Abstract
Purpose
Citral is composed of a random mixture of two geometric stereoisomers geranial (trans-citral) and neral (cis-citral) yet few studies have directly compared their in vivo antitumor properties. A micelle formulation was therefore developed.
Methods
Geranial and neral were synthesized. Commercially-purchased citral, geranial, and neral were formulated in PEG-b-PCL (block sizes of 5000:10000, Mw/Mn 1.26) micelles. In vitro degradation, drug release, cytotoxicity, flow cytometry, and western blot studies were conducted. The antitumor properties of drug formulations (40 mg/kg and 80 mg/kg based on MTD studies) were evaluated on the 4T1 xenograft mouse model and tumor tissues were analyzed by western blot.
Results
Micelles encapsulated drugs with >50% LE at 5-40% drug to polymer (w/w), displayed sustained release (t1/2 of 8-9 hours), and improved drug stability at pH 5.0. The IC50 of drug formulations against 4T1 cells ranged from 1.4-9.9 μM. Western blot revealed that autophagy was the main cause of cytotoxicity. Geranial at 80 mg/kg was most effective at inhibiting tumor growth.
Conclusions
Geranial is significantly more potent than neral and citral at 80 mg/kg (p<0.001) and western blot of tumor tissues confirms that autophagy and not apoptosis is the major mechanism of tumor growth inhibition in p53-null 4T1 cells.
Keywords: citral, geranial, neral, micelle, breast cancer
Introduction
Citral (3,7-dimethyl-2,6-octadienal) is a naturally occurring bioactive compound found in the essential oils of plants such as citrus fruits, lemon grass and ginger, and is composed of a mixture of the two terpenoid geometric stereoisomers geranial (trans-citral) and neral (cis-citral), Fig. 1. Citral has been used as a natural additive to food, cosmetics, and beverages due to its intense lemon aroma and flavor.(1, 2) Essential oils containing citral have been shown to exhibit antimicrobial, antifungal and antiparasitic properties,(3-7) making citral a natural preservative.
Fig. 1.
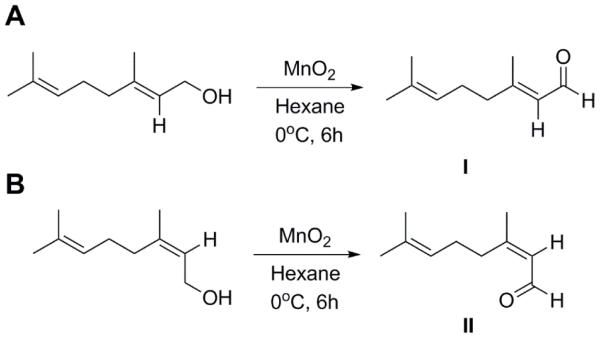
Geraniol was reacted with manganese dioxide to generate pure geranial (I) with a yield of 95% yield. Nerol was reacted with manganese dioxide to generate pure neral (II) with a yield of 90%.
In mammals, the first report of citral as an inducer of caspase 3 activity was reported in 2005 in the leukemic U937 and HL60 cell lines.(8) We have recently demonstrated that the steam distilled extracts of ginger are actually composed of 30-40% citral and that this drug could induce caspase 3 activity, activate p53 and decrease Bcl-2 expression, resulting in apoptosis in two endometrial cancer cell lines (ECC-1 and Ishikawa) at IC50 of 15-25 μM.(9) The anticancer mechanism and respective potency of citral and its isomers have not yet been completely elucidated, but citral represents an emerging class of natural products which demonstrates activity against p53-expressing ovarian cancer cells (9). In addition, here we report on evidence of geranial as the more potent isomer of citral, being capable of inducing autophagy and not apoptosis as the primary mechanism of cell death both in vitro and in vivo, in mouse p53-null 4T1 breast cancer cells.
To properly evaluate the anticancer properties of citral and its isomers in vivo, a safe intravenous formulation is required. Citral has a known aqueous solubility of ca. 590 μg/ml at 25 °C and has previously been shown to degrade at acidic pH and under oxidative stress into various oxidative products (e.g. p-cymene, p-cresol, p-methylacetophenone, 8-hydroperoxy-p-cymene) dependent on pH, temperature, and presence of oxygen.(10, 11) The majority of citral chemical stabilization methods reported in the literature have relied on encapsulating the compound in O/W emulsions with or without the addition of naturally-occurring antioxidants (e.g. ascorbic acid, β-carotene, black tea extract).(11) For example, in the absence of antioxidants, negatively-charged SDS and positively-charged chitosan were used to create layers of SDS-chitosan complexes around emulsion droplets (ca. 0.41 μm in diameter) and this barrier was shown to effectively retard oxidation-induced citral degradation products, whereas gum arabic stabilized O/W emulsions (ca. 1.1 μm in diameter) seemed more effective at protecting citral from acid-induced degradation.(12) Citral has also been successfully incorporated into the hydrophobic environment of micelles formed from non-ionic polyoxyethylene-type surfactants,(1, 13) micelles formed from Tween 80,(14) and reverse micelles formed from polyglycerol polyricinoleate for enhanced protection against acid-induced degradations.(14) Most of these formulations have been tailored to improve citral stability in the context of food, fragrance, cosmetics or beverage applications.(2) However, none of these reported formulations are ideal for cancer therapeutic applications since biomaterial safety, micelle size, drug encapsulation and protection are all critical parameters to control in an i.v. formulation. NPs such as micelles can typically take advantage of the EPR effect(15) to successfully extravasate through leaky blood vessels into the solid tumor.
Here, we report on the formulation of citral, geranial, and neral into biocompatible PEG-b-PCL micelles. PCL is an attractive polymer for drug delivery due to the biocompatible nature of the degradation products (16) and PCL is currently approved by the FDA for use in humans. It has been observed that unstable micelles can fall apart rapidly in plasma (17) and lead to excessive drug loss. However, recent work has suggested that PEG-b-PCL micelles can maintain sustained drug release in the plasma due to enhanced circulation (18, 19) and low CMCs, indicative of high stability. There are also recent studies which have confirmed that PEG-b-PCL micelles are indeed relatively stable in vivo (20, 21). PEG-b-PCL micelles were able to encapsulate citral, geranial, and neral with high loading efficiency (>50% between 5-40% w/w drug to polymer ratio), displayed sustained release (t1/2 of 8-9 hours), and improved drug stability at pH 5.0. We also investigated the cytotoxic properties of the formulations in vitro against the mouse p53-null 4T1 breast cancer cells. The antitumor properties of the formulations at two concentrations (40 and 80 mg/kg based on MTD studies) were evaluated in vivo on the 4T1 xenograft mouse model and tumor tissues were collected at termination of animal experiments for analysis by western blot.
Materials and Methods
Materials
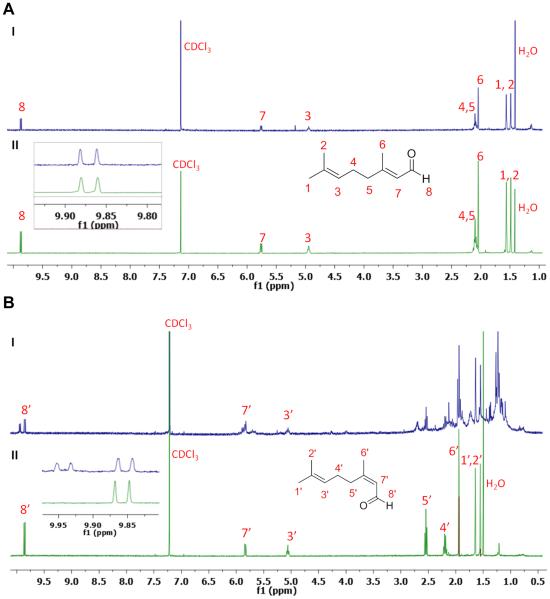
Commercial citral, geraniol, nerol and active manganese (IV) oxide (MnO2) were purchased from Sigma-Aldrich (Milwaukee, WI). Citral was analyzed by 1H-NMR and determined to have a 2:1 ratio of geranial to neral (see supplemental, Fig. 1S). PEG-b-PCL (Mn of PEG = 5000 g/mol; Mn of PCL = 10,000 g/mol; Mw/Mn= 1.26) was purchased from Polymer Source (Quebec, Canada). Dialysis tubing (MWCO = 3500) was obtained from SpectraPor. DMEM, PBS, FBS, trypsin-EDTA (0.05% trypsin, 0.48 mM EDTA in HBSS) and penicillin-streptomycin were purchased from Cellgro (MediaTech, Herndon, VA). The 4T1 cells (mouse breast cancer) were obtained from ATCC and cultured according to ATCC protocols. The Annexin V-FITC apoptosis detection kit I containing propidium iodide to detect for apoptosis and necrosis was purchased from BD Biosciences, San Jose, CA. For the western blot, primary antibodies against LC3B and Atg5 proteins were purchased from Cell Signaling (Danvers, MA). Secondary antibodies against rabbit were purchased from Jackson Immunoresearch (West Grove, PA).
1H-NMR spectra were obtained with a Varian Unity-Inova 400 MHz NMR spectrometer (Palo Alto, CA), with temperature regulation of 25°C or otherwise as indicated. Chemical shifts are reported in ppm with respect to the deuterated solvent used.
All aspects of the animal studies were performed in accordance with the guidelines defined by the Animal Research Committee of the University of Wisconsin. Female BALB/c mice (6-7 week old) were obtained from Jackson Laboratory and divided into six groups (5 mice per group) for xenograft tumor studies. General anesthesia to animals was induced with 1.5% isoflurane/oxygen. Tumor volume (volume = 0.5 × l × w2) and animal BW were monitored daily.
Results are presented as mean ± SEM, n=3-5. To compare between data sets, Graphpad Prism 5 Software was used to perform one way analysis of variance (ANOVA). A p<0.01 and p<0.001 were considered significant and denoted by * or ** respectively to indicate statistical differences.
Methods
Synthesis and geometric stability of geranial and neral
Citral isomers, geranial or neral, were prepared from geraniol or nerol via oxidation of the primary alcohol group to aldehyde (Fig. 1).(22) To a flask containing 1.15 g (13.2 mmol) of activated MnO2, a solution of 100 mg (0.65 mmol) of geraniol dissolved in 16 ml of hexane was added dropwise at 0 °C, while vigorously stirring. The reaction was allowed to proceed for 6 h at 0 °C, followed by filtration. The filtrate was then concentrated to yield the final product, which was oily, with 95% yield. Neral was synthesized in the same manner, using nerol as the primary alcohol, with 90% yield. The chemical structures of pure geranial and neral were confirmed by 1H-NMR (see supplemental, Fig. 1S). Geranial and neral were respectively incubated at 25 °C or 37 °C for up to a week, and the resulting geometric stability of geranial (Fig. 2A) and neral (Fig. 2B) were characterized by 1H-NMR.
Fig. 2.
1H-NMR of geranial and neral in CDCl3 after incubating for 7 days at 25 °C or 37 °C. There is no change in the spectrum of geranial (A) after incubating at 25 °C (II) or 37 °C (I) for up to 7 days. Similarly, there was no evidence of change from neral to geranial (B) incubated at 25 °C (II) for up to 7 days; however two new peaks at ca. 9.95 ppm characteristic of geranial begin to appear on day 7 with incubation at 37 °C (I). The ratio of these two new peaks were integrated with respect to the neral peaks at ca. 9.85 ppm and was determined to be about 1:1 indicative of 50% neral conversion to geranial by day 7 at 37 °C.
Preparation and characterization of geranial, neral, or citral in PEG-b-PCL micelles
The micelles were prepared as previously reported;(23) typically 0.5 mM of diblock polymer PEG-b-PCL was dissolved in 1 ml of acetone, then 1 ml of ddH2O was quickly added to the polymer suspension while vigorously stirring. Geranial-loaded micelles (geranial/NP), neral-loaded micelles (neral/NP) and citral-loaded micelles (citral/NP) were prepared by first dissolving the drug at various concentrations with the polymer in acetone and then adding ddH2O. Note that citral here was commercially purchased from Sigma-Aldrich and not purified from ginger as previously done during earlier studies; this batch of citral contained ca. 2:1 geranial to neral (this ratio varies depending on the commercial source). The drug spontaneously loads into the micelle during self-association of the polymer chains and acetone was removed by rotary evaporation. Micelles were centrifuged at 10,000 rpm for 5 min and passed through a 0.2 μm nylon filter to remove un-encapsulated drug and/or aggregates.
Particle size was characterized at 25°C using a Zeta sizer Nano-ZS (Malvern Instruments, UK) equipped with a He-Ne ion laser (λ= 633 nm). The hydrodynamic diameters of samples were obtained by cumulant method and are reported as Z-average diameters.
The concentration of drugs in the micelles was measured by reverse-phase HPLC(23, 24) equipped with a Symmetry Shreld RP18 column from Waters, and monitoring geranial, neral, or citral by UV detection at 254 nm (the diblock polymer PEG-b-PCL did not absorb at this wavelength). The mobile phase used was a cocktail of 40% water and 60% acetonitrile containing 0.1% acetic acid. The concentration of drug was increased from 5%, 10%, 20% to 40% (w/w) while keeping the concentration of the diblock polymer PEG-b-PCL constant at 0.5 mM. Drug loaded and LE were calculated using the following equations:
In vitro cytotoxicity
4T1 cells were seeded in a 96-well plate at 5,000 cells per well for 24 h. The next day, the medium was replaced with 10 μl of formulations containing either empty NP, citral/NP, neral/NP or geranial/NP; formulations were added to wells at a final concentration of 0.01 μM-1 mM drug. The cells were incubated for 72 h and cell viability (as measured by metabolic activity) was monitored with the resazurin assay.(25) The IC50s were obtained by curve fitting with GraphPad Prism 5 Software.
Western blot
4T1 cells were seeded at 25,000 cells per well in a 6-well plate for 24 h. Cells were then treated with 25 μM of citral/NP, neral/NP or geranial/NP for an additional 48 h, using PBS and NP as control groups. Cell lysates were collected and loaded onto a 12% SDS-PAGE gel and the gel was run at 100 V for 55 min. The protein bands were then transferred to a PVDF membrane at 100 V for 1 hour, blocked with 5% BSA for 1 hour, and the autophagy proteins LC3B and Atg5 were simultaneously detected by incubating the membrane with appropriate primary antibodies overnight at 4°C. The membrane was developed through the use of secondary antibodies conjugated to horseradish peroxidase by incubating at room temperature for 1 hour before imaging.
Flow Cytometry
4T1 cells were seeded in 60 cm2 cell culture dishes and incubated overnight at 37°C/5% CO2 for 24 h. Cells were then treated with either 25 μM geranial/NP (9), PBS, or NP as the negative control for 24 and 48 h. At each time point, both dead and live cells were collected, washed with PBS, and re-suspended in 1x binding buffer (10 mM HEPES/NaOH, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2) to a final concentration of 1x106 cells/ml. Next, 1x105 cells were stained with the Annexin V-FITC apoptosis detection kit I by incubation at room temperature for 15 min. Afterwards, 400 μl of binding buffer was added to each sample and the cells were analyzed on a FACSCalibur flow cytometer. The data was analyzed with FlowJo® software (version 10.0.6) by gating n = 20,000 cells. Note that gated apoptosis data reported are the sum of both apoptotic and late apoptotic cells.
pH degradation studies
For degradation studies, 10 mM of either geranial/NP, free geranial, neral/NP, free neral, citral/NP or free citral in 0.5 mL final volumes were respectively incubated at pH 5.0 and pH 7.4 for up to 24 h at room temperature; drug stability was monitored by HPLC at time t=0, 2, 4, 8, 12, and 24 h. Empty micelles incubated under these conditions did not show instability or evidence of PEG-b-PCL polymer degradation (data not shown). Note that in this assay, we did not identify degradation products for citral nor its isomers geranial and neral since it was not the focus of this work; if interested, citral degradation products have already previously been meticulously reported.(10, 11) Differences between degradation profiles at pH 5.0 and 7.4 for geranial, neral, and citral were monitored by HPLC (column temperature of 40 °C, mobile phase consisted of water: acetonitrile at 40:60, v/v, flow rate of 0.5 ml/min) to investigate drug stability when formulated in micelles.
Animal studies
For MTD studies, we made an educated guess by starting with a single 100 mg/kg dose of citral/NP via retro-orbital injections into normal Balb/c mice (n=3 animals/dose). Generally, about 200 μl of the citral/NP was injected into animals (n=3) which were then monitored over time. This assayand drugconcentrations tested weredonesequentially, meaningthat wedidn’t inject the next dose into animals until we knew whether the dose tested had been acutely toxic to animals based on changes in behavior, bleeding, and overall appearance. Final concentrations utilized for the MTD studies were 100, 90, and 80 mg/kg.
For tumor-bearing studies, approximately 1x106 4T1 cells were inoculated subcutaneously into the right flanks of anesthetized mice. When the tumor size reached about 50 mm3, 200 μl of the various formulations (saline, empty NP, C40, C80, G40, G80, N40, and N80) were injected into the animals on days 13, 16, 19 and 22. The C, G, or N stands for either citral, geranial, or neral, and the number represents the concentration injected, either 40 mg/kg or 80 mg/kg; for example, C40 means that 40 mg/kg of citral was injected. The tumor size and BW of animals were monitored daily. The animals were sacrificed when tumors in the control group reached about 2000 mm3. Tumor tissues were dissected, cut into small pieces and placed immediately on ice. Next, 250 μl of RIPA buffer with protease inhibitor was added to 5 mg of minced tissue and the samples were sonicated on ice to break the tissue further. The protein lysates from tumor tissues were then loaded onto a 12% SDS-PAGE gel for western blot analysis of autophagy proteins LC3B (14 kDa) and Atg5 (55 kDa).
Results
Synthesis and geometric stability of geranial and neral
The two isomers of citral were synthesized by oxidation of alcohol using MnO2, converting geraniol to geranial, and nerol to neral (Fig. 1). The geometric structures of geranial and neral were confirmed by 1H-NMR (see supplemental, Fig. 1S). The characteristic peaks of geranial and neral at ca. 10 and ca. 9.9 ppm respectively were used to determine the ratio of each isomer present in the citral purchased from Sigma-Aldrich, with results revealing that the ratio of geranial to neral was 2:1 in this particular batch (see supplemental, Fig. 1S).
The geometric stability of free geranial and neral was investigated by incubation at 25 °C and 37 °C for up to 7 days with 1H-NMR. For geranial, there was no apparent change to neral found in the 1H-NMR after incubating at both 25 °C (II) and 37 °C (I) for up to 7 days (Fig. 2A). For neral, we observed no apparent change in the 1H-NMR after incubating at 25 °C (II) for up to 7 days but there was an obvious peak shift observed in the 1H-NMR of neral after incubation at 37 °C (I) on day 7 (Fig. 2B). More specifically, we noted the appearance of peaks characteristic of geranial at ca. 10 ppm; by integrating characteristic neral and geranial peaks, we obtained a ratio of 1:1 suggesting that 50% of neral had converted to geranial by day 7 when incubated at 37°C.
Preparation and characterization of geranial, neral, or citral in PEG-b-PCL micelles
Micelles were prepared from PEG-b-PCL (5000:10000) diblock copolymer by nanoprecipitation. The amount of drug encapsulated into the micelles was measured by HPLC, and the LE ranged between 54-99% for geranial, 55-97% for neral, and 51-97% for citral at drug to polymer (w/w) ratio of 40-5% (Table I). Resulting micelle sizes were characterized with DLS and ranged between 77-85 nm for citral/NP, 88-66 nm for geranial/NP, and 74-79 nm for neral/NP at 40-5% w/w polymer to drug ratio (see supplemental, Table IS).
Table I.
Characterization of geranial, neral, and citral loading levels into 0.5 mM PEG-b-PCL micelles. On a w/w ratio of drug to polymer, the drug concentration was then increased from 5% to 40%.
| w/w | Initial drug (mg/ml) |
Drug solubilized (mg/ml) | Efficiency % loaded | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| geranial | neral | citral | geranial | neral | citral | ||
|
| |||||||
| 5% | 0.170 | 0.168 ± 0.013 | 0.166 ± 0.029 | 0.165 ± 0.005 | 98.8 ± 1.6 | 97.4 ± 3.7 | 96.7 ± 2.9 |
| 10% | 0.341 | 0.289 ± 0.012 | 0.202 ± 0.012 | 0.260 ± 0.022 | 84.7 ± 1.5 | 59.3 ± 2.7 | 76.2 ± 6.6 |
| 20% | 0.682 | 0.380 ± 0.018 | 0.383 ± 0.026 | 0.397 ± 0.024 | 55.7 ± 3.2 | 56.2 ± 1.5 | 58.3 ± 3.5 |
| 40% | 1.364 | 0.739 ± 0.016 | 0.748 ± 0.009 | 0.692 ± 0.015 | 54.2 ± 1.6 | 54.8 ± 1.8 | 50.7 ± 1.1 |
For the release study, drug-loaded micelles were prepared at the 5% w/w ratio of drug to polymer to maximize drug encapsulation within the PCL core rather than at the interfaces of micelles. The micelle formulations were diluted with respect to drug concentration to 0.1 mg/ml in ddH2O and loaded into a dialysis cassette. The release study was conducted by dialyzing the cassettes against ddH2O at 37°C since citral (and consequently geranial and neral) has a water solubility of ca. 590 μg/mL. At various time points, 20 μl of suspension was withdrawn from the dialysis cassettes for citral analysis by RP-HPLC to quantify the % of drug left and 20 μl of ddH2O was added to replenish the volumes in the cassettes. For citral/NP we obtained a t1/2 of ca. 8.5 h to 50% drug release; for geranial/NP we obtained a t1/2 of ca. 8.3 h and for neral/NP, we obtained a t1/2 of ca. 8.9 h (see supplemental, Fig. 2S).
In vitro cytotoxicity
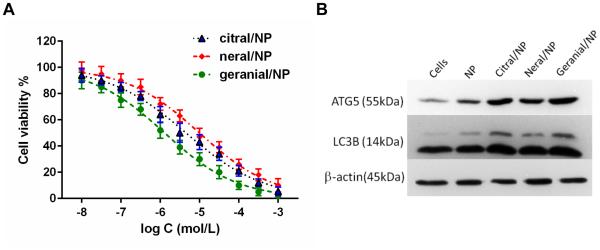
To compare the cytotoxicity of geranial, neral and citral (the commercial mixture of geranial and neral at ratio of 2:1 w/w, Sigma-Aldrich), 4T1 cells were seeded in 96-well plates for 24 h. The next day, cells were treated with 0.01 μM-1 mM drugs and allowed to incubate for 72 h. Cell viability was measured by the resazurin assay. As shown in Fig. 3A, the IC50 of geranial/NP at 72 h was found to be 1.4 μM, neral/NP cytotoxicity was characterized by an IC50 of 9.9 μM, and citral/NP cytotoxicity was characterized by an IC50 of 4.5 μM. There was no apparent cytotoxicity to cells incubated with empty NP (i.e. micelles) compared to cells-only controls (data not shown).
Fig. 3.
Cytotoxicity assay on 4T1 cells after incubating with citral/NP, neral/NP and geranial/NP for 72 h (A). At the highest concentrations of drug tested, empty NP were not toxic to cells with >90% cell viability, data not shown on graph. The IC50 for citral/NP, neral/NP and geranial/NP was calculated to be 4.5, 9.9, and 1.4 μM respectively by curve fitting with Graph Pad Prism 5 Software. After 24 h incubation, western blot reveals increased expression of Atg5 (55k Da) and LC3B (14 kDa) proteins in 4T1 cells treated with 25 μM of each formulation, indicative of autophagy (B).
Western blot
4T1 cells were treated with 25 μM of citral/NP, neral/NP, or geranial/NP for 48 hours and then autophagy proteins LC3B and Atg5 were detected by western blot (Fig. 3B). The strongest LC3B and Atg5 band intensities were observed in cells treated with geranial/NP, followed by citral/NP, and then neral/NP, in comparison to untreated or NP-only treated cells (see supplemental, Fig. 3S) and confirm that p53-null 4T1 mouse breast cancer cells preferably underwent autophagy upon incubation with the drugs.
Flow cytometry
Since cytotoxicity and western blot studies demonstrated that geranial was more potent than neral or citral, only geranial/NP was investigated for evidence of apoptosis. Flow cytometry revealed that there were no significant differences between the control groups and the geranial/NP-treated cells after 24 or 48 h incubation, indicating that the cell death mechanism induced by geranial was not apoptosis (see supplemental, Fig. 4S).
pH degradation studies
Solutions containing neral and neral/NP (Fig. 4A, Fig. 4B), geranial and geranial/NP (Fig. 4C, Fig. 4D), or citral and citral/NP (Fig. 4E, Fig. 4F) at final concentrations of 10 mM were incubated at room temperature at pH 5.0 and pH. 7.4 for up to 24 h. The pH degradation of the drug was monitored via HPLC by UV detection of the drug at 254 nm. HPLC data for neral, geranial, and citral incubation at pH 7.4 reveals that all the drugs and micelle formulated drugs were relatively stable at this pH, with little evidence of drug degradation up to the 24 h monitored (see supplemental, Fig. 5S).
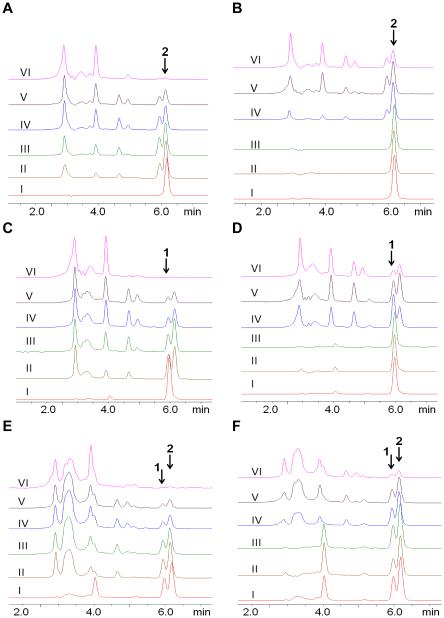
Fig. 4.
The acid-induced degradation of neral, geranial, and citral was monitored via HPLC by incubating drugs at pH 5.0. The profiles for neral (A) and neral/NP (B) at t=0 show the characteristic peaks for this isomer at ca. 5.9 min (peak 1); the profiles for geranial (C) and geranial/NP (D) at t=0 show the characteristic peaks for this isomer at ca. 6.1 min (peak 2); the profiles for citral (E) and citral/NP (F) at t=0 show the characteristic peaks for this drug at ca. 5.9 min (peak 1) and ca. 6.1 min (peak 2) corresponding to both neral and geranial respectively. Note that incubation at pH 7.4 for all the drugs did not show evidence of degradation up to the 24 h monitored (see supplemental, Fig. 5S). With increasing incubation time, 0h (I), 2h (II), 4h (III), 8h (IV), 12h (V) and 24h (VI), we begin to see more evidence of drug degradation (as evidenced by the increasing intensity of peaks observed between 0 and 5 min) compared to the decreasing characteristic drug peaks at t=0 (I).
At pH 5.0, the degradation of pure neral (Fig. 4A), peak 1 at approx. 5.9 min, occurred much faster than neral/NP (Fig. 4B) over the 0, 2, 4, 8, 12 and 24 h monitored (I-VI respectively). For example, at 4 h incubation there is evidence of free neral conversion to geranial (due to appearance of the geranial peak 2 at approx. 6.1 min) and presence of more degradation products than neral/NP. Similarly, the HPLC profile for geranial at pH 5.0 shows the characteristic peak 2 at t=0 for this isomer at ca. 6.1 min and with increasing time (II-VI), we begin to see more evidence of geranial degradation products compared to geranial/NP. For example, peak 1 corresponding to neral can be readily observed after 2 h incubation with free geranial (Fig. 4C), but this peak is not observed with geranial/NP until after 8 h incubation (Fig. 4D). At pH 5.0 and t=0, citral is characterized by peaks 1 and 2 (Fig. 4E) corresponding to neral and geranial respectively. Degradation of these peaks occurred much faster for free citral than for citral/NP over the 0, 2, 4, 8, 12 and 24 h monitored (I-VI respectively). For example, at t=8 h, there was evidence of more degradation products in citral than in citral/NP, which still revealed two strong peaks for neral and geranial (Fig. 4F).
Animal studies
For MTD studies, at dosage of 100 mg/kg we observed that 2 mice died immediately after the injection and 1 mouse died the next day. When we decreased the dose to 90 mg/kg, we saw that 1 mouse died the next day and 2 mice survived. At 80 mg/kg citral/NP, all three animals survived and looked normal for up to 2 weeks based on signs of acute toxicities (e.g. changes in behavior, bleeding, and overall appearance). This was deemed the MTD for citral and its respective isomers.
The xenograft tumor model was established in mice by subcutaneously injecting 4T1 mouse breast cancer cells into the right flanks of animals. C40, C80, G40, G80, N40, or N80 was injected i.v. via retro-orbital injections to animals on day 13, 16, 19, and 22; the control groups received either empty NP or saline. The tumor size and BW of animals were monitored daily to assess the therapeutic efficacy and acute toxicity of the treatments in animals. The empty NP treatments did not inhibit tumor growth similar to saline (p>0.05), whereas all the drug formulations at 40 and 80 mg/kg did slow down growth of this aggressive xenograft tumor model to different extents when compared to controls (p<0.01). The anti-tumor efficacy of C40, G40, and N40 formulations were all effective with a tumor volume reduction of approximately 32-49% compared to the NP control (Fig. 5A). More significantly, the results for G80 revealed 92% tumor volume reduction by day 24 compared to the NP vehicle (p<0.001) and G40 displayed a 49% tumor volume reduction by day 24 compared to the NP vehicle (p<0.001). Comparing G80 to C80 and N80, there was a statistically significant difference in tumor volume reduction between G80 and C80 (p<0.001), and G80 and N80 (p<0.001). The formulations did not generate acute toxicity in animals based on consistent animal weights throughout the treatment period compared to controls (Fig. 5B); a BW loss of ± 20% would have indicated acute toxicities to animals.
Fig. 5.

Mice (n=5) received four retro-orbital injections on days 13, 16, 19, and 22 of either saline, empty NP, C40, N40, G40, C80, N80, or G80. The saline and NP treatments did not inhibit tumor growth (p>0.05), whereas C40, N40 and G40 were relatively all effective at reducing tumor burden 32-49% compared to NP controls by day 24. The most significant tumor regression was observed with G80. There was significant difference in tumor volume reduction between G80 and C80 (p<0.001), and G80 and N80 (p<0.001) (A) and suggest that geranial is more potent than neral at 80 mg/kg. The formulations did not generate acute toxicity in animals based on consistent animal weights throughout the treatment period (B). **p<0.001
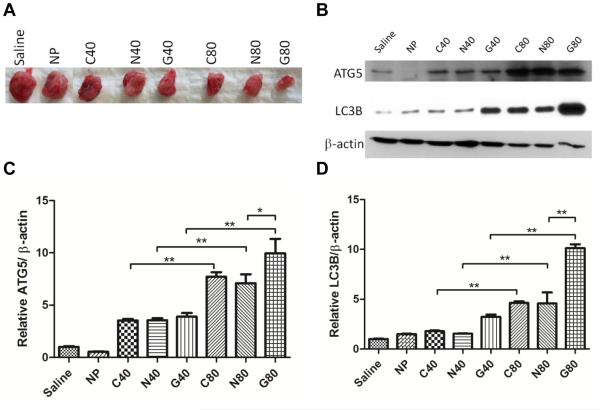
At the end of the study (day 24), animals were all promptly euthanized and tumors were excised (Fig. 6A, see supplemental Fig. 6S) for western blot analyses. Western blot analyses of tumor tissues reveals that autophagy is indeed the major mechanism of tumor growth inhibition for animals treated with the formulations (Fig. 6B). At 40 mg/kg treatment concentrations, we see evidence of autophagy proteins Atg5 and LC3B being increasingly expressed across all formulations (C40, N40 and G40, C80, N80, G80). More specifically, there was statistically more expression of Atg5 and LC3B comparing G80 to G40, N80 to N40, and C80 to C40, p<0.001; there was also more expression of Atg5 and LC3B in G80-treated animals compared to N80-treated animals, p<0.001, suggesting that geranial is more effective at inducing autophagy (Fig. 6C, Fig. 6D).
Fig. 6.
Animals were euthanized and tumors were excised (A) for western blot analysis of autophagy proteins Atg5 and LC3B (B). There was more expression of Atg5 and LC3B in G80 tumors compared to G40 tumors, and there was also more expression of Atg5 (C) and LC3B (D) in G80 tumors compared to N80 tumors, suggesting that geranial is more effective than neral or citral at inducing autophagy at the same concentrations. The degree of tumor inhibition appears to correlate with increasing induction of autophagy in tumor tissues. **p<0.001
Discussion
We have recently demonstrated that the steam distilled extracts of ginger composed of 30-40% citral could induce caspase 3 activity, activate p53 and decrease Bcl-2 expression, resulting in apoptosis in two endometrial cancer cell lines (ECC-1 and Ishikawa) at IC50 of 15-25 μM.(9) The anticancer mechanism of citral has not yet been completely elucidated, but citral represents an emerging class of natural products which has potential to be developed as a chemotherapeutic agent against several forms of cancer.
Although citral is known to be composed of a mixture of geranial (trans-citral) and neral (cis-citral), little work has been done to investigate the anticancer properties of these respective isomers. For example, the potent anticancer agent cisplatin can kill cancer cells by binding to DNA and interfering with cell repair mechanisms but its trans isomeric form transplatin binds differently to DNA and is therefore inactive.(26, 27) Before formulation of citral was investigated, we set about synthesizing pure geranial and neral in the lab (Fig. 1) to compare each isomer’s relative cytotoxicity with respect to commercially purchased citral from Sigma, containing a ratio of approximately 2:1 geranial to neral based on 1H-NMR (see supplemental, Fig. 1S). The yield for syntheses of geranial and neral were 95% and 90% respectively. We incubated each isomer for up to 7 days at temperatures of 25°C and 37°C, and found that the geranial (trans-citral) isomer was stable with no detectible conversion to neral (cis-citral) for either temperature (Fig. 2A). Similarly, citral (cis-citral) did not display evidence of change to geranial after incubating at 25 °C up to 7 days; however, we did observe 50% conversion of cis-citral to trans-citral by day 7 at higher incubation temperature of 37°C (Fig. 2B).
We successfully encapsulated citral and its isomers geranial and neral into PEG-b-PCL micelles. The drug encapsulation levels for geranial, neral, and citral were similar, with all drug loading above 50% at 5-40% (w/w) drug to polymer ratio (Table I). Drug loaded micelles were also similarly sized, ranging between 66-88 nm in diameter (see supplemental, Table IS) and are small enough to take advantage of the EPR effect for extravasation through leaky blood vessels into the solid tumor tissue. Clearly, the geometric conformation of geranial and neral does not appear to play a critical role in LE of the isomers into PEG-b-PCL micelles. Citral was previously successfully encapsulated into micelles formed from non-ionic polyoxyethylene alkylether surfactants and was also found to preferentially solubilize citral within the alkylether core of C12H25-PEO4 micelles,(1) but to the best of our knowledge this is the first report of drug loading properties comparing geranial to neral. The in vitro release study of geranial, neral, and citral from PEG-b-PCL micelles was performed by the dialysis cassette method at 37°C against water for 48 h and demonstrated comparable sustained release properties for all the drugs, characterized by a half-life of ca. 8.5 hrs for citral, 8.3 h for geranial, and 8.9 h for neral (see supplemental, Fig. 2S).
When we evaluated the cytotoxicity of geranial/NP, neral/NP, and citral/NP on 4T1 mouse breast cancer cells, the IC50 of geranial was calculated to be 1.4 μM, 9.9 μM for neral, and 4.5 μM for citral. Empty NP (i.e. PEG-b-PCL micelles) did not display evidence of cytotoxicity to cells at the highest concentration of drugs tested (>90% cell viability) so we did not investigate cytotoxicity of the polymer for lower concentrations (data could not be plotted in Fig. 3 so it is not shown). It should also be noted that PCL is well-known to be biocompatible (16), being currently approved by the FDA for use in humans (e.g. biodegradable sutures). When 4T1 cells were treated with 25 μM citral/NP, neral/NP, or geranial/NP, western blot detected up-regulation of LC3B and Atg5 proteins (Fig. 3B), which are known to be involved in the regulation of autophagy.(28) Autophagy is a self-digestion mechanism that is activated to ensure cell survival under stress and there is strong evidence of a link between cancer and regulation of autophagy as either a tumor suppressor or a tumor promoter.(29) One possible role for autophagy-induced recycling in established cancers is to maintain a functional pool of mitochondria after mitochondrial damage,(28, 29) which may explain the up-regulation of autophagy proteins and decrease in metabolism (cell viability) detected in 4T1 cells treated with the drugs. Although we had found in previous studies that the mechanism of cell death by citral in two endometrial cancer cell lines was mainly due to apoptosis via activation of p53,(9) this seemed unlikely to be the case for 4T1 cells since they do not express p53 (p53-null).(30) This was verified by treating 4T1 cells with 25 μM of geranial/NP for up to 48 h and assaying cells for evidence of apoptosis by flow cytometry (see supplemental, Fig. 4S); data indeed confirmed that the mechanism of cell death was not apoptosis.
The acid-induced degradation of neral, geranial, and citral formulated in micelles was investigated at pH 5.0 by incubating samples at room temperature for up to 24 h (Fig. 4). It’s important to note that none of the drugs, in free form or when formulated in micelles, showed evidence of degradation when incubated at room temperature at pH 7.4 (see supplemental, Fig. 5S). This data does agree with our previous NMR stability studies conducted at 25 °C which had revealed that both geranial and neral remained stable geometric stereoisomers at this temperature (Fig. 2). At pH 5.0, all the drugs were indeed better protected from acid-induced degradation compared to the free drugs (Fig. 4), with degradation correlating to drug release from micelles. Empty micelles incubated under these conditions did not show evidence of micelle instability or polymer degradations during the course of the study (data not shown). Protection from acid-degradation is most likely a result of the drug preferentially partitioning into the hydrophobic core of PEG-b-PCL micelles.
For the animal studies, mice (5 per group) received four retro-orbital injections on days 13, 16, 19, and 22 of either saline, empty NP, C40, N40, G40, C80, N80, or G80. The tumor size and BW of animals were monitored daily to assess the therapeutic efficacy and acute toxicity of the treatments in animals. The saline and NP treatments did not inhibit tumor growth (p>0.05), whereas C40, N40 and G40 were relatively all effective at reducing tumor burden 32-49% compared to NP controls by day 24. The most significant tumor regression was observed with G80. Comparing G80 to C80 and N80, there was a statistically significant difference in tumor volume reduction between G80 and C80 (p<0.001), and G80 and N80 (p<0.001) (Fig. 5A). In vivo results suggest that geranial is more potent than neral at 80 mg/kg, with a 92% reduction in tumor volume by day 24 compared to NP controls. Since C80 contains approximately 2:1 geranial to neral in the formulation, it makes sense that its antitumor properties would be slightly less effective than G80 but better than N80. In addition, the formulations did not generate acute toxicity in animals based on consistent animal weights throughout the treatment period (Fig. 5B). In vivo results suggest that geranial is therefore more potent than neral at 80 mg/kg.
At the end of the study (day 24), all animals were euthanized and tumors were excised (Fig. 6A, see supplemental Fig. 6S) for western blot analysis of autophagy proteins Atg5 and LC3B. Confirming the in vitro western blot data (Fig. 3B), western of tumor tissues revealed that autophagy was indeed the major mechanism of tumor growth inhibition for animals treated with the formulations (Fig. 6B). Interestingly, there was statistically more expression of Atg5 and LC3B for G80-treated animals compared to G40, p<0.001, and there was also more expression of Atg5 and LC3B in G80-treated animals compared to N80-treated animals, p<0.001, suggesting again that geranial is more effective than neral or citral at inducing autophagy at the same concentrations (Fig. 6C, Fig. 6D). The degree of tumor inhibition appears to correlate with increasing induction of autophagy in tumor tissues.
Conclusions
Citral is a naturally occurring bioactive compound found in the essential oils of plants such as citrus fruits, lemon grass and ginger, and is composed of a mixture of the two terpenoid isomers geranial (trans-citral) and neral (cis-citral). PEG-b-PCL micelles were able to encapsulate citral, geranial and neral with >50% loading efficiency at 40-5% drug/polymer (w/w) and displayed similar patterns of sustained drug release (t1/2 of 8-9 hours). The IC50 of geranial, citral and neral formulation against p53-null 4T1 cells ranged from 1.4 to 4.5 to 9.9 μM respectively; western blot revealed in vitro evidence of autophagy as the primary mechanism of cell growth inhibition in 4T1 cells. Flow cytometry revealed that apoptosis/necrosis did not play a role in inhibiting cell growth. The rate of drug degradation decreased at pH 5.0 up to 24 h when formulated in micelles compared to free drugs; there was no evidence of drug degradation (free or formulated into NP) at pH 7.4 up to 24 h. In vivo studies on mice bearing 4T1 xenograft tumors revealed that geranial is the more potent isomer of citral and western blot of tumor tissues confirmed that autophagy was the major mechanism of tumor growth inhibition in p53-null 4T1 cells. None of the formulations tested exhibited evidence of adverse acute toxicity to animals based on body weights.
Supplementary Material
Acknowledgement
This research was supported by NIH grant R01DK099596 and startup funds from the University of Wisconsin-Madison, School of Pharmacy.
Abbreviations
- BW
body weight
- CMC
critical micelle concentration
- DLS
dynamic light scattering
- DMEM
Dulbecco’s Modified Eagle Medium
- EPR
enhanced Permeability and Retention
- FBS
fetal bovine serum
- geranial
trans-citral
- i.v.
intravenous
- LE
loading efficiency
- MTD
maximum tolerated dose
- neral
cis-citral
- NP
nanoparticle
- O/W
oil-in-water
- PBS
phosphate buffered saline
- PCL
polycaprolactone
- PDI
polydispersity index
- PEG-b-PCL
poly(ethylene glycol)-block-polycaprolactone
- SDS
sodium dodecyl sulfate
References
- 1.Maswal M, Dar AA. Inhibition of citral degradation in an acidic aqueous environment by polyoxyethylene alkylether surfactants. Food Chem. 2013;138(4):2356–2364. doi: 10.1016/j.foodchem.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 2.Kimura K, Nishimura H, I I, J M. Deterioration mechanism of lemon flavor. 2. Formation of off-odor substances arising from citral. J Agric Food Chem. 1983;31:801–804. [Google Scholar]
- 3.Onawunmi GO. Evaluation of the antimicrobial activity of citral. Lett Appl Microbiol. 1989;9:105–108. [Google Scholar]
- 4.Korenblum E, Regina de Vasconcelos Goulart F, de Almeida Rodrigues I, Abreu F, Lins U, Alves PB, Blank AF, Valoni E, Sebastian GV, Alviano DS, Alviano CS, Seldin L. Antimicrobial action and anti-corrosion effect against sulfate reducing bacteria by lemongrass (Cymbopogon citratus) essential oil and its major component, the citral. AMB Express. 2013;3(1):44. doi: 10.1186/2191-0855-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado M, Pires P, Dinis AM, Santos-Rosa M, Alves V, Salgueiro L, Cavaleiro C, Sousa MC. Monoterpenic aldehydes as potential anti-Leishmania agents: activity of Cymbopogon citratus and citral on L. infantum, L. tropica and L. major. Exp Parasitol. 2012;130(3):223–231. doi: 10.1016/j.exppara.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Khan MS, Malik A, Ahmad I. Anti-candidal activity of essential oils alone and in combination with amphotericin B or fluconazole against multi-drug resistant isolates of Candida albicans. Med Mycol. 2012;50(1):33–42. doi: 10.3109/13693786.2011.582890. [DOI] [PubMed] [Google Scholar]
- 7.Helal GA, Sarhan MM, Abu Shahla AN, Abou El-Khair EK. Antimicrobial activity of some essential oils against microorganisms deteriorating fruit juices. Mycobiology. 2006;34(4):219–229. doi: 10.4489/MYCO.2006.34.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dudai N, Weinstein Y, Krup M, Rabinski T, Ofir R. Citral is a new inducer of caspase-3 in tumor cell lines. Planta Med. 2005;71(5):484–488. doi: 10.1055/s-2005-864146. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Whelan RJ, Pattnaik BR, Ludwig K, Subudhi E, Rowland H, Claussen N, Zucker N, Uppal S, Kushner DM, Felder M, Patankar MS, Kapur A. Terpenoids from Zingiber officinale (Ginger) induce apoptosis in endometrial cancer cells through the activation of p53. PLoS One. 2012;7(12):e53178. doi: 10.1371/journal.pone.0053178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liang CP, Wang M, Simon JE, Ho CT. Antioxidant activity of plant extracts on the inhibition of citral off-odor formation. Mol Nutr Food Res. 2004;48(4):308–317. doi: 10.1002/mnfr.200400027. [DOI] [PubMed] [Google Scholar]
- 11.Yang X, Tian H, Ho CT, Huang Q. Inhibition of citral degradation by oil-in-water nanoemulsions combined with antioxidants. J Agric Food Chem. 2011;59(11):6113–6119. doi: 10.1021/jf2012375. [DOI] [PubMed] [Google Scholar]
- 12.Djordjevic D, Cercaci L, Alamed J, McClements DJ, Decker EA. Chemical and physical stability of citral and limonene in sodium dodecyl sulfate-chitosan and gum arabic-stabilized oil-in-water emulsions. J Agric Food Chem. 2007;55(9):3585–3591. doi: 10.1021/jf063472r. [DOI] [PubMed] [Google Scholar]
- 13.Park S, Hong C, Choi S. Citral degradation in micellar structures formed with polyoxyethylene-type surfactants. Food Chem. 2015;170:443–447. doi: 10.1016/j.foodchem.2014.08.089. [DOI] [PubMed] [Google Scholar]
- 14.Choi SJ, Decker EA, Henson L, Popplewell LM, McClements DJ. Inhibition of citral degradation in model beverage emulsions using micelles and reverse micelles. Food Chem. 2010;122:111–116. [Google Scholar]
- 15.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1-2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 16.Woodward SC, Brewer PS, Moatamed F, Schindler A, Pitt CG. The intracellular degradation of poly(epsilon-caprolactone) J Biomed Mater Res. 1985;19(4):437–444. doi: 10.1002/jbm.820190408. [DOI] [PubMed] [Google Scholar]
- 17.Kwon GS, Yokoyama M, Okano T, Sakurai Y, Kataoka K. Biodistribution of micelle-forming polymer-drug conjugates. Pharmaceutical research. 1993;10(7):970–974. doi: 10.1023/a:1018998203127. [DOI] [PubMed] [Google Scholar]
- 18.Soo P, Luo L, Maysinger D, Eisenberg A. Incorporation and release of hydrophobic probes in biocompatible polycaprolactone-block-poly(ethylene oxice) micelles: implications for drug delivery. Langmuir. 2002;18:9996–10004. [Google Scholar]
- 19.Park Y, Lee J, Chang Y, Jeong J, Chung J, Lee M, Park K, Lee S. Radioisotope carrying polyethylene oxide-caprolactone copolymer micelles for targetable bone imaging. Biomaterials. 2003;23:873–879. doi: 10.1016/s0142-9612(01)00196-x. [DOI] [PubMed] [Google Scholar]
- 20.Liu J, Zeng F, Allen C. In vivo fate of unimers and micelles of a poly(ethylene glycol)-block-poly(caprolactone) copolymer in mice following intravenous administration. Eur J Pharm Biopharm. 2007;65(3):309–319. doi: 10.1016/j.ejpb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 21.Savic R, Azzam T, Eisenberg A, Maysinger D. Assessment of the integrity of poly(caprolactone)-b-poly(ethylene oxide) micelles under biological conditions: a fluorogenic-based approach. Langmuir. 2006;22(8):3570–3578. doi: 10.1021/la0531998. [DOI] [PubMed] [Google Scholar]
- 22.Tsuboi S, Ishii N, Sakai T, Tari I, Utaka M. OXIDATION OF ALCOHOLS WITH ELECTROLYTIC MANGANESE-DIOXIDE - ITS APPLICATION FOR THE SYNTHESIS OF INSECT PHEROMONES. Bulletin of the Chemical Society of Japan. 1990;63(7):1888–1893. [Google Scholar]
- 23.Zeng S, Xiong MP. Trilayer micelles for combination delivery of rapamycin and siRNA targeting Y-box binding protein-1 (siYB-1) Biomaterials. 2013;34(28):6882–6892. doi: 10.1016/j.biomaterials.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(epsilon-caprolactone) micelles. J Control Release. 2006;110(2):370–377. doi: 10.1016/j.jconrel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Xiong MP, Forrest ML, Ton G, Zhao A, Davies NM, Kwon GS. Poly(aspartate-g-PEI800), a polyethylenimine analogue of low toxicity and high transfection efficiency for gene delivery. Biomaterials. 2007;28(32) doi: 10.1016/j.biomaterials.2007.07.043. [DOI] [PubMed] [Google Scholar]
- 26.Rosenberg B, Van Camp L, Grimley EB, Thomson AJ. The inhibition of growth or cell division in Escherichia coli by different ionic species of platinum(IV) complexes. J Biol Chem. 1967;242(6):1347–1352. [PubMed] [Google Scholar]
- 27.Cleare M, Hoeschele J. Studies on the antitumor activity of group VIII transition metal complexes. Part I. Platinum (II) complexes Bioinorg Chem. 1973;2:187–210. [Google Scholar]
- 28.Mai S, Muster B, Bereiter-Hahn J, Jendrach M. Autophagy proteins LC3B, ATG5 and ATG12 participate in quality control after mitochondrial damage and influence lifespan. Autophagy. 2012;8(1):47–62. doi: 10.4161/auto.8.1.18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12(6):401–410. doi: 10.1038/nrc3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parajuli P, Pisarev V, Sublet J, Steffel A, Varney M, Singh R, LaFace D, Talmadge JE. Immunization with wild-type p53 gene sequences coadministered with Flt3 ligand induces an antigen-specific type 1 T-cell response. Cancer Res. 2001;61(22):8227–8234. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.