Abstract
Background
Aromatase inhibitor (AI) use results in low estrogen levels which in turn affect bone mineral density (BMD). Periodontitis, alveolar bone loss, and tooth loss are associated with low BMD. The goal of this study was to assess the prevalence of periodontitis, perceived oral health, and evaluate salivary biomarkers in postmenopausal women who are early stage (I-IIIA) breast cancer (BCa) survivors and receive adjuvant AI therapy.
Methods
Participants included 58 postmenopausal women; 29 with BCa on AIs and 29 controls without BCa diagnoses. Baseline periodontal status was assessed with: (1) periodontal pocket depth (PD); (2) bleeding on probing (BOP); and (3) attachment loss (AL). Demographic and dental utilization information was gathered by questionnaire. Linear regression modeling was used to analyze the outcomes.
Results
No differences in mean PD or the number of teeth were found. The AI group had significantly more sites with BOP (27.8 vs. 16.7; p = 0.02), higher worst-site AL (5.2 mm vs. 4.0 mm; p < 0.01) and more sites with dental calculus than did controls (18.2 vs. 6.4; p < 0.001). Linear regression adjusted for income, tobacco use, and dental insurance, and previous radiation and chemotherapy exposure demonstrated AI use increased CAL over 2 mm (95% CI: 0.46 -3.92). Median salivary osteocalcin and Tumor Necrosis Factor levels were significantly higher in the BCa group than the control group.
Conclusions
This first investigation of the periodontal status of women initiating adjuvant AI therapy identifies this population as having an increased risk for periodontitis (NCT1272570).
Keywords: Breast neoplasms, Postmenopause, Aromatase inhibitors, Periodontal attachment loss, Women Health, Biological markers
Introduction
Approximately 230,000 women were diagnosed with breast cancer (BCa) in 2013 in the U.S. 1 With an increase in early detection and improved therapies, more of these women have become survivors.2 Nearly 75% of all BCa occur in postmenopausal (PM) women and 66-80% of these BCa are hormone-receptor positive and therefore amenable to hormone adjuvant therapy.3 Use of tamoxifen, a synthetic selective estrogen modulator for estrogen receptor positive BCa was the drug of choice until the recent emergence of the aromatase inhibitors (AIs).4 Third generation AIs (anastrozole, exemestane and letrozole) are recommended as a component of the care plan in PM women with hormone-receptor-positive early BCa due to their superior efficacy in reducing tumor recurrence5 and their general tolerability. AIs inhibit the conversion of androgen to estrogen in peripheral tissues, leading to a marked reduction in circulating estrogen. This pharmacological induced drop in circulating estrogen levels is associated with negative effects on bone health. Loss of BMD and increased risk of fragility fracture are well documented toxicities of adjuvant AI therapy.6-9
The density of the bones in the oral cavity is one aspect of systemic BMD and correlates with a risk for osteoporosis10, 11 and hip fracture in older women.12 Currently, the potential impact of AI therapy on the periodontal health of BCa survivors is unknown. With an increasing use of AI in adjuvant therapy and potentially in the preventive setting as well,13 the oral toxicities of AI use need to be better understood.
Periodontal diseases, alveolar bone loss, and tooth loss are associated with both the low estrogen states of menopause and osteoporosis. 14, 15 While site specific variations are known, osteoporosis is a systemic condition resulting in the loss of bone mass and microarchitecture. In general, patients diagnosed with osteopenia or osteoporosis have reduced jaw bone mass16 and changes in dental radiographs are correlated with hip fractures in PM women.12 Proinflammatory biomarkers detected in the saliva have been associated with periodontitis including interleukins (IL-6, IL-1, IL-8, tumor necrosis factor-alpha (TNF-a) and matrix metalloproteinases (MMP)-8 and -9)). 17-19
The effects of AIs on oral health is a neglected topic, particularly in light of the known systemic effects of AIs on bone remodeling among PM women - leading to a net loss of bone. Evidence supports the role of low skeletal BMD and osteoporosis as risk indicators for reduced alveolar crestal height and attachment loss (AL).20-24 As low levels of circulating estrogen are an important risk factor for the development of osteoporosis, the role of AIs as possible risk factors for oral conditions among PM women needs to be evaluated. The objectives were to explore the (1) prevalence and of periodontitis (2) perception of oral health, and (3) salivary biomarkers in PM women initiating adjuvant AI therapy and control participants.
Materials and Methods
This study was reviewed and approved by the Institutional Board at the University of Michigan prior to enrolling patients and was registered through National Institutes of Health ClinicalTrials.gov (Identifier no. NCT01272570). This paper conforms to the STROBE guidelines for observational studies.25
Participants
Data were collected from 29 women with ER + BCa who had been on AI therapy for 2-11 months and 29 PM women without Bca not using AI therapy. This sample size was chosen for feasibility, rather than to statistically power a specific hypothesis for the baseline data. Nonetheless, based upon longitudinal pilot data of AL in non-cancer patients, we found that 58 patients would supply at least 80% power (with a Type I error rate of 5%) to detect a 10-point difference (i.e .20 vs. .10) in the 18 month change in percentage of sites with 3mm or more of AL between the two groups of participants (AI therapy vs. control).
All 58 patients provided informed consent prior to participation. PM women with and without BCa diagnoses and having > 15 teeth26 (based upon a previously published report) were eligible to participate in the study. The number of teeth needed for inclusion was based upon Participants were recruited between April 2009 and September 2010. Menopausal status was determined using the National Comprehensive Cancer Network (NCCN) criteria.27 PM women with a histopathologic confirmed diagnosis of early stage (I-IIIA) BCa newly on any adjuvant AI therapy (within 1-11 months of start) were recruited from the Breast Medical Oncology Clinic of the University of Michigan Comprehensive Cancer Center. AI prescriptions were provided by each patients’ oncologist as clinically indicated (AIs included anastrozole, exemestane or letrozole). Participants may have had a history of Tamoxifen use, chemotherapy and/or radiation therapy. Women were excluded if they received a diagnosis of metastatic BCa.
The control group consisted of PM women without BCa diagnoses (or any other cancer than thyroid or basal cell cancer) and not on AI therapy. This group was chosen as women with ER + BCa on tamoxifen may not be appropriate controls. Tamoxifen use is associated with an increase in bone mineral density and thus may preclude the ability to examine how menopause and the loss of estrogen have an impact on alveolar bone changes.28 These women were recruited from the University of Michigan Breast Imaging clinic at the time of routine mammography. Additional exclusion criteria for both groups included: 1) uncontrolled diabetes (A1c, >7.2) as determined from self-reported Ac1 screening within last 2 months; and 2) the use of medications that affect periodontal status (calcium antagonists, anti-convulsives, and immune-suppressive such as > prednisone 7.5mg daily). NSAIDS (occasional use only) and bisphosphonate usage were allowed.
Examination Procedures
All dental examinations were performed at the Michigan Center for Oral Health Research (MCOHR). Two trained and calibrated dental examiners (Karen Essell and Alaina Robinson from the University of Michigan) were blinded to the patient's status completed a full mouth comprehensive periodontal examination, excluding third molars using a mouth mirror and periodontal probe. Study measurements included probing depth (PD), gingival recession, AL, bleeding upon probing (BOP), plaque scores, missing teeth and presence of calculus on all teeth for each participant. PD was measured from the gingival margin (GM) to the base of the gingival sulcus/pocket with a 0.5 mm diameter, calibrated North Carolina probe. Supra-gingival plaque, was coded as 0 - no plaque and 1 - plaque; supragingival calculus was defined as supra-gingival calcified deposits on tooth crowns and roots and was measured as 0 - no supra-gingival calculus and 1 - supragingival calculus. PD was measured on 6 sites per tooth (mesio-buccal, mid-buccal, disto-buccal, mesio-lingual, mid-lingual and disto-lingual). All measurements were rounded to the lowest whole millimeter. AL was calculated using the same sites by first measuring the distance from the cemento-enamel junction (CEJ) to the GM, then subtracting this distance from the PD. Values for interproximal sites were used to calculate the worst PD and AL sites. Periodontitis was defined as a loss of 3 mm or more AL. The presence of gingival bleeding was determined while obtaining the PD measurements. Gingival bleeding was coded as- 0 no bleeding and 1 bleeding and was noted 10 seconds after removal of the periodontal probe.
Radiographic Measures
Standardized peri-apical digital radiographs were taken in the posterior dentition of all participants using a parallel technique. The radiographs were standardized with the use of bite registration material and an aluminum step wedge of known density 29 while the same settings were used (63 kV, 8 mA, 0.1 s). ∥ An average of the distance in pixel measurement was used to establish the distance of the step wedge. Linear bone measurements were taken between the CEJ or on apical border of a restoration on the mesial and distal surfaces of the first molars for the determination of alveolar bone height. A higher value for the alveolar bone height indicates greater bone loss and worse periodontitis. The radiographs were analyzed by a trained examiner with the use of a computer software measurement tool . 30
Examiner training and calibration for clinical measurements
The dental examiners were calibrated prior to the study. Examiners demonstrated at least 94% of PD measurements within 1 mm of each other with a 95% confidence interval of (0.84, 0.95) and at least 85% of AL measurements within 1 mm of each other with a 95% confidence interval of (0.72, 0.93). Examiners were masked to the cancer history of the patient.
Saliva Biomarkers
Unstimulated whole saliva was collected via passive drooling into a sterile plastic tube from all study participants as previously described by Mandel and Wotman.31 Saliva collection was stopped once a total of 2 ml was collected or 15 minutes had elapsed whichever occurred first. The sample was immediately placed on ice, aliquoted, and supplemented with a proteinase inhibitors aprotinin and Phenylmethane-sulfonyl fluoride (PMFS) stored at -80 C. 18 Saliva samples were analyzed for IL-α IL-1b, I IL-6, IL-8, IL-10, IL-17, IL-18, as well as TNF-a, C-Reactive Proteins (CRP) , MMP-8, MMP-9, osteocalcin (OCN) , osteoprotegerin (OPG), vascular endothelial growth factor (VEGF), TNF-related activation-induced cytokine (TRANCE) and stromal cell-derived factor 1 (SDF-1 or CXCL12). Protein biomarker levels were determined through a custom human array-based multiplex sandwich ELISA system¶ as previously reported. 19
Questionnaire
A survey was used to collect demographic information such as age (years), ethnicity/race (white, other), education (less than high school, high school, >high school), income as well as oral health-related behaviors and dental care utilization, and prior periodontal therapy or scaling and root planning. To measure the participants’ perceptions of oral health, four questions were included regarding the perception of the health of their teeth and gums, importance of oral health as well as perception of mouth dryness. 32 The patient responses to the two questions ‘How would you describe the health of your teeth?” and “How would you describe the health of your gums?” were given on 5 point rating scales ranging from 1 = “poor” to 5= “Excellent.” Respondents were asked to rate the importance of their dental health given on 5 point rating scales ranging from 1 = “not at all important” to 5= “Very Important.” Finally, respondents were asked to rate the dryness of their mouth. The 5 point scale ranged from 1= “Very little saliva”=1 to 5= “Perfect amount of saliva.” The average response to these items was used as an assessment of their oral health perceptions. The questionnaire was pretested with 10 patient volunteers from the University of Michigan Comprehensive Cancer Center. Feedback concerning the clarity of some questions was used to finalize the survey. Cancer-related data such as the diagnosis, time since cancer diagnosis, cancer treatments, medical conditions, and medication use were obtained from the patient's medical chart.
Statistical Analyses
All site-specific measures were averaged within-participant before being analyzed, and all biomarker measures examined for normality before being analyzed. Between-group differences in demographic and behavioral characteristics were assessed with a chi-squared test of association (categorical) or Wilcoxon Rank Sum test (continuous). Between-group differences in clinical measures were assessed with either a chi-squared test of association (categorical) or two-sample t-test (continuous). Between-group differences in biomarker levels were assessed with the Wilcoxon Rank Sum test. Between-group differences in self-perceived oral health measures were assessed with an independent sample t-test. Between-group differences in maximum AL and percentage of sites with BOP were further assessed using multiple linear regression to adjust for possible confounders using a manual backward selection technique. A full model with all candidate confounders was fit; then, the predictor with the lowest p-value was removed and the model was re-fit. The next least significant predictor was removed, and so forth, until all predictors in the model were statistically significant or clinically relevant. We explored AI duration, local factors such as dental plaque and calculus oral health behaviors (brushing and flossing), past periodontal treatment (deep cleanings), socio-demographic factors and bisphosphonate use. However as they were not informative, we dropped them from the final model. Although chemotherapy and radiation exposure did not show statistical significance, as they may have a negative impact on a patient's periodontal health we retained them as possible confounders in the linear regression models. Data analyses were performed using statistical analysis software package.# Statistical significance is defined as a p-value less than 0.05.
Results
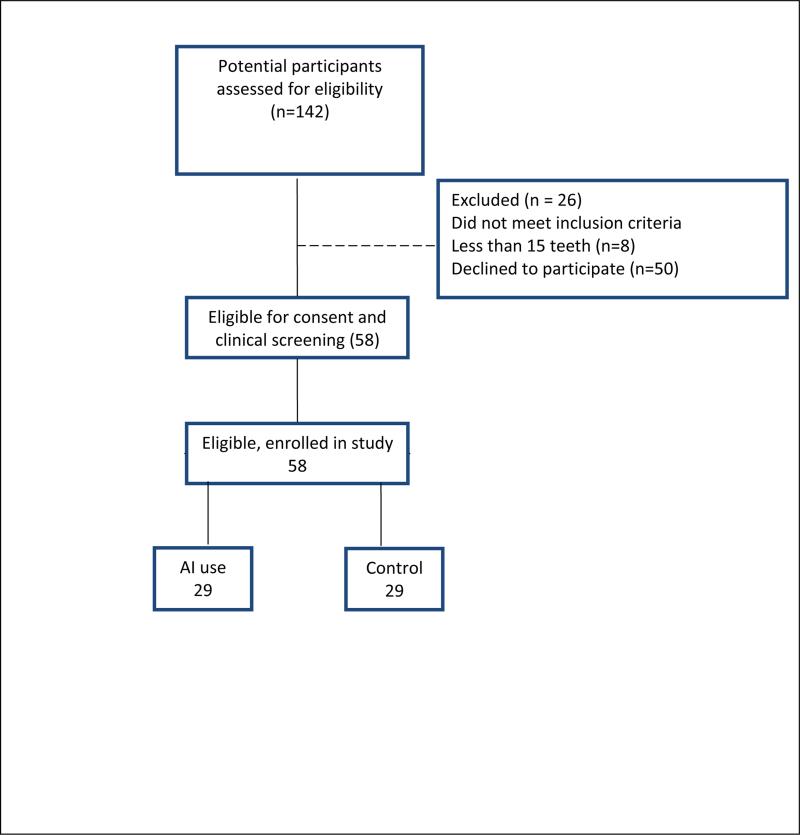
The study met its target accrual of 58 PM; 29 with BCa on AI and 29 controls (Figure 1). Descriptive characteristics for our sample stratified by AI status are presented in Table 1. The majority of participants were white and married. The mean age for both groups was 61 years. Characteristics were similar in the AI vs. control group regarding education, income level, dental visits, and dental insurance status. Bisphosphonate use was reported in 38% of the AI users as compared to 17% of the controls (p=0.11). Oral health behaviors and lifestyle behaviors such as tobacco and alcohol use and tooth brushing behaviors were not statistically different between study arms. Of note, 20-28% of all participants did not have dental insurance. The two groups did not differ in regards to having received periodontal treatment or periodontal cleanings (p=0.44; data not tabulated).
FIGURE 1.
SCHEMATIC OF ENROLLMENT
Table 1.
Demographic and Behavioral Characteristics of 58 Postmenopausal Women Stratified by AI status
| No AI Use N=29 | AI Use N=29 | ||||
|---|---|---|---|---|---|
| Analysis variable | Obs | % | Obs | % | P Value |
| Age (years) Mean ± SE | 61.6 (5.4) | 61.7 (7.6) | 0.92* | ||
| Ethnicity | |||||
| White | 26 | 89.6 | 26 | 89.7 | |
| Non White | 3 | 10.4 | 3 | 10.3 | 0.92† |
| Education | |||||
| Less than high school | 5 | 17.8 | 3 | 10.5 | |
| High school diploma | 5 | 17.9 | 6 | 20.7 | |
| More than high school | 18 | 64.3 | 20 | 68.8 | 0.70† |
| Income | |||||
| No income to $19,999 | 8 | 28.6 | 5 | 17.9 | |
| $20,000-$39,999 | 5 | 17.9 | 3 | 10.7 | |
| $40,000-$59,999 | 3 | 10.7 | 3 | 10.7 | |
| $60,000-$74,999 | 2 | 7.1 | 6 | 21.4 | |
| over $75,000 | 10 | 35.7 | 11 | 39.3 | 0.22† |
| Marital status | |||||
| Married | 18 | 62.1 | 21 | 72.4 | |
| Not married | 11 | 37.9 | 8 | 27.6 | 0.29† |
| Has dental insurance | |||||
| Yes | 23 | 79.3 | 21 | 72.4 | |
| No | 6 | 20.7 | 8 | 27.6 | 0.76† |
| Last dental visit | |||||
| Within last 6 months | 27 | 93.1 | 25 | 89.3 | |
| More than 6 months | 2 | 6.90 | 3 | 10.6 | 0.67† |
| Smoking status | |||||
| Current | 1 | 3.4 | 1 | 3.4 | |
| Past | 10 | 34.4 | 16 | 55.0 | |
| Never | 18 | 62.2 | 12 | 41.6 | 0.11† |
| Bisphosphonate use | |||||
| Yes | 5 | 17.2 | 11 | 37.9 | |
| No | 24 | 82.8 | 18 | 62.1 | 0.07† |
| Diabetes | |||||
| Yes | 1 | 3.4 | 4 | 13.8 | |
| No | 28 | 96.6 | 25 | 86.2 | 0.16† |
| Frequency of brushing | |||||
| Every day | 10 | 34.5 | 12 | 41.4 | |
| More than once a day | 19 | 65.5 | 17 | 58.6 | 0.58† |
| Frequency of flossing | |||||
| Every day | 14 | 48.3 | 12 | 41.5 | |
| Nearly every day | 12 | 41.4 | 11 | 37.9 | |
| Occasionally | 3 | 10.3 | 6 | 20.6 | 0.20† |
| Alcohol | |||||
| Yes | 19 | 65.52 | 16 | 57.1 | |
| No | 10 | 34.48 | 12 | 42.9 | 0.51† |
Two sample t-test
Chi-square test of association
The mean age at Bca diagnosis was 59.3 years (± 7.1 SD; range 42-73 years). A total of 51.7% had been diagnosed with Stage I and 31% with Stage II estrogen receptor positive BCa. The time since diagnosis was 1.3 years (± 6.1 months; range 8 months-1.7 years). Adjuvant cancer treatments included, chemotherapy (37.9%), radiation therapy (86.9%), and 17.2% had received tamoxifen prior to treatment with an AI. The distribution of AI medications were as follows: 20 women indicated anastrozole use, 2 indicated exemestane use and, 7 women indicated letrozole use. The median time of AI duration was 5.7 months (±3.1 months; 2-11months) (data not tabulated).
A comparison of periodontal measures in AI users and controls is shown in Table 2. Participants receiving AI therapy as compared to the control group had a significantly higher mean number of gingival bleeding sites (27.8 vs. 16.7; p < 0.02), higher mean worst-site AL (5.2 mm vs. 4mm; p < 0.01), and nearly 3 times the number of sites with dental calculus and dental plaque. No difference between the two groups was found concerning the mean PD and the number of teeth present. The percentage of women with 6 mm or greater of AL was significantly higher in the AI group compared to the control group (31.0% vs. 6.9%; p =0.03) (Table 2). Radiographic linear mean bone levels were not statistically different between the two groups (on AI: 2.65 ±0.63mm vs. no AI: 2.69±0.45 mm (p=0.057).
Table 2.
Periodontal Measures Among Study Participants by AI Use
| Periodontal Characteristic | No AI use N=29 | AI use N=29 | P-Value† |
|---|---|---|---|
| Mean ± SD* | Mean ± SD* | ||
| Number of teeth | 26.6 ± 1.6 | 26.1 ± 2.3 | 0.39 |
| Number of plaque /biofilm sites | 16.3 ± 6.6 | 55.4 ± 3.4 | 0.03 |
| Number of calculus sites | 6.4 ± 1.7 | 18.2 ± 3.0 | 0.001 |
| Number of gingival bleeding sites | 16.7 ± 12.3 | 27.8 ± 23.4 | 0.02 |
| Pocket depth (mm)‡ | 2.0 ± 0.29 | 2.0 ± 0.27 | 0.95 |
| Worst-site pocket depth∥ (mm)‡ | 4.2 ± 1.4 | 4.6 ± 0.75 | 0.21 |
| Attachment loss (mm)‡ | 1.4 ± 0.39 | 1.5 ± 0.75 | 0.56 |
| Worst-site attachment loss (mm)‡ | 4.0 ± 1.0 | 5.2 ± 2.3 | 0.01 |
| Gingival recession | 0.28 ± 0.44 | 0.36 ± 0.67 | 0.06 |
| Radiographic bone (Mean bone height) | 2.69 ± 0.46 | 2.65 ± 0.63 | 0.06 |
| Women with: | n(%) | n (%) | P Value‡ |
| Gingival Recession (GR), percent (%) | |||
| % with GR of 0-1 mm§ | 8 (27.6) | 2 (6.9) | |
| % with GR of 2 mm§ | 7 (24.1) | 11(37.9) | |
| % with GR of 3 mm§ | 11(37.9) | 10 (34.5) | |
| % with GR of 4 mm§ | 3 (10.3) | 6 (20.7) | 0.35 |
| Periodontitis± | |||
| % with AL = 3 mm§ (Mild) | 8 (27.5) | 5 (17.2) | |
| % with AL >=4 mm§ but < 6 mm (Moderate) | 17 (58.3) | 14 (48.3) | |
| % with of AL >= 6 mm (Severe) | 2 (6.9) | 9 (31.0) | 0.03 |
Standard deviation of the mean
Two sample t-test
Chi-square test of association
mm, millimeters
Worst site was calculated using interproximal values
One patient in each group did not have periodontitis resulting in an n=28 for this measure.
Table 3 compares the self-reported oral health perceptions of the two groups. AI users had a lower perception of the health of their teeth as compared to controls although it did not research statistical significance (on a scale of 1 [poor] to 5[excellent]: AI users 3.14, control 3.69; p=0.056). The two groups did not differ in their perceptions for either the health of their teeth, or the health of their gums, or in the perception of the importance placed on dental health. Concerning the respondents perception of the amount saliva or dryness of their mouths, there was no difference between groups.
Table 3.
Study Participant's Self-Perceived Oral Health, Level of Saliva and Importance of Dental Health
| Perceived Oral Health | No AI use (n=29) | AI use (n=29) | P-Value* |
|---|---|---|---|
| Mean SD | Mean SD | ||
| How would you describe the health of your teeth? | 3.69 ± 0.96 | 3.14 ± 1.18 | 0.056 |
| How would you describe the health of your gums? | 3.34± 1.04 | 2.97 ± 1.29 | 0.22 |
| How much saliva do you have? | 4.34± 0.97 | 4.03 ±1.08 | 0.25 |
| How important is your dental health? | 4.97± 0.18 | 4.72 ± 0.75 | 0.09 |
Two sample t-test
Multivariate analyses describing the periodontal health of AI users and non-users are shown in Table 4. AIs users have significantly higher prevalence of worst site AL values than women who do not use AIs after adjusting for income, tobacco use, dental insurance status, and previous radiation and chemotherapy treatments. Furthermore, when examining BOP, a linear regression model demonstrated that AI use was significantly correlated with the presence of bleeding. On average, those women using an AI had 12 more sites of bleeding than those not on AIs after controlling for AI status, presence of dental insurance status, tobacco use, and income level.
Table 4.
Multiple Linear Regression Models for BOP and CAL in Postmenopausal Women
| Mean % of Sites with BOP | Worst-site CAL (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| Independent Variables | Coefficient (B) | Std. Error | P value | 95% CI | Coefficient | Std. Error | P Value | 95% CI |
| AI use | ||||||||
| No | REF | REF | ||||||
| Yes | 11.22 | 4.28 | 0.02 | (1.63, 22.00) | 2.03 | 0.99 | 0.02 | (0.46, 3.92) |
| Income | ||||||||
| < $19,000 | REF | REF | ||||||
| >=$20,000 - $75,000 | −6.81 | 6.77 | 0.30 | (−20.58, 6.77) | −0.612 | 0.55 | 0.15 | (−1.73, 0.51) |
| > $75,000 | −1.50 | 7.10 | 0.83 | (−15.67, 12.71) | −1.05 | 0.58 | 0.07 | (−2.22, 0.15) |
| Tobacco Use | ||||||||
| No | REF | REF | ||||||
| Yes | −5.08 | 0.44 | 0.41 | (−15.9, 5.75) | 0.22 | 0.52 | 0.61 | (−0.66, 1.11) |
| Dental insurance | ||||||||
| No | REF | |||||||
| Yes | −5.14 | 6.31 | 0.36 | (−17.4, 7.55) | −1.10 | 0.53 | 0.09 | (−1.10, 1.03) |
| Radiation treatment | ||||||||
| No | REF | |||||||
| Yes | 4.82 | 12.0 | 0.69 | (19.3, 29.0) | −0.88 | 1.11 | 0.43 | (−3.13, 1.35) |
| Chemotherapy | ||||||||
| No | REF | |||||||
| Yes | −11.61 | 8.68 | 0.18 | (−29.0,5.85) | −1.09 | 0.71 | 0.13 | (−2.52, 0.34) |
Data are shown as mean ± SE (95% CI).
Table 5 provides the data on the biomarker results. The two groups differed significantly in the level of salivary TNFa, with the AI group exhibiting higher levels than the control group (9 (0, 632 ) pg/ml vs. 2, (0,27 ) pg/ml; p < 0.003) as well as osteocalcin (182 (72,323) pg/ml vs. 121 (47,405) pg/ml; p=0.03). Salivary levels of RankL (p=0.058) showed a trend toward significance. No other markers suggested differences between the study groups.
Table 5.
Salivary Biomarkers (pg/ml) Identified in AI Users and Controls
| Biomarker (pg/ml) | Group (n=29 per group) | Median | Range | P Value* |
|---|---|---|---|---|
| CRP | Not on AI | 888 | 12 to 10,513 | |
| On AI | 1,481 | 25 to 10,137 | 0.13 | |
| IL-1a | Not on AI | 214 | 44 to 4,794 | |
| On AI | 262 | 44 to 3,561 | 0.28 | |
| IL-1b | Not on AI | 69 | 0 to 1,224 | |
| On AI | 1,51 | 4 to 1,319 | 0.89 | |
| IL-6 | Not on AI | 59 | 0 to 239 | |
| On AI | 42 | 2 to 332 | 0.90 | |
| IL-8 | Not on AI | 265 | 56 to 1,253 | |
| On AI | 335 | 50 to 978 | 0.71 | |
| IL-10 | Not on AI | 214 | 0 to 552 | |
| On AI | 220 | 96 to 488 | 0.82 | |
| IL-17 | Not on AI | 32 | 0 to 103 | |
| On AI | 25 | 0 to 85 | 0.48 | |
| IL-18 | Not on AI | 320 | 35 to 12,038 | |
| On AI | 463 | 34 to 13,085 | 0.48 | |
| MCP-1 | Not on AI | 1,421 | 280 to 6,335 | |
| On AI | 1,842 | 428 to 4,736 | 0.76 | |
| MMP-8 | Not on AI | 7,206 | 3,875 to 9,840 | |
| On AI | 7,076 | 4,550 to 8,929 | 0.98 | |
| MMP-9 | Not on AI | 38,883 | 21,159 to 53,477 | |
| On AI | 38,034 | 13,646 to 52,003 | 0.54 | |
| OPG | Not on AI | 4,174 | 896 to 32,616 | |
| On AI | 4,848 | 123 to 41,012 | 0.67 | |
| Osteocalcin | Not on AI | 121 | 47 to 405 | |
| On AI | 182 | 72 to 323 | 0.03† | |
| SDF-1α | Not on AI | 270 | 23 to 15,415 | |
| On AI | 508 | 35 to 16,803 | 0.59 | |
| TNFa | Not on AI | 2 | 0 to 27 | |
| On AI | 9 | 0 to 632 | 0.003† | |
| RANKL | Not on AI | 1,110 | 0 to 7,028 | |
| On AI | 1,861 | 0 to 5,194 | 0.058 | |
| VEGF | Not on AI | 3,410 | 1,714 to 7,957 | |
| On AI | 3,276 | 539 to 7,683 | 0.59 |
Wilcoxon test of rank sum used to test the median of means
Significant differences between groups
IL, Interleukin; MCP-1, Monocyte Chemoattractant Protein-1; MMP, matrix metalloproteinases; SDF-1α, stromal cell-derived factor 1α; TNFα, Tumor necrosis factor; RANKL, Receptor activator of nuclear factor-kappa β ligand.
Discussion
AIs are an important therapy in the management of estrogen receptor positive (ER+) PM early stage BCa. As use of AIs are often recommended for 5 years, and these women are being treated for a cure, it is important to assess the potential impact of these drugs on oral health. The present study is the first to specifically investigate the effects of AI treatments on oral health. We found that AI use is associated with an increased prevalence of periodontitis.
PM participants in this study had good periodontal health with regard to mean whole mouth PD, AL and radiographic bone height measures. However, women on adjuvant AI therapy for a median time of use of 5.7 months, demonstrated significantly more localized AL compared to the women in the control group (worst site AL 5.2 mm vs 4mm; p < 0.01). The relationship between AI use and worst site of AL held after adjustment for dental insurance status, tobacco use, income and previous radiation and chemotherapy exposure. It has been reported that whole mouth mean values for periodontal measures may not reflect the level of disease at individual affected sites 33 thus supporting the examination of the worst site mean values. Our data showed that worst sites mean values for AL and PD were 2-3 fold greater than whole mouth measures, and nearly one third of AI users had 6mm or greater of AL.
As noted previously, AI use in PM patients with BCa demonstrates enhanced rates of skeletal bone loss34 estimated between 2.6- 5.3% within the first 6 to 12 months of use 7, 35,36 as compared with untreated PM women whose rate is estimated to be at 2%.37 To our knowledge, there has not been a report on the use of AIs and periodontal health to which to compare our findings. However, the reduction of endogenous estrogen following the cessation of menses and the resulting low estrogen state among PM women has been shown to play a role in the progression of oral bone loss and AL.38-40 The differences in AL were supported by the salivary bone turnover markers results. Significantly higher levels of TNFα (p=0.003) and osteocalcin (p=0.03) were observed in the AI group as compared to controls. Salivary levels of RANKL (p=0.06) were higher in the AI group but did not reach statistical significance. TNFα is an important cytokine involved in many inflammatory responses including bone metabolism in health and disease.41 Likewise, osteocalcin and RANKL are bone associated proteins associated with osteoblast activity.42 The higher biomarker values noted in those treated with an AI suggests that the osteoblastic/osteoblastic activities may be increased. 43 Given that osteoblast signaling is integral to osteoclastic activity, it is hypothesized that these cytokine results reflect enhanced bone and connective tissue turnover and destruction.44.
In a more general sense osteoclast formation, locally in periodontitis and systemically in PM osteoporosis, shares many similar pathways for activation and function in pathogenic situations.45 The salivary findings herein support previous investigations of serum bone turnover biomarkers in blood or urine which noted increased TNFα concentrations in both estrogen deficient46 PM women as well as women undergoing AI therapy.6 In treatment-naïve PM women with BCa, AIs have been shown to increase the serum osteocalcin, TNFα, RankL and IL-6 markers levels by 10–35% in comparison with baseline PM levels.47
At present the mechanism connecting AI use with loss of periodontal structures is unclear, as duration of AI use was not found to play a significant role. One mechanism which could play a role are changes related to the oral microflora. Previous reports have linked changes in Bacteroides and other species associated with periodontal disease progression to changes in local or systemic estrogen and progesterone levels.48 However the direct link between oral microfloral changes and AI usage is limited to reports focused on mucosal lesions which cannot directly be attributed to AI usage alone independent of chemotherapy.49 A second mechanism which may explain the differences in AL and PD, may be the effect of AIs on oral wound healing. Our findings demonstrate no changes in MMP-8 or MMP-9 levels in saliva, but did show significant changes in osteocalcin levels between the two study populations. These results suggest, although do not prove, that the tissue turnover phase of wound healing may not, in and of itself, be altered. Thus, the repair phase of wound healing may be different in AI users. Yet, here too none of the three cytokines that we assayed which are associated with vascular remodeling (VEGF and SDF-1, but not IL-8) approached significance for being altered in the AI users over controls. Thus how tissue turnover differences in AI users verses controls could contribute to the clinical differences we observed, remains unclear. A third mechanism which could account for our observations is that immune response is altered in AI users. As estrogen has been shown to inhibit the expression of bone-resorbing cytokines such as IL-1, TNF-a and IL-6, for AI users, who are in a severely estrogen deficient state, higher amounts of these cytokines may be produced leading to the enhanced progression of bone loss in AI users as compared to non AI users. 45 Clearly further investigation is warranted to discern the mechanism(s) responsible for the periodontal impact of AI use.
Women on AIs demonstrated a trend toward lower perception of their oral health compared to PM controls (p=.056). These findings are in contrast to a population based analysis of the NHANES 1999-2004 data of PM women which reported that women with a diagnosis of BCa had a significantly higher perception of their oral health as compared to women without a BCa diagnosis (Taichman et al., unpublished observations). One possible reason for the difference in results is the NHANES analysis did not have complete data on anti-estrogen therapy exposure. Patients on AIs frequently report disturbed sleep, joint pain and arthralgia, and mood disturbances which result in higher levels of psychological distress-anxiety and depression. 50 This increased psychological anxiety in turn could impact these women's subjective oral health perceptions. Our observations in women on AIs may indicate an AI specific change in oral health perceptions and will require further investigation.
Interestingly there were no significant differences between AI users and controls regarding the question “How important is your dental health?” We were surprised by this finding given the psychological stress and anxiety that a diagnosis of cancer can bring to an individual. Yet, the number of sites demonstrating significant biofilms and calculus were significantly higher in the AI user groups. From these two sets of data we conclude that while dental/oral health is of great importance to women on AIs, they may not be able to achieve optimal oral care. The barriers to achieving optimal oral care amongst AI users is being evaluated in our ongoing clinical trials.
The strengths of this study include comprehensive whole mouth periodontal examinations and biomarker data. Furthermore, an extensive collection of the participant's demographic; cancer characteristics and treatments, oral health behaviors, and lifestyle behaviors have been established. However, this study also has some limitations. First, the homogeneity of the study participants related to race/ethnicity and other socio-demographic characteristics requires careful interpretation and caution when generalizing to other groups. Second, these data only consider information of up to 12 months of use of AIs. Future research should consider the effects of long term AI use on these women's periodontal health. Finally, this study is limited by its cross-sectional design which prevents the determination of causality between AI use and periodontal parameters. An ongoing clinical study (NCT01693731) is being conducted to address some of these concerns.
Conclusions
Oral health has significant implications for overall systemic health, thus oral health is an important component of BCa survivorship care. We have shown for the first time that adjuvant AI use is associated with increased AL and gingival bleeding in PM early stage ER + BCa survivors. Since long-term survival rates are high in patients with early-stage BCa who receive AIs, and treatment may continue for many years, the complications arising from therapy in this patient population can have long-term effects and may ultimately impact patients’ quality of life. Additional prospective clinical studies with women on adjuvant AI are needed.
Summary.
Aromatase inhibitor use in early stage breast cancer survivors is associated with an increase in periodontal conditions and requires further investigation.
Acknowledgements
The study was supported by a pilot grant from the Michigan Center for Oral Health Research and the Michigan Institute for Clinical and Health Research UL1RR024986 (University of Michigan, Ann Arbor, MI) and the National Institute of Dental & Craniofacial Research (NIDCR) grants 1K23DEO21779 and5K22 DE20197 (Bethesda, Maryland).
Footnotes
Quantibody Human Cytokine Custom Array, RayBiotech, Inc, Norcross, GA, USA
STATA Statistics and Data Analysis, Version 11, STATA Corporation
The authors report no conflicts of interest related to this study.
References
- 1.American Cancer Society . Breast Cancer Facts & Figures 2009-2010. American Cancer Society, Inc; Atlanta: 2009. [August 22, 2014]. Available at http://www.cancer.org/cancer/breastcancer/detailedguide/breast-cancer-key-statistics. [Google Scholar]
- 2.Ries LA YJ, Keel GE, Eisner MP, Lin YD, Horner MJ. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program; Bethesda, MD: 2007. [July 22, 2013]. Available at http://seer.cancer.gov/publications/survival. [Google Scholar]
- 3.Anderson WF, Chatterjee N, Ershler WB, Brawley OW. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat. 2002;76:27–36. doi: 10.1023/a:1020299707510. [DOI] [PubMed] [Google Scholar]
- 4.Howell A, Cuzick J, Baum M, et al. Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer. Lancet. 2005;365:60–62. doi: 10.1016/S0140-6736(04)17666-6. [DOI] [PubMed] [Google Scholar]
- 5.Winer EP, Hudis C, Burstein HJ, et al. American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report 2004. J Clin Oncol. 2005;23:619–629. doi: 10.1200/JCO.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 6.Eastell R, Hannon RA, Cuzick J, et al. Effect of an aromatase inhibitor on bmd and bone turnover markers: 2-year results of the anastrozole, tamoxifen, alone or in combination (ATAC) trial. J Bone Miner Res. 2006;21:1215–1223. doi: 10.1359/jbmr.060508. [DOI] [PubMed] [Google Scholar]
- 7.Eastell R, Adams JE, Coleman RE, et al. Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial. J Clin Oncol. 2008;26:1051–1057. doi: 10.1200/JCO.2007.11.0726. [DOI] [PubMed] [Google Scholar]
- 8.Lonning PE, Geisler J. Aromatase inhibitors--socio-economical issues. J Steroid Biochem Mol Biol. 2005;95:137–142. doi: 10.1016/j.jsbmb.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Perez EA, Josse RG, Pritchard KI, et al. Effect of letrozole versus placebo on bone mineral density in women with primary breast cancer completing 5 or more years of adjuvant tamoxifen: a companion study to NCIC CTG MA.17. J Clin Oncol. 2006;24:3629–635. doi: 10.1200/JCO.2005.05.4882. [DOI] [PubMed] [Google Scholar]
- 10.von Wowern N, Klausen B, Kollerup G. Osteoporosis: a risk factor in periodontal disease. J Periodontol. 1994;65:1134–1138. doi: 10.1902/jop.1994.65.12.1134. [DOI] [PubMed] [Google Scholar]
- 11.Bozic M, Ihan Hren N. Osteoporosis and mandibles. Dentomaxillofac Radiol. 2006;35:178–84. doi: 10.1259/dmfr/79749065. [DOI] [PubMed] [Google Scholar]
- 12.White SC, Atchison KA, Gornbein JA, et al. Change in mandibular trabecular pattern and hip fracture rate in elderly women. Dentomaxillofac Radiol. 2005;34:168–174. doi: 10.1259/dmfr/32120028. [DOI] [PubMed] [Google Scholar]
- 13.Olin JL, St Pierre M. Aromatase Inhibitors in Breast Cancer Prevention. Ann Pharmacother. 2014;48:1605–10. doi: 10.1177/1060028014548416. [DOI] [PubMed] [Google Scholar]
- 14.Tezal M, Wactawski-Wende J, Grossi SG, Dmochowski J, Genco RJ. Periodontal disease and the incidence of tooth loss in postmenopausal women. J Periodontol. 2005;76:1123–1128. doi: 10.1902/jop.2005.76.7.1123. [DOI] [PubMed] [Google Scholar]
- 15.Wactawski-Wende J. Periodontal diseases and osteoporosis: association and mechanisms. Ann Periodontol. 2001;6:197–208. doi: 10.1902/annals.2001.6.1.197. [DOI] [PubMed] [Google Scholar]
- 16.Hildebolt CF. Osteoporosis and oral bone loss. Dentomaxillofac Radiol. 1997;26:3–15. doi: 10.1038/sj.dmfr.4600226. [DOI] [PubMed] [Google Scholar]
- 17.Miller CS, King CP, Jr., Langub MC, Kryscio RJ, Thomas MV. Salivary biomarkers of existing periodontal disease: a cross-sectional study. J Am Dent Assoc. 2006;137:322–329. doi: 10.14219/jada.archive.2006.0181. [DOI] [PubMed] [Google Scholar]
- 18.Ramseier CA, Kinney JS, Herr AE, et al. Identification of pathogen and host-response markers correlated with periodontal disease. J Periodontol. 2009;80:436–446. doi: 10.1902/jop.2009.080480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinney JS, Morelli T, Braun T, et al. Saliva/pathogen biomarker signatures and periodontal disease progression. J Dent Res. 2011;90:752–758. doi: 10.1177/0022034511399908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tezal M, Wactawski-Wende J, Grossi SG, et al. The relationship between bone mineral density and periodontitis in postmenopausal women. J Periodontol. 2000;71:1492–1498. doi: 10.1902/jop.2000.71.9.1492. [DOI] [PubMed] [Google Scholar]
- 21.Wactawski-Wende J, Grossi SG, Trevisan M, et al. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67:1076–1084. doi: 10.1902/jop.1996.67.10s.1076. [DOI] [PubMed] [Google Scholar]
- 22.Wactawski-Wende J, Hausmann E, Hovey K, et al. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76:2116–2124. doi: 10.1902/jop.2005.76.11-S.2116. [DOI] [PubMed] [Google Scholar]
- 23.Hildebolt CF, Vannier MW, Shrout MK, et al. Periodontal disease morbidity quantification. II. Validation of alveolar bone loss measurements and vertical defect diagnosis from digital bite- wing images. J Periodontol. 1990;61:623–32. doi: 10.1902/jop.1990.61.10.623. [DOI] [PubMed] [Google Scholar]
- 24.Krall EA. Osteoporosis and the risk of tooth loss. Clin Calcium. 2006;16:287–290. [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Taguchi A, Sanada M, Krall E, et al. Relationship between dental panoramic radiographic findings and biochemical markers of bone turnover. J Bone Miner Res. 2003;18:1689–94. doi: 10.1359/jbmr.2003.18.9.1689. [DOI] [PubMed] [Google Scholar]
- 27.National Comprehensive Cancer Network (NCCN) Practice Guidelines in Oncology-v.1 2010. [December 17, 2014];Invasive breast cancer. Available at http://www.nccn.org/professionals/physicians_gls/PDF/breast.pdf.
- 28.Powles TJ, Hickish T, Kanis JA, Tidy A, Ashley S. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- 29.Duckworth JE, Judy PF, Goodson JM, Socransky SS. A method for the geometric and densitometric standardization of intraoral radiographs. J Periodontol. 1983;54:435–440. doi: 10.1902/jop.1983.54.7.435. [DOI] [PubMed] [Google Scholar]
- 30.Rasband WS. ImageJ Image Processing and Analysis in Java software. U. S. National Institutes of Health; Bethesda, Maryland, USA: 1997-2014. [March 3, 2013]. Available at http://imagej.nih.gov/ij/. [Google Scholar]
- 31.Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;8:25–47. [PubMed] [Google Scholar]
- 32.Centers for Disease Control and Prevention (CDC) National Health and Nutrition Examination Protocol. U.S.: Department of Health and Human Services, Centers for Disease Control and Prevention; Hyattsville, MD: [August 29, 2014]. National Center for Health Statistics. Avaliable at http://www.cdc.gov/nchs/nhanes/nhanes_questionnaires.htm. [Google Scholar]
- 33.LaMonte MJ, Hovey KM, Genco RJ, et al. Five-year changes in periodontal disease measures among postmenopausal females: the Buffalo OsteoPerio study. J Periodontol. 2013;84:572–584. doi: 10.1902/jop.2012.120137. [DOI] [PubMed] [Google Scholar]
- 34.Burstein HJ, Prestrud AA, Seidenfeld J, et al. American Society of Clinical Oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor- positive breast cancer. J Clin Oncol. 2010;28:3784–96. doi: 10.1200/JCO.2009.26.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eastell R. Aromatase inhibitors and bone. J Steroid Biochem Mol Biol. 2007;106:157–161. doi: 10.1016/j.jsbmb.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Coleman RE, Banks LM, Girgis SI, et al. Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the Intergroup Exemestane Study (IES): a randomised controlled study. Lancet Oncol. 2007;8:119–27. doi: 10.1016/S1470-2045(07)70003-7. [DOI] [PubMed] [Google Scholar]
- 37.Kanis JA, McCloskey EV, Powles T, et al. A high incidence of vertebral fracture in women with breast cancer. Br J Cancer. 1999;79:1179–1181. doi: 10.1038/sj.bjc.6690188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Payne JB, Reinhardt RA, Nummikoski PV, Patil KD. Longitudinal alveolar bone loss in postmenopausal osteoporotic/osteopenic women. Osteoporos Int. 1999;10:34–40. doi: 10.1007/s001980050191. [DOI] [PubMed] [Google Scholar]
- 39.Pilgram TK, Hildebolt CF, Dotson M, et al. Relationships between clinical attachment level and spine and hip bone mineral density: data from healthy postmenopausal women. J Periodontol. 2002;73:298–301. doi: 10.1902/jop.2002.73.3.298. [DOI] [PubMed] [Google Scholar]
- 40.Pilgram TK, Hildebolt CF, Yokoyama-Crothers N, et al. Relationships between longitudinal changes in radiographic alveolar bone height and probing depth measurements: data from postmenopausal women. J Periodontol. 1999;70:829–833. doi: 10.1902/jop.1999.70.8.829. [DOI] [PubMed] [Google Scholar]
- 41.Walsh MC, Kim N, Kadono Y, et al. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 42.Katagiri T, Takahashi N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral Dis. 2002;8:147–59. doi: 10.1034/j.1601-0825.2002.01829.x. [DOI] [PubMed] [Google Scholar]
- 43.Folkestad L, Bjarnason NH, Bjerregaard JK, Brixen K. The effect of aromatase inhibitors on bone metabolism. Basic Clin Pharmacol Toxicol. 2009;104:3–10. doi: 10.1111/j.1742-7843.2008.00337.x. [DOI] [PubMed] [Google Scholar]
- 44.Kwan Tat S, Padrines M, Theoleyre S, Heymann D, Fortun Y. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev. 2004;15:49–60. doi: 10.1016/j.cytogfr.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 45.Lerner UH. Inflammation-induced bone remodeling in periodontal disease and the influence of post-menopausal osteoporosis. J Dent Res. 2006;85:596–607. doi: 10.1177/154405910608500704. [DOI] [PubMed] [Google Scholar]
- 46.Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- 47.Szulc P. The role of bone turnover markers in monitoring treatment in postmenopausal osteoporosis. Clin Biochem. 2012;45:907–19. doi: 10.1016/j.clinbiochem.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 48.Tarkkila L, Kari K, Furuholm J, Tiitinen A, Meurman JH. Periodontal disease-associated micro-organisms in peri-menopausal and post-menopausal women using or not using hormone replacement therapy. A two-year follow-up study. BMC Oral Health. 2010;10:10. doi: 10.1186/1472-6831-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jensen SB, Mouridsen HT, Bergmann OJ, et al. Oral mucosal lesions, microbial changes, and taste disturbances induced by adjuvant chemotherapy in breast cancer patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:217–26. doi: 10.1016/j.tripleo.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 50.Henry NL, Pchejetski D, A'Hern R, et al. Inflammatory cytokines and aromatase inhibitor- associated musculoskeletal syndrome: a case-control study. Br J Cancer. 2009;103:291–6. doi: 10.1038/sj.bjc.6605768. [DOI] [PMC free article] [PubMed] [Google Scholar]