Abstract
Both lifespan and healthspan are influenced by nutrition, with nutritional interventions proving to be robust across a wide range of species. However, the relationship between nutrition, health and aging is still not fully understood. Caloric restriction is the most studied dietary intervention known to extend life in many organisms, but recently the balance of macronutrients has been shown to play a critical role. In this review, we discuss the current understanding regarding the impact of calories and macronutrient balance in mammalian health and longevity and highlight the key nutrient-sensing pathways that mediate the effects of nutrition on health and ageing.
Keywords: calories, macronutrients, health, lifespan, longevity
Introduction
Aging is one of the greatest societal challenges in the modern world. Lifestyle choices, improved technology and modern medicine have contributed to a rapidly growing aging population (Partridge 2014). While we live longer on average than our ancestors, increased lifespan is not without its drawbacks. The primary problem with living longer is that with increasing age comes a heightened risk of chronic diseases such as cancer, type II diabetes, stroke, dementia and cardiovascular disorders, leading to disability and related mortality (de Cabo and Le Couteur 2015; Fontana, et al. 2010; Piper, et al. 2011). Currently, the major focus of modern medicine is treating specific age-related diseases; but with a growing number of older people encumbered with multiple chronic conditions (Fontana, et al. 2014), this approach is problematic, e.g. leading to complications arising from multiple medications for different conditions (de Cabo and Le Couteur 2015). Rather than treat the symptoms of aging, a logical alternative approach would be to intervene in the aging process itself (Partridge 2014).
Interventions that slow the rate of aging and increase healthspan and lifespan have been of considerable interest over the past 80 years. While genetic and pharmaceutical interventions have been widely explored in laboratory models (Baur, et al. 2006; Kenyon, et al. 1993; Mitchell, et al. 2014), translating such approaches to humans is difficult (Fontana and Partridge 2015). Nutritional manipulations, however, have proven to be similarly robust across multiple animal models and humans, with profound impacts on reproduction, health and aging. The complex relationship between nutrition and age-related health is not fully understood, however. A growing body of evidence has pointed to dietary restriction as an important mediator of health and lifespan (Masoro 2000, 2003; Miller, et al. 2005; Piper et al. 2011). But what does dietary restriction actually mean? Throughout the literature, dietary restriction is often used interchangeably with caloric restriction (CR). Whereas dietary restriction can involve different feeding regimens such as intermittent fasting or alternate day feeding (Ingram and Roth 2015), CR refers more specifically to the reduction of total calorie intake by 20-50% without malnutrition (Masoro 2005; Weindruch, et al. 1986). Recent evidence, however, suggests that the balance of macronutrients, rather than total energy intake, plays a larger role in lifespan extension than previously attributed (Mair, et al. 2005; Solon-Biet, et al. 2014; Zimmerman, et al. 2003). Whether calories or specific nutrients affect aging is a critically important issue to resolve, with important implications for aging research (Simpson and Raubenheimer 2007). In this review, we discuss the current understanding and impact of both calories and macronutrients on health and lifespan based on studies in invertebrate and mammalian models, and highlight the use of nutritional geometry as a framework to help disentangle the complex relationship between diet and healthy aging.
Dietary restriction
There is widespread consensus in aging research that eating fewer calories results in a longer, healthier life. To date, CR has been the primary focus of most non-genetic nutritional interventions (Ingram, et al. 2004; Mattison, et al. 2003; Sinclair 2005). Yeasts, nematode worms, fruit flies, rodents and even non-human primates have been used as models for the study of CR and aging, suggesting that these effects must act via evolutionarily conserved mechanisms (Figure 1) (Lin, et al. 2000; Weindruch et al. 1986). Since the first account of the life-extending effects of CR in rats in the early 1930’s, there has been a substantial amount of research into the dietary basis of aging (McCay, et al. 1935). It was not until the early 1980s that the idea of CR as a viable model for aging and the study of age-related diseases really came to fruition (Masoro 1991; Masoro, et al. 1982; Walford, et al. 1992). Despite the great strides made towards understanding the mechanisms of CR, much still remains unknown. Initial work exploring CR as a robust nutritional intervention for aging began with yeasts, worms and flies. In yeast (Saccharomyces cerevisiae), CR is mediated by reduced glucose levels, extending both overall lifespan and replicative lifespan (Bonawitz, et al. 2007; Kaeberlein, et al. 2005; Powers, et al. 2006) and in the worm Caenorhabditis elegans and the fruit fly Drosophila melanogaster, some forms of food restriction via nutrient manipulation also successfully extended lifespan (Partridge, et al. 2005; Taormina and Mirisola 2014). Interestingly, the beneficial effect of CR does not appear to be universal. Although experiments in rodents produces a net beneficial effect to overall metabolic health in laboratory animals, (Bordone and Guarente 2005; Ingram et al. 2004; Masoro 2000) the effects on lifespan extension are highly dependent on various other factors such as strain and sex (Festing and Blackmore 1971; Liao, et al. 2010; Yuan, et al. 2009). 40% CR extends maximal lifespan in male B6D2F1 mice by 20% relative to ad libitum fed controls (Wolf, et al. 1995); however, whether this effect also extends to females remains to be seen. We do know that CR extends lifespan in genetically heterogeneous mice created from four inbred strains (BALB/c, C57BL/6, C3H, and DBA2), although more than 90% died of cancer which may not be representative of the human situation (Miller, et al. 2011). Translation into longer-lived mammals has continued to show conflicting results (Liao et al. 2010; Mattison, et al. 2012). Two long term studies in non-human primates were initiated in the early 1980s in order to address this question. Although both studies confirm the findings that CR delays the onset of age-associated diseases, CR monkeys from the National Institute on Aging (NIA) did not live longer than their ad libitum fed counterparts, which is in contrast to results obtained in the Wisconsin cohort (Colman, et al. 2009; Colman, et al. 2014; Mattison et al. 2012). These results were attributed to possible discrepancies in diet design and diet composition (Mattison et al. 2012). NIA monkeys were fed a diet rich in natural ingredients such as protein derived primarily from plant sources while the Wisconsin monkeys were fed a semi-purified diet with protein derived from lactalbumin (Ingram, et al. 1990; Ramsey, et al. 2000). Carbohydrate quality also differed between studies with the NIA diets containing significantly less sucrose than the Wisconsin study (Mattison et al. 2012).
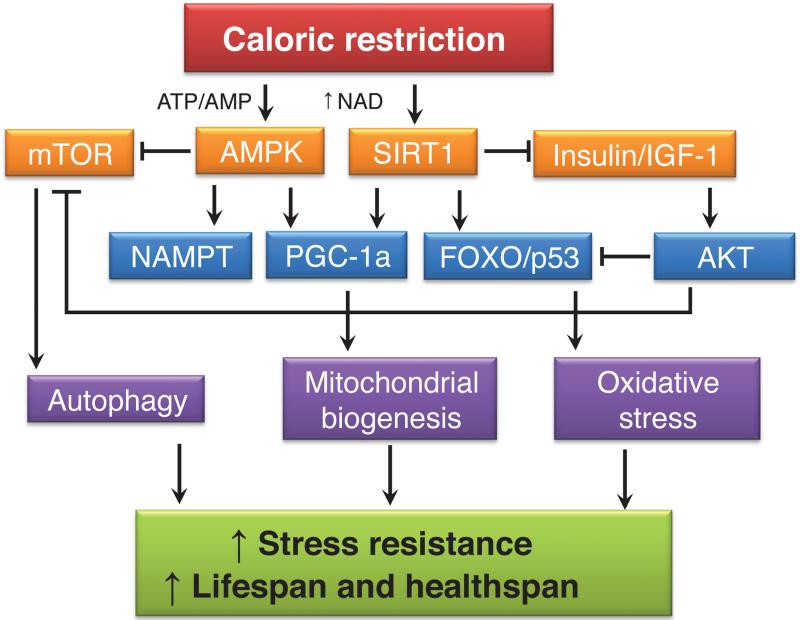
Figure 1.
The complex metabolic network of potential players in the mechanism of caloric restriction (CR). A reduction in energy intake influences cellular energy levels, activating the AMPK and SIRT1 pathways. Antagonistic responses include the inhibition of the anabolic pathways mTOR and insulin/IGF-1. Downstream effects result in increased stress resistance and improved lifespan and healthspan.
Such studies highlight the question of whether CR per se is solely responsible for extended longevity or if particular macronutrients or a balance of macronutrients is more important (Table 1). While the effect of CR on human lifespan is yet to be determined, CR has been shown to improve several markers of health (Fontana et al. 2010; Heilbronn, et al. 2006). But despite these benefits, a central limitation is that compliance to lifetime CR is challenging in humans and the risk of missing essential nutrients can be detrimental to reproduction, bone structure and overall metabolic health (Fontana and Partridge 2015; Ingram and Roth 2015). Hence, dietary interventions involving ad libitum access to diets designed to prolong healthspan would be of greater utility than CR.
Table 1.
Experiments on a range of organisms exploring CR, protein restriction and macronutrient balance.
| Animal | lifespan increase | beneficial health effects | Reproductive effects |
Reference |
|---|---|---|---|---|
| Caloric restriction | ||||
| Yeast | Increase in mean and maximum lifespan with glucose depletion |
Increased mitochondrial respiration | Biomass production impaired on low glucose treatments |
Lin, et al. (2004); Wu, et al. (2013); Bonawitz et al. (2007) |
| Worms | Up to 50% with bacteria-free media |
Increase oxidative and thermal stress resistance |
Number of eggs laid per worm increased |
Hosono, et al. (1989); Houthoofd, et al. (2002) |
| Flies | Up to 50% at 40% CR | Not reported | Egg production decreased with CR |
Partridge et al. (2005) |
| Rhesus Monkeys | Trend seen. Conflicting results about lifespan extension |
Delay in age-related disease, improved metabolic health and decrease in cancer. Reduction in cardiovascular disease and brain atrophy |
Unknown |
Colman et al. (2009); Mattison et al. (2012) |
| Humans | Unknown | Reduced risk of age-related disease such as diabetes, stoke, cardiovascular disease, obesity, metabolic disorders, cancer. |
Late reproductive maturity, suppressed ovarian function, impaired fecundity |
Fontana and Klein (2007); Heilbronn and Ravussin (2003); Mercken, et al. (2012) |
| Protein restriction | ||||
| Flies | Yeast-restricted flies also show median and maximal lifespan extension. |
Not reported | CR reduces lifetime fecundity. |
Grandison et al. (2009); Mair et al. (2005) |
| Mice | Maximal lifespan extended by methionine restriction |
Reduced IGF-1, insulin, glucose and thyroid hormone levels. Delayed immune impairment and cataracts |
Not reported | Miller et al. (2005) |
| Rats | Mean and maximal lifespan extended by methionine restriction |
Reduced body weight | Not reported | Richie et al. (1994) |
| Humans | Decreased mortality by 25% in people aged 50-65. Higher protein intake associated with reduced mortality in people over 65. |
Four-fold decrease in cancer death risk in people aged 50-65. Five-fold decrease in diabetes overall. |
Not reported | Levine et al. (2014) |
|
Macronutrient
balance |
||||
| Flies | 3 fold on diets with a low protein to carbohydrate ratio |
Increased triglyceride storage on low protein diets |
Higher protein to carbohydrate ratios optimized reproduction |
Lee et al. (2008); Fanson et al. (2009); Jensen et al. (2015); Bruce et al. (2013) ; Skorupa, et al. (2008) |
| Crickets | Up to 3.5 fold on diets with a low protein to carbohydrate ratio |
Not reported | Responses vary by sex. Reproduction optimized at higher protein to carbohydrate ratios females only. |
Maklakov et al. (2008) |
| Mice | ~30% on diets with a low protein to carbohydrate ratio |
Improved blood pressure, lipid profiles, mitochondrial function, insulin sensitivity, HOMA and immune function |
Reproduction optimized in diets with a higher protein to carbohydrate ratio compared to lifespan |
Solon-Biet et al. (2014); Solon-Biet et al. (2015a) Solon-Biet et al. (2015b); Le Couteur et al. (2014) |
Calorie restriction or protein restriction?
Recent studies have suggested that the beneficial effects of CR on lifespan may be due to the reduced intake of specific dietary components such as proteins, rather than total energy intake (Mair et al. 2005; Pamplona and Barja 2006; Piper, et al. 2005; Zimmerman et al. 2003) with these effects acting largely through the same evolutionarily conserved signaling pathways (Figure 2). The restriction of protein intake, rather than energy, may offer a more feasible nutritional intervention in humans. Work by McCay as early as 1929 reported that a low protein diet extended the lifespan of trout (McCay, et al. 1929). Since then, it has been shown that the restriction of essential amino acids can increase lifespan in honeybees (Paoli, et al. 2014), and the restriction of particular amino acids, such as methionine, can extend lifespan in mice (Sun, et al. 2009) and rats (Orentreich, et al. 1993; Richie, et al. 1994), and lower the levels of serum IGF-I, insulin, glucose and thyroid hormone in (BALB/cJ × C57BL/6 J) F1 mice (Miller et al. 2005). Recently, the restriction of essential amino acids (Robertson, et al. 2015) and the sulfur amino acids methionine and cysteine (Robertson et al. 2015) have been shown to protect against hepatic ischemia reperfusion injury by preconditioning against oxidative stress, complications of cardiovascular surgery (Robertson et al. 2015) and mediating hydrogen sulfide production (H2S) (Hine, et al. 2015). H2S production under protein restriction exerts a hormetic response, acting on brain signaling and the vascular system to reduce blood pressure and trigger the same signaling response cascade observed in animals fed protein-restricted diets via activation of GCN2, eIF2α and ATF4 and repression of mTOR (Figure 2) (Hine et al. 2015; Robertson et al. 2015). Moreover, a meta-analysis of animal studies of caloric restriction and aging conclude that the restriction of protein, rather than caloric restriction, appeared to have the greatest effect on delaying aging (Nakagawa, et al. 2012). Data in humans indicate that reduced protein intake may become an important component of anticancer and anti-aging dietary interventions (Fontana, et al. 2008; Levine, et al. 2014).
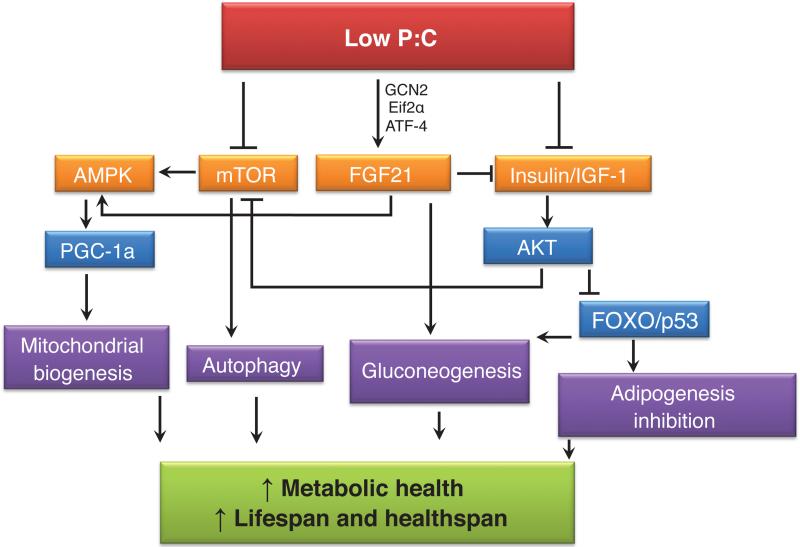
Figure 2.
The complex metabolic network of potential players in the mechanism of low protein:carbohydrate (P:C) diets. Low P:C diets activate GCN2 and FGF21 and inhibit activation of mTOR and Insulin/IGF-1. Inhibition of these pathways activates AMPK and AKT, resulting in improved metabolic health, lifespan and healthspan.
Macronutrient balance
While both CR and protein restriction have been shown to impact aging, a fundamental limitation of these two one-variable-at-a-time approaches is that they cannot disentangle the interactive effects of nutrients and calories (Simpson, et al. 2015). Recent studies have begun to tackle these interactions and shown the importance of the balance of macronutrients on health and aging. Such evidence has been derived using the Geometric Framework for nutrition (GF; Simpson and Raubenheimer 2009, Simpson and Raubenheimer 2012). In the GF, nutrition is represented in an n-dimensional space, in which the components of n represent focal dietary components (e.g. macronutrients). Various phenotypic responses (e.g. lifespan) can be modelled onto this n-dimensional space, providing a detailed landscape of the effects of nutrition. Using this framework allows the use of nutritional geometry to simultaneously interpret the effects of energy, individual macronutrients (or other focal dietary components) and the interactions within and between nutrients (Piper et al. 2011; Simpson and Raubenheimer 2012). This framework has helped to resolve conflicting ideas about the nutritional determinants of health and aging, and to reconcile views on resource-mediated trade-offs between reproduction and longevity (Jensen, et al. 2015; Lee, et al. 2008; Solon-Biet, et al. 2015b; Tatar, et al. 2014).
Studies in both invertebrates and mice show that reproduction and longevity do not trade-off against one another; rather, these responses have different nutritional requirements. In the field cricket Teleogryllus commodus and fruit fly Drosophila melanogaster, the macronutrient blend that maximized lifespan was markedly different from diets which maximized reproductive variables (Jensen et al. 2015; Maklakov, et al. 2008). Maximal longevity occurred on low protein (P), high carbohydrate (C) diets in both males and females, while a higher P:C ratio was better for reproduction in females only. Consuming a low proportion of protein in the diet relative to carbohydrate, not total calories, extended lifespan in ad libitum-fed flies (Bruce, et al. 2013; Lee et al. 2008), while diets with a higher proportion of protein shortened lifespan but improved reproduction (Lee et al. 2008). This result has been replicated in several other insect species (Dussutour and Simpson 2009; Fanson, et al. 2009; Grandison, et al. 2009; Lee et al. 2008; Piper et al. 2011) and consistently indicates that the balance of macronutrients is the chief nutritional cue that directs metabolism towards longevity or reproduction (Wilder, et al. 2012). A recent study in mice showed that ad libitum low protein, high carbohydrate diets fed short-term improved several markers of health including insulin, HOMA, glucose tolerance and triglycerides to a level comparable to CR, but without at 40% reduction in total calorie intake (Solon-Biet, et al. 2015a). Long-term investigations in ad libitum-fed mice across 25 different diets varying in macronutrient composition support these findings, showing that latelife health and longevity were optimized not by reducing energy intake, but by low P:C diets (Solon-Biet et al. 2014). In an attempt to stabilize protein intake, mice displayed a compensatory increase in food intake on low protein diets, resulting in increased energy intake and greater adiposity, but experienced a significant increase in lifespan, improved blood pressure, lipid profiles, mitochondrial function, insulin sensitivity (Solon-Biet et al. 2014) and immune function (T and B cell populations) measured at 15 months (Le Couteur, et al. 2014). These health and longevity consequences were shown to be related to circulating branched chain amino acid (BCAA) levels, which, interestingly, were the only amino acids to be positively correlated to protein intake under chronic feeding conditions. BCAA levels were the lowest in mice on the low protein, high carbohydrate diets correlating to diet treatments that yielded the longest health and lifespan.
Reports about the role of BCAAs in aging and health are seemingly divergent. Some suggest that elevated BCCAs are harmful because they are linked with obesity and diabetes, while others suggest that BCAAs should be supplemented to increase mitochondrial biogenesis (D’Antona, et al. 2010). For example, in a major review, Newgard (2012) noted that human epidemiological studies and animal studies show that elevated BCAAs are associated with and predict diabetes, obesity and heart disease, while animal and cell studies show that BCAA supplementation increases activation of certain nutrient signaling pathways which are detrimental for aging (Chotechuang, et al. 2009). In another review, Valerio, et al. (2011) argue that BCAAs increase mitochondrial biogenesis and muscle function, thus BCAA supplementation should be considered as a treatment for older people. While the exact roles of BCAAs in health and lifespan are yet to be determined, evidence suggest that BCAAs may be an important mediator of key molecular pathways that link nutrition with aging.
Nutrient-sensing pathways
Nutrient-sensing pathways that mediate the effects of nutrition on health and aging have been explored in many experimental models (Chantranupong, et al. 2015; Fontana et al. 2010; Hubbard and Sinclair 2014). These include the evolutionarily conserved key regulators mTOR, AMPK, insulin/IGF-1 and sirtuins. Both calories and macronutrients influence these pathways which have evolved to respond to periods of famine by switching cells and organism from their focus on growth and reproduction, towards survival and resilience (Figure 3) (Kapahi, et al. 2010; Le Couteur, et al. 2012; Speakman and Mitchell 2011). Although there are at least four key nutrient-sensing pathways implicated in longevity, these interact and share many downstream targets that regulate cell processes involved in aging, including mitochondrial biogenesis, cellular metabolism, autophagy, DNA repair and expression, and translation.
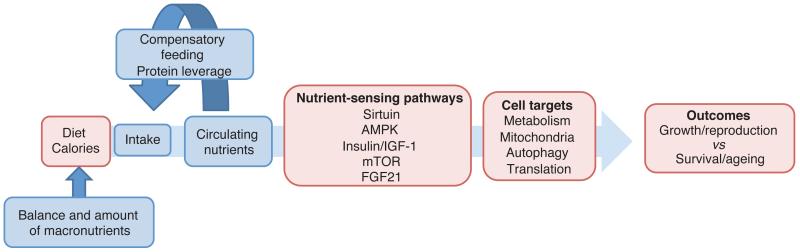
Figure 3.
Schema showing the mechanism for the beneficial effects of caloric restriction on aging. The red boxes show that standard view based on energy intake. The impact of the balance of macronutrients and compensatory feeding in ad libitum diets in relation to this pathway (blue boxes). This more closely approaches real-life feeding in animals and humans that have unlimited access to food across a wide range of macronutrient compositions.
Mechanistic Target of Rapamycin (mTOR)
In eukaryotic cells, mTOR is highly conserved and acts as a central regulator of growth and metabolism in response to nutrient and growth factor cues (Stanfel, et al. 2009). This pathway is involved in anabolic processes including protein and lipid synthesis (Efeyan, et al. 2015). mTOR integrates input from various pathways, including insulin and IGF-1, and responds to dietary protein, particularly BCAAs (Chotechuang et al. 2009; Solon-Biet et al. 2014). In addition, mTOR responds to changes in cellular energy levels, altered genetic makeup, gene manipulations and pharmacological interventions that affect lifespan (Arsham, et al. 2003; Tato, et al. 2011; Wang and Proud 2009). In mammals, mTOR has two structurally and functionally distinct multiprotein complexes: mTORC1 and mTORC2 which are differentiated by their accessory proteins, Raptor and Rictor (Jacinto, et al. 2004). mTORC1 is the only complex sensitive to amino acids (Yuan, et al. 2013) and is the primary modulator of protein, lipid, nucleotide synthesis and autophagy while mTORC2 is involved in cell proliferation and survival (Chantranupong et al. 2015). In animal models, it has been demonstrated that inhibition of mTOR protects against metabolic dysfunction, obesity, cancer and neurodegeneration (Stanfel et al. 2009) which can be achieved through pharmaceutical interventions such a rapamycin (Harrison, et al. 2009; Miller et al. 2011) or nutritional interventions such as alterations in the ratio of dietary P:C (Solon-Biet et al. 2014). In mice, mTOR was activated most strongly by the ratio of circulating BCAA to glucose (i.e. the P:C), providing a key mechanistic link from the longevity and health impacts of low P:C diets to the mTOR pathway. Reducing mTOR signaling is critical for improved health and lifespan.
5’ adenosine monophosphate-activated protein kinase (AMPK)
AMPK regulates cellular uptake of glucose, β-oxidation of fatty acids, the glucose transporter 4 and mitochondrial biogenesis. Activation of AMPK has been proposed as one of the mechanisms through which CR has beneficial effects on lifespan and healthspan (Cantó and Auwerx 2011). AMPK is a serine/threonine protein kinase, which is activated by cellular stresses that alter the AMP/ATP ratio resulting in depletion of ATP. As a consequence, ATP-consuming pathways are turned off, while ATP generation is turned on (Dagon, et al. 2006). AMPK is a heterotrimeric protein comprised of one catalytic (α) and two regulatory (β and γ) subunits containing the kinase domain which when phosphorylated results in increased AMPK activity (Dagon et al. 2006). Recently, Mair and colleagues showed that cyclic AMP-responsive element binding protein (CREB)-regulated transcriptional co-activator (CRTC-1) is an essential target for AMPK-mediated lifespan extension in C. elegans (Mair, et al. 2011). Longevity via transcriptional regulation of AMPK occurred through CRTC-1 downregulation, with neuronal CRTC-1 playing a primary role in regulating longevity and mitochondrial metabolism in peripheral tissues (Burkewitz, et al. 2015; Mair et al. 2011). In mammals, hepatic AMPK activation acts to slow gluconeogenesis and down-regulate key genes such as G6Pase and PEPCK, while in the muscle, it stimulates glucose uptake by increasing expression of glucose transporters such as GLUT-4 (McCarty 2004). The cardioprotective effects of short-term CR are thought to be mediated through AMPK activation (Shinmura, et al. 2007). Administration of the drug Metformin enhances lifespan in mice and this is accompanied by an increase in AMPK activity (Martin-Montalvo, et al. 2013), hence AMPK modulation represents an attractive target for inducing CR-like benefits. By activating this nutrient sensor, AMPK can extend healthspan and lifespan by restoring energy balance via catabolic responses such as fatty acid oxidation, proteolysis and inhibiting processes not essential for survival such as cell growth and proliferation (Canto, et al. 2009). These responses have been shown to underlie the beneficial effects of CR. Whether AMPK activation reflects the balance of dietary macronutrients as well as measures of energy status remains to be seen, but has been postulated (Simpson and Raubenheimer 2009).
Sirtuin pathway: SIRT1
Sirtuins have been shown to regulate the aging process and mediate CR-induced longevity in organisms including S. cerevisiae, C. elegans and D. melanogaster (Guarente and Kenyon 2000). Sirtuins are class III histone deacetylases that require NAD+ as a cosubstrate. CR increases cellular NAD+ as a consequence of reduced energy intake, thereby activating sirtuins. In mammals, there are seven homologs (SIRT1-7) which have been identified. SIRT1 remains perhaps the best and most studied, which is likely due to it sharing the most sequence similarity with the yeast Sir2 (Allard, et al. 2009; Frye 2000). SIRT1 has multiple functions, some of which are outlined in Figure 1, and include deacetylation of a large number of transcription factors (Boily, et al. 2008; Guarente 2006; Longo and Kennedy 2006), and regulation of PGC-1α (Gerhart-Hines, et al. 2007; Rodgers, et al. 2005; Sun, et al. 2007). In middle aged rats, CR has been reported to increase the expression of SIRT1 protein in brain, fat, kidney, and liver (Cohen, et al. 2004; Nisoli, et al. 2005). In young CR mice, SIRT1 protein expression was increased in muscle and fat but markedly reduced in the liver (Chen, et al. 2008). The SIRT1 protein, but not its increased expression, is essential for lifespan extension in CR mice (Mercken, et al. 2013). There is a number of pharmacological agents that allosterically activate SIRT1 and delay aging, including resveratrol and SRT2014 (Baur et al. 2006; Howitz, et al. 2003; Mercken, et al. 2014; Milne, et al. 2007; Sinclair and Guarente 2014). Notably, resveratrol increased lifespan in mice fed a high fat diet but not in mice on standard chow where only health benefits were observed (Baur et al. 2006; Pearson, et al. 2008). This suggests that activation of the SIRT1 pathway may have its greatest effect on aging where there is high energy intake and greatest inhibition of SIRT activity.
Insulin/IGF-1
Lower levels of insulin and IGF-1 induced by CR or low P:C diets are associated with improved health and increased lifespan across taxa including humans (Fontana et al. 2010; Levine et al. 2014; Miller et al. 2011). Mice with mutations along the growth hormone (GH)-IGF-1-insulin pathway have been shown to be long-lived (Flurkey, et al. 2001; Hsieh, et al. 2002) and low IGF-1 levels in humans can predict survival in people with exceptional longevity (Milman, et al. 2014). The balance of macronutrients, namely low P:C, reduces insulin levels and HOMA in mice (Solon-Biet et al. 2014) supporting findings that inhibiting this pathway through diet is important for healthspan and lifespan extension. Moderating insulin secretion either by diet or administration of Metformin can reduce insulin/IGF-1 signaling via activation of AMPK (McCarty 2004), facilitating glucose uptake into the cell, reducing glucose, insulin, and IGF-1 levels, leading to the prevention, or even reversal, of insulin resistance (Minor, et al. 2010).
FGF21: an emerging key regulator?
A recent potential addition to these four classical nutrient sensing pathways is Fibroblast Growth Factor 21 (FGF21), which is emerging as an endocrine signal associated with metabolic control. It is increased in response to acute starvation but also in the obese/diabetic condition, with a recent study showing that low protein intake is the major stimulant for its expression in liver and subsequent increase in the circulation (Laeger, et al. 2014). FGF21 regulates several metabolic functions (gluconeogenesis, mitochondrial activity, ketogenesis, lipid metabolism, energy expenditure) which would be expected to be beneficial for age-related health. Similar effects have been reported in response to dietary methionine restriction (Lees, et al. 2014; Stone, et al. 2014). Although circulating FGF21 derives primarily from liver, it is also expressed in other metabolically important tissues, including white and brown adipose tissue, skeletal muscle, heart and pancreas (exocrine and β cells). Such a pattern of expression is indicative of a role for this hormone in metabolic control. Just as for the four other nutrient sensing pathways discussed above are highly interconnected, FGF21 too, plays a communicated role in nutrient signaling and has been shown to activate AMPK and SIRT1 (Chau, et al. 2010), suggesting a role for FGF21 in linking nutrition and aging.
Outlook
This review has focused primarily on the relationships between calories and macronutrients and their effects on health and aging. Although both CR and macronutrient balance have profound impacts on health and lifespan, it is important to note that other dietary regimens such as intermittent fasting and time-restricted feeding, also have beneficial effects in both mice and humans (Fontana and Partridge 2015; Mattson, et al. 2014). The fact that results of Solon-Biet et al (2014) show that limiting energy intake by dilution under ad libitum conditions has no benefit, yet under CR protocols it does, must suggest that it is not just the restriction per se that matters, but also the timing of intake (Simpson et al. 2015). Exactly how the complex network of nutrient signaling pathways interact to mediate the effects of various feeding regimens remains to be investigated. Although considerable research has gone into understanding these underlying mechanisms, none have yet studied it as a function of multiple nutrient dimensions. As highlighted in a recent review (Simpson et al. 2015), different nutritional interventions will have different effects on these pathways and understanding exactly how multiple nutrient dimensions affect these pathways can only be done using a framework that integrates these components simultaneously. The Geometric Framework is such a tool. Exactly how calories and macronutrients, and the interplay of both, influence these pathways is a fundamental question to resolve. A better understanding can have important implications for diet management, disease prevention and pharmaceutical interventions.
Acknowledgments
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Footnotes
Declaration of interest
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
REFERENCES
- Allard JS, Perez E, Zou S, de Cabo R. Dietary activators of Sirt1. Molecular and Cellular Endocrinology. 2009;299:58–63. doi: 10.1016/j.mce.2008.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. Journal of Biological Chemistry. 2003;278:29655–29660. doi: 10.1074/jbc.M212770200. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, et al. SirT1 Regulates Energy Metabolism and Response to Caloric Restriction in Mice. Plos One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metabolism. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: Understanding longevity. Nature. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Bruce KD, Hoxha S, Carvalho GB, Yamada R, Wang H-D, Karayan P, He S, Brummel T, Kapahi P, Ja WW. High carbohydrate-low protein consumption maximizes Drosophila lifespan. Experimental Gerontology. 2013;48:1129–1135. doi: 10.1016/j.exger.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkewitz K, Morantte I, Weir HJ, Yeo R, Zhang Y, Huynh FK, Ilkayeva OR, Hirschey MD, Grant AR, Mair WB. Neuronal CRTC-1 governs systemic mitochondrial metabolism and lifespan via a catecholamine signal. Cell. 2015;160:842–855. doi: 10.1016/j.cell.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Auwerx J. Calorie Restriction: Is AMPK a Key Sensor and Effector? Physiology. 2011;26:214–224. doi: 10.1152/physiol.00010.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantranupong L, Wolfson Rachel L, Sabatini David M. Nutrient-Sensing Mechanisms across Evolution. Cell. 2015;161:67–83. doi: 10.1016/j.cell.2015.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chau MD, Gao J, Yang Q, Wu Z, Gromada J. Fibroblast growth factor 21 regulates energy metabolism by activating the AMPK-SIRT1-PGC-1alpha pathway. Proceedings of the National Academy of Sciences. 2010;107:12553–12558. doi: 10.1073/pnas.1006962107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin S-J, Cheng H-L, Alt FW, Guarente L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes & Development. 2008;22:1753–1757. doi: 10.1101/gad.1650608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotechuang N, Azzout-Marniche D, Bos C, Chaumontet C, Gausseres N, Steiler T, Gaudichon C, Tome D. mTOR, AMPK, and GCN2 coordinate the adaptation of hepatic energy metabolic pathways in response to protein intake in the rat. American Journal of Physiology. Endocrinology and Metabolism. 2009;297:E1313–1323. doi: 10.1152/ajpendo.91000.2008. [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, et al. Caloric Restriction Delays Disease Onset and Mortality in Rhesus Monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Kemnitz JW, Johnson SC, Weindruch R, Anderson RM. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nature Communications. 2014;5 doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, et al. Branched-Chain Amino Acid Supplementation Promotes Survival and Supports Cardiac and Skeletal Muscle Mitochondrial Biogenesis in Middle-Aged Mice. Cell Metabolism. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Dagon Y, Avraham Y, Berry EM. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2Œ± in adipocytes. Biochemical and Biophysical Research Communications. 2006;340:43–47. doi: 10.1016/j.bbrc.2005.11.159. [DOI] [PubMed] [Google Scholar]
- de Cabo R, Le Couteur DG. The Biology of Aging. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J, editors. Harrison’s Principles of Internal Medicine. edn 19 McGraw-Hill Professional; 2015. [Google Scholar]
- Dussutour A, Simpson SJ. Communal Nutrition in Ants. Current Biology. 2009;19:740–744. doi: 10.1016/j.cub.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature. 2015;517:302–310. doi: 10.1038/nature14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanson BG, Weldon CW, Pérez-Staples D, Simpson SJ, Taylor PW. Nutrients, not caloric restriction, extend lifespan in Queensland fruit flies (Bactrocera tryoni) Aging Cell. 2009;8:514–523. doi: 10.1111/j.1474-9726.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- Festing MF, Blackmore DK. Life span of specified-pathogen-free (MRC category 4) mice and rats. Laboratory Animals. 1971;5:179–192. doi: 10.1258/002367771781006564. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proceedings of the National Academy of Sciences. 2001;98:6736–6741. doi: 10.1073/pnas.111158898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Kennedy BK, Longo VD, Seals D, Melov S. Medical research: treat ageing. Nature. 2014;511:405–407. doi: 10.1038/511405a. [DOI] [PubMed] [Google Scholar]
- Fontana L, Partridge L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell. 2015;161:106–118. doi: 10.1016/j.cell.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Weiss EP, Villareal DT, Klein S, Holloszy JO. Long-term effects of calorie or protein restriction on serum IGF-1 and IGFBP-3 concentration in humans. Aging cell. 2008;7:681–687. doi: 10.1111/j.1474-9726.2008.00417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochemical and Biophysical Research Communications. 2000;273:793–798. doi: 10.1006/bbrc.2000.3000. [DOI] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1alpha. The Embo Journal. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature. 2009;462:1061–1064. doi: 10.1038/nature08619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente L. Sirtuins as potential targets for metabolic syndrome. Nature. 2006;444:868–874. doi: 10.1038/nature05486. [DOI] [PubMed] [Google Scholar]
- Guarente L, Kenyon C. Genetic pathways that regulate ageing in model organisms. Nature. 2000;408:255–262. doi: 10.1038/35041700. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Journal of the American Medical Association. 2006;295:1539–1548. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hine C, Harputlugil E, Zhang Y, Ruckenstuhl C, Lee BC, Brace L, Longchamp A, Trevino-Villarreal JH, Mejia P, Ozaki CK, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hsieh C-C, DeFord JH, Flurkey K, Harrison DE, Papaconstantinou J. Effects of the Pit1 mutation on the insulin signaling pathway: implications on the longevity of the long-lived Snell dwarf mouse. Mechanisms of Ageing and Development. 2002;123:1245–1255. doi: 10.1016/s0047-6374(02)00037-4. [DOI] [PubMed] [Google Scholar]
- Hubbard BP, Sinclair DA. Small molecule SIRT1 activators for the treatment of aging and age-related diseases. Trends in Pharmacological Sciences. 2014;35:146–154. doi: 10.1016/j.tips.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram DK, Anson RM, De Cabo R, Mamczarz J, Zhu MIN, Mattison J, Lane MA, Roth GS. Development of Calorie Restriction Mimetics as a Prolongevity Strategy. Annals of the New York Academy of Sciences. 2004;1019:412–423. doi: 10.1196/annals.1297.074. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Culter RG, Weindruch R, Renquist DM, Knapka JJ, April M, Belcher CT, Clark MA, Hatcherson CD, Marriott BM, et al. Dietary restriction and aging: the initiation of a primate study. Journals of Gerontology Series A: Biologcial and Medical Sciences. 1990;45:B148–163. doi: 10.1093/geronj/45.5.b148. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Roth GS. Calorie restriction mimetics: Can you have your cake and eat it, too? Ageing Research Reviews. 2015;20C:46–62. doi: 10.1016/j.arr.2014.11.005. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, R√ºegg MA, Hall A, Hall MN. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nature Cell Biology. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- Jensen K, McClure C, Priest NK, Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell. 2015 doi: 10.1111/acel.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Powers RW, 3rd, Steffen KK, Westman EA, Hu D, Dang N, Kerr EO, Kirkland KT, Fields S, Kennedy BK. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196. doi: 10.1126/science.1115535. [DOI] [PubMed] [Google Scholar]
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW-L, Thomas EL, Kockel L. With TOR, Less Is More: A Key Role for the Conserved Nutrient-Sensing TOR Pathway in Aging. Cell Metabolism. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366:461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, et al. FGF21 is an endocrine signal of protein restriction. Journal of Clinical Investigation. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. Journals of Gerontology Series A: Biologcial and Medical Sciences. 2012;67:168–174. doi: 10.1093/gerona/glr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Couteur DG, Tay SS, Solon-Biet SM, Bertolino P, McMahon AC, Cogger VC, Pichaud N, Horan M, Correa C, Melvin RG, et al. The Influence of Macronutrients on Splanchnic and Hepatic Lymphocytes in Aging Mice. Journals of Gerontology Series A: Biologcial and Medical Sciences. 2014:1–9. doi: 10.1093/gerona/glu196. [DOI] [PubMed] [Google Scholar]
- Lee KP, Simpson SJ, Clissold FJ, Brooks R, Ballard JW, Taylor PW, Soran N, Raubenheimer D. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proceedings of the National Academy of Sciences. 2008;105:2498–2503. doi: 10.1073/pnas.0710787105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees EK, Krol E, Grant L, Shearer K, Wyse C, Moncur E, Bykowska AS, Mody N, Gettys TW, Delibegovic M. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell. 2014;13:817–827. doi: 10.1111/acel.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine Morgan E, Suarez Jorge A, Brandhorst S, Balasubramanian P, Cheng C-W, Madia F, Fontana L, Mirisola Mario G, Guevara-Aguirre J, Wan J, et al. Low Protein Intake Is Associated with a Major Reduction in IGF-1, Cancer, and Overall Mortality in the 65 and Younger but Not Older Population. Cell Metabolism. 2014;19:407–417. doi: 10.1016/j.cmet.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao C-Y, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell. 2010;9:92–95. doi: 10.1111/j.1474-9726.2009.00533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S-J, Defossez P-A, Guarente L. Requirement of NAD and SIR2 for Life-Span Extension by Calorie Restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Longo VD, Kennedy BK. Sirtuins in Aging and Age-Related Disease. Cell. 2006;126:257–268. doi: 10.1016/j.cell.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Mair W, Morantte I, Rodrigues AP, Manning G, Montminy M, Shaw RJ, Dillin A. Lifespan extension induced by AMPK and calcineurin is mediated by CRTC-1 and CREB. Nature. 2011;470:404–408. doi: 10.1038/nature09706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mair W, Piper MDW, Partridge L. Calories Do Not Explain Extension of Life Span by Dietary Restriction in Drosophila. PLoS Biology. 2005;3:e223. doi: 10.1371/journal.pbio.0030223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maklakov AA, Simpson SJ, Zajitschek F, Hall MD, Dessmann J, Clissold F, Raubenheimer D, Bonduriansky R, Brooks RC. Sex-specific fitness effects of nutrient intake on reproduction and lifespan. Current Biology. 2008;18:1062–1066. doi: 10.1016/j.cub.2008.06.059. [DOI] [PubMed] [Google Scholar]
- Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, et al. Metformin improves healthspan and lifespan in mice. Nature Communications. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoro EJ. Use of rodents as models for the study of “normal aging”: conceptual and practical issues. Neurobiology of Aging. 1991;12:639–643. doi: 10.1016/0197-4580(91)90114-y. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric restriction and aging: an update. Experimental Gerontology. 2000;35:299–305. doi: 10.1016/s0531-5565(00)00084-x. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Subfield History: Caloric Restriction, Slowing Aging, and Extending Life. Science of Aging Knowledge Environment. 2003;2003:re2. doi: 10.1126/sageke.2003.8.re2. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Overview of caloric restriction and ageing. Mechanisms of Ageing and Development. 2005;126:913–922. doi: 10.1016/j.mad.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, Yu BP, Bertrand HA. Action of food restriction in delaying the aging process. Proceedings of the National Academy of Sciences. 1982;79:4239–4241. doi: 10.1073/pnas.79.13.4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattison JA, Lane MA, Roth GS, Ingram DK. Calorie restriction in rhesus monkeys. Experimental Gerontology. 2003;38:35–46. doi: 10.1016/s0531-5565(02)00146-8. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Roth GS, Beasley TM, Tilmont EM, Handy AM, Herbert RL, Longo DL, Allison DB, Young JE, Bryant M, et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature. 2012;489:318–321. doi: 10.1038/nature11432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FAJL, et al. Meal frequency and timing in health and disease. Proceedings of the National Academy of Sciences. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty MF. Chronic activation of AMP-activated kinase as a strategy for slowing aging. Medical Hypotheses. 2004;63:334–339. doi: 10.1016/j.mehy.2004.01.043. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowell MF, Maynard LA. The Effect of Retarded Growth Upon the Length of Life Span and Upon the Ultimate Body Size. The Journal of Nutrition. 1935;10:63–79. [PubMed] [Google Scholar]
- McCay CM, Dilley WE, Crowell MF. Growth Rates of Brook Trout Reared upon Purified Rations, upon Dry Skim Milk Diets, and upon Feed Combinations of Cereal Grains. The Journal of Nutrition. 1929;1:233–246. [Google Scholar]
- Mercken EM, Hu J, Krzysik-Walker S, Wei M, Li Y, McBurney MW, de Cabo R, Longo VD. SIRT1 but not its increased expression is essential for lifespan extension in caloric restricted mice. Aging Cell. 2013 doi: 10.1111/acel.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercken EM, Mitchell SJ, Martin-Montalvo A, Minor RK, Almeida M, Gomes AP, Scheibye-Knudsen M, Palacios HH, Licata JJ, Zhang YQ, et al. SRT2104 extends survival of male mice on a standard diet and preserves bone and muscle mass. Aging Cell. 2014;13:787–796. doi: 10.1111/acel.12220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell. 2005;4:119–125. doi: 10.1111/j.1474-9726.2005.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. Journals of Gerontology Series A: Biologcial and Medical Sciences. 2011;66:191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milman S, Atzmon G, Huffman DM, Wan J, Crandall JP, Cohen P, Barzilai N. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13:769–771. doi: 10.1111/acel.12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Allard JS, Younts CM, Ward TM, de Cabo R. Dietary Interventions to Extend Life Span and Health Span Based on Calorie Restriction. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2010;65A:695–703. doi: 10.1093/gerona/glq042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell Sarah J, Martin-Montalvo A, Mercken Evi M, Palacios Hector H, Ward Theresa M, Abulwerdi G, Minor Robin K, Vlasuk George P, Ellis James L, Sinclair David A, et al. The SIRT1 Activator SRT1720 Extends Lifespan and Improves Health of Mice Fed a Standard Diet. Cell Reports. 2014;6:836–843. doi: 10.1016/j.celrep.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Lagisz M, Hector KL, spencer HG. Comparative and meta-analytic insights into life-extension via dietary restriction. Aging Cell. 2012;11:401–409. doi: 10.1111/j.1474-9726.2012.00798.x. [DOI] [PubMed] [Google Scholar]
- Newgard CB. Interplay between lipids and branched-chain amino acids in development of insulin resistance. Cell Metabolism. 2012;15:606–614. doi: 10.1016/j.cmet.2012.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. Journal of Nutrition. 1993;123:269–274. doi: 10.1093/jn/123.2.269. [DOI] [PubMed] [Google Scholar]
- Pamplona R, Barja G. Mitochondrial oxidative stress, aging and caloric restriction: the protein and methionine connection. Biochimica et Biophysica Acta. 2006;1757:496–508. doi: 10.1016/j.bbabio.2006.01.009. [DOI] [PubMed] [Google Scholar]
- Paoli P, Wakeling L, Wright G, Ford D. The dietary proportion of essential amino acids and Sir2 influence lifespan in the honeybee. AGE. 2014;36:1239–1247. doi: 10.1007/s11357-014-9649-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. Intervening in ageing to prevent the diseases of ageing. Trends in Endocrinology and Metabolism. 2014;25:555–557. doi: 10.1016/j.tem.2014.08.003. [DOI] [PubMed] [Google Scholar]
- Partridge L, Piper MD, Mair W. Dietary restriction in Drosophila. Mechanisms of Ageing and Development. 2005;126:938–950. doi: 10.1016/j.mad.2005.03.023. [DOI] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metabolism. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MD, Partridge L, Raubenheimer D, Simpson SJ. Dietary restriction and aging: a unifying perspective. Cell Metabolism. 2011;14:154–160. doi: 10.1016/j.cmet.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MDW, Mair W, Partridge L. Counting the Calories: The Role of Specific Nutrients in Extension of Life Span by Food Restriction. Journals of Gerontology Series A: Biologcial and Medical Sciences. 2005;60:549–555. doi: 10.1093/gerona/60.5.549. [DOI] [PubMed] [Google Scholar]
- Powers RW, 3rd, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes and Development. 2006;20:174–184. doi: 10.1101/gad.1381406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresel TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Experimental Gerontology. 2000;35:1131–1149. doi: 10.1016/s0531-5565(00)00166-2. [DOI] [PubMed] [Google Scholar]
- Richie J, Jr, Leutzinger Y, Parthasarathy S, Malloy V, Orentreich N, Zimmerman J. Methionine restriction increases blood glutathione and longevity in F344 rats. Federation of American Societies for Experimental Biology. 1994;8:1302–1307. doi: 10.1096/fasebj.8.15.8001743. [DOI] [PubMed] [Google Scholar]
- Robertson LT, Trevino-Villarreal JH, Mejia P, Grondin Y, Harputlugil E, Hine C, Vargas D, Zheng H, Ozaki CK, Kristal BS, et al. Protein and calorie restriction contribute additively to protection from renal ischemia reperfusion injury partly via leptin reduction in male mice. Journal of Nutrition. 2015 doi: 10.3945/jn.114.199380. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Shinmura K, Tamaki K, Saito K, Nakano Y, Tobe T, Bolli R. Cardioprotective Effects of Short-Term Caloric Restriction Are Mediated by Adiponectin via Activation of AMP-Activated Protein Kinase. Circulation. 2007;116:2809–2817. doi: 10.1161/CIRCULATIONAHA.107.725697. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Le Couteur DG, Raubenheimer D. Putting the balance back in diet. Cell Metabolism. 2015;161:18–23. doi: 10.1016/j.cell.2015.02.033. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Caloric Restriction and Aging Revisited: The Need for a Geometric Analysis of the Nutritional Bases of Aging. Journals of Gerontology Series A: Biologcial and Medical Sciences. 2007;62:707–713. doi: 10.1093/gerona/62.7.707. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. Macronutrient balance and lifespan. Aging. 2009;1:875–880. doi: 10.18632/aging.100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson SJ, Raubenheimer D. The nature of nutrition. A unifying framework form animal adaption to human obesity. Princeton University Press; Princeton: 2012. [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mechanisms of Ageing and Development. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. Small-Molecule Allosteric Activators of Sirtuins. Annua Review of Pharmacology and Toxicology. 2014;54:363–380. doi: 10.1146/annurev-pharmtox-010611-134657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet Samantha M, McMahon Aisling C, Ballard JWilliam O, Ruohonen K, Wu Lindsay E, Cogger Victoria C, Warren A, Huang X, Pichaud N, Melvin Richard G, et al. The Ratio of Macronutrients, Not Caloric Intake, Dictates Cardiometabolic Health, Aging, and Longevity in Ad Libitum-Fed Mice. Cell Metabolism. 2014;19:418–430. doi: 10.1016/j.cmet.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Mitchell SJ, Coogan SCP, Cogger VC, Gokarn R, McMahon AC, Raubenheimer D, De Cabo R, Simpson SJ, Le Couteur DG. Dietary protein to carbohydrate ratio and caloric restriction: comparing metabolic outcomes in mice. Cell Reports. 2015a doi: 10.1016/j.celrep.2015.05.007. in press http://dx.doi.org/10.1016/j.celrep.2015.1005.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon-Biet SM, Walters KA, Simanainen UK, McMahon AC, Ruohonen K, Ballard JWO, Raubenheimer D, Handelsman DJ, Le Couteur DG, Simpson SJ. Macronutrient balance, reproductive function, and lifespan in aging mice. Proceedings of the National Academy of Sciences. 2015b;112:3481–3486. doi: 10.1073/pnas.1422041112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Molecular Aspects of Medicine. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochimica et Biophysica Acta. 2009;1790:1067–1074. doi: 10.1016/j.bbagen.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone KP, Wanders D, Orgeron M, Cortez CC, Gettys TW. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes. 2014;63:3721–3733. doi: 10.2337/db14-0464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 Improves Insulin Sensitivity under Insulin-Resistant Conditions by Repressing PTP1B. Cell Metabolism. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Sun L, Sadighi Akha AA, Miller RA, Harper JM. Life-Span Extension in Mice by Preweaning Food Restriction and by Methionine Restriction in Middle Age. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A:711–722. doi: 10.1093/gerona/glp051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taormina G, Mirisola MG. Calorie restriction in mammals and simple model organisms. Biomed Research International. 2014;2014:308690. doi: 10.1155/2014/308690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends in Endocrinology and Metabolism. 2014 doi: 10.1016/j.tem.2014.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tato I, Bartrons R, Ventura F, Rosa JL. Amino Acids Activate Mammalian Target of Rapamycin Complex 2 (mTORC2) via PI3K/Akt Signaling. Journal of Biological Chemistry. 2011;286:6128–6142. doi: 10.1074/jbc.M110.166991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio A, D’Antona G, Nisoli E. Branched-chain amino acids, mitochondrial biogenesis, and healthspan: an evolutionary perspective. Aging. 2011;3:464–478. doi: 10.18632/aging.100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proceedings of the National Academy of Sciences. 1992;89:11533–11537. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Proud CG. Nutrient control of TORC1, a cell-cycle regulator. Trends in Cell Biology. 2009;19:260–267. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The Retardation of Aging in Mice by Dietary Restriction: Longevity, Cancer, Immunity and Lifetime Energy Intake. Journal of Nutrition. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Wilder SM, Le Couteur DG, Simpson SJ. Diet mediates the relationship between longevity and reproduction in mammals. Age. 2012;35:921–927. doi: 10.1007/s11357-011-9380-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf NS, Penn PE, Jiang D, Fei RG, Pendergrass WR. Caloric Restriction: Conservation of in Vivo Cellular Replicative Capacity Accompanies Life-Span Extension in Mice. Experimental Cell Research. 1995;217:317–323. doi: 10.1006/excr.1995.1092. [DOI] [PubMed] [Google Scholar]
- Yuan H-X, Xiong Y, Guan K-L. Nutrient Sensing, Metabolism, and Cell Growth Control. Molecular Cell. 2013;49:379–387. doi: 10.1016/j.molcel.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan R, Tsaih S-W, Petkova SB, De Evsikova CM, Xing S, Marion MA, Bogue MA, Mills KD, Peters LL, Bult CJ, et al. Aging in inbred strains of mice: study design and interim report on median lifespans and circulating IGF1 levels. Aging Cell. 2009;8:277–287. doi: 10.1111/j.1474-9726.2009.00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman JA, Malloy V, Krajcik R, Orentreich N. Nutritional control of aging. Experimental Gerontology. 2003;38:47–52. doi: 10.1016/s0531-5565(02)00149-3. [DOI] [PubMed] [Google Scholar]