Summary
Haemophilia A carriers have historically been thought to demonstrate normal haemostasis. However, recent data demonstrates that despite normal factor VIII, haemophilia A carriers demonstrate an increased bleeding tendency. We tested the hypothesis that obligate haemophilia carriers demonstrate an increase in bleeding symptoms. A cross sectional study was performed comparing haemophilia A carriers to normal women. Questionnaire assessment included a general bleeding questionnaire, condensed MCMDM-1VWD bleeding assessment tool and Pictorial Bleeding Assessment Chart (PBAC). Laboratory assessment included complete blood count, prothrombin time, activated partial thromboplastin time, fibrinogen activity, FVIII activity (FVIII:C), von Willebrand factor antigen level, ristocetin cofactor, platelet function analyser-100™ and ABO blood type. 44 haemophilia A carriers and 43 controls were included. Demographic features were similar. Laboratory results demonstrated a statistically significant difference only in FVIII:C (82.5 versus 134%, p value < 0.001). Carriers reported a higher number of bleeding events, and both condensed MCMDM-1 VWD bleeding scores (5 versus 1, p value < 0.001) and PBAC scores (423 versus 182.5, p value = 0.018) were significantly higher in carriers. Haemophilia A carriers exhibit increased bleeding symptoms when compared to normal women. Further studies are necessary to fully understand the bleeding phenotype in this population and optimize clinical management.
Keywords: coagulation, haemostasis, Haemophilia, factor VIII
Introduction
Haemophilia A, the result of reduced plasma factor VIII (FVIII) activity, is an X-linked recessive bleeding disorder, affecting one in 5,000 males born in the US (Lee et al, 2010). Diagnosis and severity classification of haemophilia A is based on residual FVIII activity. Given the genetic inheritance pattern, the majority of affected patients are male. Female family members are more commonly heterozygous for the gene mutation, and referred to as carriers. Haemophilia A carriers are typically identified through analysis of family pedigree and can be verified by family history and more recently via factor VIII genotype.
Historically, haemophilia carriers were thought to have a limited bleeding phenotype, as the majority have plasma factor VIII levels (0.40–0.60 IU/mL) that are considered adequate for haemostasis (Graham et al, 1986), however this notion has been challenged by recent literature. Mounting evidence supports the notion that haemophilia A carriers may exhibit an increased bleeding tendency despite FVIII activity levels within the normal laboratory range (greater than 2 standard deviations below the population mean). Haemophilia A carriers report higher rates of bleeding than predicted by their physicians (Paroskie et al, 2014), and regardless of their plasma factor levels, haemophilia A carriers may be at an increased risk of bleeding if their male relatives demonstrated a more severe bleeding phenotype and had severe disease (Miesbach et al, 2010). Furthermore, haemophilia A and B carriers were noted to have increased bleeding scores when compared to controls (Olsson et al, 2014). Finally, in a pilot study of obligate haemophilia A carriers with FVIII activities within the normal range, 65% (22 of 34) demonstrated soft tissue and/or osteochondral joint changes corroborating the findings of reduced joint range of motion in haemophilia A carriers enrolled in the UDC project (Sidonio et al, 2014; Gilbert et al, 2014). These studies all suggest an increased bleeding tendency in haemophilia A carriers despite a normal FVIII activity.
To better define the extent of excessive bleeding symptoms in haemophilia A carriers, we have tested the hypothesis that adult obligate haemophilia A carriers will have an increase in clinically relevant bleeding symptoms, as measured by the condensed MCMDM-1VWD bleeding assessment tool and Pictorial Bleeding Assessment Chart (PBAC), when compared to a control population.
Materials and Methods
We conducted a cross-sectional study after obtaining Institutional Review Board (IRB) approval at Vanderbilt University in Nashville, Tennessee. We recruited adult haemophilia A carriers who were mothers and aunts of children with haemophilia A, who received care at The Vanderbilt University Haemostasis Clinic. Genetically verified or obligate haemophilia A carriers age 18 to 60 years were eligible for inclusion in the study as a case. Normal women were mothers of children with cancer who received care at Children’s Hospital at Vanderbilt. Exclusion criteria for both cases and controls included a personal history of another bleeding disorder, inherited or acquired thrombophilia, pregnancy or autoimmune disorder. Laboratory testing was performed to exclude the possibility of a concomitant bleeding disorder. We compiled a list of all eligible obligate haemophilia A carriers and initially contacted them in an alphabetical order. Three haemophilia A carriers declined participation in this study while ten normal women declined participation.
Clinical Assessment Procedures
Clinical assessment tools were performed by trained interviewers, and included the following: 1) demographics questionnaire, 2) pre-laboratory questionnaire, eliminating medications that could alter laboratory results, 3) bleeding inventory, querying past bleeding history (number of bleeding events, duration and severity of menstrual cycle, combined oral contraceptive (COC) use or alternative medical intervention to treat heavy menstrual bleeding, anemia and blood product or factor product use) 3) condensed MCMDM-1VWD bleeding assessment tool and 4) Pictorial Bleeding Assessment Chart (PBAC), current and historic evaluation of menstrual bleeding patterns were obtained retrospectively. The condensed MCMD-1 VWD bleeding assessment score is a bleeding assessment tool used to score bleeding symptoms (individual components scored from −1 to 4) and predict when those symptoms are suggestive of a bleeding disorder; it has been used in the assessment of heavy menstrual bleeding and is noted to be correlate inversely with von Willebrand factor levels (Azzam et al, 2012). The PBAC is a semi-quantitative visual assessment of menstrual bleeding; a score of greater than 100 is highly correlating with the presence of an inherited bleeding disorder, most notably von Willebrand disease (Higham et al, 1990). Neither bleeding assessment tools were modified for purposes of this study in haemophiila carriers. Laboratory testing, which was completed in full by 72% of all participants, included complete blood count (CBC), prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen activity, one-stage coagulation assay (STA-R Evolution®) for FVIII activity (FVIII:C), von Willebrand factor antigen (vWF:Ag), ristocetin cofactor assay (vWF:RCof), platelet function analyser-100™ (PFA-100) and blood type (ABO & RH-D). Evaluation for Factor V Leiden mutation, prothrombin 20210 gene mutation, protein S and C activity, antithrombin activity was performed on 72% of all participants.
Data Management and Analysis
Data was entered into REDCap, a secure web accessible tool for data storage residing on a network central server. Data were analyzed using IBM SPSS Statistics version 22 software. Descriptive analyses for categorical variables included frequencies, and for continuous variables included mean, median, minimum and maximum values. Categorical and continuous variables were compared using Chi-Square and Mann-Whitney U tests respectively.
Results
Demographics
A total of 87 women were recruited for participation in the study, 44 Haemophilia A carriers and 43 normal women. Age was similar between the two groups; the overall median age was 36.8 years (24.5 – 59.6). The two groups were similar when comparisons were made for ethnicity, education, employment and health insurance status (table I). Differences were only noted in insurance coverage: Haemophilia A carriers were more likely to have HMO insurance coverage and less likely to have PPO coverage.
Table I.
demographic information for all women in the study, Haemophilia A carriers and normal women, all of whom were negative for inherited bleeding and thrombotic disorders.
| Haemophilia A Carriers (n = 44) (n (%)) | Normal Women (n = 43) (n (%)) | ||
|---|---|---|---|
|
| |||
| Ethnicity | Non-Hispanic | 40 (90.9) | 35 (81.4) |
| Hispanic | 1 (2.3) | 4 (9.3) | |
| Other | 3 (6.8) | 2 (4.7) | |
|
| |||
| Education | Primary / secondary school | 9 (20.5) | 7 (16.3) |
| Technical school | 3 (6.8) | 0 (0.0) | |
| Completed college | 15 (34.1) | 12 (27.9) | |
| Advanced degree | 2 (4.5) | 5 (11.6) | |
| Other | 15 (34.1) | 17 (39.5) | |
|
| |||
| Employment | Full time | 14 (31.8) | 17 (39.5) |
| Part time | 7 (15.9) | 4 (9.3) | |
| Homemaker | 21 (47.7) | 20 (46.5) | |
| Other | 2 (4.7) | 2 (4.7) | |
|
| |||
| Blood Type | O | 12 (27.3) | 16 (37.2) |
| AB | 2 (4.5) | 1 (2.3) | |
| B | 5 (11.4) | 3 (7.0) | |
| A | 18 (40.9) | 14 (32.6) | |
| Data Missing | 7 (15.9) | 9 (20.9) | |
Clinical history
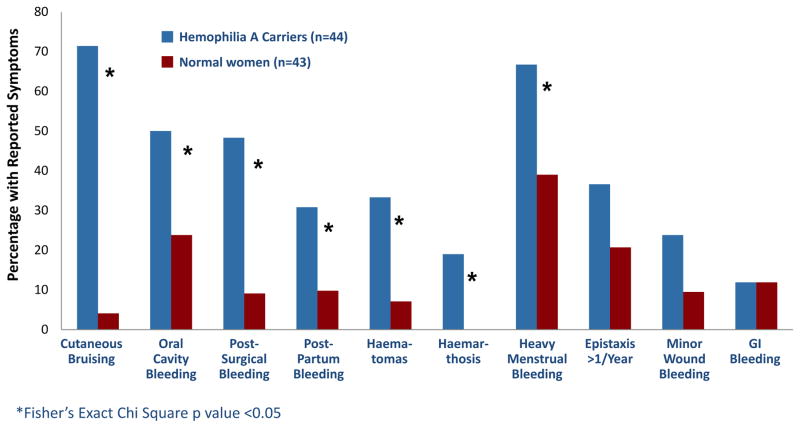
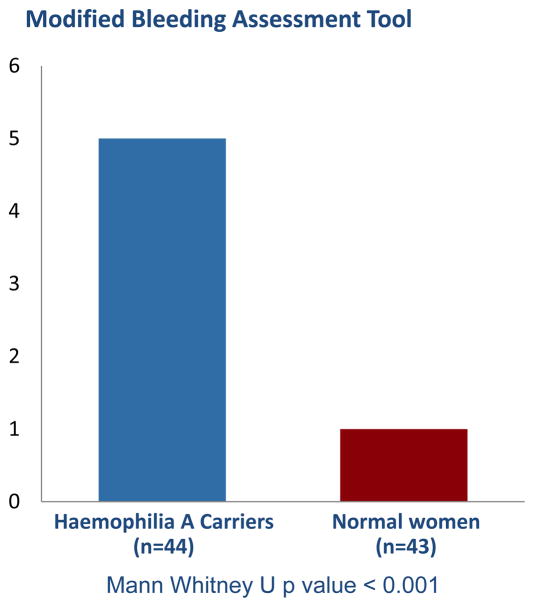
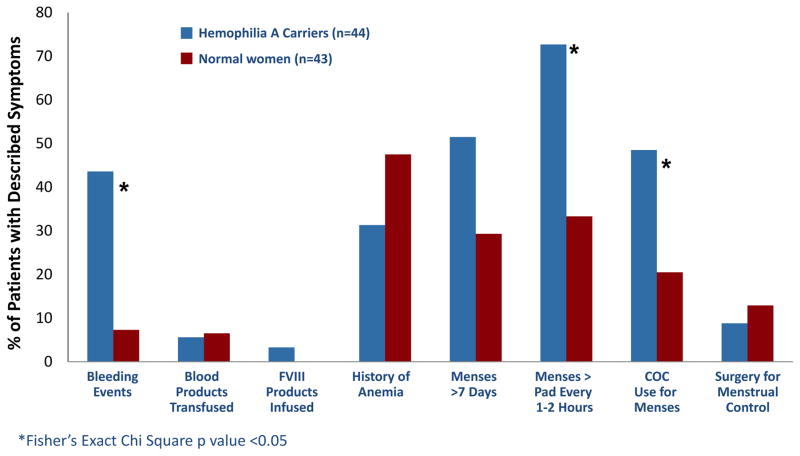
Haemophilia A carriers reported more bleeding symptoms as determined by our bleeding assessment tools. Haemophilia A carriers reported a higher incidence of bleeding events (figure 1), although these events occurred at the same age as our normal comparison population (13.5 versus 14.0 years, p value = 0.779). The condensed MCMDM-1VWD bleeding assessment tool score was higher in Haemophilia A carriers (5 versus 1, p value < 0.001) (figure 2) indicating a higher bleeding tendency. In an analysis of the individual components of the condensed bleeding assessment tool, haemophilia A carriers reported increased cutaneous bruising, post-surgical bleeding, post-partum haemorrhage, haematomas, atraumatic haemarthrosis and heavy menstrual bleeding compared to controls (figure 3).
Figure 1.
Comparison of reported bleeding history between haemophilia A carriers and normal women. Statistical analysis performed with Fisher’s Exact Chi Square test.
Figure 2.
Condensed MCMDM-1 VWD score for Haemophilia A carriers versus normal women. Statistical analysis performed with Mann Whitney U test.
Figure 3.
Comparison of individual components of the condensed MCMDM-1VWD bleeding assessment tool between Haemophilia A carriers and normal women. Statistical analysis performed with Fisher’s Exact Chi Square test.
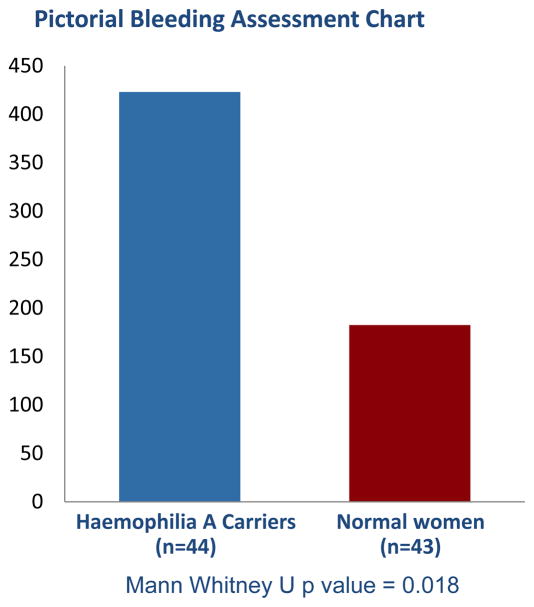
Haemophilia A carriers had increased menstrual bleeding, and reported more frequent use of COCs as a consequence of heavy menstrual bleeding, as well as increased severity of bleeding as demonstrated by changing pads every 1–2 hours (figure 3). Furthermore, carriers had significantly higher historic PBAC scores compared to normal women (423 versus 182.5, p value = 0.018) (figure 4). Contrarily, carriers did not have increased duration of menses nor require surgical gynecologic intervention at an increased frequency (figure 3). Furthermore, our cohort did not report use of antifibrinolytics for clinical management of heavy menstrual bleeding.
Figure 4.
Historical pictorial bleeding assessment chart score for Haemophilia A carriers versus normal women. Statistical analysis performed with Mann Whitney U test.
Laboratory values
Laboratory results of the two groups were similar in all testing except FVIII:C, which was reduced in haemophilia A carriers. ABO blood typing was consistent with the expected frequency in the US population (O 44%, A 42%, B 10%, AB 4%) (table I). Thrombophilia testing was normal in all participants who received the testing (72%). Laboratory values that are known to affect haemostasis, notably haemoglobin, platelet-count, PT, vWF:Ag, VWF:RCof and PFA-100 were not significantly different between the two groups; aPTT was significantly prolonged in carriers compared to controls (table II). While within the normal references ranges for all except one case subject (FVIII:C 37%), FVIII:C was significantly lower in the haemophilia A carriers (82.5 versus 134%, p value < 0.001) (table II). Platelet aggregometry was not performed on any of the subjects as all participants had normal PFA-100 closure times.
Table II.
Comparison of laboratory data in Haemophilia A carriers compared to normal women. Statistical analysis performed with Mann Whitney U test.
| Haemophilia A Carriers (n = 34) (median (range)) | Normal Women (n = 29) (median (range)) | p-value | |
|---|---|---|---|
| Hemoglobin (g/l) | 132 (107 – 162) | 131 (119 – 144) | 0.360 |
| Platelet-count (×109/l) | 277 (193 – 380) | 256 (179 – 378) | 0.264 |
| PT (seconds) | 12.9 (11.8 – 15.2) | 12.9 (11.9 – 13.8) | 0.920 |
| aPTT (seconds) | 33.1 (26.1 – 38.5) | 29.7 (25.1 – 37.6) | 0.015 |
| Fibrinogen (g/l) | 3.41 (2.46 – 5.44) | 3.33 (2.14 – 7.69) | 0.628 |
| FVIII activity (%) | 82.5 (37 – 195) | 134 (68 – 242) | < 0.001 |
| VWF antigen (%) | 102 (47 – 212) | 94 (52 – 214) | 0.612 |
| Ristocetin Cofactor (%) | 110 (37 – 200) | 92 (41 – 196) | 0.879 |
| PFA-100 ADP (seconds) | 90.5 (62 – 138) | 91 (57 – 159) | 0.666 |
| PFA-100 Epinephrine (seconds) | 126.5 (68 – 188) | 130 (80 – 300) | 0.135 |
Discussion
An increase in bleeding symptoms in haemophilia A carriers has been previously reported by our group (Paroskie et al, 2014) as well as others, however, this is the first prospective study to assess the bleeding phenotype of haemophilia A carriers utilizing multiple validated questionnaires and utilizing normal women for comparison. In agreement with mounting evidence, our investigation identified an increase in multiple bleeding symptoms, including heavy menstrual bleeding, atraumatic haemarthrosis, cutaneous bruising, haematomas and post-surgical and peri-partum haemorrhage in haemophilia A carriers.
The frequency of bleeding symptoms reported by haemophilia A carriers with FVIII:C >40% in our cohort was similar to previous reports with 19% of our cohort reporting haemarthrosis while 14% reported joint bleeding in previous studies (Plug et al, 2006). Epistaxis was reported in 43% of carriers in previous studies similar to our cohort rate of 37%. Furthermore, heavy menstrual bleeding was common in both groups but more common in the carriers. Our cohort did not report use of NSAIDs or anti-fibrinolytics for clinical management of their heavy menstrual bleeding. A majority (67%) of our cohort reported heavy menstrual bleeding similar to a previously published rate of 57%. There was some discrepancy in our findings with previously published data particularly in procedural bleeding. Bleeding following dental extraction was reported in 27% of haemophilia A carriers while 72% of our cohort reported this symptom. A similar discrepancy was seen in post surgical bleeding with 29% previously reporting this bleeding symptom compared to 48% in our cohort. Our study is unique in that it used a control group that tested negative for common bleeding disorders (qualitative platelet disorders, as detected by an abnormal PFA-100, and vWD). Of note, a single normal patient was excluded from analysis due to heterozygosity for Factor V Leiden mutation, which could theoretically lead to a reduced bleeding phenotype.
Despite an established normal FVIII:C range, our findings suggest that in haemophilia A carriers we may consider viewing FVIII:C as more of a continuum with increased bleeding noted in the low-normal range and worsening as the level drops. A similar relationship has been suggested between VWF:Ag and reported bleeding and provides the evidence that consideration of medical management of patients with low VWF is reasonable (Nichols et al, 2008). Despite having only one single case subject (FVIII:C 37%) in our cohort having a FVIII:C more than two standard deviations below the population mean, haemophilia A carriers in this cohort reported increased bleeding symptoms as measured by both PBAC and the condensed MCMCDM-1 VWD bleeding assessment tool.
Our study has a number of limitations. First, this study represents a small population of haemophilia A carriers. The inclusion of a normal group for comparison strengthens our argument, however these findings should be validated in a larger cohort so that we can further understand the bleeding tendencies in the general population. While the bleeding rate appears high, it is comparable to previously reported rates and, FVIII or FIX is not likely a good predictor of bleeding phenotype in carriers (Olsson et al, 2014). Second, we did not have full genotype results in this population of obligate haemophilia A carriers. It is possible that certain genetic mutations portend a more severe phenotype and thus deserve closer clinical monitoring. Further investigation of the genotype predicting a bleeding tendency is under investigation and to be reported in subsequent studies. Third, our laboratory testing for FVIII activity was performed using a one-stage coagulation assay (STA-R Evolution®) as opposed to the potentially more precise chromogenic method. The chromogenic method could provide results free from influence by impurities and activated FVIII, which could be advantageous. Investigation of a discrepancy between FVIII:C and chromogenic assay results in haemophilia A carriers is also currently being investigated. We feel that given the consistency of testing between all participants still allows for comparisons and conclusions, and the chromogenic assay is not as widely available as is the one stage clotting assay. Finally, inherent in cross sectional studies and questionnaires is the concern for recall bias. It is possible that haemophilia A carriers who agreed to be included in the study were more likely to recall increased bleeding symptoms than those carriers that were asymptomatic, in addition, carriers are likely to be more aware of their bleeding symptoms due to their association with family members with more significant bleeding. We acknowledge the potential bias for over-reporting of historical bleeding symptoms and have plans for prospective evaluation in subsequent studies (Plug et al, 2006, Mauser-Bonschoten et al, 2008). This bias was decreased by our use of a normal population for comparison, as well as the use of multiple validated bleeding assessment tools. In addition we compiled a list of all eligible obligate haemophilia A carriers at Vanderbilt University and contacted them in an alphabetical order in an effort to be objective in our communication with families.
Conclusion
Our study demonstrates that haemophilia A carriers have clinically significant bleeding as determined by a higher condensed bleeding assessment tool score, Pictorial Bleeding Assessment Chart score and individual bleeding symptoms such as increased cutaneous bruising, post-surgical bleeding, post-partum haemorrhage, haematomas, atraumatic haemarthrosis and heavy menstrual bleeding compared to a normal control population. These findings also support the notion that FVIII activity at the lower end of the laboratory normal range in genetically verified or obligate haemophilia A carriers may lead to an increased bleeding phenotype. This data suggests the need for validation of these findings in a larger cohort and reassessment of guidelines for medical management and surveillance in this underserved patient population.
Acknowledgments
Funding Sources: All phases of the study were financially supported by the following grants. The funding sources had no input into the study design; collection, analysis and interpretation of data; writing of the report; or decision to submit the article for publication.
UL1 TR000445 from NCATS/NIH
Vanderbilt CTSA grant 1 UL1 RR024975 from NCRR/NIH
CA154267 from NIH, Conducting Research in Pediatric Hematology/Oncology
Vanderbilt Clinical & Translational Research Scholars Program, KL2
Investigator-initiated grant from Grifols.
Hemostasis and Thrombosis Research Society (HTRS) Mentored Research Award
Hemophilia of Georgia Clinical Scientist Development Grant
Allison Paroskie, MD MSCI conceptualized and designed the study, performed data collection, carried out the initial analyses, drafted the initial manuscript, and approved the final manuscript submitted. Dave Gailani, MD, Michael R. DeBaun, MD MPH, and Robert F Sidonio Jr, MD MSC all contributed to the design of the study, reviewed and revised the manuscript and approved the final manuscript as submitted. We would also like to thank all of the patients and family members who participated in this study.
Footnotes
Financial Disclosure: The authors declare that they have no financial conflicts.
Conflict of Interest: The authors declare that they have no conflicts of interest relevant to the manuscript. Robert Sidonio has received honoraria for participation in advisory boards for Grifols. Baxter, Bayer, Pfizer, Biogen, CSL Behring and Kedrion.
Contributor’s Statement: Allison Paroskie, MD MSCI conceptualized and designed the study, performed data collection, carried out the initial analyses, drafted the initial manuscript, and approved the final manuscript submitted. Dave Gailani, MD, Michael R. DeBaun, MD MPH, and Robert F Sidonio Jr, MD MSC all contributed to the design of the study, reviewed and revised the manuscript and approved the final manuscript as submitted.
References
- Azzam HA, Goneim HR, El-Saddik AM, Azmy E, Hassan M, El Sharawy S. The condensed MCMDM-1 VWD bleeding questionnaire as a predictor of bleeding disorders in women with unexplained menorrhagia. Blood Coagulation Fibrinolysis. 2012;23(4):311–5. doi: 10.1097/MBC.0b013e32835274d9. [DOI] [PubMed] [Google Scholar]
- Gilbert L, Rollins L, Hilmes M, Luo Y, Gailani D, Debaun MR, Sidonio RF. Haemophilia A carriers demonstrate pathological and radiological evidence of structural joint changes. Haemophilia : the official journal of the World Federation of Haemophilia. 2014;20(6):e426–9. doi: 10.1111/hae.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham JB, Rizza CR, Chediak J, Mannucci PM, Briet E, Ljung R, et al. Carrier detection in Haemophilia A: a cooperative international study. I. The carrier phenotype. Blood. 1986;67(6):1554–9. [PubMed] [Google Scholar]
- Higham JM, O’Brien PMS, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. British Journal of Obstetrics and Gynecology. 1990;97:734–739. doi: 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- Lee C, Berntorp EA, Hoots WK. Textbook Of Haemophilia. 2. Oxford, UK: Wiley-Blackwell; 2010. [Google Scholar]
- Mauser-Bunschoten EP, Creveldkliniek V Haematology University Medical Centre, Utrecht. World Federation of Hemophilia. 2008. Symptomatic Carriers of Hemophilia; p. 46. [Google Scholar]
- Miesbach W, Alesci S, Geisen C, Oldenburg J. Association between phenotype and genotype in carriers of haemophilia A. Haemophilia. 2011;17(2):246–51. doi: 10.1111/j.1365-2516.2010.02426.x. [DOI] [PubMed] [Google Scholar]
- Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA) Haemophilia. 2008;14(2):171–232. doi: 10.1111/j.1365-2516.2007.01643.x. [DOI] [PubMed] [Google Scholar]
- Olsson A, Hellgren M, Berntorp E, Ljung R, Baghaei F. Clotting factor level is not a good predictor of bleeding in carriers of haemophilia A and B. Blood coagulation & fibrinolysis. 2014;25(5):471–5. doi: 10.1097/MBC.0000000000000083. [DOI] [PubMed] [Google Scholar]
- Paroskie A, Oso O, Almassi B, DeBaun MR, Sidonio RF. Both hemophilia health care providers and hemophilia a carriers report that carriers have excessive bleeding. Journal Pediatric Hematology / Oncology. 2014;36(4):e224–30. doi: 10.1097/MPH.0000000000000022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plug I, Mauser-Bunschoten EP, Brocker-Vriends AH, van Amstel HK, van der Bom JG, van Diemen-Homan JE, et al. Bleeding in carriers of Hemophilia. Blood. 2006;108(1):52–6. doi: 10.1182/blood-2005-09-3879. [DOI] [PubMed] [Google Scholar]
- Sidonio RF, Mili FD, Li T, Miller CH, Hooper WC, DeBaun MR, et al. Females with FVIII and FIX deficiency have reduced joint range of motion. American journal of hematology. 2014;89(8):831–6. doi: 10.1002/ajh.23754. [DOI] [PMC free article] [PubMed] [Google Scholar]