Abstract
The aged population suffers increased morbidity and higher mortality in response to episodes of acute kidney injury (AKI). Aging is associated with telomere shortening, and both telomerase reverse transcriptase (TerT) and RNA (TerC) are essential to maintain telomere length. To define a role of telomerase deficiency in susceptibility to AKI, we used ischemia/reperfusion injury in wild type mice or mice with either TerC or TerT deletion. Injury induced similar renal impairment at day 1 in each genotype, as assessed by azotemia, proteinuria, acute tubular injury score and apoptotic tubular epithelial cell index. However, either TerC or TerT knockout significantly delayed recovery compared to wild type mice. Electron microscopy showed increased autophagosome formation in renal tubular epithelial cells in wild type mice but a significant delay of their development in TerC and TerT knockout mice. There were also impeded increases in the expression of the autophagosome marker LC3 II, prolonged accumulation of the autophagosome protein P62, an increase of the cell cycle regulator p16, and greater activation of the mTOR pathway. The mTORC1 inhibitor, rapamycin, partially restored the ischemia/reperfusion-induced autophagy response, without a significant effect on either p16 induction or tubule epithelial cell proliferation. Thus, muting the maintenance of normal telomere length in mice impaired recovery from AKI, due to an increase in tubule cell senescence and impairment of mTOR-mediated autophagy.
Keywords: Telomerase T, telomerase C, renal ischemia reperfusion, autophagy, proximal tubule, mammalian target of rapamycin (mTOR), senescence, p16
Introduction
AKI frequently results from tubule injury by acute ischemic or toxic exposure to the kidney1, 2,3, with higher morbidity and increasing mortality seen in aged patients (≥ 65 yrs.)4–7. Age-related renal morphological changes, functional alterations and accompanying comorbidities may all contribute to the vulnerability of the aged population to either acute or chronic renal injury8–10. However, intrinsic underlying predisposing molecular and genetic mechanisms related to aging per se remain incompletely studied.
Telomeres become shorter each time a cell divides and are shortened in an age-dependent manner in human kidney, particularly in renal cortex11, and telomere shortening reduces regenerative capacity after renal injury12. Telomerase is a reverse transcriptase enzyme complex that adds DNA sequence repeats (TTAGGG) to the 3' end of DNA strands in the telomere regions at the ends of eukaryotic chromosomes. There are two major components in the transcriptase ribonucleoprotein complex: the RNA-directed DNA polymerase, TerT and the RNA template, TerC. TerC or TerT gene mutations are invariably associated with marked telomere shortening, resulting in Dyskeratosis Congenita and inherited bone marrow failure syndromes in humans13 and are risk factors for a range of other human telomeric syndromes, including aplastic anemia, idiopathic pulmonary fibrosis, and acute myeloid leukemia14. Telomerase participates in chromosomal repair; de novo synthesis of telomere repeats may occur at double-stranded breaks15.
Absence of telomerase leads to telomere shortening progressively during successive generations of TerC or TerT deficient mice16–19. To investigate the impact of telomerase on renal tubular injury and regeneration, we induced acute renal damage by clamping both renal pedicles in G4 mice with either TerT or TerC deficiency to compare tubular injury and regeneration with wild type mice and to explore underlying mechanisms. Since non-telomerase functions have also been linked to TerT20, we used both TerC and TerT KO mice in the current study to clarify the role of telomerase deficiency. Short telomeres in those mice were confirmed by our co-author, Dr. Lawson and colleagues previously21.
Results
I/R led to Renal Injury in Each Genetic Group, but with Delayed Recovery in Mice with TerC/ TerT KO
I/R induced elevation of BUN with a peak at day 1 in mice from each genetic group (Fig. 1A). BUN quickly returned to normal within 14 days in the wild type mice but was delayed in both the TerC or TerT KO mice (Fig. 1A). Serum creatinine levels were consistent with the BUN results (Suppl. Fig. 1A). Since sham operation did not cause significant differences in renal function (data not shown), subsequent studies only investigated mice subjected to I/R. I/R induced both increased albuminuria (Suppl. Fig. 1B) and low molecular weight proteinuria (so called tubular proteinuria)22 (Suppl. Fig. 1C); albuminuria and total proteinuria persisted longer in mice with either TerC KO or TerT KO.
Fig. 1. Delayed Recovery of BUN and Proteinuria in TerC/TerT KO Mice after Ischemia-Reperfusion Injury.
A) I/R induced increased BUN in mice from each genetic group, but delayed recovery in TerC and TerT KO mice. n=8–10, *: P<0.05, compared with basal level; #: P<0.05 TerC and TerT KO mice compared to Wt mice. B) Delayed renal tubular restoration from I/R in TerC and TerT KO mice. Representative photos from day 0 (D0), 1, 3 and 14 were selected. Scale bar = 200 µm. C) Tubular injury score further supports the delayed recovery from I/R in mice with telomerase deficiency. n=4, *: P<0.05. D) TUNEL staining indicated more extensive renal tubule apoptosis in mice with telomerase deficiency. Representative photos from 4 independent experiments. E) Semi- quantitation of TUNEL-positive tubular cells. n=4, *: P<0.05. F) Increased renal active cleaved caspase-3 in TerC/TerT KO mice following I/R. Immunoblot demonstrated increased active cleaved caspase-3 following I/R in TerC and TerT KO mice. Representative photos from 3 independent experiments.
Renal histopathology assessed with PAS staining further confirmed I/R-induced renal injury, predominantly in proximal tubules. All mice subjected to I/R demonstrated significant tubular damage, including loss of brush border, shedding of both necrotic and viable epithelial cells into the tubular lumen, tubular dilation, cast formation, and cell lysis (Fig. 1B). A semi-quantitative acute tubular injury score (ATI) indicated that in wild type mice, morphology began to recover from day 3 and had almost returned to normal at day 14, while slower histologic evidence of recovery was seen in either TerC or TerT KO mice (Fig. 1C). Only mild fibrosis by Masson's trichrome stain was detected within the observation period in all groups (data not shown). KIM-1 immunohistology was increased in all groups post I/R, but had returned to baseline levels by day 14 in wild type mice while remaining elevated in TerC or TerT KO mice (data not shown).
Telomerase Deficiency was Linked to More Severe Apoptosis, Lower Proliferation and up-regulated cell-cycle inhibitor, p16 in TECs after I/R
I/R induced significant apoptosis from day 1 in each group, but there was evidence for prolonged apoptosis in the TerC KO and TerT KO mice (Fig. 1D and E); significance differences were seen at day 5 and 7 (Fig. 1D & E). Apoptosis was predominantly detected in proximal tubules (Fig. 1D). Determination of active cleaved caspase-3 further confirmed the extended apoptosis in TerC/TerT KO mice following I/R (Fig. 1F). Cell proliferation evaluated by Ki67 staining indicated that Ki67 positivity, predominantly in proximal tubule, appeared as early as day 1, with a peak at day 3 in wild type mice. In contrast, there was delayed and blunted Ki 67 positivity in TerC and TerT KO mice (Fig. 2A–B).
Fig. 2. Inhibition of Proliferation after I/R of TECs in Mice with Telomerase Deficiency.
A) Representative Ki67 Staining; B) Quantitation of Ki 67 Positive cells. n=4, *: P<0.05.
Previous studies have indicated that in the presence of dysfunctional telomeres, environmental stress leads to induction of the cell-cycle regulator, p1623. Few positive p16 cells were detected in kidneys from wild type mice at baseline, and I/R induced only moderate up-regulation at day 1, especially in proximal tubule epithelial cells (Fig. 3A). There was higher basal expression and stronger I/R stimulation of p16 in both TerC and TerT KO mice, and increased p16 expression was still present 7 days post I/R (Fig. 3B & C).
Fig. 3. I/R and Telomerase Deficiency Up-regulated p16.
A) Representative image. Scattered p16+ TECs were detected at baseline in TerC and TerT KO mice, indicated by red arrows. I/R induced p16 predominantly in renal tubules. B) Representative renal p16 immunoblotting. C) Semi-quantitation of p16 immunoblotting, normalized by β actin.
Telomerase Deficiency Blunted Autophagic Responses to I/R
Autophagy is an important cellular housekeeping process and has been proposed to be renoprotective24,25 and influenced by age26,27. Autophagosomes, detected by EM, increased in wild type mice, beginning as early as day 3. In contrast, there was a delay in the onset of autophagosome development in in either TerC or TerT KO mice, and increased autophagosomes were detected as late as day 14 after I/R injury (Fig. 4A). Consistent with the delayed appearance of autophagosomes, the LC3 II/I ratio significantly increased by day 3 after I/R in wild type mice, but only at day 14 after I/R TerC or TerT KO mice (Fig.4B & 4D). There was also prolonged accumulation of P62 following I/R injury in mice with telomerase deficiency (Fig. 4C & 4D).
Fig. 4. Autophagy Impairment During I/R Injury in Mice with Telomerase Deficiency.
A) Representative image of I/R-induced autophagosome formation by electron microscopy, Scale bar = 2 µm, The areas in D3 from Wt and D14 from TerC and TerT KO mice are composed of the enlarged autophagosomes; B) Immunoblot of LC3 indicating I/R-induced conversion from LC3 I to II; C) Prolonged P62 expression in mice with telomerase deficiency after I/R. D) Semiquantitation of the LC3 II /I ratio and P62 expression. n=4.
mTOR Activation was associated with Autophagy Impairment in Telomerase Deficient Mice during I/R
mTOR signaling is crucial for cellular functions and plays important roles in aging28. mTOR activation has been reported in genetic telomerase-deficient (K5TRF2/TerC−/−) mice29. Following I/R injury, phospho-mTOR expression persisted in either TerC or TerT KO mice compared to wild type mice (Fig. 5A–D). In isolated TECs, there was also increased phosphorylation of mTOR, along with its downstream targets, p70-S6 kinase and 4E-BP1 in response to an anoxic insult (Suppl. Fig. 2A).
Fig. 5. I/R Activated mTOR in Mice with Telomerase Deficiency.
A & B) Immunoblotting indicated up-regulation of mTOR phosphorylation by I/R in Wt and TerC or TerT KO mice, with higher p-mTOR and more prolonged expression in mice with telomerase deficiency. C–D) Semiquantitative data (from A–B respectively) further support the above finding, n=4. Dotted line indicated for mTOR/ β Actin; solid line for p-mTOR/ β Actin.
To determine the role of mTOR in autophagy impairment, we inhibited mTORC1 activity in response to anoxia by administration of rapamycin in primary cultured TECs. In TECs from wild type mice, moderate increases in mTOR activation were detected at 24 hrs, which returned to normal at 48 hrs, but mTOR activation was still detected in TerC KO mice at 48 hrs after the anoxic insult (Suppl. Fig. 2B). There was also delayed conversion of the autophagic marker LC3 I to II and persistent P62 in TECs with TerC deletion (Fig. 6A, B), both of which were reversed by rapamycin (Fig. 6C, D). Anoxia induced conversion of LC3 as early as 6 hours post I/R in wild type mice and reached peak levels at 24h. In the wild type mice, the response to rapamycin was more significant at 6 hours instead of 24 hours following transient anoxia (Suppl. Fig. 3).
Fig. 6. Impaired Autophagy in Mouse Renal Tubular Cells with TerC deletion in response to anoxia, which was rescued by rapamycin.
A) Delayed increase of LC3 II /I and prolonged P62 expression in tubular epithelial cells (TECs) from TerC KO mice. B) The mTORC1 inhibitor, rapamycin, increased LC3 II /I, and decreased P62 in TECs. C) Rapamycin stimulated the conversion of LC3 I to II in TECs with TerC deletion. D) Rapamycin promoted P62 normalize in TECs with TerC deficiency. n=4.
To examine further the effect of mTOR in telomerase deficient kidney during I/R, we treated TerC mice with the mTORC1 inhibitor, Rapamycin. Rapamycin promoted conversion of LC3 from I to II in TerC KO mice, which was most obvious on day 3 to 5 following I/R (Fig.7), when LC3 II was still very low without treatment. Since I/R induced LC3 conversion earlier in wild type mice, a stimulation response by rapamycin in these mice was seen on day 1 (Fig.7). Correspondingly, rapamycin reduced P62 accumulation in TerC KO mice as well as wild type mice. The time course of rapamycin in TerC KO and wild type is shown in Fig. Suppl. 4. These results further support that mTOR activation can lead to inhibition to autophagy during I/R. However, notably there was no significant impact of rapamycin on BUN recovery (Suppl. Fig. 4), prompting further investigation into the role of cell senescence on altered recovery.
Fig. 7. Rapamycin stimulates Autophagy during I/R.
Rapamycin (0.16 mg/kg) intraperitoneally daily administration following surgery led to increase conversion of LC3 from I to II and decreased accumulation of P62 in TerC KO mice significantly at Day 3–5 and wild type at day 1. Representative photo of TerC KO mice were from three independent mice experiments; wild type from two independent mice experiments.
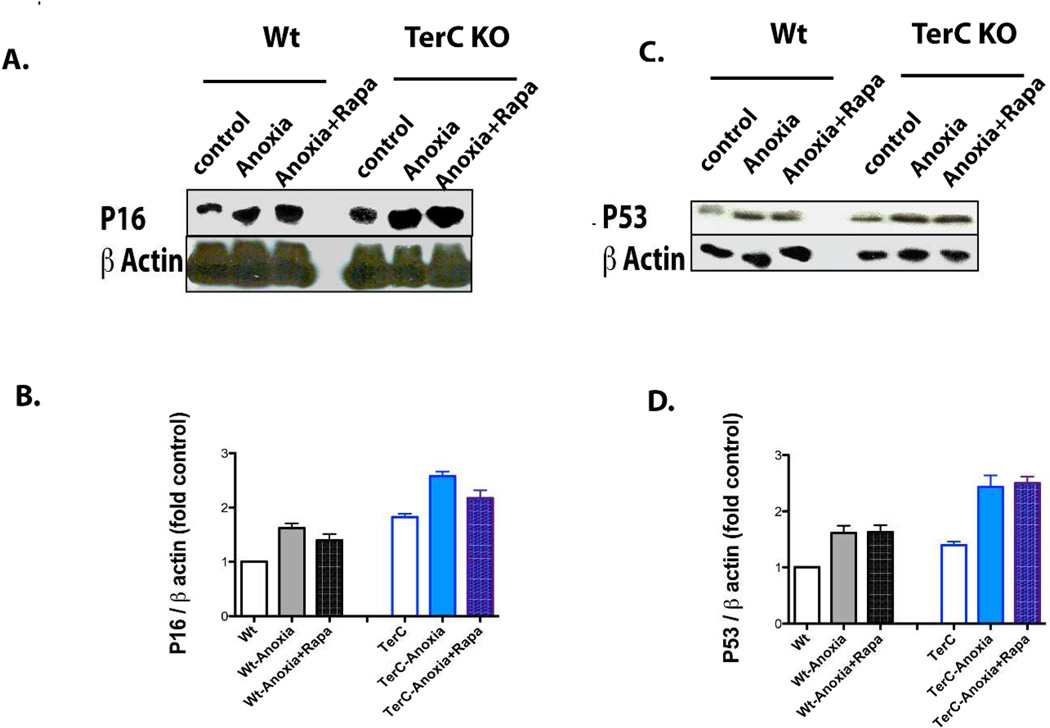
Rapamycin did not significantly reverse the up-regulation of p16 or proliferation defect in Injured TECs by I/R
TerC-deficient TECs had increased p16 at baseline, with further increased expression in response to the anoxic insult (Fig. 8A & B). Rapamycin treatment had no significant inhibitory effect on p16 expression (Fig. 8A & B) or p53 (Fig. 8C & D) expression in either wild type- or TerC-deleted TECs. There was increased proliferation of wild type TECs compared to TerC deficient TECs in response to the anoxic insult (Suppl. Fig. 5A, B). Rapamycin partially inhibited proliferation in the wild type cells but did not significantly affect the proliferation of senescent TECs from TerC KO mice (Suppl. Fig 5A, B).
Fig. 8. Rapamycin did not Significantly Affect p16 or P53 in TECs following Anoxia.
A) Immunoblotting indicating up-regulation of p16 by I/R and higher p16 expression with TerC deficiency; B) Semiquantitation of p16 expression, n=4. C) Immunoblotting indicating up-regulation of p53 by anoxia, with greater up-regulation with TerC deficiency; D) Semiquantitation of p53 expression, n=4.
Discussion
The current studies using mouse models of telomerase deficiency suggested that deletion of either TerC or TerT delayed renal tubular regeneration and increased apoptosis in tubular epithelial cells after I/R injury. This delay was accompanied with impairment of autophagy. In addition, the telomerase deficient mice had increased expression at baseline of p16, which has been associated with cell senescence, with further increases in response to I/R injury. Telomerase deficiency also increased mTOR activation in renal epithelial cells at baseline, with further increases in response to I/R injury. mTORC1 inhibition led to partial recovery of the I/R-induced autophagy response in primary cultured renal TECs with TerC deletion but did not alter the proliferation defect or increased expression of p16 or P53. Rapamycin treatment stimulated the autophagic response to I/R in kidney from TerC mice, but did not accelerate functional recovery.
Telomerase contains two major components: the catalytic protein, TerT, and the RNA component, TerC, which prevents telomere shortening by adding telomeric DNA repeats to chromosome ends30. Telomeres become shorter with aging, influenced by environmental factors and genetic alterations like telomerase mutations31. Genetic deletion of either TerC or TerT in mice does not lead to significant phenotypic abnormalities at an early age in the first generations, but does lead to telomere shortening by G4, with premature loss of viability and decreased lifespan associated with a number of degenerative pathologies16,32,33, 34. Telomere shortening has been described in human kidneys from aged patients, without significant association between telomere length and renal function11. However, shorter telomere length observed at the time of transplantation of a renal allograft was35.
A recent study with TerC KO mice suggested that murine kidneys with critically short telomeres were prone to acute cell death and reduced long-term regeneration12. The prevalence of chronic kidney disease with age also supports a potential role of telomere length and telomerase activity in its progression36. Our data demonstrated delayed recovery from renal I/R in mice with deletion of either TerC or TerT, further suggesting an influence of telomerase on renal regeneration after injury.
Telomerase deficiency mice, as a model for accelerated aging, have been shown to modulate a number of signaling pathways and genes, including the up-regulation of mTOR29. mTOR (especially mTORC1), is a ubiquitous kinase that regulates many different cellular processes, including possibly mediating the process of regeneration and recovery following acute injury37. Previous studies suggested that I/R induced mTOR activation and inhibition by rapamycin delayed renal tubular recovery38, 39. On the other hand, autophagy has been suggested to be renal protective following I/R injury, and mTOR is known to inhibit autophagy, leaving unresolved whether inhibiting mTORC1 could increase autophagic flux during AKI40,24,41,42,43,44, 45. Recently it has been reported that mTORC1-deficient mice have decreased renal recovery from injury46, suggesting an essential role in maintaining renal tubular homeostasis.
mTOR signaling may also have significant effects on aging28. Activation of this pathway may convert cellular quiescence into senescence47,48 and promote cellular and organismal aging49,50. Inhibition of mTOR expression may increase mammalian lifespan51. It is important to understand the multiple impacts of mTOR in renal ischemia, especially in the telomerase deficient state.
We found that in wild type mice renal mTOR activity was low at baseline, but increased after ischemia-reperfusion injury, and the increases seen in telomerase deficient mice were greater and more prolonged. To examine the role of mTOR signaling specifically on renal tubules, we isolated TECs and confirmed that mTOR activity was higher in cells from mice with telomerase deficiency than wild type mice, even at basal levels. Inhibition of mTORC1 activity by rapamycin partially restored the defect in autophagy in response to acute injury in cells from telomerase deficient mice. However, activation of mTOR signaling may be the secondary response to I/R. Its inhibition by rapamycin also repressed proliferation in wild type TECs. In TECs from TerC KO mice, telomerase deficiency inhibited proliferation with or without rapamycin, which may be related to p16 mediated cell senescence23.
p16 protein, a member from the INK4 family (p16INK4a), is a cyclin-dependent kinase (CDK) inhibitor that inhibits the cell cycle by blocking progression from G1 phase to S phase. It is a robust biomarker and a possible effector in mammalian renal aging52. Deletion of p16 results in improved kidney regeneration and decreased capillary rarefaction after I/R53. Our data confirmed previous reports that I/R or dysfunctional telomeres induces p1653,12, and further demonstrated that rapamycin did not significantly suppress p16 up-regulation in our cultured telomerase-deficient TECs.
Therefore, I/R accelerates senescence and autophagy impairment in mice with telomerase deficiency, probably triggered by endogenous and exogenous stimuli that potentially induce DNA-damage (e.g. oxidative stress). Autophagy controls the quality of cellular components to prevent cell senescence54,55. Meanwhile, senescent cells increase lysosomal enzymes directed towards lipofuscin-rich lysosomes, but these enzymes lose effective autophagic degradation, leaving the lipofusicin non-degradable, which further decreases autophagy in senescent post mitotic cells56.
In summary, we found that telomerase deficiency delayed renal recovery from I/R injury in mice, which was accompanied by impairment of renal tubular autophagy, mediated in part by over-activation of mTOR pathway. Proliferation was also decreased in telomerase deficient mice, which was associated with increased expression of the senescence marker, p16 and p53. Thus, telomerase-deficient dependent renal tubular senescence and autophagy impairment may synergistically contribute to delayed renal tubular regeneration in TerC/TerT KO mice.
Methods
Materials
Rabbit anti-p62, anti-LC3 and anti-phospho-mTOR (Ser2448) antibodies were purchased from Sigma-Aldrich Co. (St. Louis, MO); rabbit anti-mTOR, anti-4E-BP1, anti-phospho-4E-BP1 (Ser65), anti-p70 S6 Kinase (Thr389) and Cleaved Caspase-3 (Asp175) antibody were from Cell Signaling Technology (Danvers, MA); anti-β actin antibody was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); rabbit anti-TIM antibody was from ProSci. Inc. (Poway, CA), rabbit ant-Ki67 antibody was from Abcam (Cambridge, MA); CDKN2A/p16INK4A antibody was from LifeSpan Biosciences, Inc. (Seattle, WA); TUNEL apoptosis detection kits were from Upstate (Lake Placid NY). Rapamycin, L-arginine and other reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Animal Experiments
Mice with TerT or TerC deficiency on the C57BL/6J background were originally from The Jackson Laboratory (Bar Harbor, Maine). Age-matched (10–12 weeks old) male mice were used in each genetic group: Wild type (C57BL/6J); TerC KO or TerT KO mice were subjected to standard renal ischemia-reperfusion protocols. Briefly, Mice were anesthetized with ketamine (100 mg/kg ip) and xylazine (10 mg/kg ip). Both renal pedicles were clamped for 25 min. with nontraumatic microsurgical vascular clips (Aesculap, Tuttlingen, Germany). After removal of the vascular clamp and closing the surgical wounds, mice recovered on a thermo-regulated pad to maintain body temperature at 37°C and received analgesia. As controls, sham-operation was performed in each group by anesthesia and laparotomy only. At day 0, 1, 3, 7 and 14 post surgery blood and urine were collected; kidneys were removed in each group at the indicated end points. A subgroup of wild type and TerC KO mice were either administrated rapamycin (0.16 mg/kg) or its vehicle (0.1 ml 0.3% DMSO) intraperitoneally daily following surgery (8 TerC KO and 6 wild type mice were treated with rapamycin).
The Animal Care and Use Committee of Vanderbilt University Medical Center approved all animal procedures.
Assessment of renal injury
Renal injury was assessed histologically with light microscopy by H&E, PAS, Masson's trichrome stain and by electron microscopy (EM). Tissue damage was assessed in a blinded manner and scored using a tubular damage score as previously described57. Briefly, injury was scored according to the percentage of damaged tubules (loss of brush border, shedding of both necrotic and viable epithelial cells into the tubular lumen, tubular dilation, cast formation, and cell lysis): 1) less than 25% damaged; 2) 25–50% damaged; 3) 50–75% damaged; and 4) more than 75% damaged. EM samples were collected from the border of cortex and outer medulla and reviewed by a renal pathologist (Dr. Paueksakon). Autophagosomes were assessed semi-quantitatively from 10 fields in each sample.
Proteinuria, BUN and Creatinine
Urinary albumin levels were determined by ELISA using a murine microalbuminuria ELISA kit, AlbuwellM™ (Exocell Inc., Philadelphia, PA, USA). The urine creatinine concentration was measured with a microplate assay kit, Creatinine Companion (Exocell Inc., Philadelphia, PA, USA). All measurements were performed in duplicate and albuminuria was determined as the ratio of urinary albumin (µg/ml) to creatinine (mg/ml). For electrophoresis, urine was centrifuged at 17,000 × g for 15 min at 4 °C to remove urinary sediment including whole cells, large membrane fragments, and other debris. 3 µl of the remaining suspension was added to an equal volume of 2 × Laemmli sample buffer and run on a 15% gel under non-reducing conditions. The gel was stained with Coomassie stain (BioRad). BUN was detected with QuantiChrom™ Urea Assay Kit (DIUR-500) (BioAssay Systems, Hayward, CA) according to manufacturer’s instructions. Serum creatinine was detected with (Enzymatic) Reagent Set from Pointe Scientific, Inc. (Canton, MI).
Immunohistochemistry
Under deep anesthesia with Nembutal (70 mg/kg i.p.), mice were exsanguinated with 50-ml/100 g heparinized saline (0.9% NaCl, 2 U/ml heparin, 0.02% sodium nitrite) through a transcardial aortic cannula and fixed with glutaraldehyde-periodate acid saline (GPAS) as previously described. GPAS contains final concentrations of 2.5% glutaraldehyde, 0.011 M sodium metaperiodate, 0.04 M sodium phosphate, 1% acetic acid and 0.1 M NaCl and provides excellent preservation of tissue structure and antigenicity. Antigens were retrieved in 0.01 M citrate buffer pH 6.0 by microwave for 2 minutes, followed by steam for 25 minutes. The second antibody was localized using Vectastain ABC-Elite (Vector, Burlingame, CA) with diaminobenzidine as the chromogen, followed by a light counterstain with toluidine blue. The fixed kidneys were dehydrated through a graded series of ethanols, embedded in paraffin, sectioned at 4 µm thickness and mounted on glass slides.
Primary Culture of Mouse Renal Tubular Epithelial Cells (TECs) and in vitro study
Renal tubules were isolated immediately after sacrifice, using successive sieving similarly to previous descriptions58, 59. Briefly, kidneys were removed and washed with medium. The kidney cortices from Wt and TerC KO mice were cut into pieces and digested with collagenase (3 mg/ml) at 37°C for 25 minutes, followed by medium washing. The kidney digest was washed through a series of sieves or cell strainer (mesh diameters of 100, 70, and 40 µm). The cortical tubular cells were spun down at 300 g for 5 minutes, further washed, collected and cultured. Exclusion of glomeruli was confirmed under low-power microscopy.
For in vitro studies, renal TECs were rendered transiently anoxic by immersing the cellular monolayer in mineral oil according to the protocol of Meldrum et al.60 (Wu, 2007 #3562}. Briefly, renal TECs were placed in serum-free K1 medium61 for 24 hours, washed twice with PBS, and immersed in mineral oil (Sigma-Aldrich) for 60 minutes at 37°C in humidified air with 5% CO2 to form an epithelial cell sheet. At the end of immersion, TECs were returned to normal medium after extensive washing. This immersion simulated ischemia by restricting cellular exposure to either oxygen or nutrients. In each genetic group, a subset of cells remained untreated as control; another subset with anoxia was incubated with rapamycin (20 nM) in recovery medium. Cultured monolayer TECs proliferation was counted at 48 h with or without rapamycin (20 nM) as previously described39. Cellular nucleic acids synthesis was also detected with CyQUANT® NF Cell Proliferation Assay Kit (Invitrogen Corporation, Carlsbad, CA) according to the manufacturer’s instructions.
Immunoblotting
Cultured cells or mouse kidneys were homogenized as described previously62. Proteins were re-suspended in SDS-sample buffer, diluted in SDS buffer containing 2-mercaptoethanol (Sigma Chemical Co., St. Louis, MO, USA) and boiled for 10 minutes before loading. The samples were run on 8% SDS-PAGE gels under reducing conditions and transferred onto polyvinylidene fluoride (PVDF) membrane (Immobilion-P; Millipore Co., Bedford, MA, USA). After blocking with 5% non-fat milk in TTBS, the membranes were exposed to the primary antibody overnight at 4°C, followed by HRP-conjugated secondary antibodies. The HRP signal was enhanced using the ESL method and the images were developed on high performance autoradiography film, Hyperfilm™ MP (Amersham Biosciences, Buckinghamshire, UK). Membranes were re-hybridized with goat anti-β-actin antibody (Santa Cruz, CA) to normalize protein loading.
Apoptosis detection
Measurements utilized a TUNEL Apoptosis detection kit (UPSTATE, Lake Placid, NY). Propidium iodide (PI) was used for counter stain. The percentage of apoptotic tubular epithelial cells in 400 total cells from the same field was determined for quantification.
Statistical Analyses
All values are presented as mean ±SEM. ANOVA and Bonferroni t tests were used for statistical analysis, and differences were considered significant when p<0.05.
Supplementary Material
Acknowledgements
This work was supported by funds from the Department of Veterans Affairs and National Institutes of Health Grants DK51265, DK62794, and DK95785.
Footnotes
Disclosure: The authors declared no competing interests.
References
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. The Journal of clinical investigation. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chertow GM, Burdick E, Honour M, et al. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. Journal of the American Society of Nephrology : JASN. 2005;16:3365–3370. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 3.Chawla LS, Amdur RL, Shaw AD, et al. Association between AKI and Long-Term Renal and Cardiovascular Outcomes in United States Veterans. Clinical journal of the American Society of Nephrology : CJASN. 2013 doi: 10.2215/CJN.02440213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pascual J, Liano F, Ortuno J. The elderly patient with acute renal failure. Journal of the American Society of Nephrology : JASN. 1995;6:144–153. doi: 10.1681/ASN.V62144. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Kader K, Palevsky PM. Acute kidney injury in the elderly. Clin Geriatr Med. 2009;25:331–358. doi: 10.1016/j.cger.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitt R, Coca S, Kanbay M, et al. Recovery of kidney function after acute kidney injury in the elderly: a systematic review and meta-analysis. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2008;52:262–271. doi: 10.1053/j.ajkd.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 7.Hsu RK, McCulloch CE, Dudley RA, et al. Temporal changes in incidence of dialysis-requiring AKI. Journal of the American Society of Nephrology : JASN. 2013;24:37–42. doi: 10.1681/ASN.2012080800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin JE, Sheaff MT. Renal ageing. J Pathol. 2007;211:198–205. doi: 10.1002/path.2111. [DOI] [PubMed] [Google Scholar]
- 9.Chronopoulos A, Cruz DN, Ronco C. Hospital-acquired acute kidney injury in the elderly. Nature reviews Nephrology. 2010;6:141–149. doi: 10.1038/nrneph.2009.234. [DOI] [PubMed] [Google Scholar]
- 10.Anderson S, Eldadah B, Halter JB, et al. Acute kidney injury in older adults. Journal of the American Society of Nephrology : JASN. 2011;22:28–38. doi: 10.1681/ASN.2010090934. [DOI] [PubMed] [Google Scholar]
- 11.Melk A, Ramassar V, Helms LM, et al. Telomere shortening in kidneys with age. Journal of the American Society of Nephrology : JASN. 2000;11:444–453. doi: 10.1681/ASN.V113444. [DOI] [PubMed] [Google Scholar]
- 12.Westhoff JH, Schildhorn C, Jacobi C, et al. Telomere shortening reduces regenerative capacity after acute kidney injury. Journal of the American Society of Nephrology : JASN. 2010;21:327–336. doi: 10.1681/ASN.2009010072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du HY, Pumbo E, Ivanovich J, et al. TERC and TERT gene mutations in patients with bone marrow failure and the significance of telomere length measurements. Blood. 2009;113:309–316. doi: 10.1182/blood-2008-07-166421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calado RT, Young NS. Telomere maintenance and human bone marrow failure. Blood. 2008;111:4446–4455. doi: 10.1182/blood-2007-08-019729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong KK, Chang S, Weiler SR, et al. Telomere dysfunction impairs DNA repair and enhances sensitivity to ionizing radiation. Nature genetics. 2000;26:85–88. doi: 10.1038/79232. [DOI] [PubMed] [Google Scholar]
- 16.Blasco MA, Lee HW, Hande MP, et al. Telomere shortening and tumor formation by mouse cells lacking telomerase RNA. Cell. 1997;91:25–34. doi: 10.1016/s0092-8674(01)80006-4. [DOI] [PubMed] [Google Scholar]
- 17.Chiang YJ, Hemann MT, Hathcock KS, et al. Expression of telomerase RNA template, but not telomerase reverse transcriptase, is limiting for telomere length maintenance in vivo. Molecular and cellular biology. 2004;24:7024–7031. doi: 10.1128/MCB.24.16.7024-7031.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Snow BE, Hande MP, et al. The telomerase reverse transcriptase is limiting and necessary for telomerase function in vivo. Curr Biol. 2000;10:1459–1462. doi: 10.1016/s0960-9822(00)00805-8. [DOI] [PubMed] [Google Scholar]
- 19.Chiang YJ, Calado RT, Hathcock KS, et al. Telomere length is inherited with resetting of the telomere set-point. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:10148–10153. doi: 10.1073/pnas.0913125107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi J, Southworth LK, Sarin KY, et al. TERT promotes epithelial proliferation through transcriptional control of a Myc- and Wnt-related developmental program. PLoS Genet. 2008;4:e10. doi: 10.1371/journal.pgen.0040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Degryse AL, Xu XC, Newman JL, et al. Telomerase deficiency does not alter bleomycin-induced fibrosis in mice. Experimental lung research. 2012;38:124–134. doi: 10.3109/01902148.2012.658148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hall CL, Hardwicke J. Low molecular weight proteinuria. Annu Rev Med. 1979;30:199–211. doi: 10.1146/annurev.me.30.020179.001215. [DOI] [PubMed] [Google Scholar]
- 23.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 24.Kimura T, Takabatake Y, Takahashi A, et al. Autophagy protects the proximal tubule from degeneration and acute ischemic injury. Journal of the American Society of Nephrology : JASN. 2011;22:902–913. doi: 10.1681/ASN.2010070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang M, Wei Q, Dong G, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney international. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. The Journal of biological chemistry. 2000;275:31505–31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 27.Donati A, Cavallini G, Paradiso C, et al. Age-related changes in the regulation of autophagic proteolysis in rat isolated hepatocytes. J Gerontol A Biol Sci Med Sci. 2001;56:B288–B293. doi: 10.1093/gerona/56.7.b288. [DOI] [PubMed] [Google Scholar]
- 28.Hands SL, Proud CG, Wyttenbach A. mTOR's role in ageing: protein synthesis or autophagy? Aging. 2009;1:586–597. doi: 10.18632/aging.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoeftner S, Blanco R, Lopez de Silanes I, et al. Telomere shortening relaxes X chromosome inactivation and forces global transcriptome alterations. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:19393–19398. doi: 10.1073/pnas.0909265106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell. 1985;43:405–413. doi: 10.1016/0092-8674(85)90170-9. [DOI] [PubMed] [Google Scholar]
- 31.Calado RT, Young NS. Telomere diseases. The New England journal of medicine. 2009;361:2353–2365. doi: 10.1056/NEJMra0903373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrera E, Samper E, Martin-Caballero J, et al. Disease states associated with telomerase deficiency appear earlier in mice with short telomeres. The EMBO journal. 1999;18:2950–2960. doi: 10.1093/emboj/18.11.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudolph KL, Chang S, Lee HW, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 34.Samper E, Flores JM, Blasco MA. Restoration of telomerase activity rescues chromosomal instability and premature aging in Terc−/− mice with short telomeres. EMBO Rep. 2001;2:800–807. doi: 10.1093/embo-reports/kve174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koppelstaetter C, Schratzberger G, Perco P, et al. Markers of cellular senescence in zero hour biopsies predict outcome in renal transplantation. Aging Cell. 2008;7:491–497. doi: 10.1111/j.1474-9726.2008.00398.x. [DOI] [PubMed] [Google Scholar]
- 36.Wills LP, Schnellmann RG. Telomeres and telomerase in renal health. J Am Soc Nephrol. 2011;22:39–41. doi: 10.1681/ASN.2010060662. [DOI] [PubMed] [Google Scholar]
- 37.Lieberthal W, Levine JS. The role of the mammalian target of rapamycin (mTOR) in renal disease. Journal of the American Society of Nephrology : JASN. 2009;20:2493–2502. doi: 10.1681/ASN.2008111186. [DOI] [PubMed] [Google Scholar]
- 38.Lieberthal W, Fuhro R, Andry CC, et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. American journal of physiology Renal physiology. 2001;281:F693–F706. doi: 10.1152/ajprenal.2001.281.4.F693. [DOI] [PubMed] [Google Scholar]
- 39.Lieberthal W, Fuhro R, Andry C, et al. Rapamycin delays but does not prevent recovery from acute renal failure: role of acquired tubular resistance. Transplantation. 2006;82:17–22. doi: 10.1097/01.tp.0000225772.22757.5e. [DOI] [PubMed] [Google Scholar]
- 40.Jiang M, Liu K, Luo J, et al. Autophagy is a renoprotective mechanism during in vitro hypoxia and in vivo ischemia-reperfusion injury. The American journal of pathology. 2010;176:1181–1192. doi: 10.2353/ajpath.2010.090594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber TB, Edelstein CL, Hartleben B, et al. Emerging role of autophagy in kidney function, diseases and aging. Autophagy. 2012;8:1009–1031. doi: 10.4161/auto.19821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J, Kundu M, Viollet B, et al. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ganley IG, Lam du H, Wang J, et al. ULK1.ATG13.FIP200 complex mediates mTOR signaling and is essential for autophagy. The Journal of biological chemistry. 2009;284:12297–12305. doi: 10.1074/jbc.M900573200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosokawa N, Hara T, Kaizuka T, et al. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell. 2009;20:1981–1991. doi: 10.1091/mbc.E08-12-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lieberthal W, Levine JS. Mammalian target of rapamycin and the kidney. II. Pathophysiology and therapeutic implications. American journal of physiology Renal physiology. 2012;303:F180–F191. doi: 10.1152/ajprenal.00015.2012. [DOI] [PubMed] [Google Scholar]
- 46.Grahammer F, Haenisch N, Steinhardt F, et al. mTORC1 maintains renal tubular homeostasis and is essential in response to ischemic stress. Proc Natl Acad Sci U S A. 2014;111:E2817–E2826. doi: 10.1073/pnas.1402352111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blagosklonny MV. TOR-centric view on insulin resistance and diabetic complications: perspective for endocrinologists and gerontologists. Cell Death Dis. 2013;4:e964. doi: 10.1038/cddis.2013.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leontieva OV, Natarajan V, Demidenko ZN, et al. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:13314–13318. doi: 10.1073/pnas.1205690109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging. 2009;1:357–362. doi: 10.18632/aging.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leontieva OV, Paszkiewicz GM, Blagosklonny MV. Mechanistic or mammalian target of rapamycin (mTOR) may determine robustness in young male mice at the cost of accelerated aging. Aging. 2012;4:899–916. doi: 10.18632/aging.100528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu JJ, Liu J, Chen EB, et al. Increased Mammalian Lifespan and a Segmental and Tissue-Specific Slowing of Aging after Genetic Reduction of mTOR Expression. Cell Rep. 2013;4:913–920. doi: 10.1016/j.celrep.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krishnamurthy J, Torrice C, Ramsey MR, et al. Ink4a/Arf expression is a biomarker of aging. The Journal of clinical investigation. 2004;114:1299–1307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lee DH, Wolstein JM, Pudasaini B, et al. INK4a deletion results in improved kidney regeneration and decreased capillary rarefaction after ischemia-reperfusion injury. American journal of physiology Renal physiology. 2012;302:F183–F191. doi: 10.1152/ajprenal.00407.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubinsztein DC, Marino G, Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Araya J, Kojima J, Takasaka N, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. American journal of physiology Lung cellular and molecular physiology. 2013;304:L56–L69. doi: 10.1152/ajplung.00213.2012. [DOI] [PubMed] [Google Scholar]
- 56.Kurz T, Terman A, Gustafsson B, et al. Lysosomes in iron metabolism, ageing and apoptosis. Histochem Cell Biol. 2008;129:389–406. doi: 10.1007/s00418-008-0394-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brooks C, Wei Q, Cho SG, et al. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. The Journal of clinical investigation. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang JL, Cheng HF, Zhang MZ, et al. Selective increase of cyclooxygenase-2 expression in a model of renal ablation. Am J Physiol. 1998;275:F613–F622. doi: 10.1152/ajprenal.1998.275.4.F613. [DOI] [PubMed] [Google Scholar]
- 59.Wu H, Chen G, Wyburn KR, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. The Journal of clinical investigation. 2007;117:2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meldrum KK, Meldrum DR, Hile KL, et al. A novel model of ischemia in renal tubular cells which closely parallels in vivo injury. J Surg Res. 2001;99:288–293. doi: 10.1006/jsre.2001.6201. [DOI] [PubMed] [Google Scholar]
- 61.Wuthrich RP, Glimcher LH, Yui MA, et al. MHC class II, antigen presentation and tumor necrosis factor in renal tubular epithelial cells. Kidney international. 1990;37:783–792. doi: 10.1038/ki.1990.46. [DOI] [PubMed] [Google Scholar]
- 62.Cheng HF, Harris RC. Cyclooxygenase-2 Expression in Cultured Cortical Thick Ascending Limb of Henle Increases in Response to Decreased Extracellular Ionic Content by Both Transcriptional and Post-transcriptional Mechanisms. ROLE OF p38-MEDIATED PATHWAYS. J Biol Chem. 2002;277:45638–45643. doi: 10.1074/jbc.M206040200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.