in the first 72 hrs post injury and that inhibition of neointima formation is not dependent on long term treatment with E2.
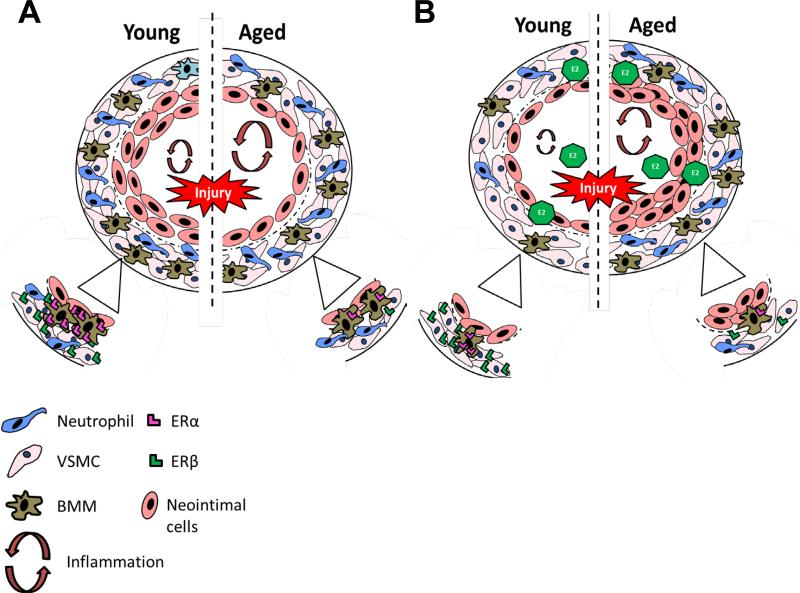
Figure 2. 17 β-Estradiol (E2) Effects on the Acute Vascular Injury Response are Age Dependent.
(A) Cross section of an injured carotid artery from young and aged rodent's shows young rodents have robust neointima formation compared to aged rodents. The insert shows that vascular smooth muscle cells (VSMCs) and bone marrow macrophages (BMMs) from younger rodents have higher levels estrogen receptors (ER), ERβ and ERα respectively, compared to aged rodents. (B) Cross section of an injured carotid artery from young and aged rodents pretreated with E2 shows that E2 decreases the inflammatory response in young rodents by decreasing inflammatory cytokines, such as IL-8, and MCP-1, and decreasing leukocyte (neutrophils and bone marrow macrophages) recruitment to the injured vessel compared to aged mice. In aged rodents, E2 treatment can exacerbate the inflammatory response, increase leukocyte infiltration to the vessel and may cause an increase in neointima formation.
We then examined the effects of E2 on the early phase of the injury response. There were robust increases in mRNA levels of inflammatory mediators, leukocyte infiltration and adventitial fibroblast proliferation and migration toward the lumen in injured arteries of untreated or placebo treated OVX rats [38]. E2 treatment resulted in major decreases in mRNA expression of inflammatory cytokines and chemokines, specifically CINC-2β (rat homolog to IL-8, neutrophil chemoattractant) and MCP-1 (monocyte chemoattractant protein-1), attenuation of leukocyte infiltration, and inhibition of adventitial fibroblast migration and proliferation compared to vehicle treated controls [38]. E2 reduced CINC-2β protein expression within 2 hrs of injury and led to decreased neutrophil and monocyte infiltration in the injured artery at the 24 hr time point [43, 44]. Further, E2 decreased expression of the adhesion molecules VCAM-1, ICAM-1 and P-selectin and the cytokines MCP-1, IL-1β, IL-6 at 2 and 24 hrs post injury [44].
E2 inhibits TNF- α induced inflammation in isolated rat aortic smooth muscle cells
In order to elucidate the molecular mechanisms by which E2 inhibits injury-induced inflammation in arteries, we utilized vascular smooth muscle cells (VSMCs) isolated from rat aortae and treated with the inflammatory mediator TNF-α as an in vitro model. TNF-α dose dependently increased CINC-2β mRNA and protein expression, as well as mRNA expression of the inflammatory mediators MCP-1, ICAM-1, VCAM-1 and P-selectin within 24hrs post treatment [45]. Pretreatment with E2 decreased expression of all inflammatory mediators. We assessed the functional significance of the increased CINC2β expression on neutrophil chemotaxis using a modified Boyden Chamber migratory assay. Conditioned media from TNFα treated VSMCs stimulated neutrophil migration, and this effect was inhibited by E2 pretreatment via an ER-β dependent mechanism, as shown by decreased neutrophil chemotaxis in response to pretreatment with the selective ER-β agonist diarylpropiolnitrile (DPN), but not the selective ER-α agonist pyrazole triol (PPT).
Subsequent studies demonstrated that TNF-α induced upregulation of CINC-2β and MCP-1 in isolated VSMCs is mediated via NFκB signaling and that this signaling pathway is inhibited by E2 [46]. Iκβα is phosphorylated in response to TNF-α targeting it for degradation. This effectively removes the inhibition of NFκB which translocates to the nucleus and stimulates CINC-2β and MCP-1 mRNA expression, thus producing an inflammatory phenotype within the cell. E2 inhibits this inflammatory cascade by two distinct mechanisms, 1) by promoting Iκβα production to bind NFκB and inhibit its translocation and 2) by directly inhibiting the binding of NFκB binding to the DNA promotor region of CINC-2β and MCP-1, thus inhibiting production of these pro-inflammatory mediators. Together, these data elucidate important mechanisms by which E2 attenuates inflammation in acute vascular injury.
E2 decreases vascular smooth muscle cell and fibroblast proliferation and migration in vivo and in vitro
Acute vascular injury induces VSMC proliferation, increasing cell number, and activation, releasing cytokines and chemokines that reach the periadventitial space and cause leukocyte migration and further inflammation within the injured artery [39, 47, 48, 49]. E2 treatment in OVX rats attenuates VSMC proliferation as depicted by a reduction in BrdU staining in the injured artery at 7 days post treatment [47]. A Boyden chamber migratory assay demonstrated that conditioned media from rat VSMCs caused an increase in adventitial fibroblast migration that was attenuated by pretreatment with E2 for 24 hrs [48]. Adventitial fibroblast chemotaxis is dependent on integrin-β3 and osteopontin produced by VSMCs [49]. E2 inhibits adventitial fibroblast migration by decreasing osteopontin production in VSMCs which, at least in part, explains its role in the attenuation of neointima formation in acute vascular injury.
E2 has age dependent effects on neointima formation and inflammation in vivo and in vitro
The studies summarized above that demonstrated inhibitory effects of E2 on inflammation and neointima formation in injured arteries were all preformed on young (10-12 week old) Sprague Dawley rats. In an effort to model the effects of MHT on arteries of aged postmenopausal women, we studied the modulation by E2 of the acute vascular injury response to its modulation by E2 treatment in aged (52 weeks) female rats (Figure 2) [50]. We observed that E2 treatment no longer attenuated the inflammatory response and neointima formation in the aged animals.
To further elucidate the differential effects of E2 in young and aged animals, we used the C-reactive protein transgenic mice (CRPtg) and ERα and ERβ knock out mouse models. The CRPtg mouse model expresses human CRP in a manner similar to the human condition [51, 52, 53]. During basal conditions, CRP is expressed at low levels but after injury, human CRP production quickly increases resulting in a more robust neointima formation in response to acute vascular injury [51]. In young (12 week) CRPtg OVX mice, E2 attenuated the increased neointima formation after acute vascular injury [51, 52, 53]. In contrast, E2 treatment did not attenuate and may have increased neointima formation and inflammatory cytokine production in aged CRPtg mice (52 weeks old) (Figure 2) [54]. The increases in inflammation and neointima formation in to E2 treated aged mice provides additional evidence supporting the timing hypothesis, which proposes MHT treatment in late menopausal women may increase CVD risk [6].
In vitro studies using bone marrow macrophages (BMMs) and VSMCs derived from young and old mice showed increases in inflammatory cytokines in response to CRP treatment for 24hrs [54]. E2 attenuated the CRP mediated inflammatory responses in BMMs derived from young mice by decreasing mRNA expression of CCL3, CCL4, IL-1, and C3 and in VSMCs derived from young mice by decreasing mRNA expression of ICAM, VCAM, and p-selectin. In contrast, E2 exhibited no attenuation or increased inflammation in response to CRP in BMMs and VSMC derived from aged mice. These data indicate age dependent, anti-inflammatory effects of E2. Further, to test the ER dependent anti-inflammatory effects of E2, we used BMMS and VSMCs derived from ERα and ERβ knock out mice and demonstrated that the anti-inflammatory effects of E2 were dependent on ERα in BMMs and ERβ in VSMCs (Figure 2). We have shown that BMMs derived from aged mice express less ERα compared to those from young mice, which may explain the decreased efficacy of E2 in aged BMMs.
Further studies that pretreated the cells with the G-protein coupled estrogen receptor (GPER) agonist, G1 showed significant attenuation of CRP induced inflammation in young and old BMMs [54]. Studies by Chakrabarti and Davidge have also demonstrated G1, but not E2, has anti-inflammatory effects in TNF-α activated human umbilical vein endothelial cells [55]. GPER also plays a role in atherosclerotic plaque formation and GPER knock out mice have increased atherosclerotic plaque progression when fed an atherogenic diet compared to wild type controls [56]. These data suggest that GPER may be a novel target for CVD prevention and/or treatment in older postmenopausal women in whom estrogen is not cardioprotective.
Translational Studies
Macrophage studies in women
We are currently testing the effects of ERα expression and its anti-inflammatory role in macrophages derived from premenopausal and postmenopausal women [57]. Preliminary studies in human macrophages show a decrease in ERα mRNA and protein levels in postmenopausal women compared to premenopausal women. Importantly, continuous MHT from the time of menopause maintains ERα expression at premenopausal levels. Further, E2 attenuates CRP-driven inflammation in macrophages derived from premenopausal women and postmenopausal women on MHT but not those from postmenopausal women not on MHT. We hypothesize that maintaining ERα expression in macrophages conserves the anti-inflammatory effects of E2 in these cells. Decreases in ER levels in aging women may play a role in the deleterious effects of estrogen therapy in older, postmenopausal women.
Human uterine arteries have differential responses to E2 dependent on time since menopause
An ex vivo assay carried out in uterine arteries from 68 postmenopausal women, 3 months to 10+ years after menopause, showed differential inflammatory responses to E2 dependent on years since menopause [58]. Arteries were sectioned and treated for 24 hrs for with E2 or vehicle. Direct correlations were established between years after menopause and protein expression levels of inflammatory markers IL-1β, VEGF, TNFα, MCP-1, s ICAM, and sVCAM. Treatment with E2 decreased IL-1β, VEGF and TNFα at all ages post menopause. When arteries were grouped into <5, 5-10, and >10 years post menopause, MCP-1, sICAM, sVCAM, IL-6 and IL-8 were all decreased by E2 treatment (<5 years), unchanged by E2 (5-10 years), and increased by E2 (>10 years).
Protein levels of ERβ increased in uterine arteries with increasing years post menopause, while ERα levels remained unchanged. Further, ERβ protein expression and protein levels of IL-β, VEGF, TNFα, MCP-1, sICAM, sVCAM, IL-6 and IL-8 following 24 hrs of E2 treatment were positively correlated. These data suggest than an increased ERβ mediated response to E2 in VSMCs can play a role in the deleterious effects of estrogen therapy in older menopausal women. The increasing pro-inflammatory response to E2 of the uterine arteries with increasing time post menopause supports the timing hypothesis. Further mechanistic studies are needed to determine the role of altered ER expression and function in menopause and the age related response to E2.
Conclusions and Future Directions
Large randomized clinical trials with concurrent basic science studies testing the timing hypothesis are needed to determine the mechanisms behind the protective effects of MHT and bridge bench to bedside research. Further mechanistic studies are needed to elucidate the cellular and molecular pathways through which estrogen mediates its anti-inflammatory and cardioprotective actions. The effects of estrogen deprivation on inflammation need to be further studied. Translational animal to human studies should be considered for evaluating the effects of aging on cellular responses to estrogen. Importantly, fundamental studies elucidating aging-affected pathways may reveal novel strategies for estrogen rescue. Together these studies will help to determine the role of E2 is CVD prevention.
Acknowledgements and Sources of Funding
This work was supported, in part, National Heart, Lung, and Blood Institute (NHLBI) grant R01 HL087980 (Oparil S), T32 HL07457 (Oparil S, Giordano, S); by Veterans Affairs Biomedical Laboratory Research & Development Service Merit Award OMB 4040-0001 (Hage FG).
References
- 1.Mozaffarian D, et al. AHA statistical update heart disease and stroke statistics—2015 update. Circulation 2015. 131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Van der Schow YT, van der Graaf Y, Steyerberg EW, Eijkemans MJC, Banga JD. Age at menopause as a risk factor for cardiovascular mortality. Lancet. 1996;347:714–718. doi: 10.1016/s0140-6736(96)90075-6. [DOI] [PubMed] [Google Scholar]
- 3.Bowling M, Oparil S, Hage F, Hilgers R, Xing D. Chapter 1: Sex hormones and vascular function. Sex Hormones. DOI:10.5772/1313. 2012. Pg 1-31. [Google Scholar]
- 4.Humphrey LL, Chan BKS, Sox HC. Postmenopausal hormone replacement therapy and the primary prevention of cardiovascular disease. Annals of Internal Medicine. 2002;137:273–284. doi: 10.7326/0003-4819-137-4-200208200-00012. [DOI] [PubMed] [Google Scholar]
- 5.Magliano DJ, Rogers SL, Abramson MJ, Tonkin AM. Hormone therapy and cardiovascular disease: a systemic review and meta-analysis. BJOG. 2006;113:5–14. doi: 10.1111/j.1471-0528.2005.00797.x. [DOI] [PubMed] [Google Scholar]
- 6.Phillips LS, Langer RD. Postmenopausal hormone therapy: Critical reappraisal and unified hypothesis. Fertility and Sterility. 2005;83:558–566. doi: 10.1016/j.fertnstert.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Barrett-Conner E, Grady D. Hormone replacement therapy, heart disease, and other considerations. Annual Reviews of Public Health. 1998;19:55–72. doi: 10.1146/annurev.publhealth.19.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Grodstein F, Manson JE, Colditz GA, Willett WC, Speizer FE, Stampfer MJ. A prospective, observational study of postmenopausal hormone therapy and primary prevention of cardiovascular disease. Annals of Internal Medicine. 2000;133:933–941. doi: 10.7326/0003-4819-133-12-200012190-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hannan EL. Randomized clinical trials and observational studies: Guidelines for assessing respective strengths and limitations. Journal American College Cardiology Intervention. 2008;1:211–217. doi: 10.1016/j.jcin.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Barrett-Connor E. Postmenopausal estrogen and prevention bias. Ann Intern Med. 1991;115(6):455–456. doi: 10.7326/0003-4819-115-6-455. [DOI] [PubMed] [Google Scholar]
- 11.Grodstein F, Stampfer M. The epidemiology of coronary heart disease and estrogen replacement in postmenopausal women. Progress in Cardiovascular Disease. 1995;3:199–210. doi: 10.1016/s0033-0620(95)80012-3. [DOI] [PubMed] [Google Scholar]
- 12.Matthews KA, Kuller LH, Wing RR, Meilahn EN, Plantinga P. Prior to use of estrogen replacement therapy, are users healthier than non-users? American Journal of Epidemiology. 1996;143:971–978. doi: 10.1093/oxfordjournals.aje.a008678. [DOI] [PubMed] [Google Scholar]
- 13.Hulley S, Grady D, Bush T, Furberg C, Herrington DM, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 14.Grady D, Herrington DM, Bittner V, Blumenthal R, Davidson M, Hlatky M, Hsia J, Hulley S, Herd A, Khan S, Newby LK, Waters D, Vittinghoff E, Wenger N, the HERS Research Group Cardiovascular disease outcomes during 6.8 years of hormone replacement: Heart and estrogen/progestin replacement study follow-up (HERS II). JAMA. 2002;288:49–57. doi: 10.1001/jama.288.1.49. [DOI] [PubMed] [Google Scholar]
- 15.Herrington DM. The HERS trial results: Paradigms lost? Annals of Internal Medicine. 1999;131:463–466. doi: 10.7326/0003-4819-131-6-199909210-00012. [DOI] [PubMed] [Google Scholar]
- 16.Writing Group for the Women's Health Initiative Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principle results from the Women's Health Initiative randomized controlled trial. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 17.Writing Group for the Women's Health Initiative Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: The Women's Health Initiative randomized controlled trial. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 18.Alving B. NIH News, NIH asks participants in Women's Health Initiative Estrogen-Alone study to stop study pills, begin follow up phase. 2004 Mar 2; doi: 10.1097/01.SMJ.0000125549.20351.BB. http://www.nhlbi.nih.gov/whi/pr_04-3-2.pdf. [DOI] [PubMed]
- 19.LaCroix AZ, Chlebowski RT, Manson JE, Aragaki AK, Johnson KC, Martin L, Margolis KL, Stefanick ML, Bryski R, Curb JD, Howard BV, Lewis CE, Wactawski-Wendi J. Health outcomes after stopping conjugated equine estrogen among postmenopausal women with prior hysterectomy: A randomized controlled trial. JAMA. 2011;305:1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hersh AL, Stefanick ML, Stafford R. National use of postmenopausal hormone therapy: Annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 21.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, Grady D, Haan CK, Hayes SN, Judelson DR, Keenan NL, McBride P, Oparil S, Ouyang P, Oz MC, Mendelsohn ME, Pasternack RC, Pinn VW, Robertson RM, Scheneck-Gustafsson K, Sila CA, Smith SC, Sopko G, Taylor AL, Walsh BW, Wneger NK, Williams CL. Evidence-Based Guidelines for Cardiovascular Disease Prevention of in Women. JACC. 2004;43:900–921. doi: 10.1016/j.jacc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Dubey RK, Imthurn B, Zacharia LC, Jackson EK. Hormone replacement therapy and cardiovascular disease. What went wrong and where do we go from here? Hypertension. 2004;44:789–795. doi: 10.1161/01.HYP.0000145988.95551.28. [DOI] [PubMed] [Google Scholar]
- 23.Rossouw JE, Prentice RL, Manson JE, Wu LL, Barad D, Barnabei VM, Ko M, LaCroix AZ, Margolis K, Stefanick ML. Postmenopausal hormone therapy and risk of cardiovascular disease by age and years since menopause. JAMA. 2007;297:1465–1426. doi: 10.1001/jama.297.13.1465. [DOI] [PubMed] [Google Scholar]
- 24.Toh S, Hernández-Díaz S, Logan R, Rossouw JE, Hernán MA. Coronary heart disease in postmenopausal recipients of estrogen plus progestin therapy: does the increased risk ever disappear? A randomized trial. Ann Intern Med. 2010;152(4):211–217. doi: 10.1059/0003-4819-152-4-201002160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harman SM, Vittinghoff E, Brinton EA, Budoff MJ, Cedars MI, Lobo RA, Merriam GR, Miller VM, Naftolin F, Pal L, Santoro N, Taylor HS, Black DM. Timing and duration of menopausal hormone treatment may affect cardiovascular outcomes. Am J Med. 2011;124(3):199–205. doi: 10.1016/j.amjmed.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manson JE, Chlebowski RT, Stefanick ML, Aragaki AK, Rossouw JE, Prentice RL, Anderson G, Howard BV, Thomson CA, LaCroix AZ, Wactawski-Wende J, Jackson RD, Limacher M, Margolis KL, Wassertheil-Smoller S, Beresford SA, Cauley JA, Eaton CB, Gass M, Hsia J, Johnson KC, Kooperberg C, Kuller LH, Lewis CE, Liu S, Martin LW, Ockene JK, O'Sullivan MJ, Powell LH, Simon MS, Van Horn L, Vitolins MZ, Wallace RB. Menopausal hormone therapy and health outcomes during the intervention and extended post stopping phases of the Women's Health Initiative randomized trials. JAMA. 2013 Oct 2;310(13):1353–68. doi: 10.1001/jama.2013.278040. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shufelt CL, Merz NB, Prentice RL, Pettinger MB, Rossouw JE, Aroda VR, Kaunitz AM, Lakshminarayan K, Martin LW, Phillips LS, Manson JE. Hormone therapy, dose, formulation, route of delivery, and risk of cardiovascular events in women: findings from the Women's Health Initiative Observational Study. Menopause. 2014;21:260–266. doi: 10.1097/GME.0b013e31829a64f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarrel PM, Nijike VY, Vinante V, Katz DL. The mortality toll of estrogen avoidance: an analysis of excess deaths among hysterectomized women aged 50-59. American Journal of Public Health. 2013;103:1583–1588. doi: 10.2105/AJPH.2013.301295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schierbeck LL, Rejnmark L, Tofteng CL, Stilgren L, Eiken P, Mosekilde L, Kober L, Jensen JEB. Effect of hormone replacement on cardiovascular events in recently postmenopausal women: randomized trial. BMJ. 2012;345:e6409. doi: 10.1136/bmj.e6409. [DOI] [PubMed] [Google Scholar]
- 30.Miller VM, Black DM, Brinton EA, Budoff MJ, Cedars MI, Hodis HN, Lobo RA, Manson JE, Merriam GR, Naftolin F, Santoro N, Taylor HS, Harman SM. Using basic science to design a clinical trial: Baseline characteristics of women enrolled in the Kronos Early Prevention Study (KEEPS). Journal of Cardiovascular Translational Research. 2009;2:228–239. doi: 10.1007/s12265-009-9104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harman SM, Brinton EA, Cedars M, Lobo R, Manson JE, Merriam GR, Miller VM, Naftolin F, Santoro N. KEEPS: The Kronos Early Estrogen Prevention Study. Climacteric. 2005;8:2–12. doi: 10.1080/13697130500042417. [DOI] [PubMed] [Google Scholar]
- 32.Harman SM, Black DM, Naftolin F, Brinton EA, Budoff MJ, Cedars MI, Hopkins PN, Lobo RA, Manson JE, Merriam GR, Miller VM, Neal-Perry G, Santoro N, Taylor HS, Vittinghoff E, Yan M, Hodis HN. Arterial imaging outcomes and cardiovascular risk factors in recently menopausal women: A randomized trial. Annals of Internal Medicine. 2014;161:249–260. doi: 10.7326/M14-0353. [DOI] [PubMed] [Google Scholar]
- 33.Miller VM, Petterson TM, Jeavons EN, Lnu AS, Rider DN, Heit JA, Cunningham JM, Huggins GS, Hodis HN, Budoff MJ, Santoro N, Hopkins PN, Lobo RA, Manson JE, Naftolin F, Taylor HS, Harman SM, de Andrade M. Genetic polymorphisms associated with carotid intima—media thickens and coronary artery calcification in women of the Kronos Early Estrogen Prevention Study. Physiol Genomics. 2013;45:79–88. doi: 10.1152/physiolgenomics.00114.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Quinones MP, Martinez HG, Jimenez F, Estrada CA, Dudley M, Willmon O, Kulkarni H, Reddick RL, Fernandes G, Kuziel WA, Ahuja SK, Ahuja SS. CC chemokine receptor 5 influences late stage atherosclerosis. Atherosclerosis. 2007;195:e92–e103. doi: 10.1016/j.atherosclerosis.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 35.Braunersreuther V, Zernecke A, Araud C, Liehn EA, Steffens S, Shagdarsuren E, Bidzhekov K, Burger F, Pelli G, Lucklow B, Mach F, Weber C. Ccr5 but not Ccr1 deficiency reduces development of diet-induced atherosclerosis in mice. ATVB. 2007;27:373–379. doi: 10.1161/01.ATV.0000253886.44609.ae. [DOI] [PubMed] [Google Scholar]
- 36.Heit JA, Armasu SM, Asmann YW, Cunningham JM, Matusumoto ME, Petterson TM, De Andrade M. A genome-wide association study of venous thromboembolism identifies risk variants in chromosomes 1q24.2 and 9q. J Thromb Haemost. 2012;10:1521–1531. doi: 10.1111/j.1538-7836.2012.04810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hodis HN, Mack WJ, Shoupe D, Azen SP, Stanczyk FZ, Hwang-Levine J, Budoff MJ, Henderson VW. Abstract 13283: Testing the menopausal hormone therapy Timing Hypothesis: Early versus late intervention trial with estradiol. 2014;130:A13283. doi: 10.1097/GME.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing D, Nozell S, Chen YF, Hage F, Oparil S. Estrogen and Mechanisms of Vascular Protection. ATVB. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen SJ, Li H, Durand J, Oparil S, Chen YF. Estrogen reduces myointimal proliferation after balloon injury of rat carotid artery. Circulation. 1996;93:577–584. doi: 10.1161/01.cir.93.3.577. [DOI] [PubMed] [Google Scholar]
- 40.Levine R, Chen SJ, Durand J Chen YF, Oparil S. Medroxyprogesterone attenuated estrogen-mediated inhibition of neointima formation after balloon injury of the carotid artery. Circulation. 1996;94:2221–2227. doi: 10.1161/01.cir.94.9.2221. [DOI] [PubMed] [Google Scholar]
- 41.Sunday L, Tran MM, Krause DN, Duckles SP. Estrogen and progestogens differentially modulate vascular pro-inflammatory factors. Am J Physiol. 2006;291:E261–E267. doi: 10.1152/ajpendo.00550.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori T, Durand J, Chen YF, Thompson A, Bakir S, Oparil S. Effects of short-term estrogen treatment on the neointimal response to the balloon injury of rat carotid artery. American Journal of Cardiology. 2000;85:1276–1279. doi: 10.1016/s0002-9149(00)00748-7. [DOI] [PubMed] [Google Scholar]
- 43.Xing D, Miller A, Novak L, Rocha R, Chen YF, Oparil S. Estradiol and progestins differentially modulate leukocyte infiltration after vascular injury. Circulation. 2004;109:234–241. doi: 10.1161/01.CIR.0000105700.95607.49. [DOI] [PubMed] [Google Scholar]
- 44.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation. 2004;110:1664–1669. doi: 10.1161/01.CIR.0000142050.19488.C7. [DOI] [PubMed] [Google Scholar]
- 45.Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, Blalock E, Oparil S. Estrogen modulates TNF-induced inflammation in rat aortic smooth muscle cells through estrogen receptor- activation. Am J Physiol. 2007;292:H2607–H2612. doi: 10.1152/ajpheart.01107.2006. [DOI] [PubMed] [Google Scholar]
- 46.Xing D, Oparil S, Yu H, Gong K, Feng W, Black J, Chen YF, Nozell S. Estrogen modulates NFκB signaling by enhancing Iκβα levels and blocking p65 binding at the promotors of inflammatory genes via estrogen receptor-β. PLoS ONE. 2012;7:e36890. doi: 10.1371/journal.pone.0036890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oparil S, Chen SJ, Chen YF, Durand JN, Allen L, Thompson JA. Estrogen attenuates the adventitial contribution to neointima formation in injured rat carotid arteries. Cardiovascular Res. 1999;44:608–614. doi: 10.1016/s0008-6363(99)00240-0. [DOI] [PubMed] [Google Scholar]
- 48.Li G, Chen YF, Greene GL, Oparil S, Thompson JA. Estrogen inhibits vascular smooth muscle cell-dependent adventitial fibroblast migration in vitro. Circulation. 1999;100:1639–1645. doi: 10.1161/01.cir.100.15.1639. [DOI] [PubMed] [Google Scholar]
- 49.Li G, Chen YF, Kelpke S, Oparil S, Thompson JA. Estrogen attenuates integrin-β3-dependent adventitial fibroblast migration after inhibition of osteopontin production in vascular smooth muscle cells. Circulation. 2000;101:2949–2955. doi: 10.1161/01.cir.101.25.2949. [DOI] [PubMed] [Google Scholar]
- 50.Miller AP, Xing D, Feng W, Fintel M, Chen YF, Oparil S. Aged rats lose vasoprotective and anti-inflammatory effects of estrogen in injured arteries. Menopause. 2007;14:251–260. doi: 10.1097/01.gme.0000235366.39726.f6. [DOI] [PubMed] [Google Scholar]
- 51.Xing D, Hage FG, Chen YF, McCroy MA, Feng W, Skibinski GA, Majid-Hassan E, Oparil S, Szalai AJ. Exaggerated neointima formation in human C-reactive protein transgenic mics is IgG Fc receptor type 1 (Fc gamma RI)-dependent. Am J Path. 2008;172:22–30. doi: 10.2353/ajpath.2008.070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang D, Oparil S, Chen YF, McCrory MA, Skibinski GA, Fen W, Szalai A. Estrogen treatment abrogates neointima formation in human C-reactive protein transgenic mice. ATVB. 2005;25:2094–2099. doi: 10.1161/01.ATV.0000179602.85797.3f. [DOI] [PubMed] [Google Scholar]
- 53.Hage FG, Oparil S, Xing D, Chen YF, McCroy MA, Szalai AJ. C-reactive protein-mediated vascular injury requires complement. ATVB. 2010;30:1189–1195. doi: 10.1161/ATVBAHA.110.205377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bowling MR, Xing D, Kapadia A, Chen YF, Szalai AJ, Oparil S, Hage FG. Estrogen effects on vascular inflammation are age dependent: Role of estrogen receptors. ATVB. 2014;34:1477–1485. doi: 10.1161/ATVBAHA.114.303629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chakrabarti S, Davidge ST. G-protein coupled receptor 30 (GPR30): a Novel regulator of endothelial inflammation. PLOS One. 2012;12:e52357. doi: 10.1371/journal.pone.0052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, Amann K, Arterburn JB, Baron M, Prossnitz ER. G-Protein coupled estrogen receptor protects from atherosclerosis. Scientific Reports. 2014;4:7564. doi: 10.1038/srep07564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hage FG, Xing D, Guo Y, Colon C, Szalai AJ, Chen YF, Oparil S. Estrogen-induced vasoprotection is preserved after prolonged estrogen deprivation. Circulation. 2014;130:A14875. [Google Scholar]
- 58.Novella S, Heras M, Hermenegildo C, Dantas AP. Effects of estrogen on vascular inflammation: A Matter of timing. ATVB. 2012;32:2035–2042. doi: 10.1161/ATVBAHA.112.250308. [DOI] [PubMed] [Google Scholar]