Abstract
Purpose
The interleukin-11 receptor (IL-11R) is an established molecular target in primary tumors of bone, such as osteosarcoma, and in secondary bone metastases from solid tumors such as prostate cancer. However, its potential role in management of hematopoietic malignancies has not yet been determined. Here we evaluated the IL-11R as a candidate therapeutic target in human leukemia and lymphoma.
Experimental Design and Results
First, we show that the IL-11R protein is expressed in a variety of human leukemia- and lymphoma derived cell lines and in a large panel of bone marrow samples from leukemia and lymphoma patients, while expression is absent from non-malignant control bone marrow. Moreover, a targeted peptidomimetic prototype (termed BMTP-11) specifically bound to leukemia and lymphoma cell membranes, induced ligand-receptor internalization mediated by the IL-11R, and resulted in a specific dose-dependent cell death induction in these cells. Finally, a pilot drug lead-optimization program yielded a new myristoylated BMTP-11 analog with an apparent improved anti-leukemia cell profile.
Conclusion
These results indicate (i) that the IL-11R is a suitable cell surface target for ligand-directed applications in human leukemia and lymphoma and (ii) that BMTP-11 and its derivatives have translational potential against this group of malignant diseases.
Keywords: cancer, IL-11 receptor, leukemia, phage display, targeted drug delivery
Introduction
Leukemia cells express unique surface receptors that may be leveraged towards targeted delivery of diagnostic and therapeutic agents (1–4). In vivo phage display is one approach that can potentially identify and validate functional ligand-mimics binding to relevant membrane receptors that promote cell internalization within the context of the tumor microenvironment. Our group has pioneered the direct screening of phage display random peptide libraries in cancer patients to enable unbiased discovery of tumor targets (5–6). In previous work with this platform technology, we isolated a ligand that mimics interleukin-11 (IL-11) motif (cyclic peptide CGRRAGGSC) and have demonstrated that the interleukin-11 receptor (IL-11R) is a tumor target in primary tumors of bone, such as osteosarcoma, and in secondary bone metastases from solid tumors such as prostate cancer (7–10). Based on these findings, we have designed and produced a new ligand-directed agent, Bone Metastasis Targeting Peptidomimetic-11 (BMTP-11). BMTP-11 consists of the selected IL-11R-targeting motif synthesized in tandem to the sequence D(KLAKLAK)2, a peptidomimetic motif that induces cell death via mitochondrial membrane disruption upon cell internalization. The efficacy and toxicology of various ligand-directed versions of D(KLAKLAK)2 have been extensively evaluated in pre-clinical models of human diseases with a vascular component such as cancer, obesity and retinopathies (7,10–14).
Given the marked expression of the IL-11R in the bone marrow within the context of primary or metastatic solid tumors, along with its absence from normal bone marrow (7,8,10), we reasoned that the IL-11R might also be a suitable target in human leukemia.
Here we evaluate the protein expression of the IL-11R in a panel of leukemia cell lines and patient-derived bone marrow and peripheral blood samples. Moreover, we assess the effectiveness of the prototype BMTP-11 for inducing cell death in human leukemia cell lines and the clonogenic potential in patient-derived leukemia samples. We also introduce a lead-optimized myristoylated BMTP-11 analog with an improved anti-leukemia profile. Together, these data indicate that the IL-11R is a relevant molecular target in human leukemia. Given the results presented here, along with extensive toxicology studies and a first-in-human trial in prostate cancer patients, to be reported in Pasqualini et al, in press (15), the parental BMTP-11 in consort with its derivatives merit attention as targeted drug leads against human leukemia.
Materials and Methods
Leukemia and lymphoma cell lines and tissue culture
A panel of human cell lines was obtained from the Leukemia Cell and Tissue Bank of the Department of Leukemia at The University of Texas M.D. Anderson Cancer Center (UTMDACC). No authentication was done. The panel (n=12) included cryopreserved samples of MOLT-4 (T-cell acute lymphoblastic leukemia), CCRF-CEM (T-cell acute lymphoblastic leukemia), HL-60 (acute promyeolocytic leukemia), OCI-AML3 (acute myelogenous leukemia), THP-1 (monocytic acute leukemia), K562 and KBM7 (chronic myelogenous leukemia), SR-786 (anaplastic large T-cell lymphoma), U937 and TUR (monocytic lymphoma), TF-1 (erythroleukemia), and RPMI-8226 (myeloma). Cells were maintained in humidified hypoxia chambers (HeraCell 150, Thermo Electron Corporation) with 5% CO2 and 5% oxygen at 37°C in RPMI1640 containing 10% fetal bovine serum (FBS), L-glutamine (0.292 mg/ml), penicillin (100 units/ml), and streptomycin (100 units/ml) [complete RPMI-1640].
Leukemia and lymphoma patient-derived and control tissue samples
Primary samples from leukemia patients who had signed written informed consent were obtained from the Leukemia Cell and Tissue Bank of the Department of Leukemia at the University of Texas M. D. Anderson Cancer Center (UTMDACC). Normal blood and bone marrow samples were commercially obtained (AllCells). Cells were maintained in humidified hypoxia chambers (HeraCell 150, Thermo Electron Corporation) with 5% CO2 and 5% oxygen at 37°C in StemPro34 SFM (Life Technologies), L-glutamine (0.292 mg/ml), penicillin (100 units/ml), and streptomycin (100 units/ml).
Blast percentage analysis and white blood cell counts
Available Wright-Giemsa-stained peripheral blood and bone marrow aspirate smears, hematoxylin-eosin-stained bone marrow aspirate clot and trephine biopsy specimens were reviewed. In the bone marrow, the blast percentage was derived from a 500-manual cell differential of all nucleated cells in the aspirate smears. WBC counts were produced by a multichannel hematology analyzer (Sysmex XE; Sysmex America Inc., Brea, Calif.).
BMTP-11 synthesis, manufacturing and drug lead-optimization
BMTP-11 is a synthetic peptidomimetic composed of an IL-11R-binding cyclic motif (containing natural L-residues, sequence CGRRAGGSC). A glycinylglycine linker was added to the targeting motif as a spacer to prevent steric hindrance and fused in tandem to the D-residue of cell death-inducing motif D(KLAKLAK)2 (8,10–14). Parental BMTP-11 and all its derivative peptidomimetics were generated by commercial vendors (AnaSpec, Jitsubo or PolyPeptide, as indicated) through solid-phase peptide synthesis assembly process, cyclization by air oxidation, purification by reverse-phase high-performance liquid chromatography and isolation by lyophilization to our specifications. Final identification of the sequences was carried out by analysis with matrix-assisted laser desorption time-of-flight mass spectrometry. Peptide and peptidomimetic sequences were analyzed and confirmed (Supplemental Table 1).
Flow cytometry analysis
Exponentially growing leukemia cells were harvested, washed with PBS containing 1% FBS and 2mM EDTA and re-suspended in ice-cold wash buffer. Mononuclear cells from peripheral blood and bone marrow samples obtained from healthy donors and AML patients were isolated via Ficoll-Paque PLUS (GE Healthcare) purification. Human Fc Receptor Blocking Reagent (Miltenyi Biotec) was added to each cell suspension (20 μl per 1 × 107 cells) and incubated for 10 min on ice. Next, 20 μl of either anti-human IL11Rα-PE labeled antibody (Clone N-20) (Santa Cruz) or PE-conjugated rabbit IgG isotype control (GeneTex) was added per 1 × 106 cells in 100 μl of FACS buffer and incubated at 4° C in the dark for 1 h. To detect the IL-11Rα-expression, the cells were double-stained with antibodies against the cell type specific CD markers: anti-human CD3-APC (eBioscience), anti-human CD19-APC-Cy7 (BD Biosciences), anti-human CD14-PE-Cy7 (eBiosciences), anti-human CD33-PE-Cy7 (eBioscience), and anti-human CD34-APC (BD Bioscience) as instructed by the manufacturer. Subsequently, cells were washed with FACS buffer and analyzed using a BD LSR Fortessa flow cytometer (BD Biosciences).
Immunohistochemistry of leukemia and lymphoma patient-derived samples
Immunohistochemistry was performed on formalin-fixed, paraffin-embedded sections of bone marrow aspirate clot or biopsy specimens. The sections were de-paraffinized, re-hydrated and subjected to heat-induced epitope retrieval process using Diva buffer (Biocare Medical, CA) and a Decloaking Chamber (pressure cooker) (Biocare Medical, CA). The slides were incubated with an anti-IL11R mouse monoclonal antibody at a dilution of 1:10 (Clone 4D12; Santa Cruz Biotechnology, CA) or a negative control mouse IgG at RT in a dark chamber for 1h. Detection of the primary antibody was achieved with the Mach4 kit (Biocare Medical, CA), used according to the manufacturer’s instruction. Chromogen development was performed with DAB+ (DakoCytomation). Slides were counterstained with hematoxylin, dehydrated, mounted and cover-slipped. A specimen from a prostate carcinoma, metastatic to the bone marrow, was used as a positive control for the immunohistochemical (IHC) staining (8). Two observers (KK and CBR), without prior knowledge of the clinical data, evaluated the IHC results. We used 40x magnification to determine the percentage of tumor cells that exhibited positive immunoreactivity against the IL-11R antibody. At least 500 neoplastic cells were counted in each case. The presence of membrane and/or cytoplasmic staining for IL-11R in ≥ 5% cells was scored as positive. The intensity of the staining also varied between specimens and we have indicated the weak-staining pattern as weak positive.
Cytospins of healthy bone marrow and leukemia patient-derived samples
Cytospins were prepared with CD34+ cells, air-dried and fixed with buffered 10% formalin. The cytospins were briefly washed with Tris-buffered saline with 0.1% Tween (which was also used throughout the procedure in washing steps) to remove formalin residue and then subjected to heat-induced epitope retrieval process using Diva buffer (Biocare Medical, CA) and a Decloaking Chamber (pressure cooker) (Biocare Medical, CA). The cytospins were incubated with an anti-IL11R mouse monoclonal antibody at a dilution of 1:10 (4D12; Santa Cruz Biotechnology, CA) at room temperature in a dark chamber for 1h. Detection of the primary antibody was achieved with the Mach4 kit (Biocare Medical, CA) used according to the manufacturer’s instruction. Chromogen development was performed with DAB+ (DakoCytomation). Slides were counterstained with hematoxylin, air-dried and cover-slipped.
Cell viability and proliferation assays
Human leukemia cells (2 × 104 cells/well) were plated in 96-well dishes containing complete RPMI-1640. Cells were incubated with increasing concentrations of either BMTP-11 or an admixture of CGRRAGGSC plus D(KLAKLAK)2 (negative control) at equimolar concentrations overnight at 37°C. Leukemia cell viability was determined through counting of viable cells after trypan blue staining by an automated cell counter (TC10 automated cell counter, BioRad) or through measuring of cytoplasmic lactate dehydrogenase (LDH) enzymatic activity with a commercial kit (DHL™ Cell Viability & Proliferation Assay Kit; AnaSpec). This standard assay enables one-step counting and continuous monitoring of leukemia cell proliferation over time by measuring cytoplasmic LDH activity. Resazurin serves as a sensitive indicator converted to the fluorescent resorufin by cytoplasmic LDH.
Cell death assay
Human leukemia cells (2 × 105 cells per well in 1 ml) were plated in 6-well plates containing complete RPMI-1640. The cells were incubated with concentrations indicated of either BMTP-11 or an admixture of CGRRAGGSC plus D(KLAKLAK)2 (negative control) overnight at 37 °C, or with BMTP-11 structural analogs and negative controls for 4 h at 37 °C. Untreated leukemia cells served as a control to measure background levels of cell death under these conditions. After the incubation, the cells were stained with FITC-conjugated AnnexinV antibody and propidium iodide (PI) with a standard cell death detection kit (Sigma). The leukemia cells were subsequently analyzed by flow cytometry as described above.
Clonogenic assay
Clonogenic potential of human hematopoietic progenitor cells was assessed via the human colony-forming cell (CFC) assay using Complete Methocult methylcellulose-based media (Stemcell Technology). The bone marrow derived cells were plated at the concentration of 2 × 104 cells per 35 mm dish in the presence of either 100 μM of BMTP-11, 30 μM of BMTP-11A#4 or 100 μM of negative control (D(KLAKLAK)2). The cultures were incubated for 16 days after which the colonies were counted manually with an inverted microscope.
Statistical analysis
All data are reported as the average mean ± standard error of the mean (SEM). Student’s t-test or Fisher’s exact test were used to determine statistical significance as indicated. P-values that were considered statistically significant are indicated with asterisks as follows: less than 0.05 (*), less than 0.01 (**), or less than 0.001 (***). We used the following categories for Fisher’s exact test. Group A: positive white blood cells (≥ 5% nuclei positive) and negative white blood cells (< 5% nuclei positive) in healthy bone marrow and diseased bone marrow. Group B: positive white blood cells plus endothelial cells (≥ 5% nuclei and vessels positive) and negative white blood cells plus endothelial cells (< 5% nuclei and vessels positive) in healthy bone marrow and diseased bone marrow.
Results
IL-11R is expressed in leukemia and lymphoma cell lines and patient-derived bone marrow specimens
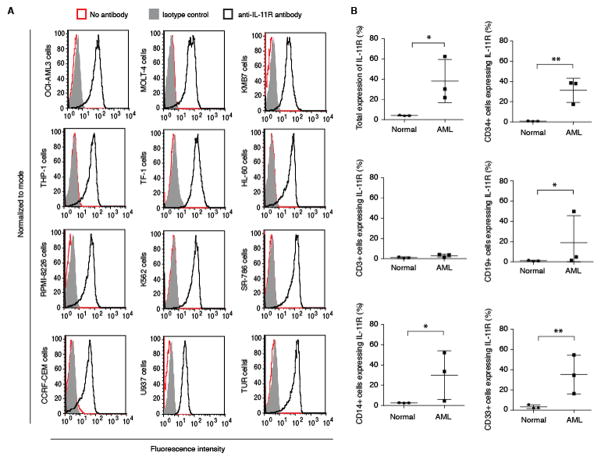
To evaluate IL-11R as a membrane target in leukemia, we analyzed the surface expression of IL-11R on leukemia cells. Positive anti-IL-11R antibody staining was observed on all the human leukemia, myeloma and lymphoma cell lines tested, namely: MOLT-4, OCI-AML3, K562, KMB7, THP-1, HL-60, CCRF-CEM, TF-1, SR-786, U937, TUR and RPMI-8226 (Fig. 1A). The cell surface expression of IL-11Rα was subsequently studied via flow cytometry (Fig. 1B & Supplemental Fig. 1). Different hematopoietic cell populations from both healthy (n=3) and leukemic tissues were analyzed. The overall expression of IL-11Rα was increased in all the AML samples tested compared to the normal bone marrow or peripheral blood tested. At the cell population level, the surface expression was consistently high in CD34+ (3/3 cases) and CD33+ cell populations (3/3 cases), as well as in CD14+ population (2/3 cases). One AML sample also stained positive in CD19+ cells (Fig. 1B & Supplemental Fig. 1). Cell surface membrane expression of IL-11Rα was also confirmed via cytospin analysis of CD34+ bone marrow cells derived from healthy individuals as well as patients with AML (Fig. 1B & Supplemental Fig. 2).
Figure 1.
Flow cytometry analysis of cell surface expression of IL-11R. (A) The expression of IL-11R was studied in a panel of leukemia cell lines (n=12). Cell samples were stained as follows: unstained cells (red line), control IgG (solid grey), anti-IL-11R antibody (black line). (B) The expression of IL-11R was studied in the patient derived AML samples and hematopoietic cells from healthy donors. The expression levels were analyzed at the total mononuclear blood cell population level, as well as in individual peripheral blood cell populations, identified by relevant CD markers.
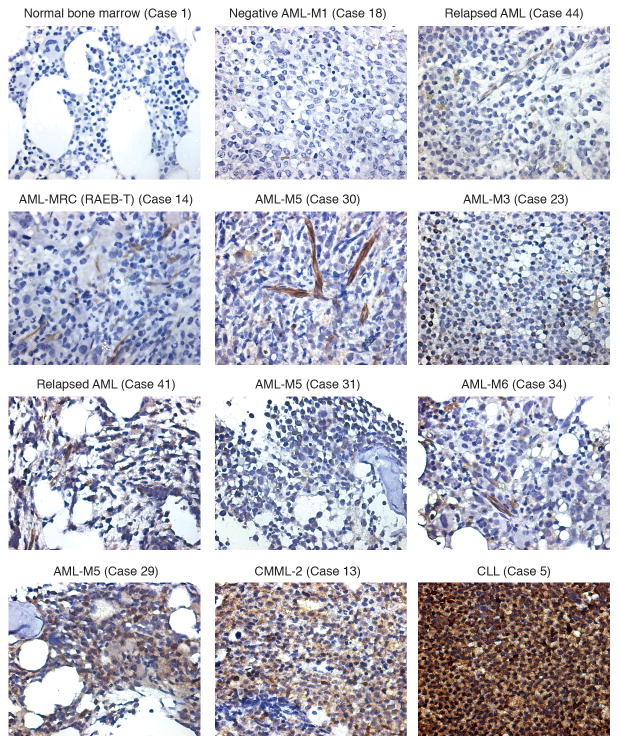
Next, we examined levels of IL-11R in bone marrow samples from a panel (n=43) of leukemia patients via immunohistochemistry (Table 1). This panel of patient-derived samples was obtained from clinically annotated cases including acute myeloid leukemia (AML) (n=33), myelodysplastic syndrome (n=4), myeloproliferative syndrome (n=2) and B-cell malignancies (n=4). The expression of IL-11R in bone marrow specimens involved by leukemia (leukemia cells and vessels) was higher than in normal bone marrow (Fisher’s exact test, p=0.0448), with over half of the leukemia blasts (23 of 43, 53%) staining positively. Notably, all the leukemic bone marrow samples with evaluable blood vessels (16 of 17, 94%) had positive IL-11R vascular staining, whereas there was no detectable expression in the normal vasculature (Fisher’s exact test, p=0.0158), regardless of subtype and corresponding blast cell positivity. A majority of cases with leukemic bone marrow (~99%) had > 20 % disease involvement, and most of the cases had > 40% disease involvement with very few normal bone marrow components such as megakaryocytes. Specifically, 24 AML bone marrow specimens out of 33 had > 40% disease involvement and many (16 cases) had > 60% disease involvement. However, the bone marrow specimens with myelodysplastic syndrome (MDS) presented only partial disease involvement (focal involvement). From these six cases, only two (cases 12 & 13) had > 10% disease involvement, and only one of them (case 13) stained positive. These data indicate that the IL-11R staining is specific and occurs only in certain bone marrow specimens with significant disease involvement. Representative samples for immunohistochemical staining of IL-11R in normal and diseased bone marrow are shown (Fig. 2). A 1000-fold original magnification picture of acute myeloid leukemia subtype 5 (AML-M5) expressing membrane and cytoplasmic IL-11R is shown as an example (Supplemental Fig. 2a). Moreover, we further analyzed the IL-11R expression by preparing cytospins of isolated CD34+ cell populations from healthy bone marrow specimens (n=2) and bone marrow specimens from patients with AML (n=2). The expression of IL11R in normal CD34 positive myeloblasts is rare and the fraction of positive cells in two samples of CD34+ blasts purified by immunomagnetic isolation were 1% or less. A distinct membranous IL11R expression was noted in CD34 positive myeloblasts in one of two cases of AML (Supplemental Fig. 2b).
Table 1.
IL-11R expression in leukemia and lymphoma bone marrow samples.
| Case#: | Diagnosis: | WBC | BM disease involvement | IL-11Rα WBC | IL-11Rα vessels | Other comments: |
|---|---|---|---|---|---|---|
| 1 | Normal BM | N/A | 0% | neg | neg | |
| 2 | Normal BM | N/A | 0% | neg | neg | |
| 3 | Normal BM | N/A | 0% | neg | N/A | |
|
| ||||||
| 4 | CLL | 16.9 | 61% | pos | N/A | 59% lymphocytes |
| 5 | CLL | 4.7 | 88% | pos | N/A | 88% lymphocytes |
| 6 | Low-grade B-Lymphoma | 4.4 | 30% | neg | pos wk | 12% lymphocytes |
| 7 | B-ALL | 13.3 | 89% | neg | pos | |
|
| ||||||
| 8 | Therapy related MDS | 16.5 | 6% | neg | neg | History of prostate, Merkel, basal, and squamous cell cancer |
| 9 | MDS-RARS | 4.2 | 1% | neg | N/A | |
| 10 | MDS, RCMD | 3.9 | 1% | neg | pos | |
| 11 | MDS-RCMD | 5.9 | 2% | neg | pos | |
| 12 | CMML-2 | 113 | 18% | neg | N/A | History of breast cancer |
| 13 | CMML-2 | 85.8 | 11% | pos | pos | |
|
| ||||||
| 14 | AML-MRC (RAEB-T) | 2.9 | 23% | neg | pos | AML with myelodysplasia related changes (WHO) |
| 15 | AML evolve from MPN | 1.1 | 30% | pos | pos | MPN in 2000, JAK2 negative, AML not treated yet |
| 16 | AML-MO | 10.2 | 55% | wk pos | N/A | |
| 17 | AML-M1 | 194.4 | 97% | neg | N/A | NPM1 mutation, FLT3 internal tandem duplication |
| 18 | AML-M1 | 20.9 | 66% | neg | N/A | |
| 19 | AML-M1 | 0.7 | 87% | pos | N/A | Complex cytogenetics/karyotype, FLT3 wild type |
| 20 | AML-M1 | 12.8 | 85% | pos | pos | Untreated |
| 21 | AML-M2 | N/A | 30% | neg | N/A | |
| 22 | AML-M3/APL | 1.6 | 32% | neg | N/A | Untreated |
| 23 | AML-M3/APL | 40.7 | 91% | focal pos | N/A | |
| 24 | AML-M3/APL | 5.5 | 55% | focal pos | pos | |
| 25 | AML-MRC (M4) | 39.7 | 61% | neg | N/A | Newly diagnosed, Untreated |
| 26 | AML-M4 | 25.9 | 78% | neg | N/A | Untreated |
| 27 | AML-M4 | 88.9 | 33% | pos | N/A | Arising from CMML |
| 28 | AML-M4 | 5.7 | 26% | wk pos | N/A | FLT3 internal tandem duplication |
| 29 | Therapy related AML-M5 | 43.6 | 30% | pos | N/A | History of prostate adenocarcinoma, radiation only |
| 30 | AML-M5 | 2.9 | 68% | wk pos | pos | |
| 31 | AML-M5 | 36.7 | 80% | pos | pos | Untreated |
| 32 | AML-M5 | 13.8 | 71% | pos | N/A | FLT3 internal tandem duplication |
| 33 | AML-M5 | 100.2 | 85% | wk pos | N/A | |
| 34 | AML-M6 | 3.1 | 88% | pos | pos | Untreate |
| 35 | AML | 85.2 | 36% | wk pos | N/A | Previously treated, refractory |
| 36 | AML | 1.2 | 79% | pos | N/A | |
| 37 | AML-MRC | 3.7 | 57% | neg | N/A | |
| 38 | AML-MRC | 2.8 | 25% | neg | N/A | Newly diagnosed, untreated |
| 39 | AML-MRC | 15.4 | 77% | neg | pos | Treated with chemotherapy, not responding |
| 40 | AML refractory | 1.1 | 70% | neg | N/A | Treated with 6 courses of chemotherapy |
| 41 | AML relapse | 7.7 | 90% | focal pos wk | pos | Treated ovarian carcinoma 2009, treated AML |
| 42 | AML refractory, relapsing | 3.3 | 41% | neg | pos | Treated with chemothery |
| 43 | AML inv16, relapse | 5.8 | 49% | neg | pos | Treated |
| 44 | AML relapse | 4.7 | 70% | neg | pos | Treated |
| 45 | AML refractory | 2.2 | 27% | neg | N/A | Treated |
| 46 | AML relapse | 0.5 | 44% | neg | N/A | Treated |
|
| ||||||
| Total # of specimens: 46 | ||||||
White blood cell (WBC) counts and bone marrow (BM) blast percentage of each sample are indicated. The approximate levels of IL-11R were estimated by positively staining cells in the specimens. Staining status of blood vessels is listed or marked as N/A if no blood vessels were visible in a particular sample. Previous cancer history and current treatment status, if known, are also indicated.
Abbrevations: negative (neg); positive (pos); not available (N/A); weak (wk); bone marrow (BM); chronic lymphocytic leukemia (CLL); acute lymphocytic leukemia (ALL); myelodysplastic syndrome (MDS); AML with MDS-related changes (MRC); refractory anemia with ring sideroblasts (RARS); refractory cytopenia with myltilineage dysplasia (RCMD); refractory anemia with excess blasts in transformation (RAEB-T); chronic myelomonocytic leukemia (CMML); acute myeloid leukemia (AML); myeloproliferative neoplasm (MPN); acute promyelocytic leukemia (APL).
Figure 2.
IL-11R expression in human bone marrow samples from patients with leukemia. Representative samples of immunohistochemical staining of IL-11R on CLL, AML, CMML and healthy normal bone marrow specimens are shown. The pictures are shown at 400-fold original magnification.
BMTP-11 is broadly active against human leukemia and lymphoma cells
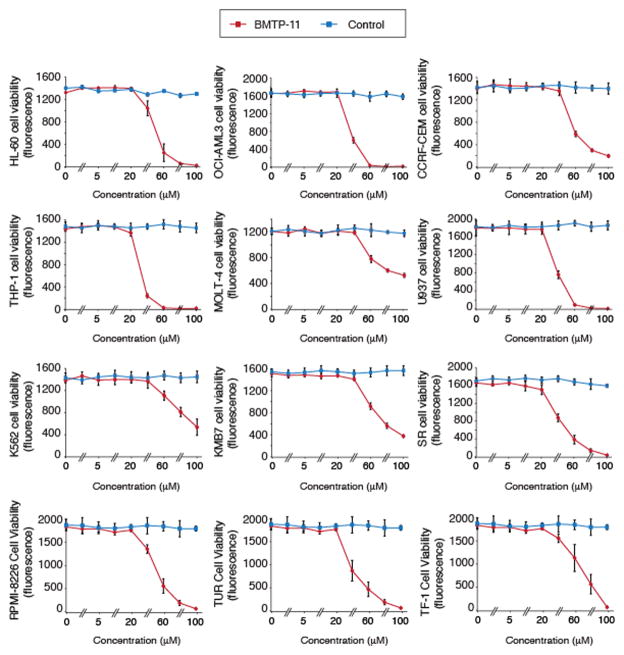
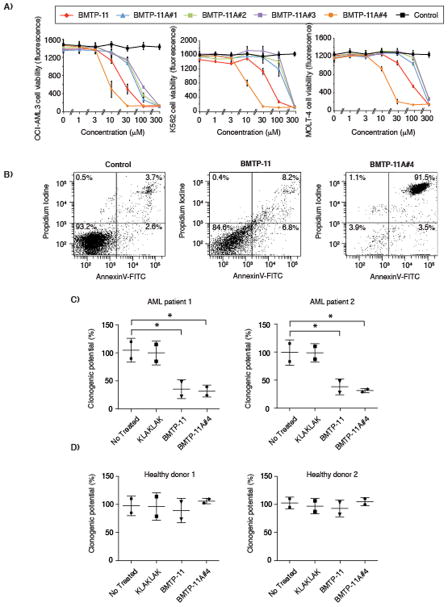
We next investigated whether IL-11R would serve as a molecular target for ligand-directed drug delivery of BMTP-11. Upon internalization by target cells via ligand directed delivery, D(KLAKLAK)2 induces cell death by disruption of mitochondrial membranes (8,10–14). BMTP-11 decreased cell viability at low concentrations (40–100 μM range) in human leukemia cell lines, and in most cases the maximum inhibitory concentration was reached within the 60–80 μM range (Fig. 3). In all cases, a negative control admixture of CGRRAGGSC plus D(KLAKLAK)2 had no detectable effect at equimolar concentrations (Fig 3). The cell viability was determined by measuring lactate dehydrogenase activity using established methods as described in Materials and Methods.
Figure 3.
Drug activity of BMTP-11 on a panel of established leukemia and lymphoma cell lines. The effect of increasing doses of BMTP-11 or control compounds are shown. Cell viability was measured via lactate dehydrogenase activity. Results shown represent the average of three independent experiments.
BMTP-11 specifically induces leukemia cell death
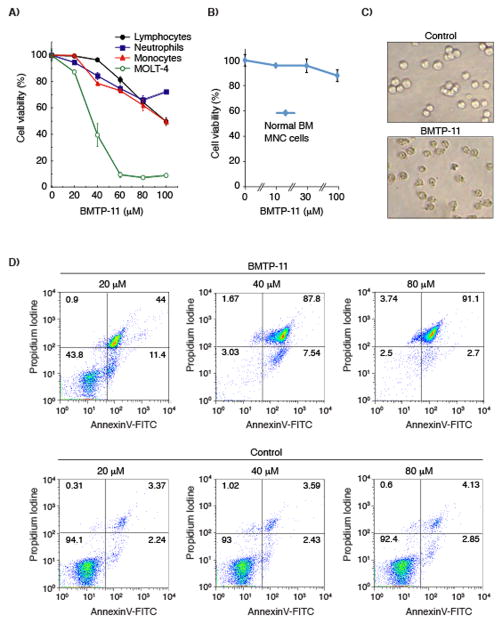
To assess the specificity of the BMTP-11, we simultaneously tested the effects of BMTP-11 on purified normal white blood cell populations including granulocytes, monocytes and lymphocytes, as well as human MOLT-4 leukemia cells. BMTP-11 had lower activity against normal white blood cells compared to MOLT-4 cells (Fig. 4A). Moreover, no detectable effects of BMTP-11 on mononuclear cells from normal bone marrow were observed (Fig. 4B). We next studied the effect of the IL-11R-targeted drug on cell morphology. Again, BMTP-11, but not a negative control admixture of CGRRAGGSC plus D(KLAKLAK)2 at equimolar concentrations, affected the morphology of human leukemia cells after an overnight incubation (Fig. 4C). The cells were further analyzed by AnnexinV-FITC/PI staining to assess the extent of cell death induced by BMTP-11 (Fig. 4D). For instance, OCI-AML3 cells treated overnight with BMTP-11 underwent a concentration-dependent cell death induction and nearly complete leukemia cell death (~90% of the cells) was observed at 40 μM or higher. In contrast, leukemia cells treated with the negative control admixture exhibited only background levels of cell death (~5%), similar to untreated control cells.
Figure 4.
Toxicity of BMTP-11 on normal white blood cells. (A) BMTP-11 exhibited no detectable toxicity on normal lymphocytes, neutrophils or monocytes in comparison to positive control leukemia cells (MOLT-4). (B) BMTP-11 had no detectable toxicity on mononuclear cells from healthy bone marrow. (C) The effect of BMTP-11 or control peptide admixture on leukemia cell morphology. (D) Anti- leukemia cell activity of BMTP-11. Untreated OCI-AML3 cells served to determine background levels of cell death under the experimental conditions used. Cells were incubated with 20, 40, or 80 μM of control admixture or the BMTP-11 overnight at 37 °C. AnnexinV-FITC/PI staining served to detect the level of cell death by flow cytometry.
BMTP-11 drug lead-optimization
To further improve the anti-leukemia activity of our drug prototype through Merrifield synthesis and chemical lead-optimization, we designed and evaluated four BMTP-11 analogs with modified structures. Cell viability of leukemia cell lines incubated with the drug candidates was determined by a lactate dehydrogenase activity assay. Three analog drugs (denominated BMTP-11A#1, BMTP-11A#2, and BMTP-11A#3) with certain L-amino acid residues changed to the cognate D-residue enantiomers (Supplemental Table 1) were produced, but found to be less active than BMTP-11 (Fig. 5A) against leukemia cell lines and were therefore not considered for further studies described here. In contrast, BMTP-11 analog #4 (BMTP-11A#4), which is myristoylated on its serine residue, had improved anti-leukemia drug activity against three different cell lines: OCI-AML3, K562 and MOLT-4 (Fig. 5A). Subsequent studies showed that BMTP-11A#4 induced cell death faster than the parental BMTP-11 at equimolar concentrations. For instance, after only four hours, BMTP-11A#4 induced cell death in over 90% of the leukemia cells, whereas BMTP-11 induced cell death in fewer than 10% of the cells (Fig. 5B). However, an extended incubation time allowed BMTP-11 to induce cell death as efficiently (Fig 4D). To further analyze the effect of BMTP-11 and BMTP-11#4 on cell viability, we counted the absolute number of viable cells after treatment with drug candidates (Supplemental Fig. 3). These results were similar to the cell viability results obtained by measuring lactate dehydrogenase activity (Fig. 3 & 5).
Figure 5.
Drug lead optimization. (A) Activities of BMTP-11 structural analogs against leukemia cell lines. (B) Induction of cell death by the parental BMTP-11 and its myristoylated analog (BMTP-11A#4) in OCI-AML3 cells after a 4 h incubation. (C–D) Clonogenic potential of patient-derived leukemia samples (C) and healthy bone marrow samples (D) in the presence and absence of BMTP-11, BMTP-11A#4, or the negative control.
Clonogenic potential of BMTP-11 and its derivative
The clonogenic potential of patient derived AML cells was assessed via methylcellulose assays and statistically significant inhibition was obtained at 100 μM concentration for CGRRAGGSC-GG-D(KLAKLAK)2 and at 30 μM with the analog #4 (Fig. 5C), while the clonogenic potential of normal bone marrow cells from healthy individuals was not altered at equivalent concentrations (Fig. 5D). The concentrations used for the individual peptides were selected based on their maximal inhibitory concentrations obtained via cell viability and proliferation assays with leukemia cell lines (Fig 5A).
Discussion
Combinatorial screening of phage display random peptide libraries in vitro, in experimental animal models and even directly in patients provides tools to identify molecular targets in the context of human disease (1–4,7–14). Using this technology, we have previously identified IL-11R as a functional target in the context of bone metastasis of prostate cancer (7–9) and primary osteosarcoma (10). In the present study, we show that IL-11R is expressed at higher levels on the cell surface of human leukemia cell lines as well as in primary bone marrow samples obtained from patients with AML and CLL, relative to control bone marrow samples obtained from healthy donors. In addition, we show an increased vascular expression of IL-11R in the blood vessels within leukemic bone marrow samples, regardless of disease subtype or percentage of blast positivity, data indicating that IL-11R is also expressed on the surface of stromal (i.e., non-malignant) cells within the tumor microenvironment. We have used an established targeting motif (7–10) and in vitro functional assays to show that the proapoptotic peptidomimetic D(KLAKLAK)2 (8,10–14), when ligand-directed to the IL-11R, is internalized by leukemia cells and induces concentration-dependent programmed cell death.
Several previous studies have implicated the IL-11/IL-11R signaling pathway in the pathogenesis of leukemia (16–18), particularly in AML-M5 blast cells and in B-cells from CLL. For instance, AML-M5 cells showed an enhanced clonal proliferation in response to stimulation by IL-11 and granulocyte colony-stimulating factor (16). Moreover, the expression level of IL-11R is elevated in B-cell CLL compared to peripheral blood lymphocytes from normal donors (17). IL-11R transcription has been detected in K562 (CML), Mo7E (megakaryocytes) and TF1 (erythroleukemia) cells (18), suggesting broad expression.
Therefore, we first evaluated the cell surface expression of IL-11R in a panel of leukemia cell lines by flow cytometry. An anti-IL-11R antibody strongly stained all the human leukemia cell lines tested, indicating broad and robust cell surface receptor expression in established human leukemia cell lines regardless of original disease subtype. We subsequently assessed the IL-11R cell surface expression in AML patient derived samples and in blood and bone marrow samples from healthy donors. The analysis at the cell population level revealed that the IL-11R expression was not significant in the normal samples, and was mainly found in the mature and immature myeloid cell populations, as well as in progenitor cell populations, in the samples from AML patients, with the exception of the B-cell population staining positive in one AML patient sample. In addition, the membrane expression of IL-11R in the isolated CD34+ cell population from AML patient samples was detected in one of two investigated cases via cytospin analysis, while the isolated CD34+ population from two different normal bone marrow samples did not show such staining. We next studied IL-11R protein levels by immunohistochemistry on a large and diverse panel of bone marrow samples derived from leukemia patients. Healthy donors were used as controls. IL-11R staining of leukemia blasts was noted in over half of the human leukemia samples of varying subtypes. Most notably, all the leukemic bone marrow samples containing evaluable blood vessels – including AML, myelodysplastic or myeloproliferative syndromes and CLL, but not normal bone marrow – stained positive for IL-11R. These results indicate that the protein expression of this receptor also occurs in the tumor microenvironment and not only in malignant cells.
When we investigated the targeted drug activity of BMTP-11 against human leukemia and lymphoma cell lines, we found that low concentrations (i.e., <100 μM) of BMTP-11 were broadly effective against the cell lines investigated, relative to an admixture of CGRRAGGSC plus D(KLAKLAK)2 at equimolar concentration. Notably, we did not observe a significant correlation between IL-11R levels and BMTP-11 sensitivity as the IL-11R expression was relatively high for all the cell lines tested. In addition, the drug sensitivity is often affected by several factors other than the expression level of the target protein. For instance, different cell types could have different levels of the p-glycoprotein 1, which would clearly alter the drug sensitivity. Similarly, the mutational landscape of a specific cancer cell plays a crucial role in treatment sensitivity, and therapeutic compound responses have been correlated to specific cancer genotypes (19).
To determine specificity, we also compared the effects of BMTP-11 on subpopulations of normal peripheral blood cells versus leukemia cells (serving as positive controls). BMTP-11 had negligible activity against normal granulocytes, monocytes and lymphocytes compared to index leukemia and lymphoma cell lines. These data suggest that BMTP-11 preferentially targets leukemia cells relative to normal white blood cells. Furthermore, these results are consistent with the finding that mice with targeted genetic disruption of the IL-11R did not display defects in hematopoiesis (20).
More than a decade ago our group used combinatorial screening in a cancer patient to isolate an IL-11 like peptide mapping to domain I of IL-11 (7). In a subsequent study, extensive structural and functional investigations uncovered a previously unrecognized receptor-binding site for human IL-11 (9). Based on these findings, we initiated a pilot drug lead-optimization program for the BMTP-11 in an attempt to find potentially superior analogs. We generated BMTP-11 analogs with modified structures. One such analog, BMTP-11A#4, a derivative with a Gly1-Cys9 thioether bond and myristoylation on Ser8 residue, had improved activity against leukemia cells on side-by-side comparisons with the parental compound BMTP-11 in the cell viability and proliferation assay with established leukemia cell lines. Both BMTP-11 and BMTP-11A#4 reduced the clonogenic potential of patient-derived AML bone marrow cells at their individual effective inhibitory concentrations, while these peptides did not have a similar effect on normal bone marrow cells at equivalent concentrations. These data demonstrate that BMTP-11 and its analog BMTP-11A#4 could potentially be used to therapeutically target leukemic progenitor cells. Myristoyl groups are highly lipophilic, an attribute that may facilitate their incorporation into phospholipid membrane bilayers and cell internalization (21). Thus, it is possible that BMTP-11A#4 has an improved ligand-directed internalization profile. However, confirmation of this working hypothesis must await formal GMP toxicology studies and X-ray crystallography of BMTP-11 and its derivatives binding to the IL-11R.
In conclusion, the expression pattern characterization and functional findings reported here establish the presence of targetable IL-11R on leukemia cells and tumor-associated vasculature within human bone marrow. To that end, we present evidence that the drug prototype BMTP-11 and its derivatives have activity against leukemia and lymphoma. Given that, in as yet unpublished work (Pasqualini et al, in press (15), we have performed an extensive formal toxicology study in rodents and primates, and conducted a first-in-human clinical trial, the BMTP-11 prototype, and perhaps one of its derivatives, may be considered experimental drug candidates for translational applications against hematopoietic malignancies.
Supplementary Material
Translational Relevance.
Despite major advances in the field of targeted therapy, management of human leukemia and lymphoma still largely relies on non-specific cytotoxic chemotherapy drugs that disrupt nucleic acid- or protein synthesis. Progress towards a ligand-directed pharmacology in hematopoietic malignancies requires the discovery, development and validation of molecular targets on the surface of tumor cells. Notably, several ligands to leukemia cell membrane receptors have been found by screening of combinatorial peptide libraries, but their corresponding receptors are either unknown or not sufficiently specific to provide differentiation between normal and leukemia cells. The morphologic and functional studies reported here demonstrate that the interleukin-11 receptor is a suitable tumor cell surface target for the delivery of a new ligand-directed drug prototype, denominated BMTP-11, against human leukemia and lymphoma. Moreover, these results establish the candidacy of BMTP-11 and/or its derivatives for further translational studies in anti-leukemia and -lymphoma drug development.
Acknowledgments
Grant Support
This work was supported by the Specialized Program in Research Excellence (SPORE) Program in Leukemia at The University of Texas M. D. Anderson Cancer Center (to R.P. and W.A), the Gillson-Longenbaugh Foundation (to R.P. and W.A). D.E.J. received support from the Kimberly Patterson Fellowship for Leukemia Research.
Footnotes
Conflict of interest: The University of Texas M. D. Anderson Cancer Center and some of its researchers (R.P. and W.A.) own equity stock in Alvos Therapeutics (Arrowhead Research Corporation; Pasadena, CA), which is subjected to certain restrictions under University Policy; the University manages and monitors the terms of these arrangements in accordance with its conflict of interest policy. W.A. and R.P. are co-inventors of issued patents filled by the University that cover BMTP-11 and are entitled to royalties that could result from commercial success of the drug. The other authors declare that they have no competing interests.
Authorship contribution
K.K., C.E.B-R., D.E.J., L.B., Y.S., A.K, W.H.D. performed research; K.K., D.E.J, C.E.B-R., L.B., A.J.Z., A.K., W.H.D., E.K., W.A., R.P. designed and analyzed the research; C.E.B-R., J.E.C., S.O’B., H.M.K., G.A.C. provided reagents; K.K., M.C-V., C.R, E.K., W.A., R.P. wrote and/or edited the article.
References
- 1.Nishimura S, Takahashi S, Kamikatahira H, Kuroki Y, Jaalouk DE, O’Brien S, et al. Combinatorial targeting of the macropinocytotic pathway in leukemia and lymphoma cells. J Biol Chem. 2008;283:11752–11762. doi: 10.1074/jbc.M708849200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McGuire MJ, Samli KN, Chang Y-C, Brown KC. Novel ligands for cancer diagnosis: selection of peptide ligands for identification and isolation of B-cell lymphomas. Exp Hematol. 2006;34:443–452. doi: 10.1016/j.exphem.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Takahashi S, Mok H, Parrott MB, Marini FC, 3rd, Andreeff M, Brenner MK, et al. Selection of chronic lymphocytic leukemia binding peptides. Cancer Res. 2003;63:5213–5217. [PubMed] [Google Scholar]
- 4.Kolonin MG, Bover L, Sun J, Zurita AJ, Do KA, Lahdenranta J, et al. Ligand-directed surface profiling of human cancer cells with combinatorial peptide libraries. Cancer Res. 2006;66:34–40. doi: 10.1158/0008-5472.CAN-05-2748. [DOI] [PubMed] [Google Scholar]
- 5.Pentz RD, Cohen CB, Wicclair M, DeVita MA, Flamm AL, Youngner SJ, et al. Ethics guidelines for research with the recently dead. Nat Med. 2005;11:1145–1149. doi: 10.1038/nm1105-1145. [DOI] [PubMed] [Google Scholar]
- 6.Pentz RD, Flamm AL, Pasqualini R, Logothetis CJ, Arap W. Revisiting ethical guidelines for research with terminal wean and brain-dead participants. Hastings Cent Rep. 2003;33:20–26. [PubMed] [Google Scholar]
- 7.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardó-Vila M, Giordano RJ, et al. Steps toward mapping the human vasculature by phage display. Nat Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 8.Zurita A, Troncoso P, Cardó-Vila M, Logothetis CJ, Pasqualini R, Arap W. Combinatorial Screening in Patients: the interleukin-11 receptor α as a candidate target in the progression of human prostate cancer. Cancer Res. 2004;64:435–439. doi: 10.1158/0008-5472.can-03-2675. [DOI] [PubMed] [Google Scholar]
- 9.Cardó-Vila M, Zurita AJ, Giordano RJ, Sun J, Rangel R, Guzman-Rojas L, et al. A ligand peptide motif selected from a cancer patient is a receptor-interacting site within human interleukin-11. PLOS One. 2008;3:e3452. doi: 10.1371/journal.pone.0003452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis VO, Ozawa MG, Deavers MT, Wang G, Shintani T, Arap W, et al. The interleukin-11 receptor α as a candidate ligand-directed target in osteosarcoma: consistent data from cell lines, orthotopic models, and human tumor samples. Cancer Res. 2009;69:1995–1999. doi: 10.1158/0008-5472.CAN-08-4845. [DOI] [PubMed] [Google Scholar]
- 11.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–1038. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 12.Arap W, Haedicke W, Bernasconi M, Kain R, Rajotte D, Krajewski S, et al. Targeting the prostate for destruction through a vascular address. Proc Nat Acad Sci USA. 2002;99:1527–1531. doi: 10.1073/pnas.241655998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 14.Lahdenranta J, Sidman RL, Pasqualini R, Arap W. Treatment of hypoxia-induced retinopathy with targeted proapoptotic peptidomimetic in a mouse model of disease. FASEB J. 2007;21:3272–3278. doi: 10.1096/fj.07-8273com. [DOI] [PubMed] [Google Scholar]
- 15.Pasqualini R, Millikan RE, Christianson DR, Cardó-Vila M, Driessen WHP, Giordano RJ, et al. Targeting the Interleukin-11 Receptor α in Metastatic Prostate Cancer: A First-in-man Study. Cancer. doi: 10.1002/cncr.29344. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimura T, Sakabe H, Minamiguchi H, Fujiki H, Abe T, Kaneko H, et al. Interleukin-11 (IL-11) enhances clonal proliferation of acute myelogenous leukemia cells with strong expression of the IL-11 receptor α chain and signal transducing gp130. Leukemia. 1999;13:1018–1027. doi: 10.1038/sj.leu.2401433. [DOI] [PubMed] [Google Scholar]
- 17.Tsimanis A, Shvidel L, Klepfish A, Shtalrid M, Kalinkovich A, Berrebi A. Over-expression of the functional interleukin-11α receptor in the development of B-cell chronic lymphocytic leukemia. Leuk & Lymph. 2001;42:195–205. doi: 10.3109/10428190109097691. [DOI] [PubMed] [Google Scholar]
- 18.Cherel M, Sorel M, Lebeau B, Dubois S, Moreau JF, Bataille R, et al. Molecular cloning of two isoforms of a receptor for the human hematopoietic cytokine interleukin-11. Blood. 1995;86:2534–2540. [PubMed] [Google Scholar]
- 19.Kim N, He N, Kim C, Zhang F, Lu Y, Yu Q, et al. Systemic analysis of genotype-specific drug responses in cancer. Int J Cancer. 2012;131:2456–2464. doi: 10.1002/ijc.27529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nandurkar HH, Robb L, Tarlinton D, Barnett L, Köntgen F, Begley CG. Adult mice with targeted mutation of the interleukin-11 receptor (IL-11Ra) display normal hematopoiesis. Blood. 1997;90:2148–2159. [PubMed] [Google Scholar]
- 21.Farazi TA, Waksman G, Gordon JI. The biology and enzymology of protein N-myristoylation. J Biol Chem. 2001;276:39501–39504. doi: 10.1074/jbc.R100042200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.