Abstract
Novel agents are desperately needed for improving the quality of life and 5-year survival to more than 30% for metastatic castrate-resistant prostate cancer. Previously we showed that Nexrutine, Phellodendron amurense bark extract, inhibits prostate tumor growth in vitro and in vivo. Subsequently using biochemical fractionation we identified butanol fraction contributes to the observed biological activities. We report here that palmatine, which is present in the butanol fraction, selectively inhibits growth of prostate cancer cells without significant effect on non-tumorigenic prostate epithelial cells. By screening receptor tyrosine kinases in a protein kinase array, we identified ribosomal protein S6, a downstream target of p70S6K and the Akt/mTOR signaling cascade as a potential target. We further show that palmatine treatment is associated with decreased activation of NFκB and its downstream target gene FLIP. These events led to inhibition of invasion. Similar results were obtained using parent extract Nexrutine (Nx) suggesting that palmatine either in the purified form or as one of the components in Nx is a potent cytotoxic agent with tumor invasion inhibitory properties. Synergistic inhibition of rpS6/NFκB/FLIP axis with palmatine may have therapeutic potential for the treatment of prostate cancer and possibly other malignancies with their constitutive activation. These data support a biological link between rpS6/NFκB/FLIP in mediating palmatine-induced inhibitory effects and warrants additional preclinical studies to test its therapeutic efficacy.
Introduction
Approximately 2.8 million men are currently living with prostate cancer in the United States alone making it the most common cancer among men [1]. Five-year survival rates for localized prostate cancer patients is almost 100%; however for metastatic disease it is less than 30% [1]. The current standard of care for localized disease is surgery followed by radiation or hormone ablation therapy, while chemotherapy is reserved for metastatic disease [2]. Lack of effective curative treatment options for advanced metastatic disease underscores the need to develop alternate approaches to successfully manage advanced disease. Studies from our laboratory demonstrated that Phellodendron amurense extract, namely Nexrutine (Nx), inhibits development of prostate tumors in transgenic adenocarcinoma of mouse prostate (TRAMP) model through modulation of Akt/CREB/Cyclin D1 signaling [3–6]. Since then, its anti-cancerous activity has been confirmed in other tumor models [7,8]. Nx inhibits tumorigenesis in mouse skin and induces apoptotic cell death in human squamous carcinoma A431 and human melanoma A375 cells [7,9]. In addition, we recently demonstrated the ability of Nx to (i) reduce fibrosis in a pancreatic cancer model which may possibly occur through modulation of Stat3/NFκB/EP4 axis and (ii) inhibit autophagy [10,11]. Additionally, we showed that the butanol fraction of Nx recapitulated its proliferative inhibitory activities as well by modulating NFκB transcriptional activity in prostate cancer cells [12]. Further, using ultraperformance liquid chromatography (UPLC), we identified the presence of palmatine or closely related compounds in this butanol fraction [12]. Palmatine, a protoberberine alkaloid, is a close structural analog of berberine (structure shown in figure 1A). Palmatine’s anti-cancer activities have not been extensively investigated. We found only 10 published reports of palmatine in cancer. Palmatine has been used in the treatment of dysentery, jaundice, inflammation, hypertension and liver-related diseases [13,14]. Available reports also suggest a potential role in cell cycle regulation, modulating glucose levels, oxidative stress and metastasis [15–17]. To the best of our knowledge, neither the cytotoxic properties nor the molecular targets of palmatine have been elucidated in prostate cancer cells. Accordingly, the purpose of this study was to investigate the potential of palmatine as an anti-prostate cancer agent and try to understand the underlying mechanism(s) of action. Using in vitro cell proliferation, anchorage independent growth, and invasion assays, we report that palmatine inhibits proliferation of androgen independent C4-2B cells that mimics clinical prostate cancer progression from androgen responsiveness to androgen independence, PC-3 and DU145 cells. On the other hand, palmatine had minimal effect (<10%) on proliferation of androgen responsive LNCaP with no significant effect on non-tumorigenic RWPE-1 cells. Palmatine treatment reduces (i) levels of phosphorylated ribosomal protein S6 (prpS6), physiological target of ribosomal S6 kinase (RSK), transcriptional activity of NFκB and one of its downstream target genes c-FLIP; and (ii) reduces invasive ability of DU145 cells. Further, overexpression of androgen receptor (AR) partially protected PC-3 cells from palmatine-induced growth inhibitory effects.
Figure 1. Palmatine inhibits prostate cancer proliferation in vitro.
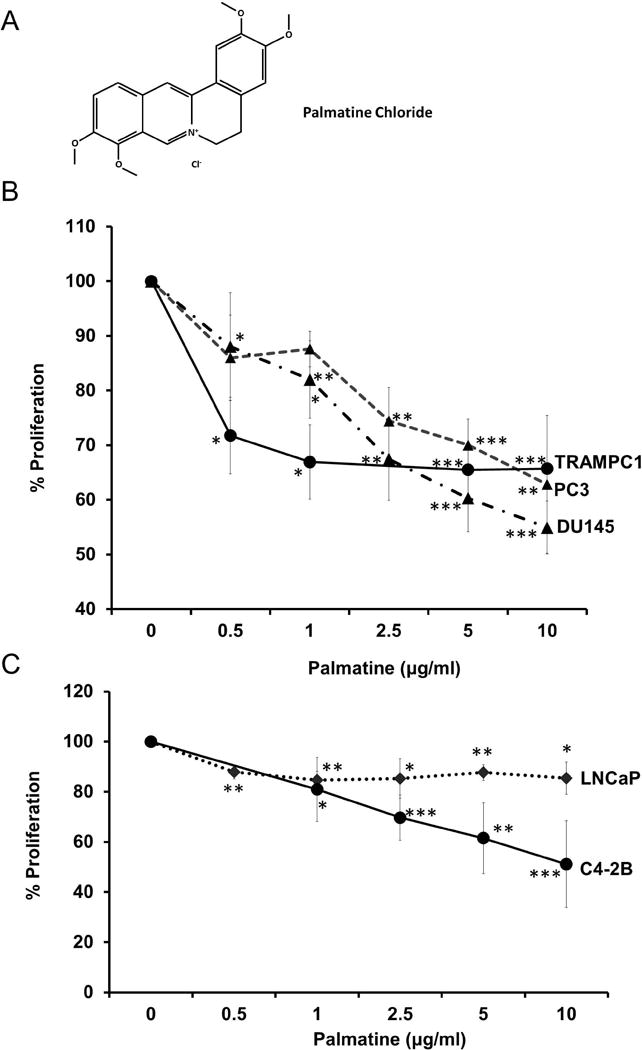
(A) Chemical structure of palmatine; (B) Cell proliferation after palmatine treatment in cell lines lacking androgen-receptor (AR), DU145, PC-3, and TRAMPC1. (C) Cell proliferation after palmatine treatment in androgen-receptor (AR) positive cell lines, LNCaP and C4-2B; Cells were treated with palmatine (0.5, 1, 2.5, 5, 10 μg/ml) for 72 hours and percent proliferation shown is compared with control wells. Data show mean values ± SD for 3 independent experiments (2 independent experiments for TRAMPC1).
Materials and methods
Reagents
Palmatine chloride hydrate was purchased from Sigma-Aldrich (St. Louis, MO), prepared as a 1 mg/ml stock solution in sterile water and stored in aliquots at −20°C. Depending on the nature of experiment, palmatine was diluted in the culture media and added to the cells to obtain the desired final concentrations.
Cell culture
Human prostate cell lines LNCaP, PC-3, DU145, and mouse TRAMPC1 were purchased from the American Type Culture Collection (Manassas, VA) and cultured in media as described previously [12]. PC-3-AR cell line was a generous gift from Dr. Ratna Vadlamudi (University of Texas Health Science Center, San Antonio, TX), and grown in F12K media supplemented with 5% charcoal stripped serum and 1% antibiotics. C4-2B cell line was a generous gift from Dr Thambi Dorai [18].
Western blotting
Whole-cell extracts were prepared using 2X SDS buffer supplemented with fresh protease and phosphatase inhibitors. Equal volumes of protein were fractionated by SDS-PAGE and transferred to nitrocellulose membranes. The following antibodies were used: Akt, mTOR, p-mTORS2448, p-mTORS2481, ribosomal protein S6, phospho-ribosomal protein S6S235/236, Rictor, Raptor, TSC2, p-TSC2S939, NFκB p50, NFκB p65, IκBα, p-IkBαS32, and IKKα (purchased from Cell Signaling Technology, Danvers, MA). β-actin antibody was purchased from Sigma-Aldrich. Anti-rabbit secondary antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Blots were developed using Western Lighting Plus ECL chemiluminescent reagents (Perkin Elmer, Waltham, MA) and Syngene G:Box Imaging System (Frederick, MD). Band quantification was performed using Syngene Genetools gel analysis software and band intensities normalized to β-actin intensity.
Cell proliferation
Cells were seeded in triplicate at a density of 4 × 103 per well in 96-well plate. Following attachment cells were treated with increasing does of palmatine (0.5, 1, 2.5, 5, 10 μg/ml) for 72 hours. Cell proliferation was measured using CellTiter 96 Aqueous One solution assay (Promega Corporation, Madison, WI) according to manufacturer’s instructions as described previously (10). Each proliferation experiment was repeated three independent times.
Invasion assay
DU145 and PC-3 cells were seeded in triplicate at a density of 1 × 106 per chamber in serum-free media or media containing palmatine at desired concentration in a 24-well plate. Cells were then incubated at 37°C, 5% CO2 for 48 hours. Experiment was carried out according to manufacturer’s instructions (Innocyte Cell Invasion Assay; EMD Millipore, Billerica, MA). Fluorescence was measured using SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) with a 485nm filter. Invasion for treatment groups is expressed as a percent of fluorescence of vehicle-treated wells. Each experiment was repeated twice using different passage cells and the data was presented as avg±sd.
Colony formation assay
Colony formation assay was performed essentially as per vendor instructions using CytoSelectTM 96-well Cell Transformation Assay (Cell Bio labs, San Diego, CA). Fluorescence was read on SpectraMax M5 plate reader (Molecular Devices) using 485/520nm filters.
Cell viability
Cell viability was evaluated using the trypan blue exclusion method after treatment with palmatine for 72 h (0, 1, 2.5, 5, or 10 μg/ml).
Protein kinase array
DU145 cells at 70–80% density were treated with solvent control or palmatine to a final concentration of 5 and 10 μg/ml for 24 h. PC-3 cells were treated with Nexrutine (0, 5, and 25 μg/ml) for 24 h. Cells were harvested in RIPA buffer, diluted, and incubated with the Human Phospho-RTK Array Kit membrane (R&D Systems, Minneapolis, MN). After washing, membrane was incubated with pan anti-phospho-tyrosine antibody conjugated to HRP and chemiluminescence was used to detect phosphorylated tyrosines on activated receptors. Quantification of spots was carried out using reference spots from the array.
Luciferase assay
Transfections were performed essentially as described previously [3]. Briefly, cells were seeded at a density of 1 × 105/well in a 24-well plate in complete media. At 70% confluency, cells were transfected with 0.5 μg of NFκB or FLIP (+1/+242) plasmid along with Renilla luciferase per well using Lipofectamine 2000 (Invitrogen) in Opti-MEM media (Invitrogen). After 6 h, cells were fed with media containing serum. Following 24 h post-transfection, cells were treated with solvent control, palmatine (5 or 10 μg/ml), or Nexrutine (10 μg/ml) for additional 24 h and cell lysates prepared. Luciferase activity was measured using Dual-Luciferase Reporter Assay System and Glomax Luminometer (Promega, Madison, WI).
Statistical analysis
Data presented are a mean ± SD performed using Student’s t-test. One-way ANOVA was used to evaluate differences between cell lines after treatment. Differences between the control and experimental groups were considered significant at p < 0.05; p < 0.01 and p < 0.001 was considered highly significant.
Results and Discussion
We examined the effect of palmatine on proliferation of androgen responsive LNCaP, androgen independent C4-2B, PC-3, and DU145 human prostate cancer cells, and mouse TRAMP1 cell lines using the CellTiter96 Aqueous one solution assay as described earlier [12]. Exponentially growing cells were treated with 0, 0.5, 1, 2.5, 5 and 10 μg/ml of palmatine and cell proliferation was measured after 72 h. As shown in figure 1B and C, palmatine had differential effects on proliferation inhibition, with DU145 being the most sensitive cell line (figure 1B, p < 0.001). For example, 10 μg/ml palmatine was sufficient to inhibit the growth of DU145 cells by about 50%; at that dose PC-3 cells showed a 38% decrease in proliferation. Further, LNCaP cells were minimally affected (<10%) by palmatine treatment under these experimental conditions (figure 1C). It is well recognized that reactivation of androgen receptor (AR) signaling plays an important role in the progression of androgen dependent to androgen independence [19]. We used C4-2B, an isogenic sub line of LNCaP that expresses AR but does not respond to exogenous androgens. Interestingly, palmatine significantly inhibited proliferation of C4-2B cells suggesting that it can prevent progression to androgen independence (figure 1C). Although it is not clear, the observed differential effects could be related to genetic differences among these cell lines including androgen receptor (AR) and wild type PTEN status of DU145 cells. However, the precise role of PTEN in palmatine-induced proliferation inhibition and why LNCaP cells are relatively resistant needs further investigation. It should be mentioned that Nx (parent extract) unlike palmatine inhibits proliferation of LNCaP, PC-3 and DU145 cells [3]. It is noteworthy to mention that according to Chinese literature Phellodendron extract contain different protoberberine alkaloids including berberine (~50–55%), palmatine, Jatrrorhizine and flavonoids. Therefore, it is plausible that other components present in the parent extract may have additive effects that are not seen in the single compound.
Since a characteristic feature of transformed cells is their ability to grow in an anchorage independent manner, we examined the effect of increasing concentrations of palmatine on to inhibit growth of colonies on soft agar. Consistent with the cell proliferation data, we found the number of colonies formed with increasing doses of palmatine treatment significantly decreased (~24–43%) in DU145, PC-3 and C4-2B cells (figure 2A,B,D). Interestingly, palmatine treatment also reduced the colony forming ability of LNCaP cells (figure 2C) despite minimal effect on proliferation (compare figure 2C with 1C). It is possible that in LNCaP cells palmatine may not affect the mitochondrial dehydrogenase that reduces tetrazolium while it may inhibit the growth and survival of cells within a colony and therefore cause reduction in number of colonies. In any event these data taken collectively show that palmatine inhibits anchorage-dependent and -independent growth of multiple prostate cancer cells, with DU145 being most sensitive.
Figure 2. Palmatine inhibits anchorage-independent growth in vitro.

Percent colonies formed by DU145 (A), PC-3 (B), LNCaP (C) and C4-2B (D) cells after palmatine treatment and incubation for 7 days on soft agar. At end-point, agar was solubilized, cells lysed and fluorescence detected using CyQuant GR dye using a plate reader. Data show mean values ± SD for 2 independent experiments. *p < 0.05; **p < 0.01;.
Since DU145 is a brain metastatic cell line and tumor invasion and metastasis are the major cause of prostate cancer related deaths, we examined the ability of palmatine to inhibit tumor cell invasion. As shown in figure 3A, palmatine significantly inhibited the invasive capacity of DU145 cells (p < 0.01). Under these experimental conditions, parent extract Nx also showed significant inhibition of invasion in these cells (figure 3B). Similar results were obtained using PC-3 cells with palmatine, although the effect was marginal albeit statistically significant (figure 3C and D). Taken together, these results show that palmatine either in the purified form or a component of Nx is a potent cytotoxic agent with tumor invasion inhibitory properties. Further, DU145 cells appear to be most sensitive. However, the underlying molecular mechanism involved is not known.
Figure 3. Palmatine inhibits prostate cancer cell invasion.

(A and C) Percent cell invasion after 0 and 5 μg/ml palmatine treatment for 48 hours in DU145 and PC-3 cells, respectively. (B and D) Percent cell invasion after 0 and 5 μg/ml Nexrutine treatment for 48 hours in DU145 and PC-3 cells, respectively. **p < 0.01.
Receptor tyrosine kinases (RTK) are known to play a critical role in wide variety of cellular functions including cell proliferation and invasion [20]. In order to understand if palmatine-mediated biological effects involve alterations in the levels and activities of RTKs, we screened phosphotyrosine kinase antibody array containing 49 different receptor tyrosine kinases using extracts prepared from solvent control and palmatine treated cells. As shown in figure 4A, these data indicates significant down regulation of IGF-IR, S6 ribosomal protein, and c-Abl in a dose-dependent manner. Of these, S6 ribosomal protein (rpS6), which is phosphorylated by RSKs at Ser235/236 was the most significantly down regulated protein. It is also well established that activation of p70S6K (mTORC1) in response to growth factors and mitogens leads to subsequent phosphorylation of S6 ribosomal protein, which regulates protein translation [21]. Previously we have shown that Nx inhibits proliferation of prostate cancer cells via modulation of Akt/mTOR signaling [3]. Given our observation showing significant down regulation of S6 ribosomal protein, we performed immunoblotting on key Akt/mTOR signaling molecules including pAkt (as a measure of mTORC2 activity), and p70S6K (as a measure of mTORC1 activity). Although most proteins involved in Akt/mTOR signaling appear to be unaffected by palmatine treatment under our experimental conditions, we consistently observed an inhibitory effect of palmatine on phosphorylated ribosomal protein S6S235/236, a key regulator of protein synthesis and direct target of S6K and mTORC1 signaling (figure 4B). Quantification of these data relative to β-actin shows significant reduction of prpS6 at both doses of palmatine (figure 4C, p < 0.05 and p < 0.01). Of note, higher doses of palmatine (25 and 50 μg/ml) did not result in significant downregulation of proteins in the Akt/mTOR pathway (data not shown). Similar results were observed with Nx treatment, suggesting that palmatine recapitulates the effects on Akt/mTOR signaling changes associated with Nx (data not shown).
Figure 4. Identification of receptor tyrosine kinase rpS6 as possible target for palmatine.
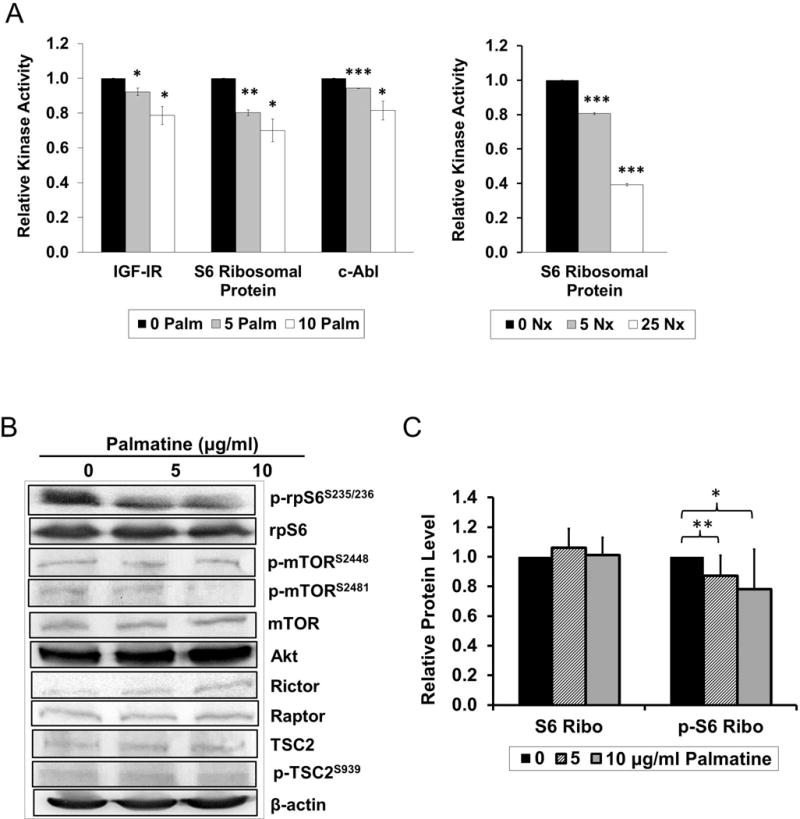
(A) Protein kinase array in DU145 after (left panel) 0, 5, or 10 μg/ml palmatine, or (right panel) 0, 5, or 25 μg/ml Nexrutine treatment for 24 hours. After probing, membrane was incubated with pan anti-phospho-tyrosine antibody conjugated to HRP and detected using chemiluminescence GBOX system. Quantification of spots was carried out using reference spots and normalized to each 0 palmatine control. (B) Akt/mTOR pathway protein levels after 0, 5 or 10 μg/ml palmatine treatment for 24 hours in DU145. (C) Quantification of rp-S6 and phopho-rp-S6S235/236 protein levels relative to β-Actin after 0, 5 or 10 μg/ml palmatine treatment for 24 hours.
It is well established that NFκB plays a critical role in prostate tumorigenesis by regulating a plethora of genes involved in various cellular process including proliferation, invasion, metastasis and apoptosis [22]. Further, stable overexpression of NFκB-regulated c-FLICE inhibitory protein (FLIP) resulted in increased tumor invasion activity in DU145 cells [23]. It is noteworthy to mention that we previously reported that Nx and its butanol fraction containing palmatine inhibits proliferation that is accompanied with transcription factor NFκB DNA binding activity and its transcriptional activity in prostate cancer cells [12]. These data implicate that palmatine could mediate its effects through NFκB-mediated inactivation of FLIP. Accordingly, we investigated whether palmatine inhibits NFκB/FLIP activation in DU145 cells. Treatment with palmatine significantly reduced promoter activity of both NFκB (5 μg/ml) and its target gene FLIP (10 μg/ml) in DU145 cells compared with control cells (figure 5A and B, **p < 0.01). Similarly, treatment with parent compound Nx (10 μg/ml) showed a significantly reduced promoter activity of FLIP (figure 5C). Immunoblot analysis showed no significant differences in the protein levels of NFκB signaling components (figure 5D). Therefore, palmatine-induced downregulation of c-FLIP via NFκB could potentially contribute to the observed inhibition of invasion in DU145 cells. However, additional studies are warranted to demonstrate this phenomenon.
Figure 5. Palmatine inhibits NFκB and FLIP reporter activity in DU145 cells.
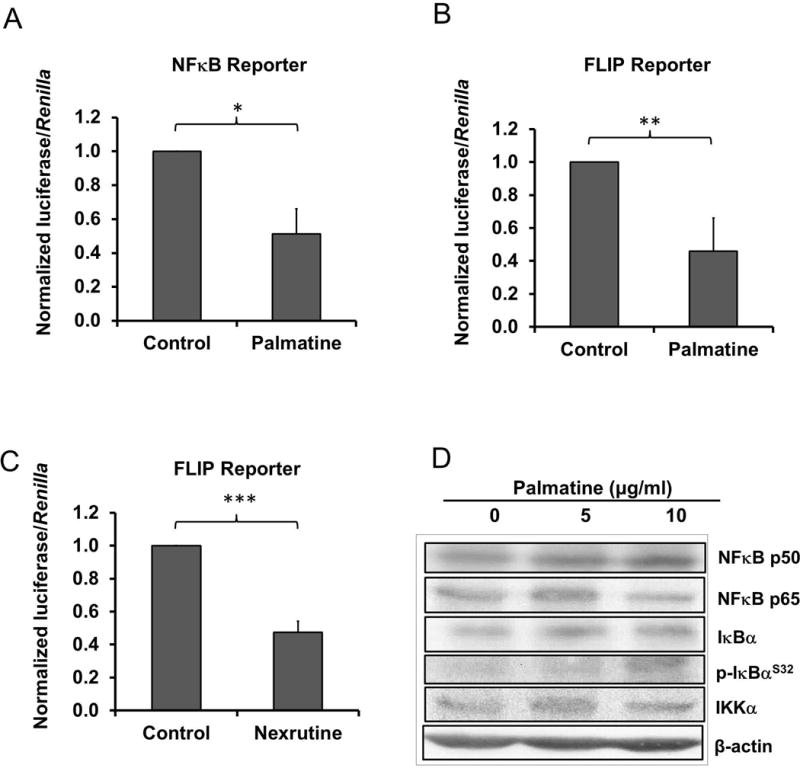
(A) Luciferase assay for NFκB promoter after 0 or 5 μg/ml palmatine treatment for 24 hours in DU145. (B) Luciferase assay for FLIP promoter (+1/+242) after 0 or 10 μg/ml palmatine treatment for 24 hours in DU145. (C) Luciferase assay for FLIP promoter (+1/+242) after 0 or 10 μg/ml Nexrutine treatment for 24 hours in DU145. (D) NFκB pathway protein levels after 0, 5 or 10 μg/ml palmatine treatment for 24 hours in DU145. A representative blot of 3 independent experiments is shown. *p < 0.05; **p < 0.01; ***p < 0.001.
One of the challenges in the clinical management of prostate cancer is progression to androgen independence and development of castrate resistance. Our findings presented here indicate the potential of palmatine to inhibit progression to androgen independence (represented by C4-2B cell line) as well as androgen independent stage (DU145 and PC-3). Although both LNCaP and C4-2B cells express AR, C4-2B cells are not sensitive to androgens. Therefore, we hypothesized that overexpression of AR in PC-3 cells could protect from palmatine induced growth inhibition. We examined the effect of palmatine on proliferation and viability of PC-3 and AR overexpressing PC-3 cells (PC-3-AR). As shown in figure 6A, consistent with our previous results, palmatine inhibited the proliferation of both PC-3 and PC-3 AR cells significantly. Similar results were observed using trypan blue exclusion assay (figure 6B). The observed differences between two cell types albeit minimal, are statistically significant at doses lower than 5 μg/ml. These data suggest a possibility that palmatine could mediate its biological effects through AR. However, additional investigations are required to text this hypothesis.
Figure 6. Palmatine-induced growth inhibitory effects in PC-3 and PC-3 AR cells.
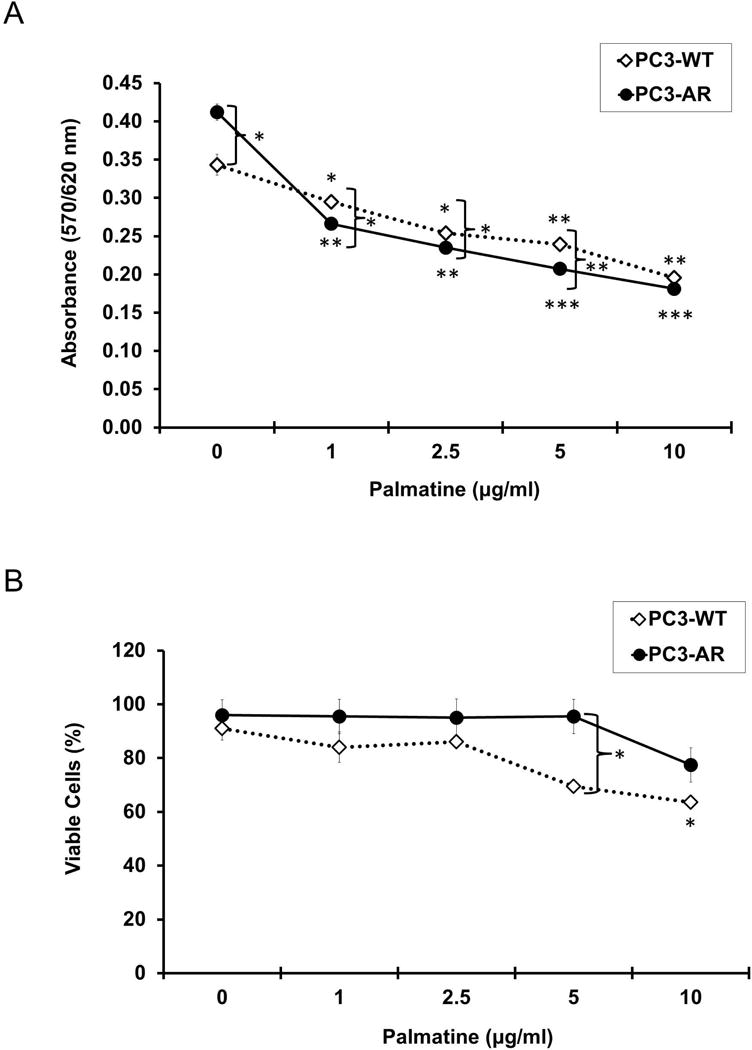
(A) Cell proliferation after palmatine treatment in PC-3 and AR overexpressing PC-3-AR cell lines. Cells were treated with palmatine (0, 1, 2.5, 5, 10 μg/ml) for 72 hours and absorbance at 570/620 nm is shown. (B) Percent cell viability after palmatine treatment in PC-3 and PC-3-AR cell lines, determined by trypan blue method. Cells were treated with palmatine (0, 1, 2.5, 5, 10 μg/ml) for 72 hours. Data presented are mean values ± SD for 2 independent experiments conducted in quadruplicate. *p < 0.05; **p < 0.01; ***p < 0.001.
Overall, we have discovered that palmatine either in the purified form or a component of Nx is a potent cytotoxic agent with growth and tumor invasion inhibitory properties. Further, palmatine (i) affected predominantly androgen-independent prostate cancer cell proliferation without affecting the proliferation of non-tumorigenic RWPE-1 or androgen responsive LNCaP cells; (ii) inhibits both anchorage-dependent and independent growth and (iii) inhibits invasion. Further, palmatine inhibits proliferation of C4-2B, a cell line representing progression to androgen independence. At the molecular level, our results show that palmatine or Nx treatment causes statistically significant decrease in the (i) levels of and activities of multiple RTKs including phospho-rps6; and (ii) transcriptional activity of NFκB and its downstream target gene c-FLIP. All these events led to inhibition of cell viability and inhibition of invasion. These data support a biological link between rpS6/NFκB/FLIP in mediating palmatine-induced inhibitory effects. Since the exact proportion of palmatine in addition to other protoberberine compounds in Nx is not entirely clear, it will be difficult to conclude whether palmatine is solely responsible for the observed biological activities associated with Nx. Nevertheless, synergistic inhibition of these pathways using palmatine may have therapeutic potential for the treatment of prostate cancer and possibly other malignancies with constitutive activation of these pathways. Both NFκB and FLIP are important and clinically relevant targets. For example, published studies from our laboratory and other investigators demonstrate elevated expression of FLIP and its transcriptional regulator NFκB in human prostate tumors [24–26]. Intervention with 2-methoxyestradiol (2-ME2), an endogenous estrogenic metabolite, prevented prostate tumor development in a preclinical animal model that was associated with reduced expression of FLIP in the prostate [27]. Further, both AR and NFκB regulate FLIP. These data suggest that modulation of AR and NFκB target gene; FLIP can potentially be developed as a therapeutic approach for management of prostate cancer.
Although our results suggest that palmatine inhibits mTORC1, it is unclear whether the inhibitory effect is a result of Akt/mTOR blockade or inhibition of ERK signaling, which also phosphorylates S6K, the kinase responsible for rpS6 phosphorylation [21]. It would be of interest in future studies to investigate a more upstream target of palmatine, which might be modulating its effects through MAPK signaling. It is important to mention that lack of significant proliferation inhibition in LNCaP cells may be related to the status of wild type p53 and/or AR activity [19]. As shown in figure 1B, palmatine effectively inhibited proliferation of the TRAMP mouse cell line TRAMPC1, suggesting that the compound has potential for in vivo and clinical development as an anticancer agent for prostate. To our knowledge, this is the first report demonstrating the growth inhibitory properties of palmatine and warrant additional investigations including preclinical and in-depth mechanistic investigations to facilitate its further development for metastatic prostate cancer treatment.
Supplementary Material
(A) Protein kinase array in DU145 after 0, 5, or 10 μg/ml palmatine treatment for 24 hours. After probing, membrane was incubated with pan anti-phospho-tyrosine antibody conjugated to HRP and detected using chemiluminescence GBOX system. Quantification of spots was carried out using reference spots and normalized to each 0 palmatine control. (B) Protein kinase array in PC-3 after 0, 5, or 25 μg/ml Nexrutine treatment for 24 hours. *p < 0.05; **p < 0.01; ***p < 0.001.
Acknowledgments
Grant Support: This work was supported in part by the funds from Veterans Affairs-Merit Award I01 BX 000766-01; and National Cancer Institute 1RO1 CA 135451, R21 CA 137578 (APK); RO1 CA 149516 (RG). We acknowledge support provided by Cancer Therapy and Research Center at University of Texas Health Science Center San Antonio through the National Cancer Institute support grant #2P30 CA 054174-17 (APK and RG) for use of flow cytometry core facility. We thank Ms. Divya Chakravarthy for providing feedback on the manuscript and Mr. Praveen Jampala for technical assistance.
Bibliography
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA-Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Huang X, Chau CH, Figg WD. Challenges to improved therapeutics for metastatic castrate resistant prostate cancer: from recent successes and failures. J Hematol Oncol. 2012;5:35. doi: 10.1186/1756-8722-5-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia GE, Nicole A, Bhaskaran S, Gupta A, Kyprianou N, Kumar AP. Akt-and CREB-mediated prostate cancer cell proliferation inhibition by Nexrutine, a Phellodendron amurense extract. Neoplasia. 2006;8(6):523–533. doi: 10.1593/neo.05745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kumar AP, Bhaskaran S, Ganapathy M, et al. Akt/cAMP-responsive element binding protein/cyclin D1 network: a novel target for prostate cancer inhibition in transgenic adenocarcinoma of mouse prostate model mediated by Nexrutine, a Phellodendron amurense bark extract. Clin Cancer Res. 2007;13(9):2784–2794. doi: 10.1158/1078-0432.CCR-06-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghosh R, Graham H, Rivas P, et al. Phellodendron amurense bark extract prevents progression of prostate tumors in transgenic adenocarcinoma of mouse prostate: potential for prostate cancer management. Anticancer Res. 2010;30(3):857–865. [PubMed] [Google Scholar]
- 6.Ghosh R, Garcia GE, Crosby K, et al. Regulation of Cox-2 by cyclic AMP response element binding protein in prostate cancer: potential role for nexrutine. Neoplasia. 2007;9(11):893–899. doi: 10.1593/neo.07502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar R, Das M, Ansari KM. Nexrutine(R) inhibits tumorigenesis in mouse skin and induces apoptotic cell death in human squamous carcinoma A431 and human melanoma A375 cells. Carcinogenesis. 2012;33(10):1909–1918. doi: 10.1093/carcin/bgs219. [DOI] [PubMed] [Google Scholar]
- 8.Yan G, Lanza-Jacoby S, Wang C. Nexrutine Inhibits Survival and Induces G1 Cell Cycle Arrest, Which Is Associated with Apoptosis or Autophagy Depending on the Breast Cancer Cell Line. Nutr Cancer. 2013;66(3):506–516. doi: 10.1080/01635581.2013.780627. [DOI] [PubMed] [Google Scholar]
- 9.Hambright HG, Meng P, Kumar AP, Ghosh R. Inhibition of PI3K/AKT/mTOR axis disrupts oxidative stress-mediated survival of melanoma cells. Pigment Cell Melanoma Res. doi: 10.18632/oncotarget.3131. (Under Revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gong J, Xie J, Rivas P, et al. Disruption of NFkB/Stat3 interaction as a potential therapeutic avenue for pancreatic cancer management. Clin Cancer Res. 2013;20(5):1259–1273. [Google Scholar]
- 11.Gong J, Munoz AR, Chan D, Ghosh R, Kumar AP. STAT3 down regulates LC3 to inhibit autophagy and pancreatic cancer cell growth. Oncotarget. 2014 Mar 15; doi: 10.18632/oncotarget.1810. [EPub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muralimanoharan SB, Kunnumakkara AB, Shylesh B, et al. Butanol fraction containing berberine or related compound from nexrutine inhibits NFkappaB signaling and induces apoptosis in prostate cancer cells. Prostate. 2009;69(5):494–504. doi: 10.1002/pros.20899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee WC, Kim JK, Kang JW, et al. Palmatine attenuates D-galactosamine/lipopolysaccharide-induced fulminant hepatic failure in mice. Food Chem Toxicol. 2010;48(1):222–228. doi: 10.1016/j.fct.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Yasukawa K, Takido M, Ikekawa T, Shimada F, Takeuchi M, Nakagawa S. Relative inhibitory activity of berberine-type alkaloids against 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice. Chem Pharm Bull (Tokyo) 1991;39(6):1462–1465. doi: 10.1248/cpb.39.1462. [DOI] [PubMed] [Google Scholar]
- 15.Semwal DK, Rawat U, Semwal R, Singh R, Singh GJ. Anti-hyperglycemic effect of 11-hydroxypalmatine, a palmatine derivative from Stephania glabra tubers. Journal of Asian natural products research. 2010;12(2):99–105. doi: 10.1080/10286020903117325. [DOI] [PubMed] [Google Scholar]
- 16.Kim YM, Ha YM, Jin YC, et al. Palmatine from Coptidis rhizoma reduces ischemia-reperfusion-mediated acute myocardial injury in the rat. J Asian Prod Nat Prod Res. 2009;47(8):2097–2102. doi: 10.1016/j.fct.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 17.Lee JW, Mase N, Yonezawa T, et al. Palmatine attenuates osteoclast differentiation and function through inhibition of receptor activator of nuclear factor-kappab ligand expression in osteoblast cells. Biol Pharm Bull. 2010;33(10):1733–1739. doi: 10.1248/bpb.33.1733. [DOI] [PubMed] [Google Scholar]
- 18.Li G, Rivas P, Bedolla R, et al. Dietary resveratrol prevents development of high-grade prostatic intraepithelial neoplastic lesions: involvement of SIRT1/S6K axis. Cancer Prev Res (Phila) 2013;6(1):27–39. doi: 10.1158/1940-6207.CAPR-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bluemn EG, Nelson PS. The androgen/androgen receptor axis in prostate cancer. Curr Opin Oncol. 2012;24(3):251–257. doi: 10.1097/CCO.0b013e32835105b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mayer BJ. Perspective: Dynamics of receptor tyrosine kinase signaling complexes. FEBS Lett. 2012;586(17):2575–2579. doi: 10.1016/j.febslet.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magnuson B, Ekim B, Fingar DC. Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks. Biochem J. 2012;441(1):1–21. doi: 10.1042/BJ20110892. [DOI] [PubMed] [Google Scholar]
- 22.Liu F, Xia Y, Parker AS, Verma IM. IKK biology. Immunol Rev. 2012;246:239–253. doi: 10.1111/j.1600-065X.2012.01107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimada K, Nakamura M, Matsuyoshi S, Ishida E, Konishi N. Specific positive and negative effects of FLIP on cell survival in human prostate cancer. Carcinogenesis. 2006;27(7):1349–1357. doi: 10.1093/carcin/bgi380. [DOI] [PubMed] [Google Scholar]
- 24.Savli H, Szendroi A, Romics I, Nagy B. Gene network and canonical pathway analysis in prostate cancer: a microarray study. Exp Mol Med. 2008;40(2):176–185. doi: 10.3858/emm.2008.40.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang L, Altuwaijri S, Deng F, et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am J Pathol. 2009;175(2):489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bedolla RG, Gong J, Prihoda TJ, et al. Predictive value of Sp1/Sp3/FLIP signature for prostate cancer recurrence. PloS One. 2012;7(9):e44917. doi: 10.1371/journal.pone.0044917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganapathy M, Ghosh R, Jianping X, et al. Involvement of FLIP in 2-methoxyestradiol-induced tumor regression in transgenic adenocarcinoma of mouse prostate model. Clin Cancer Res. 2009;15(5):1601–1611. doi: 10.1158/1078-0432.CCR-08-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Protein kinase array in DU145 after 0, 5, or 10 μg/ml palmatine treatment for 24 hours. After probing, membrane was incubated with pan anti-phospho-tyrosine antibody conjugated to HRP and detected using chemiluminescence GBOX system. Quantification of spots was carried out using reference spots and normalized to each 0 palmatine control. (B) Protein kinase array in PC-3 after 0, 5, or 25 μg/ml Nexrutine treatment for 24 hours. *p < 0.05; **p < 0.01; ***p < 0.001.


