Abstract
Human papilloma virus (HPV) infection causes cancers and their precursors (high grade squamous intraepithelial lesions) near cervical and anal squamocolumnar junctions. Recently described cervical squamocolumnar junctions cells are putative residual embryonic cells near the cervical transformation zone. These cells appear multipotential and share an identical immunophenotype (strongly CK7-positive) with over 90% of high grade squamous intraepithelial lesions and cervical carcinomas. However, because the number of new cervical cancers discovered yearly world-wide is 17-fold that of anal cancer, we posed the hypothesis that this difference in cancer risk reflects differences in the transition zones at the two sites. The microanatomy of the normal anal transformation zone (n = 37) and topography and immunophenotype of anal squamous neoplasms (n = 97) were studied. A discrete anal transition zone was composed of multi-layered CK7-positive/p63-negative superficial columnar cells and an uninterrupted layer of CK7-negative/p63-positive basal cells. The CK7-negative/p63-positive basal cells were continuous with – and identical in appearance to - the basal cells of the mature squamous epithelium. This was in contrast to the cervical squamocolumnar junction, that harbored a single-layered CK7-positive/p63-negative squamocolumnar junction cell population. Of the 97 Anal intraepithelial neoplasia/squamous cell carcinomas evaluated, only 27% (26/97) appeared to originate near the anal transition zone and only 23% (22/97) were CK7-positive. This study thus reveals two fundamental differences between the anus and cervix: 1) the anal transition zone does not harbor a single monolayer of residual un-differentiated embryonic cells and 2) the dominant tumor immuno-phenotype is in keeping with an origin in metaplastic (CK7-negative) squamous rather than squamocolumnar junction (CK7-positive) epithelium. The implication is that at birth, the embryonic cells in the anal transition zone have already begun to differentiate, presenting a less vulnerable squamous metaplasia that - like vaginal and vulvar epithelium - is less prone to HPV directed carcinogenesis. This in turn underscores the link between cancer risk and a very small and discrete population of vulnerable squamocolumnar junction cells in the cervix.
Introduction
Human papilloma virus (HPV) infection causes cervical cancer and its precursor lesions (HSIL), specifically at the squamocolumnar junction (squamocolumnar junction) near the transformation zone (1–4). For decades, this topographical preference for cervical neoplasia has remained unexplained. However, in 2011 a population of residual embryonic cells was discovered at the gastro-esophageal squamocolumnar junction that was linked to Barrett’s metaplasia (5). A subsequent study revealed a nearly identical population at the cervical squamo-columnar junction and these cells were found to share an identical immuno-phenotype (including strong staining for CK7) with over 90% of high grade squamous intraepithelial lesions and carcinomas (6). These shared properties coupled with the physical juxtaposition of squamocolumnar junction cells and cervical neoplasia support a carcinogenic sequence that initiates in the cervical squamocolumnar junction cells. In contrast, the mature metaplastic cervical epithelium, and mature squamous epithelium of the ectocervix, vulva and vagina are squamocolumnar junction marker-negative, implying carcinogenic HPV infection of non-squamocolumnar junction type basal keratinocytes. This disparity in target cell of origin between tumors in the two regions (cervical squamocolumnar junction versus lower genital tract squamous epithelium) has been postulated to explain why the number of new cervical cancers yearly world-wide (~500,000) is nearly 20-fold that of vulvo-vaginal carcinomas (~25,000) (6,7).
The anorectal junction is presumably another squamocolumnar junction similar to the cervix, where the squamous epithelium joins with the rectal mucosa at an “anal transition zone”. Epidemiological and molecular studies have shown that HPV is the causative agent of most anal carcinomas, with an estimated population attributable fraction of 88% (8–13). It is noteworthy that HPV DNA is detected at least as frequently in the anus as in the cervix (14–16). Furthermore, a history of receptive anal intercourse is not a significant risk factor for anal HPV infection in women (14,15). Finally, based on the rate of reported anal cancers in the world each year (~28,000) the cervical/anal cancer ratio is about 17:1(7). Thus, despite the high rates of anal HPV exposure there is an inexplicably low rate of anal cancer in the world relative to the cervix.
Because the incidence rate of anal cancer was so similar to that of the vulva/vagina we hypothesized that a study of the microanatomy of the cervical and anorectal transition zones might reveal differences to explain their disparate susceptibilities to HPV-related carcinogenesis. The purpose of this study was to 1) characterize the cellular phenotypes in the adult and fetal anal canals, 2) survey a spectrum of anal squamous lesions (low/high grade squamous intraepithelial lesion, squamous cell carcinoma) for squamocolumnar junction marker expression, and 3) compare the findings to the cervical squamocolumnar junction.
Materials and Methods
Rationale, Case Material and Tissue Classification
The surgical anal canal is composed of 3 discrete epithelial zones (Figure 1A): 1) colorectal zone (uninterrupted colonic mucosa), 2) anal transitional zone (mixed epithelial type) and 3) squamous zone (uninterrupted squamous epithelium that merges into the perianal skin) (17). "Normal" anal canal tissue from adults (6 abdominoperineal resections, 26 hemorrhoidectomy) and fetuses (5 fetopsies/fetal abortal examinations less than 24 weeks gestational age and considered surgical specimens; gestational age ranging from 14–22 weeks, estimated by foot length), in addition to neoplastic anal canal tissue (low grade squamous intraepithelial lesion, n = 42; HSIL, n = 36; squamous cell carcinoma, n = 19) were retrieved from the archives of Brigham and Women's Hospital (Boston, MA) and University of Arkansas Medical Center (Little Rock, AR) with the approval of the Institutional Review Board at the respective institutions. All slides were reviewed (E.Y.) and categorized into the 3 zones of the anal canal as described above. Discrepancies in interpretation were resolved by consensus review (E.Y. and C.P.C).
Figure 1.
Microanatomy of the anal transition zone. A) Schematic of the surgical anal canal. B) Representative fetal anal canal with the squamous, transitional, and colorectal zones and their staining patterns with CK7 and p63. C) Representative adult anal canal with the squamous and transitional zones and their staining patterns with CK7 and p63. The adult colorectal zone is depicted in Supplemental Figure 2.
Immunohistochemistry
Immunohistochemistry analyses were performed as previously described (5,6,18) and as routinely performed at Brigham and Women’s Hospital, Department of Pathology. The following primary antibodies were used: CK7 (clone RCK105; Thermo Scientific, Waltham, MA; used at concentration _____), p63 (Dako Corp), p16ink4 (Santa Cruz Biotechnology, Santa Cruz, CA), integrin alpha 6 (Abcam, San Francisco, CA; not routinely used for surgical pathology practice at Brigham and Women’s Hospital). Mouse and rabbit control IgG (Santa Cruz Biotechnology) were used as negative controls. CK7, p63, and p16 antibodies are
Results
Microanatomy of the Anal Transition Zone
A total of 37 anal canals (32 adults and 5 fetuses) were evaluated. There was a discrete anal transitional zone (anal transition zone) epithelium that merged into the colorectal zone proximally and the squamous zone/perianal skin distally. This transitional epithelium was composed of multi-layered columnar to cuboidal cells (Figure 1B/C, top row; ranging from one to nine layers) at the surface, with variable mucin production (scant to rare goblet cell metaplasia). Below this was a population of small basal cells that created an uninterrupted, 1–2 cell layer at the stromal interface. Anal glands with epithelium similar to the anal transition zone-type were present within the stroma. In the adult anal transition zone, there were occasional islands of squamous metaplastic epithelium (Supplemental Figure 1A/B) and scattered crypts of colorectal type. These epithelial heterogeneities (e.g. squamous, colorectal-type cells) were relatively rare in the fetal anal transition zone (one squamous metaplastic island in a 22 week gestational age fetopsy; Supplemental Figure 1C/D); however the microanatomy of the adult and fetal anal canal (regardless of gestational age) was otherwise remarkably similar. The fetal anal transition zone length correlated with gestational age, increasing in length from 95 cells (14 week gestational age) to 900 cells (22 week gestational age); (mean = 349 ± 329; n = 5). The length of the adult anal transition zone was difficult to evaluate because of frequent mucosal irregularities (e.g. mucosal ulceration, squamous metaplasia vs. true squamous zone epithelium, etc.); however, overall, the adult anal transition zone length varied greatly, ranging from abrupt transition (direct squamous to colorectal epithelial transition; n = 4), up to approximately 700 cell-lengths in a longitudinal section of an intact abdominoperineal resection specimen.
The cervical and gastroesophageal squamocolumnar junctions contain residual embryonic cells that are implicated in transition-zone specific carcinomas and precursor lesions (i.e. squamous cell carcinoma/high grade squamous intraepithelial lesion in the cervix and esophageal carcinoma/Barrett’s esophagus at the gastroesophageal junction (5,6,19,20)). These cells strongly express CK7, which is not or weakly expressed in the adjacent squamous and columnar epithelium respectively. Given that the bladder (with known CK7 expression) and anus share a common embryonic origin (10), we predicted that the anal transition zone epithelium would also be CK7 positive. The multilayered surface epithelium of the anal transition zone (present in 88%; 28 of 32 specimens) stained strongly with CK7 (Adult 100%, 28/28; Fetal 100%, 5/5; Figure 1B/C and Table 1) but was negative for p63 (0%, 0/28). Scattered CK7-positive anal transition zone cells extended into the adjacent colorectal epithelium and over the surface of the stratified squamous epithelium; however, cells of the squamous and colorectal epithelium did not express CK7 (Figure 1, Supplemental Figure 2). The basal cells underlying the surface anal transition zone cells were p63-positive, CK7-negative and were present as an uninterrupted layer at the stromal interface in all examined anal canals (100%, 28/28; Figure 1B/C, bottom row and Table 1). This basal cell population emerged at the colorectal/transition zone junction and continued into the squamous/perianal zone as basal keratinocytes. The overall CK7/p63 staining pattern was identical in the adult and fetal anal transition zone.
Table 1.
CK7/p63 staining pattern of anal canal epithelium
| Anal Canal | Adult (n=28) | Fetus (n=5) | |||
|---|---|---|---|---|---|
| CK7 | P63 | CK7 | P63 | ||
| Squamous zone /Perianal skin | 0% (0/28) | 100% (28/28*) | 0% (0/5) | 100% (5/5*) | |
| Transitional Zone | Superficial cells | 100% (28/28) | 0% (0/28) | 100% (5/5) | 0% (0/5) |
| Basal cells | 0% (0/28) | 100% (28/28) | 0% (0/5) | 100% (5/5) | |
| Colorectal zone | 0% (0/28) | 0% (0/28) | 0% (0/5) | 0% (0/5) | |
Basal and parabasal cells only; superficial squamous cells negative
Anal intraepithelial neoplasia and Invasive squamous cell carcinoma Arising from anal transition zone vs. Squamous zone/Perianal Skin
The findings are summarized in Table 2. Ninety-seven (97) total cases of HPV-related precursor lesions (n = 78) and invasive squamous cell carcinoma (n = 19) arising in the anal canal were evaluated. The lesions were divided into two micro-anatomical categories: 1) anal transition zone (based on the presence of anal transition zone-type epithelium) and 2) squamous zone/perianal skin (based on the presence of uninterrupted squamous epithelium and/or adnexal structures). Given the above criteria, 27% (26/97) of the lesions were present within the anal transition zone and 73% (71/97) were present within the squamous zone/perianal skin. Of the lesions present in the anal transition zone, 64% (7/11) of low grade squamous intraepithelial lesions, 100% (8/8) of high grade squamous intraepithelial lesions, and 86% (6/7) of squamous cell carcinomas expressed patchy to strong/diffuse CK7 (Figure 2, top 3 rows). All CK7-positive lesions were arising from anal transition zone epithelium. The four CK7-negative low grade squamous intraepithelial lesions located at the anal transition zone were not arising from the anal transition zone epithelium but from the adjacent squamous epithelium (Supplemental Figure 3). Of the lesions present in the squamous zone/perianal skin, 0% (0/31) of low grade squamous intraepithelial lesions, 0% (0/28) of high grade squamous intraepithelial lesions, and 8% (1/12) of squamous cell carcinomas were positive for CK7 (Figure 2, bottom 3 rows). We speculate that the CK7-negative squamous cell carcinoma present at the anal transition zone and CK7-positive squamous cell carcinoma present at the squamous zone/perianal skin each originated in their respective zones (as expected by their immunophenotype), but was sampled in their nonnative zones into which they invaded/expanded.
Table 2.
CK7 expression in anal squamous lesions
| CK7 Positive |
% CK7 Positivity |
||
|---|---|---|---|
| Anal Transitional Zone | 21/26 | 80% | |
| LSIL | 7/11 | 64% | |
| HSIL | 8/8 | 100% | |
| SCC | 6/7 | 86% | |
| Squamous Zone | 1/71 | 1.4% | |
| LSIL | 0/31 | 0% | |
| HSIL | 0/28 | 0% | |
| SCC | 1/12 | 8% | |
| Total (n = 97) | 22/97 | 23% | |
Figure 2.
Anal intraepithelial neoplasia and anal squamous cell carcinoma arising from the Anal transition zone and Squamous zone/Perianal Skin. The top nine panels represent low/high grade intraepithelial lesions and squamous cell carcinoma arising from the anal transition zone and their staining patterns for CK7 and p16. The lower nine panels represent low/high grade intraepithelial lesions and squamous cell carcinoma arising from the squamous zone/perianal skin and their staining patterns for CK7 and p16.
Discussion
This study has revealed distinct differences between the anus and cervix with respect to the region where the caudal squamous epithelium undergoes transition to the more cephalad columnar epithelium. Development of this region in the anus entails the emergence of cells comprising a discrete, biphasic anal transition zone epithelium composed of CK7-positive/p63-negative multilayered superficial cells and an underlying CK7-negative/p63-positive population of basal cells. The CK7-positive/p63-negative superficial cells are multilayered with columnar to cuboidal morphology, with variable mucin production, and an apparent capacity for undergoing basal expansion to produce squamous metaplasia (as seen in adult and occasional fetal anal transition zone; Supplemental Figure 2). These cells are limited to the anal transition zone, with scattered cells present in the distal colorectal zone and along the surface of the proximal squamous zone. The CK7-negative/p63-positive basal cells compose the bottom 1–2 cell layers of the anal transition zone and are in direct contact with the basement membrane and anal stroma. These basal cells appear at the distal colorectal zone, continue uninterrupted throughout the anal transition zone and merge into the squamous zone and perianal skin as basal keratinocytes. The immune-phenotypic findings are in keeping with previous studies that have evaluated the anal canal and its keratin staining patterns (17,21–23).
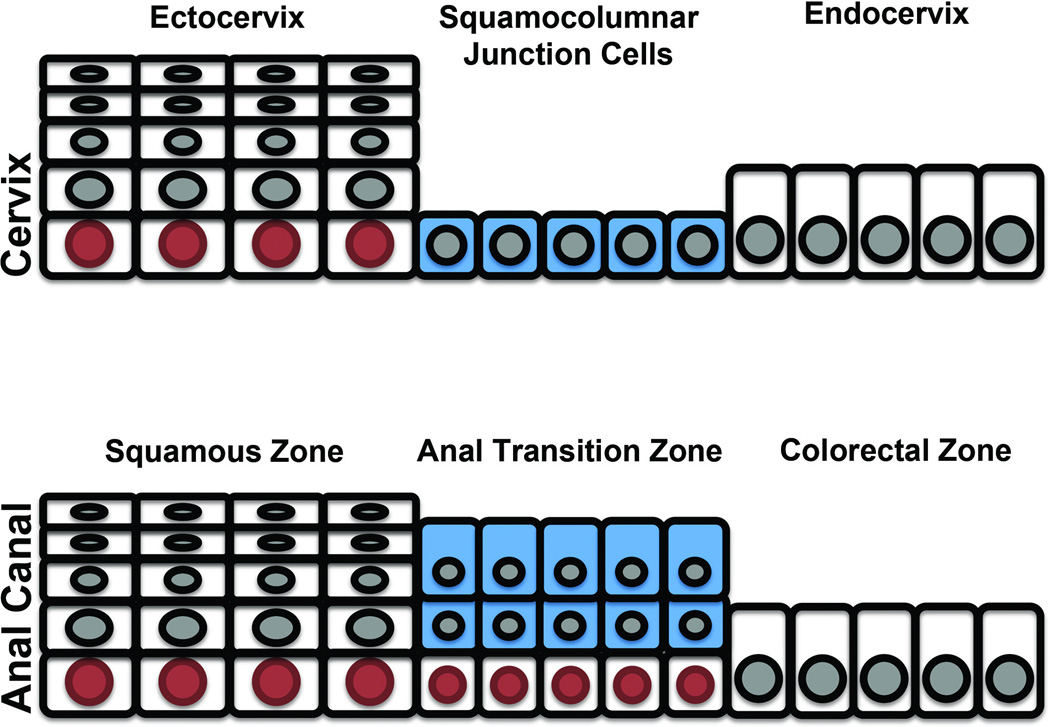
As shown in Results, the anal transition zone exhibits some similarity to the cervix transition zone. Both exhibit squamous metaplasia that appears to emerge from the CK7+ cell population. However, in distinct contrast to the cervix, the anal transition zone does not contain a monolayer of CK7+ cells (5,6,18). Unlike the anal transition zone where the CK7+ cells define zones of basal cell expansion and squamous metaplasia, the cervical squamocolumnar junction is a single cell-layered epithelium that is directly superimposed on the basement membrane. These two differentiating characteristics seen in the anal transition zone- the multilayered CK7+ cells and the obligatory underlying basal cells - are illustrated in Figure 3.
Figure 3.
Schematic representation of the Cervical squamocolumnar junction (top panel) vs. Anal transition zone (lower panel). The cervical squamocolumnar junction cells (CK7-positive) are single layered and are in direct contact with the basement membrane. The anal transition zone cells (CK7-positive) are multilayered and always have an underlying layer of p63-positive basal cells. Blue = CK7-positive junctional cells; red = p63-positive basal cells.
We propose two possible explanations for why the anal transition zone differs from the cervical transition zone. First, a wholesale proliferation of basal cells does not occur with expulsion of the CK7+ columnar cells, leaving a multilayer population capable of metaplasia without a separate solitary layer of residual embryonic cells clinging to the SC junction. (18). An alternative explanation is a collision of CK7-negative/p63-positive basal cells (migrating proximally from the squamous epithelium) with the CK7-positive/p63-negative columnar population, the latter apically displaced to form the bilayered anal transition zone epithelium; this is a developmental model analogous to the formation of the embryonic gastroesophageal junction (19). From our analysis of the fetal anal transition zone, we know that the final form of the biphasic anal transition zone is already established at 14 weeks gestational age and even earlier (9 weeks gestational age) according to previous studies (23). A detailed evaluation of the fetuses at earlier gestational ages is necessary for further insight into the mechanism of anorectal junction formation. More practically, however, the question is how these differences in microanatomy of the anal transition zone and cervical squamocolumnar junction influence HPV-related carcinogenic susceptibility.
Of the 97 anal intraepithelial neoplasia/squamous cell carcinomas evaluated, only 22 (23%) were CK7 positive, suggesting that the cells in the anal transition zone are not uniquely vulnerable to carcinogenic HPV transformation. In contrast, nearly all high grade squamous intraepithelial lesionsand squamous cell carcinomas (as well as adenocarcinomas) of the cervix express CK7 as well as other squamocolumnar junction-related biomarkers. (6,18). Two potential differences between basal keratinocytes and squamocolumnar junction cells lie in their proximity to infectious viral particles and their susceptibility to carcinogenesis. Infection of the former requires injury to the epithelium that creates access to basal cells (24). In contrast, the single-layered cervical squamocolumnar junction cells are constantly exposed, making them more physically accessible for HPV infection. In addition to viral access, HPV attachment to the cell surface is an integral step to viral infection. Injury to the squamous epithelium disrupts the integrin α6β4/laminin-5 interaction that anchors basal cells to the basement membrane. Both components are thought to mediate HPV infection: laminin-5 through the attachment and sequestration of the virus at the basement membrane (24) and integrin α6β4 as the putative HPV receptor on the cell surface (25,26). By virtue of the cervical squamocolumnar junction cells having direct contact with the basement membrane, we would expect these cells to express integrins that anchor them to the basement membrane and coincidentally make them more susceptible to HPV infection. Indeed, our preliminary data show that cervical squamocolumnar junction cells and basal cells are enriched for integrin α6 expression while minimal expression is seen in anal transition zone cells and expression is lost in the superficial keratinocytes (Supplemental Figure 4). Therefore, because the cervical squamocolumnar junction cells are 1) single layered and 2) have direct contact with the BM, they may be more susceptible to HPV infection compared to anal transition zone cells. Precisely why the squamocolumnar junction monolayer would be more susceptible to carcinogenesis remains is a question requiring further study. However, target cell phenotype as a determinant of risk following HPV infection is a theme repeated throughout the multiple susceptible mucosal sites in the human genital tract and elsewhere.
In summary, the comparative microanatomy of the anal and cervical transition zones exposes distinct topographical differences that may influence cancer outcome. This observation supports the discrepancy in the incidences of cervical and anal carcinoma, despite both lesions arising at an ostensibly similar squamocolumnar junction. However, HPV infection alone does not determine the carcinogenic potential of the lesion, as evidenced by the majority of low grade squamous intraepithelial lesions that do not progress to high grade lesions or carcinoma. Further studies investigating the differences in the cellular and molecular biology of the target cells within the cervical and anal squamocolumnar junction are needed to address these additional questions.
Supplementary Material
Squamous Metaplasia in the anal transition zone A&B) Adult anal transition zone. The Squamous metaplastic epithelium is present between the anal transition zone and colorectal epithelium. C&D) Fetal anal transition zone. The squamous metaplastic epithelium is present between two segments of anal transition zone.
Adult colorectal zone. The colorectal mucosa does not express CK7 and p63.
A) H&E of low grade squamous intraepithelial lesion arising in the anal transition zone. B) CK7-negative low grade squamous intraepithelial lesion adjacent to the CK7-positive anal transition zone epithelium.
Integrin α6 expression pattern in anal transition zone and Cervical squamocolumnar junction. Anal Transition zone: the CK7-positive/p63-negative anal transition zone epithelium does not express integrin α6; the CK7-negative/p63-positive basal cells strongly express integrin α6 (top row). Cervix: immature squamous metaplasia and cervical squamocolumnar junction cells express Integrin α6. In the ectocervix, only the basal and parabasal cells express integrin α6; the mature squamous epithelium is negative for integrin α6.
References
- 1.Bosch FX, Lorincz A, Muñoz N, Meijer CJLM, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferenczy A, Franco E. Persistent human papillomavirus infection and cervical neoplasia. Lancet Oncol. 2002;3:11–16. doi: 10.1016/s1470-2045(01)00617-9. [DOI] [PubMed] [Google Scholar]
- 3.Crum CP, Ikenberg H, Richart RM, Gissman L. Human papillomavirus type 16 and early cervical neoplasia. N Engl J Med. 1984;310:880–883. doi: 10.1056/NEJM198404053101403. [DOI] [PubMed] [Google Scholar]
- 4.Marsh M. Original site of cervical carcinoma; topographical relationship of carcinoma of the cervix to the external os and to the squamocolumnar junction. Obstet Gynecol. 1956;7:444–452. [PubMed] [Google Scholar]
- 5.Wang X, Ouyang H, Yamamoto Y, Kumar PA, Wei TS, Dagher R, Vincent M, Lu X, Bellizzi AM, Ho KY, Crum CP, Xian W, McKeon F. Residual embryonic cells as precursors of a Barrett's-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herfs M, Yamamoto Y, Laury A, Wang X, Nucci MR, McLaughlin-Drubin ME, et al. A discrete population of squamocolumnar junction cells implicated in the pathogenesis of cervical cancer. Proc Natl Acad Sci U S A. 2012;109:10516–10521. doi: 10.1073/pnas.1202684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi AK. Beyond cervical cancer: burden of other HPV-related cancers among men and women. J Adolesc Health. 2010;46:S20–S26. doi: 10.1016/j.jadohealth.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 8.Frisch M, Glimelius B, van den Brule AJ, Wohlfahrt J, Meijer CJ, Walboomers JM, et al. Sexually transmitted infection as a cause of anal cancer. N Engl J Med. 1997;337:1350–1358. doi: 10.1056/NEJM199711063371904. [DOI] [PubMed] [Google Scholar]
- 9.Tilston P. Anal human papillomavirus and anal cancer. J Clin Pathol. 1997;50:625–634. doi: 10.1136/jcp.50.8.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342:792–800. doi: 10.1056/NEJM200003163421107. [DOI] [PubMed] [Google Scholar]
- 11.Clark MA, Hartley A, Geh JI. Cancer of the anal canal. Lancet Oncol. 2004;5:149–157. doi: 10.1016/S1470-2045(04)01410-X. [DOI] [PubMed] [Google Scholar]
- 12.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer J Int Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 13.Forman D, de Martel C, Lacey CJ, Soerjomataram I, Lortet-Tieulent J, Bruni L, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–F23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 14.Palefsky JM, Holly EA, Ralston ML, Da Costa M null, Greenblatt RM. Prevalence and risk factors for anal human papillomavirus infection in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J Infect Dis. 2001;183:383–391. doi: 10.1086/318071. [DOI] [PubMed] [Google Scholar]
- 15.Williams AB, Darragh TM, Vranizan K, Ochia C, Moss AR, Palefsky JM. Anal and cervical human papillomavirus infection and risk of anal and cervical epithelial abnormalities in human immunodeficiency virus-infected women. Obstet Gynecol. 1994;83:205–211. [PubMed] [Google Scholar]
- 16.Hernandez BY, McDuffie K, Zhu X, Wilkens LR, Killeen J, Kessel B, et al. Anal Human Papillomavirus Infection in Women and Its Relationship with Cervical Infection. Cancer Epidemiol Biomark Prev. 2005;14:2550–2556. doi: 10.1158/1055-9965.EPI-05-0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fenger C. The anal transitional zone. A method for macroscopic demonstration. Acta Pathol Microbiol Scand [A] 1978;86:225–230. [PubMed] [Google Scholar]
- 18.Herfs M, Vargas SO, Yamamoto Y, Howitt BE, Nucci MR, Hornick JL, et al. A novel blueprint for “top down” differentiation defines the cervical squamocolumnar junction during development, reproductive life, and neoplasia. J Pathol. 2013;229:460–468. doi: 10.1002/path.4110. [DOI] [PubMed] [Google Scholar]
- 19.Xian W, Ho KY, Crum CP, McKeon F. Cellular origin of Barrett's esophagus: controversy and therapeutic implications. Gastroenterology. 2012;142:1424–1430. doi: 10.1053/j.gastro.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odze RD. Unraveling the mystery of the gastroesophageal junction: a pathologist’s perspective. Am J Gastroenterol. 2005;100:1853–1867. doi: 10.1111/j.1572-0241.2005.50096.x. [DOI] [PubMed] [Google Scholar]
- 21.Williams GR, Talbot IC, Northover JM, Leigh IM. Keratin expression in the normal anal canal. Histopathology. 1995;26:39–44. doi: 10.1111/j.1365-2559.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 22.Williams GR, Talbot IC, Leigh IM. Keratin expression in anal carcinoma: an immunohistochemical study. Histopathology. 1997;30:443–450. doi: 10.1046/j.1365-2559.1997.5390781.x. [DOI] [PubMed] [Google Scholar]
- 23.Fritsch H, Zehm S, Illig R, Moser P, Aigner F. New insights into the development and differentiation of the human anorectal epithelia. Are there clinical consequences? Int J Colorectal Dis. 2010;25:1231–1242. doi: 10.1007/s00384-010-0986-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- 25.Letian T, Tianyu Z. Cellular receptor binding and entry of human papillomavirus. Virol J. 2010;7:2. doi: 10.1186/1743-422X-7-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang H-S, Lambert PF. Use of an in vivo animal model for assessing the role of integrin α(6)β(4) and syndecan-1 in early steps in papillomavirus infection. Virology. 2012;433:395–400. doi: 10.1016/j.virol.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Squamous Metaplasia in the anal transition zone A&B) Adult anal transition zone. The Squamous metaplastic epithelium is present between the anal transition zone and colorectal epithelium. C&D) Fetal anal transition zone. The squamous metaplastic epithelium is present between two segments of anal transition zone.
Adult colorectal zone. The colorectal mucosa does not express CK7 and p63.
A) H&E of low grade squamous intraepithelial lesion arising in the anal transition zone. B) CK7-negative low grade squamous intraepithelial lesion adjacent to the CK7-positive anal transition zone epithelium.
Integrin α6 expression pattern in anal transition zone and Cervical squamocolumnar junction. Anal Transition zone: the CK7-positive/p63-negative anal transition zone epithelium does not express integrin α6; the CK7-negative/p63-positive basal cells strongly express integrin α6 (top row). Cervix: immature squamous metaplasia and cervical squamocolumnar junction cells express Integrin α6. In the ectocervix, only the basal and parabasal cells express integrin α6; the mature squamous epithelium is negative for integrin α6.