Abstract
Background
The risk of anterior cruciate ligament (ACL) injury is 2-10 times greater in women than men. While the role of sex on injury risk is well established, its effects on surgical outcomes remain controversial.
Purpose
To investigate whether the biomechanical outcomes of ACL reconstruction are affected by sex using an established porcine model that displays similar sex-specific differences in knee anatomy and ligament structural properties to humans. We hypothesized there are sex differences in ACL reconstruction outcomes with regards to the graft structural properties, knee laxity and cartilage damage.
Study Design
Controlled Laboratory Study.
Methods
A total of 41 (23 M, 18 F) adolescent Yucatan minipigs underwent unilateral ACL transection and ACL reconstruction using sex-matched bone-patellar tendon-bone allografts (with or without additional bio-enhancement). Graft biomechanical and histological properties, knee laxity and cartilage damage were assessed after 15 weeks. A two-factor ANOVA was used to investigate the effect of sex on all the measured outcomes after adjusting for the treatment effect.
Results
After 15 weeks of healing, female pigs had a significantly lower mean normalized graft yield load (by 18.5±7.7%; p=0.023) and linear stiffness (by 11.9±5.6%; p=0.043), compared to males. Female pigs had a significantly greater side-to-side differences in AP knee laxity at 30° (by 1.4±0.6 mm; p=0.028) and 90° (by 1.8±0.8 mm; p=0.032). Female pigs had a lower graft vascular density (by 0.8±0.3 [analog scoring];p=0.021) with similar cellular and collagen-based histologic scores in both sexes (p>0.6). Female pigs also had a significantly larger area of cartilage damage (by 43.3±14.8 mm2; p=0.014) after conventional ACL reconstruction than their male counterparts.
Conclusion
Female pigs had significantly worse outcomes (i.e., graft structural properties, knee laxity and cartilage damage) compared to males in this translational model after 15 weeks of healing.
Clinical Relevance
These data suggest that further optimization of ACL injury treatments may be needed to accommodate each sex instead of using a “one fits all” approach to improve surgical outcomes, decrease incidence of re-injury, and decrease posttraumatic osteoarthritis risk following ACL reconstruction.
Keywords: Anterior cruciate ligament, Reconstruction, Sex, Biomechanical outcomes, Posttraumatic osteoarthritis
Introduction
The anterior cruciate ligament (ACL) is one of the most frequently injured ligaments of the knee.22 In addition to pain and instability, ACL injuries are associated with other concomitant articular injuries,26 can lead to reduced functional performance33, 42 and an increased risk of early onset posttraumatic osteoarthritis (OA).7, 27, 34, 53 ACL reconstruction has been long considered as the gold standard of care for treating ACL injuries for functionally unstable knees. While many advances have been made in terms of surgical and rehabilitation interventions, patients who have suffered ACL injury continue to face long-term consequences that include:1) decreased activity levels,32, 41 2) a 10-25% incidence of reinjury within 5 years after surgery48, 51 and 3) a 50-100% incidence of OA within 10-15 years of injury.7, 27, 34, 53
The incidence of ACL injuries has been shown to be significantly influenced by sex, with women being at 2-10 fold greater risk than men when playing the same sport.24 Despite the well-described role of sex on ACL injury risk,24 the role of sex on the outcomes of ACL surgery has been a topic of considerable debate. A limited but growing number of studies have investigated the sex-specific differences in various aspects of ACL reconstruction outcomes including graft failure risk, re-injury rates, knee laxity and patient oriented outcomes.1, 3, 4, 8, 10, 13, 18, 19, 35, 39, 40, 42, 43, 47, 48 However, the findings are inconclusive as some authors reported poorer outcomes in females,1, 4, 8, 13, 18, 35, 39, 42, 43 while others noted no difference.3, 10, 19, 47, 48
Women have been reported to have significantly higher rates of graft failure compared to men by as much as 20%.35 Prior studies have reported significantly greater side-to-side differences in anteroposterior (AP) knee laxity in females than males after ACL reconstruction by as much as 1.6 mm with either bone-patellar tendon-bone or hamstring tendon grafts.4, 8, 13, 18, 35, 43 Moreover, women have been found to have significantly higher pain frequency and intensity35 with worse patient-reported outcomes compared to men after ACL reconstruction.1, 13, 18, 39 Female sex has also been associated with a lower rate of return to sport and pre-injury activity levels following an ACL surgery.35, 49 However, a recent systematic review of the thirteen studies in the literature reported no systematic differences in graft failure (7 studies), contralateral ACL injury (3 studies), knee laxity (Lachman test [5 studies], pivot-shift test [5 studies] and instrumented knee laxity [7 studies]) and patient reported outcomes (9 studies) between males and females who had undergone ACL reconstruction.47
These discrepancies in determining statistically significant sex-related outcomes of ACL surgery in clinical studies may be the result of various factors, including alack of outcome measures with the sufficient sensitivity to detect differences between the sexes. While instrumented knee laxity assessment can be performed in patients, the results can be affected by the errors in measurements caused by various factors such as soft tissue motion artifact and intra-observer variability. Direct measurements of graft healing (i.e. biomechanics and histology) are also challenging, if not impossible, in clinical trials. Most importantly, the critical outcome of posttraumatic OA risk and severity may not be detectable in a clinical cohort for a decade or more as there are currently no reliable early predictors of this disease.
Some of these limitations can be mitigated when animal models are used to study the outcomes of ACL surgery. Posttraumatic OA in animals often occurs within 1 to 12 months after injury, with smaller animals developing macroscopic changes in cartilage structure sooner than larger animals. Animal joints can be opened and evaluated to examine the integrity of the graft and cartilage. For these reasons, animal models have been long used to study ACL injuries, treatments and associated complications.2, 9, 12, 14, 15, 17, 20, 37, 38 Among those, the porcine model has been shown to be the closest to the human based on its size, anatomy and functional dependency on ACL.6, 31, 45, 55 Furthermore, it has been shown that pigs develop posttraumatic OA following ACL transection and reconstruction in a pattern similar to that reported in humans.29 Finally, the porcine knee develops posttraumatic OA at a faster rate than is seen in humans, with the findings at 12 months in the porcine model reflective of those seen at 10-15 years after ACL reconstruction in humans.29 This faster onset of posttraumatic OA allows for more rapid assessment of factors, which may influence the development of posttraumatic OA following ACL injury and treatment.
More recently, the porcine model has been validated as a sex-specific large animal surrogate model for the human knee, with similar sex differences seen for knee laxity, ACL structural properties and size, tibial slope, femoral notch size and cartilage thickness.25 Using this model, we are now poised to investigate the role of sex on the biomechanical outcomes of ACL reconstruction. We hypothesized that there are sex-specific differences in the outcomes of ACL reconstruction with regards to graft structural properties, knee laxity and cartilage damage.
Methods
Following IACUC approval, 41 normal adolescent Yucatan mini-pigs (23 Males, 18 Females; Age: 15±1months; Weight: 48.0±9.6 Kg) underwent unilateral ACL transection and were randomly assigned to one of 4 treatment groups:16 conventional ACL reconstruction (n=13; 8 males, 5 females), or bio-enhanced ACL reconstruction with 1× (n=10; 7 males, 3 females), 3× (n=9; 3 males, 6 females) and 5× (n=9; 5 males, 4 females) platelet-rich plasma (PRP). The surgical knee was randomly selected, and the contralateral ACL-intact knee served as a control. The data were obtained from a previously published study evaluating the effect of PRP concentration on the functional outcomes of the ACL surgery.16 In the current study, we analyzed this dataset to determine whether sex had any influence on the biomechanical, histological and OA outcomes of ACL surgery.
PRP Preparation
Prior to surgery, 60 mL of autologous whole blood was drawn into a syringe containing 10% acid-citratedextrose (Harvest Technologies, Plymouth, MA) and centrifuged (mean relative centrifugal force, 150 g; 6 minutes). The platelet-rich buffy coat was harvested and the platelets isolated with a second spin (mean relative centrifugal force, 500 g; 8 minutes). Platelets were counted and resuspended in a specified amount of harvested platelet-poor plasma to create a platelet concentrate of 1× (1.0±0.0), 3× (3.1±0.2) and 5× (5.2±0.3) relative to the systemic platelet count.16 Complete details of platelet and blood cell concentrations for whole blood and PRP concentrates for each group have been previously presented by Fleming et al.16 PRP concentrates were maintained at room temperature and used within 30 minutes of preparation.
Surgical Procedures
Following a medial arthrotomy, the ACL was isolated and transected at mid-substance as previously described.16 Complete transaction was confirmed by a positive Lachman test. Conventional ACL reconstruction was performed using fresh-frozen bone-patellar tendon-bone allografts from age-, weight-, and sex-matched donors.16 The entire patellar tendon (∼10 mm in width) was used for the soft tissue portion of the graft with trimmed bone plugs. Femoral and tibial tunnels were created in the standard positions for ACL reconstruction, with the tibial tunnel exiting in the center of the tibial ACL attachment and the femoral tunnel placed in the center of the femoral ACL attachment site. A 6-mm-offset femoral aimer (ACUFEX; Smith & Nephew) was used to place a 2.4 mm guidewire in the center of the femoral footprint. The femoral guidewire was overdrilled with an 8 mm reamer to create a 25×8 mm femoral socket. A 9 mm tibial tunnel was reamed over a guidepin with the exit site in the center of the ACL tibial insertion. The graft was passed into the femoral tunnel and secured with a 6×20 mm interference screw (BioSure; Smith & Nephew). The tibial bone plug was then passed retrograde through the tibial tunnel and tensioned firmly with the knee in maximum extension and then fixed to the tibia using a second 6×20 mm interference screw. Vicryl retention sutures were tied over an extracortical button on the tibia for secondary fixation.
For the bio-enhanced reconstruction groups, an extracellular matrix-based scaffold (MIACH, Boston Children's Hospital, Boston MA), was placed around the graft prior to final fixation and 3 cc of autologous PRP, manufactured as noted above, in one of three concentrations (1×, 3× and 5×) were added to the scaffold prior to wound closure.16 Surgeries were done by an experienced board-certified orthopaedic surgeon (MMM), fellowship trained in sports medicine, to ensure consistency in all surgical factors including tunnel placement and graft tension. All animals were housed in individualized pens and periodically checked for 15 weeks post-surgery. They were then euthanized, and the limbs were harvested and immediately frozen at -20°C until the biomechanical evaluation.
Physical Examination
A physical examination, to establish weight and passive knee range of motion of both knees, was performed on anesthetized animals in a supine position preoperatively and before limb harvest.
Biomechanical testing
The knees (surgically treated and the contralateral intact) were thawed to room temperature prior to biomechanical testing. Specimens were sectioned at the proximal femur and distal tibia with all soft tissues external to the joint capsule removed. The distal tibia and proximal femur were then potted for rigid attachment to a tensile testing frame (MTS 810; Material Testing Systems, Prairie Eden, MN).17 The joints were wrapped in towels saturated with physiologic saline to prevent dehydration. Investigators were blinded to the experimental group and sex during preparation and testing.
Knee Laxity
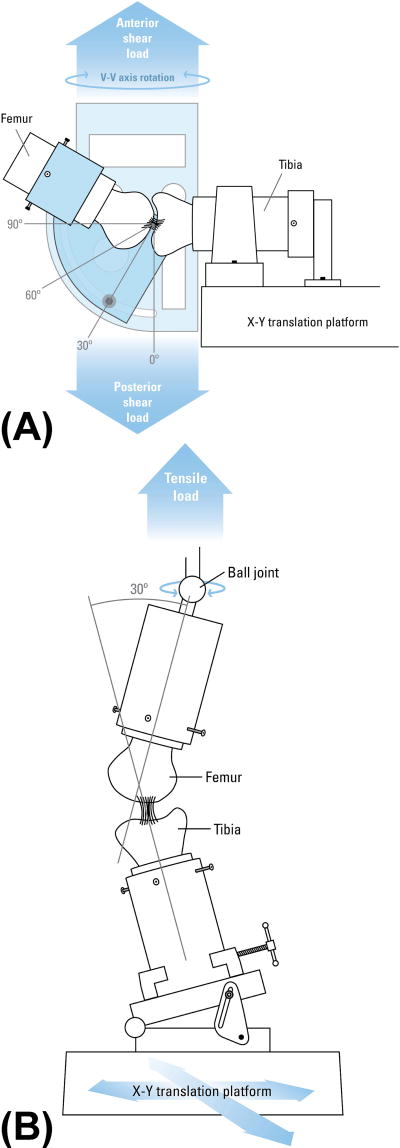
AP knee laxity values were measured using a custom fixture at 30°, 60° and 90° of knee flexion (Figure 1A).15, 17 The knees were locked at each flexion angle with axial tibial rotation constrained in the neutral position while tibial translation/rotation were unconstrained in coronal plane.17 The knees were subjected to 12 cycles of ±40 N AP shear loads at each specific flexion angle while the AP displacements were measured. AP knee laxity was defined as the overall translational motion of the femur with respect to the tibia within the AP shear load limits of ±30 N.15, 17
Figure 1.
Schematics of the test fixtures used for (A) AP laxity and (B) tensile failure testing. For AP laxity testing, the knee flexion angle was prescribed, axial tibial rotation was constrained in the neutral position, and the translations in the coronal plane were unconstrained while the AP loads were prescribed. For tensile testing (B), the knee flexion angle was initially set at 30°. The tibia was mounted to the base of the MTS via a sliding X-Y platform while the femur was unconstrained to rotations so that the specimen could seek its own position to ensure that the load was distributed over the entire graft cross section (Adopted with permission from Fleming et al15, 17).
ACL Structural Properties
Following the laxity assessment, all remaining soft tissues were dissected from the joint leaving the ACL intact. The femur-ACL-tibia constructs were then secured in a custom designed tensile testing fixture so that the mechanical axis of the ACL was collinear with the load axis of the test frame (Figure 1B).17 The femoral axial rotation was unconstrained and the tibia was connected to the test frame through a sliding X-Y table helping the specimen to seek its own physiologic position under tensile loading. Specimens were then loaded in tension to failure at 20 mm/min.15, 20, 23, 54 The recorded load-displacement data were used to quantify ACL linear stiffness, yield and maximum loads.23
Graft Histological Characteristics
Subsequently, the grafts were dissected, fixed in formalin embedded in paraffin, sectioned and then stained with Hematoxylin and Eosin or α-smooth muscle actin antibodies.44 The Ligament Maturity Index was used to assess a central sagittal slice through each graft following previously established techniques.16, 44 The Ligament Maturity Index consists of three subscores evaluating the cellular, collagen and vascular organization.44 The cellular subscore evaluated the presence of inflammatory cells, number of fibroblasts, fibroblast nuclear aspect ratio and the orientation of the fibroblast nuclei relative to the collagen fascicles. The collagen subscore considered bundle orientation and crimp appearance. The vascularity subscore assessed blood vessel density, orientation and maturity. Ligament scoring was conducted by three independent investigators (intraclass correlation coefficient [ICC]=0.92), blinded to the treatment and sex, and the values were averaged for final analysis.
Macroscopic Cartilage Assessment
After biomechanical testing, the articular cartilage across the medial and lateral femoral condyles and the medial and lateral tibial plateaus, for both the treated and contralateral intact knees, were stained using India ink to highlight surface irregularities. The length and width of all visible lesions in all 4 regions of interest, medial and lateral femoral condyles and medial and lateral tibial plateau, were measured using calipers.29 Lesion areas were estimated assuming elliptical fits. The lesion areas for each region were summed to give the total lesion area for each knee joint. Moreover, cartilage samples were scored using a common macroscopic scoring method50 with a five-point scale ranging from 0 (no damage) to four (lesions with exposed bone >10 % of the lesion area). The total score was the sum of the scores from each region. A larger score correlated with more cartilage damage. Two independent examiners (ICC=0.96), blinded to the knee, treatment and sex performed all measurements and the values were averaged.
Statistical Analysis
A two-factor analysis of variance (ANOVA) using a multivariate general linear model was used to investigate the effect of sex on all the measured outcomes. All the sex-related comparisons were adjusted for the treatment effect by adding the treatment type as a fixed factor to the model. Analyses were conducted on normalized graft structural properties (% contralateral intact), side-to-side differences in AP knee laxity (between the treated and contralateral intact knees), and graft histological properties and cartilage damage data obtained from the treated knees. In the event that any of the measured outcomes were significantly affected by the combined sex and treatment interaction, the analysis for the corresponding outcome measure was repeated on each group separately in order to isolate the effect of sex and to avoid errors due to the treatment effect. Comparisons were considered to be statistically significant for p≤0.05. All results are displayed as mean ± standard error of the mean (SEM).
Results
Animal Welfare and Physical Examination
All animals recovered well from surgery and survived the full 15-week follow-up term with no signs of infections or other complications. There were no statistically significant differences between males and females with regards to the changes in the weight or range of motion from pre-injury to post-injury levels (p>0.1 for all comparisons; Table 1).
Table 1.
Differences in physical examination outcomes.
| Measured Outcome | Adj. Meana ± SEM | Sex-Differences | ||
|---|---|---|---|---|
|
| ||||
| Male | Female | |||
| Weight (Kg) | Pre-op | 46.0±1.9 | 50.7±2.1 | P=0.112 |
| Post-opb | 56.6±1.6 | 59.8±1.7 | P=0.187 | |
| Change | 10.6±1.6 | 9.2±1.7 | P=0.525 | |
|
| ||||
| Maximum Knee Flexion (deg) | Pre-op | 137.9±2.4 | 142.1±2.4 | P=0.228 |
| Post-opb | 135.2±2.4 | 135.1±2.5 | P=0.986 | |
| Change | -2.8±3.4 | -6.9±3.6 | P=0.406 | |
|
| ||||
| Maximum Knee Extension (deg) | Pre-op | 36.0±1.7 | 37.3±1.8 | P=0.616 |
| Post-opb | 40.8±1.9 | 42.6±1.9 | P=0.518 | |
| Change | 4.7±2.9 | 5.3±3.0 | P=0.902 | |
Adjusted for treatment effect.
15 weeks after the surgery.
Biomechanical Outcomes
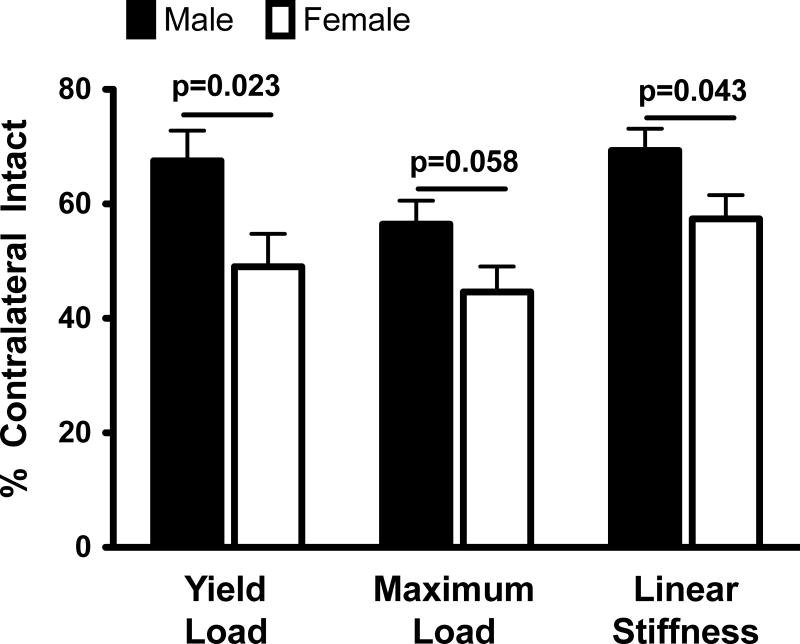
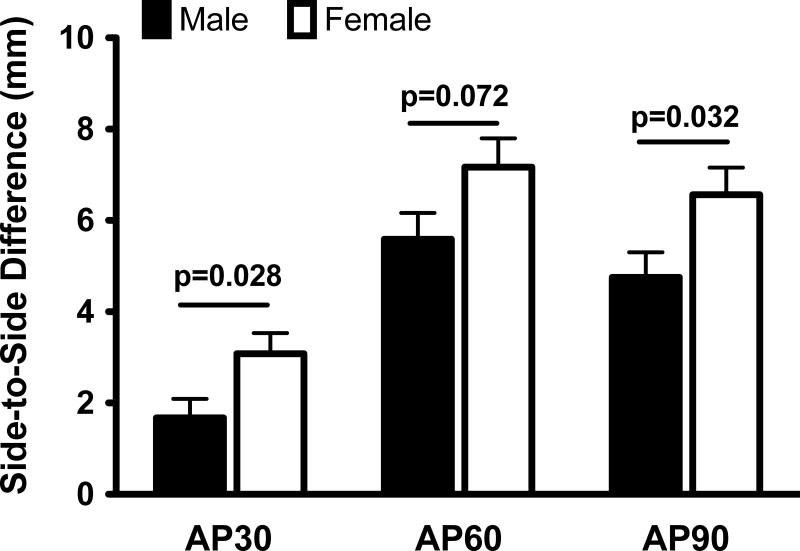
After 15 weeks of healing, the grafts in the female knees had a significantly lower normalized yield load (by 18.5±7.7%; p=0.023) and linear stiffness (by 11.9±5.6%; p=0.043) than their male counterparts (Figure 2). Females also had a lower mean normalized graft maximum load (by 11.8±6.0%) compared to the males, a difference which approached statistical significance (p=0.058). The ACL reconstructed knees in the female pigs had a greater side-to-side difference in AP knee laxity (by 1.4±0.6 mm, 1.6±0.9 mm and 1.8±0.8 mm at 30°, 60° and 90° respectively) compared to their male counterparts (Figure 3). These differences were statistically significant at 30° (p=0.028) and 90° (p=0.032), and approached statistical significance (p=0.072) at 60° of knee flexion.
Figure 2.
Sex differences in normalized graft structural properties. The values were adjusted for the treatment effect and then mean±SEM are reported.
Figure 3.
Sex differences in side-to-side differences in AP knee laxity. The values were adjusted for the treatment effect and then mean±SEM are reported.
Graft Histology
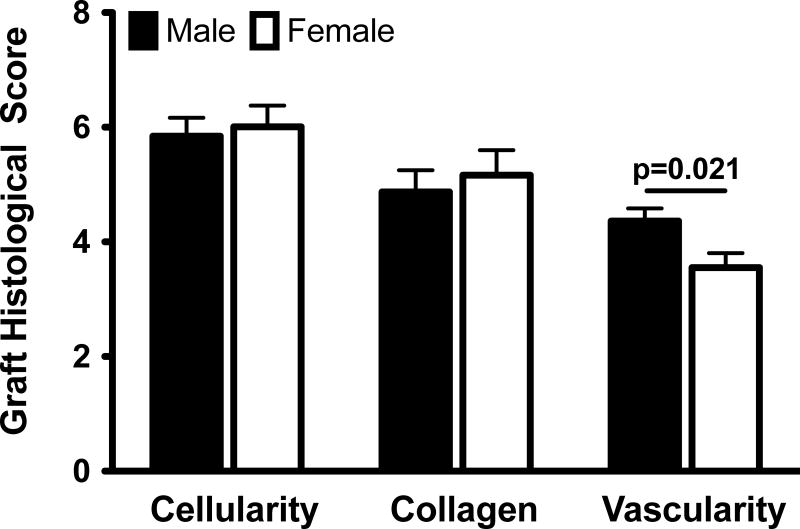
Females had a lower vascular density within the graft than the males (by 0.8±0.3 [analog scoring]; p=0.021). However, all other histologic qualitative measures were the same in both sexes with similar cell (p=0.742) and collagen (p=0.619) subscores at 15 weeks of healing (Figure 4). There was no evidence of inflammatory cells within any grafts.
Figure 4.
Sex differences in graft histological scores. The values were adjusted for the treatment effect and then mean±SEM are reported.
Cartilage Assessment
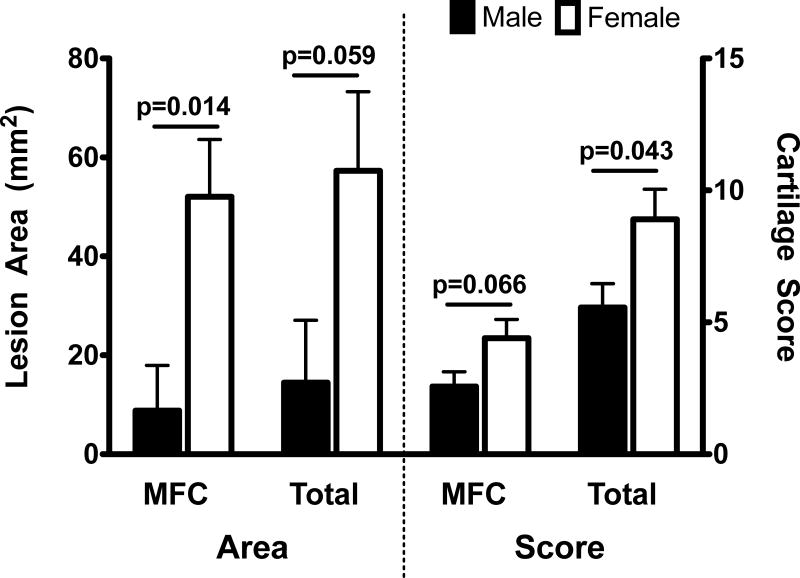
Sex-differences in cartilage damage were significantly affected by the treatment type (sex and treatment interaction p-value of ≤0.05), with significantly greater damage observed under ACL reconstruction (Table 2).16 Therefore, separate analyses were conducted on the animals that received conventional ACL reconstruction and those that underwent bio-enhanced ACL reconstruction. Female pigs had a greater degree of cartilage damage after conventional ACL reconstruction than their male counterparts (Figure 5). These differences were statistically significant for the area of cartilage damage on the medial femoral condyle (by 43.3±14.8 mm2;p=0.014) and total cartilage score for the entire knee (by 3.3±1.5 [analog scoring]; p=0.043) with differences in damaged area for the entire knee (by 42.8±20.4 mm2) and the medial femoral cartilage score (by 1.8±0.9 [analog scoring]) approaching statistical significance (p= 0.059 and p=0.066 respectively). No differences in cartilage damage were observed between male and female pigs treated with bio-enhanced ACL reconstruction (p>0.4). No differences in cartilage scoring and damage area were seen for the bio-enhanced ACL reconstruction groups (p=1.000 for all comparisons).
Table 2.
Average quantified macroscopic cartilage damage across the treatment groups.
| Cartilage Damage | Mean ± SEM | Treatment-related Differences | Sex*Treatment e-related Differences | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| ACLRa | B-ACLR (1×)b | B-ACLR (3×)c | B-ACLR (5×) d | ||||
| Lesion Area | MFC f | 25.4±9.2 | 3.6±2.6 | 7.6±6.0 | 6.9±4.6 | P=0.007 | P=0.024 |
| Total | 30.9±11.2 | 11.4±6.3 | 7.9±6.1 | 11.1±7.8 | P=0.065 | P=0.110 | |
|
| |||||||
| Cartilage Score | MFC f | 3.3±0.5 | 1.6±0.2 | 2.1±0.3 | 2.3±0.2 | P=0.006 | P=0.184 |
| Total | 6.8±0.8 | 4.3±0.4 | 4.4±0.6 | 4.3±0.6 | P=0.003 | P=0.05 | |
Conventional ACL reconstruction.
Bio-enhanced ACL reconstruction with 1× PRP.
Bio-enhanced ACL reconstruction with 3× PRP.
Bio-enhanced ACL reconstruction with 5× PRP.
Combined sex and treatment interaction term in the model.
Medial femoral condyle.
Figure 5.
Sex differences in macroscopic cartilage damage for animals undergoing conventional ACL reconstruction with allograft bone-patellar tendon-bone graft (Mean±SEM).
Discussion
Female pigs had significantly lower graft structural properties and greater AP knee laxity compared to their male counterparts 15 weeks after ACL reconstruction (with and without bio-enhancement) using bone-patellar tendon-bone allograft. In addition, ACL grafts in male knees showed significantly greater vascularization when compared to females, while other histological parameters showed no sex-related differences. Lastly, females had greater cartilage damage than males after an isolated ACL transection and conventional reconstruction. Interestingly, this difference was not seen for the groups treated with a collagen-platelet composite,16 suggesting a greater protective effect of the bio-enhancement on the cartilage of the female knees. Previous studies on human cohorts have shown sex-related differences in various functional and biomechanical outcomes of ACL reconstruction including graft failure rate,35 AP knee laxity,4, 8, 13, 18, 35, 43 and patient reported outcomes.1, 13, 18, 35, 39
Graft failure is an important determinant of outcome after ACL surgery. A recent systematic analysis by Ryan et al, including studies with a minimum two year follow up, reported overall rates of graft failure to be 4.6% in males and 5.4% in females.47 In addition, rates of failure for patellar tendon grafts were 4.0% in males and 4.7% in females. For hamstring grafts, the risks were 6.4% in males and 9.2% in females. Although, in all three comparisons the rates of failure in females was higher than that in males (by as much as 1.4 fold increase), none of these differences were found to be statistically significant in the meta-analysis and the authors concluded that there was no effect of sex on graft rupture rate.47 In contrast to their finding, a study of 39 females and 26 males with a primary focus on sex differences in ACL reconstruction outcome found a significant increase in failure rates from 4% in males to 23% in females during an average 40 month follow up.35 While no frank graft failures were noted among the pigs in this current study at 15 weeks after surgery, the weaker grafts in female knees (by ∼19%) support the previous findings of Noojin et al in higher graft failure rates in females than males (by ∼19%).35 As demonstrated by our results, a poorly healed graft can be manifested with inferior structural properties with no signs of gross failure, which can lead to lowered functional outcomes such as increased knee laxity and cartilage damage.
In terms of knee laxity, the meta-analysis by Ryan et al found no significant effect of sex on knee laxity when measured by Lachman testing or instrumented knee laxity.47 In the same study, the percentage of patients with a pivot shift test grade other than 0 was 12% in males and 20% in females. However, the meta-analysis indicated that this difference was not statistically significant.47 Other studies have reported significantly greater side-to-side differences in AP knee laxity in females than males for both the patellar tendon (up to 1 mm)13 and hamstring tendon (up to 1.6 mm) 4, 8, 13, 18, 35, 43 grafts after ACL reconstruction. These findings compare with our findings of significantly greater side-to-side differences in AP knee laxity in female pigs than males by 1.4 mm (at 30° of knee flexion).
In addition to the measured biomechanical outcomes, graft vascularity was also shown to be sex dependent, with greater vascularization in male pigs than females. Prior studies have reported greater vascularization in the graft healing process to be associated with significantly higher graft strength with respect to the graft yield and maximum loads as well as graft linear stiffness (R2≥0.54).44 Our data are consistent with those findings, as the grafts in males were significantly more vascular and stronger when compared to the grafts from the female knees. This increased vascularity of the grafts may be a key determinant in outcomes of ACL reconstruction and its link to both the graft structural properties and the sex of the animal is intriguing and will be the subject of future studies.
Patient reported outcomes are also important outcome measures for clinical studies. With regards to the patient-reported outcomes, Ryan et al suggested that the heterogeneity of the measurement tools precluded examining these outcomes as a function of sex.47 However, multiple cohorts of patients with reconstructed ACL have shown worse patient reported outcomes in females compared to males including Knee injury and Osteoarthritis Outcome Score (KOOS),1 Single Assessment Numeric Evaluation score (SANE),18 International Knee Documentation Committee score (IKDC),18 HSS radiographic score,13 and Cincinnati knee score.39 In the current study, a significant effect of sex on the development of cartilage damage after ACL reconstruction was also observed in the porcine model. The greater cartilage damage area and worse cartilage scores in the female pigs is in agreement and may be related to the findings of prior clinical trials showing an increased pain frequency and intensity,35 along with worse knee function and patient oriented outcomes in women.1, 13, 18, 35, 39 While “primary” knee OA has shown to be sex dependent, with substantially higher risk and progression rate in females than males,36, 52 the role of sex on risk of posttraumatic OA following an ACL injury has been less studied. The data here would suggest that females might be more likely to develop posttraumatic OA at a faster rate than their male counterparts. The higher rates of ACL injuries in females,24 along with the current observation of greater degree of cartilage damage when compared to males, are indicative of higher risk of posttraumatic OA development for females. Given that over 75% of young individuals develop posttraumatic OA within 14 years of an ACL injury,53 and that the peak age of ACL injury in females is 15 to 19 years,46 these data suggest that there may be a large cohort of young women who are currently at high risk for developing posttraumatic OA before the age of 35.
There are potential shortcomings with this study. The pig is a quadruped and post-operative rehabilitation is difficult to control. However, similar anatomical and biomechanical features between the pig and human have been noted.6, 45, 55 The surgeries were conducted using a fresh frozen allograft instead of autografts. Harvesting the patellar tendon autograft would compromise the extensor mechanism in a porcine model, while the hamstring autograft is not of sufficient length. It is possible that autografts would have provided different results. This is unlikely a major concern in that all treatment groups utilized the same type of allograft and the structural properties of the allografts in this study were similar to those reported for autografts in other quadruped models.11, 21 Moreover, the investigations were only conducted on adolescent pigs (15 months old). Previous studies have shown that Yucatan minipigs reach sexual and skeletal maturity at 7-10 months5 and 26-30 months28 respectively. More studies are needed to determine whether the current findings are affected by skeletal maturity, and if they will translate to younger (pre-mature) and older skeletally mature animals, since ACL healing is affected by age.30 Finally, male and female pigs were not evenly distributed within each treatment group, which might have affected the reported sex-differences. However, the fact that all the tested groups had a combination of both male and female pigs and the relatively small effect of PRP concentration on the outcomes of bio-enhanced ACL reconstruction16 has given us a reasonable statistical power to investigate the sex-specific difference in measured outcomes after adjusting for the treatment effect of PRP. A post-hoc power analysis has indicated a power of 0.8 for most of the measured outcomes. Future studies with higher sample sizes and a more specific study design to investigate the sexual dimorphism in reported outcomes are required to better understand the effect of sex on the outcomes of interest.
To our knowledge, this is the first time that sex has been demonstrated to significantly alter the outcome of ACL surgery in a large animal preclinical model. The porcine large animal model has previously been validated as a sex-specific model with similar sex differences in knee anatomy, laxity, and ACL biomechanics as to the human knee.25 Moreover, the pig knee has been shown to be the closest surrogate model for the human knee based on the anatomy, and functional dependency on ACL.6, 31, 45, 55 These support the current model as a valid approach to study the sex-specific differences in human knee pathology and surgical interventions with a special focus on ACL. Current findings further support the use of the validated pre-clinical animal models as reliable means to overcome the technical and ethical challenges associated with human trials, and enhance our abilities to measure the relevant structural outcomes of interest with sufficient resolution to further define the role of sex on the outcomes of ACL surgery.
Conclusion
The findings support our hypothesis that sex significantly affects the graft structural properties and AP knee laxity after ACL reconstruction, with female pigs having weaker and less vascular grafts and more lax knees. In addition, the female pigs had greater knee cartilage damage than their male counterparts, a difference which was ameliorated with the addition of an extracellular matrix-based scaffold loaded with autologous PRP. The results highlight the importance of further optimization of the current treatments to better fit each sex instead of a “one fits all” approach. This may in turn lead to improved surgical outcomes, decreased incidences of graft failure and re-injury, and a decreased risk of posttraumatic OA following ACL injury and reconstruction, especially among women.
Acknowledgments
The authors acknowledge funding support from the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases (RO1-AR054099; RO1-AR056834; P20-GM104937) and the Lucy Lippitt Endowment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Authors would also like to thank: Patrick Vavken from Boston Children's Hospital for helping with surgical procedures, Matthew Shalvoy, Alison Biercevics, David Paller, Sarath Koruprolu and Ryan Rich (Rhode Island Hospital Orthopaedic Foundation) for their assistance with mechanical testing, and Henry Feldman (Clinical Research Center at Boston Children's Hospital) and Harvard Catalyst (The Harvard Clinical and Translational Science Center) for helping with the statistical analysis.
Footnotes
Investigation performed at the Sports Medicine Research Laboratory, Department of Orthopaedic Surgery, Boston Children's Hospital, Harvard Medical School, Boston, MA and the Department of Orthopaedics, Rhode Island Hospital, Providence RI.
References
- 1.Ageberg E, Forssblad M, Herbertsson P, Roos EM. Sex differences in patient-reported outcomes after anterior cruciate ligament reconstruction: data from the Swedish knee ligament register. Am J Sports Med. 2010;38(7):1334–1342. doi: 10.1177/0363546510361218. [DOI] [PubMed] [Google Scholar]
- 2.Attia E, Brown H, Henshaw R, George S, Hannafin JA. Patterns of gene expression in a rabbit partial anterior cruciate ligament transection model: the potential role of mechanical forces. Am J Sports Med. 2010;38(2):348–356. doi: 10.1177/0363546509348052. [DOI] [PubMed] [Google Scholar]
- 3.Barber-Westin SD, Noyes FR, Andrews M. A rigorous comparison between the sexes of results and complications after anterior cruciate ligament reconstruction. Am J Sports Med. 1997;25(4):514–526. doi: 10.1177/036354659702500415. [DOI] [PubMed] [Google Scholar]
- 4.Bizzini M, Gorelick M, Munzinger U, Drobny T. Joint laxity and isokinetic thigh muscle strength characteristics after anterior cruciate ligament reconstruction: bone patellar tendon bone versus quadrupled hamstring autografts. Clin J Sport Med. 2006;16(1):4–9. doi: 10.1097/01.jsm.0000188040.97135.43. [DOI] [PubMed] [Google Scholar]
- 5.Bode G, Clausing P, Gervais F, et al. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62(3):196–220. doi: 10.1016/j.vascn.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: Is the porcine knee ACL dependent? J Orthop Res. 2011;29(5):641–646. doi: 10.1002/jor.21298. [DOI] [PubMed] [Google Scholar]
- 7.Chu CR, Beynnon BD, Buckwalter JA, et al. Closing the gap between bench and bedside research for early arthritis therapies (EARTH): report from the AOSSM/NIH U-13 Post-Joint Injury Osteoarthritis Conference II. Am J Sports Med. 2011;39(7):1569–1578. doi: 10.1177/0363546511411654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corry IS, Webb JM, Clingeleffer AJ, Pinczewski LA. Arthroscopic reconstruction of the anterior cruciate ligament. A comparison of patellar tendon autograft and four-strand hamstring tendon autograft. Am J Sports Med. 1999;27(4):444–454. doi: 10.1177/03635465990270040701. [DOI] [PubMed] [Google Scholar]
- 9.Cummings JF, Grood ES, Levy MS, Korvick DL, Wyatt R, Noyes FR. The effects of graft width and graft laxity on the outcome of caprine anterior cruciate ligament reconstruction. J Orthop Res. 2002;20(2):338–345. doi: 10.1016/S0736-0266(01)00119-X. [DOI] [PubMed] [Google Scholar]
- 10.Dunn WR, Lyman S, Lincoln AE, Amoroso PJ, Wickiewicz T, Marx RG. The effect of anterior cruciate ligament reconstruction on the risk of knee reinjury. Am J Sports Med. 2004;32(8):1906–1914. doi: 10.1177/0363546504265006. [DOI] [PubMed] [Google Scholar]
- 11.Dustmann M, Schmidt T, Gangey I, Unterhauser FN, Weiler A, Scheffler SU. The extracellular remodeling of free-soft-tissue autografts and allografts for reconstruction of the anterior cruciate ligament: a comparison study in a sheep model. Knee Surg Sports Traumatol Arthrosc. 2008;16(4):360–369. doi: 10.1007/s00167-007-0471-0. [DOI] [PubMed] [Google Scholar]
- 12.Fan H, Liu H, Toh SL, Goh JC. Anterior cruciate ligament regeneration using mesenchymal stem cells and silk scaffold in large animal model. Biomaterials. 2009;30(28):4967–4977. doi: 10.1016/j.biomaterials.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari JD, Bach BR, Jr, Bush-Joseph CA, Wang T, Bojchuk J. Anterior cruciate ligament reconstruction in men and women: An outcome analysis comparing gender. Arthroscopy. 2001;17(6):588–596. doi: 10.1053/jars.2001.24686. [DOI] [PubMed] [Google Scholar]
- 14.Fisher MB, Liang R, Jung HJ, et al. Potential of healing a transected anterior cruciate ligament with genetically modified extracellular matrix bioscaffolds in a goat model. Knee Surg Sports Traumatol Arthrosc. 2012;20(7):1357–1365. doi: 10.1007/s00167-011-1800-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleming BC, Carey JL, Spindler KP, Murray MM. Can suture repair of ACL transection restore normal anteroposterior laxity of the knee? An ex vivo study. J Orthop Res. 2008;26(11):1500–1505. doi: 10.1002/jor.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleming BC, Proffen BL, Vavken P, Shalvoy MR, Machan JT, Murray MM. Increased platelet concentration does not improve functional graft healing in bio-enhanced ACL reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014 doi: 10.1007/s00167-014-2932-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fleming BC, Spindler KP, Palmer MP, Magarian EM, Murray MM. Collagen-platelet composites improve the biomechanical properties of healing anterior cruciate ligament grafts in a porcine model. Am J Sports Med. 2009;37(8):1554–1563. doi: 10.1177/0363546509332257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gobbi A, Domzalski M, Pascual J. Comparison of anterior cruciate ligament reconstruction in male and female athletes using the patellar tendon and hamstring autografts. Knee Surg Sports Traumatol Arthrosc. 2004;12(6):534–539. doi: 10.1007/s00167-003-0486-0. [DOI] [PubMed] [Google Scholar]
- 19.Hettrich CM, Dunn WR, Reinke EK, Group M, Spindler KP. The rate of subsequent surgery and predictors after anterior cruciate ligament reconstruction: two- and 6-year follow-up results from a multicenter cohort. Am J Sports Med. 2013;41(7):1534–1540. doi: 10.1177/0363546513490277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt P, Scheffler SU, Unterhauser FN, Weiler A. A model of soft-tissue graft anterior cruciate ligament reconstruction in sheep. Arch Orthop Trauma Surg. 2005;125(4):238–248. doi: 10.1007/s00402-004-0643-z. [DOI] [PubMed] [Google Scholar]
- 21.Jackson DW, Grood ES, Goldstein JD, et al. A comparison of patellar tendon autograft and allograft used for anterior cruciate ligament reconstruction in the goat model. Am J Sports Med. 1993;21(2):176–185. doi: 10.1177/036354659302100203. [DOI] [PubMed] [Google Scholar]
- 22.Junkin DM, Johnson DL, Fu FH, et al. Knee Ligament Injuries. In: Kibler WB, editor. Orthopaedic Knowledge Update 4: Sports Medicine. Rosemont: American Academy of Orthopaedic Surgeons; 2009. pp. 135–153. [Google Scholar]
- 23.Katsuragi R, Yasuda K, Tsujino J, Keira M, Kaneda K. The effect of nonphysiologically high initial tension on the mechanical properties of in situ frozen anterior cruciate ligament in a canine model. Am J Sports Med. 2000;28(1):47–56. doi: 10.1177/03635465000280012001. [DOI] [PubMed] [Google Scholar]
- 24.Kiapour AM, Murray MM. Basic science of anterior cruciate ligament injury and repair. Bone Joint Res. 2014;3(2):20–31. doi: 10.1302/2046-3758.32.2000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiapour AM, Shalvoy MR, Murray MM, Fleming BC. Validation of Porcine Knee as a Sex-specific Model to Study Human Anterior Cruciate Ligament Disorders. Clin Orthop Relat Res. 2015;473(2):639–650. doi: 10.1007/s11999-014-3974-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2013;41(2):385–395. doi: 10.1177/0363546512465167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 28.Mastrangelo AN, Magarian EM, Palmer MP, Vavken P, Murray MM. The effect of skeletal maturity on the regenerative function of intrinsic ACL cells. J Orthop Res. 2010;28(5):644–651. doi: 10.1002/jor.21018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray MM, Fleming BC. Use of a bioactive scaffold to stimulate anterior cruciate ligament healing also minimizes posttraumatic osteoarthritis after surgery. Am J Sports Med. 2013;41(8):1762–1770. doi: 10.1177/0363546513483446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murray MM, Magarian EM, Harrison SL, Mastrangelo AN, Zurakowski D, Fleming BC. The effect of skeletal maturity on functional healing of the anterior cruciate ligament. J Bone Joint Surg Am. 2010;92(11):2039–2049. doi: 10.2106/JBJS.I.01368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray MM, Spindler KP, Abreu E, et al. Collagen-platelet rich plasma hydrogel enhances primary repair of the porcine anterior cruciate ligament. J Orthop Res. 2007;25(1):81–91. doi: 10.1002/jor.20282. [DOI] [PubMed] [Google Scholar]
- 32.Myklebust G, Bahr R. Return to play guidelines after anterior cruciate ligament surgery. Br J Sports Med. 2005;39(3):127–131. doi: 10.1136/bjsm.2004.010900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myklebust G, Maehlum S, Holm I, Bahr R. A prospective cohort study of anterior cruciate ligament injuries in elite Norwegian team handball. Scand J Med Sci Sports. 1998;8(3):149–153. doi: 10.1111/j.1600-0838.1998.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 34.Nebelung W, Wuschech H. Thirty-five years of follow-up of anterior cruciate ligament-deficient knees in high-level athletes. Arthroscopy. 2005;21(6):696–702. doi: 10.1016/j.arthro.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 35.Noojin FK, Barrett GR, Hartzog CW, Nash CR. Clinical comparison of intraarticular anterior cruciate ligament reconstruction using autogenous semitendinosus and gracilis tendons in men versus women. Am J Sports Med. 2000;28(6):783–789. doi: 10.1177/03635465000280060301. [DOI] [PubMed] [Google Scholar]
- 36.O'Connor MI. Sex differences in osteoarthritis of the hip and knee. J Am Acad Orthop Surg. 2007;15(Suppl 1):S22–25. [PubMed] [Google Scholar]
- 37.O'Donoghue DH, Rockwood CA, Jr, Frank GR, Jack SC, Kenyon R. Repair of the anterior cruciate ligament in dogs. J Bone Joint Surg Am. 1966;48(3):503–519. [PubMed] [Google Scholar]
- 38.Oe K, Kushida T, Okamoto N, et al. New strategies for anterior cruciate ligament partial rupture using bone marrow transplantation in rats. Stem Cells Dev. 2011;20(4):671–679. doi: 10.1089/scd.2010.0182. [DOI] [PubMed] [Google Scholar]
- 39.Ott SM, Ireland ML, Ballantyne BT, Willson JD, McClay Davis IS. Comparison of outcomes between males and females after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2003;11(2):75–80. doi: 10.1007/s00167-003-0348-9. [DOI] [PubMed] [Google Scholar]
- 40.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am J Sports Med. 2014 doi: 10.1177/0363546514530088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paterno MV, Schmitt LC, Ford KR, et al. Biomechanical measures during landing and postural stability predict second anterior cruciate ligament injury after anterior cruciate ligament reconstruction and return to sport. Am J Sports Med. 2010;38(10):1968–1978. doi: 10.1177/0363546510376053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paterno MV, Weed AM, Hewett TE. A between sex comparison of anterior-posterior knee laxity after anterior cruciate ligament reconstruction with patellar tendon or hamstrings autograft: a systematic review. Sports Med. 2012;42(2):135–152. doi: 10.2165/11596940-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinczewski LA, Lyman J, Salmon LJ, Russell VJ, Roe J, Linklater J. A 10-year comparison of anterior cruciate ligament reconstructions with hamstring tendon and patellar tendon autograft: a controlled, prospective trial. Am J Sports Med. 2007;35(4):564–574. doi: 10.1177/0363546506296042. [DOI] [PubMed] [Google Scholar]
- 44.Proffen BL, Fleming BC, Murray MM. Histological Predictors of Maximum Failure Loads Differ Between the Healing ACL and ACL Grafts After 6 and 12 Months In Vivo. Orthopaedic Journal of Sports Medicine. 2013;1(6):2325967113512457. doi: 10.1177/2325967113512457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Proffen BL, McElfresh M, Fleming BC, Murray MM. A comparative anatomical study of the human knee and six animal species. Knee. 2012;19(4):469–476. doi: 10.1016/j.knee.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Renstrom P, Ljungqvist A, Arendt E, et al. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. Br J Sports Med. 2008;42(6):394–412. doi: 10.1136/bjsm.2008.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryan J, Magnussen RA, Cox CL, Hurbanek JG, Flanigan DC, Kaeding CC. ACL reconstruction: do outcomes differ by sex? A systematic review. J Bone Joint Surg Am. 2014;96(6):507–512. doi: 10.2106/JBJS.M.00299. [DOI] [PubMed] [Google Scholar]
- 48.Salmon L, Russell V, Musgrove T, Pinczewski L, Refshauge K. Incidence and risk factors for graft rupture and contralateral rupture after anterior cruciate ligament reconstruction. Arthroscopy. 2005;21(8):948–957. doi: 10.1016/j.arthro.2005.04.110. [DOI] [PubMed] [Google Scholar]
- 49.Sandon A, Werner S, Forssblad M. Factors associated with returning to football after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2014 doi: 10.1007/s00167-014-3023-4. [DOI] [PubMed] [Google Scholar]
- 50.Schinhan M, Gruber M, Vavken P, et al. Critical-size defect induces unicompartmental osteoarthritis in a stable ovine knee. J Orthop Res. 2012;30(2):214–220. doi: 10.1002/jor.21521. [DOI] [PubMed] [Google Scholar]
- 51.Shelbourne KD, Gray T, Haro M. Incidence of subsequent injury to either knee within 5 years after anterior cruciate ligament reconstruction with patellar tendon autograft. Am J Sports Med. 2009;37(2):246–251. doi: 10.1177/0363546508325665. [DOI] [PubMed] [Google Scholar]
- 52.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 53.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woo SL, Gomez MA, Seguchi Y, Endo CM, Akeson WH. Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res. 1983;1(1):22–29. doi: 10.1002/jor.1100010104. [DOI] [PubMed] [Google Scholar]
- 55.Xerogeanes JW, Fox RJ, Takeda Y, et al. A functional comparison of animal anterior cruciate ligament models to the human anterior cruciate ligament. Ann Biomed Engin. 1998;26(3):345–352. doi: 10.1114/1.91. [DOI] [PubMed] [Google Scholar]