Abstract
Background
Knees undergoing revision ACL reconstruction (rACLR) have a high prevalence of articular cartilage lesions.
Hypothesis
The purpose of this study was to test the hypothesis that the prevalence of chondrosis at the time of rACLR is associated with meniscus status and lower extremity alignment.
Study design
Cross sectional study.
Methods
Data from the prospective Multicenter ACL Revision Study (MARS) cohort was reviewed to identify patients with pre-operative lower extremity alignment films. Lower extremity alignment was defined by the weight bearing line (WBL) as a percentage of the tibial plateau width, while the chondral and meniscal status of each weight bearing compartment was recorded at the time of surgery. Multivariable proportional odds models were constructed and adjusted for relevant factors in order to examine which risk factors were independently associated with the degree of medial and lateral compartment chondrosis.
Results
The cohort included 246 patients with lower extremity alignment films at the time of rACLR. Average (SD) patient age was 26.9 (9.5) years with a BMI of 26.4 (4.6). The medial compartment had more chondrosis (Grade 2/3: 42%, Grade 4: 6.5%) than the lateral compartment (Grade 2/3: 26%, Grade 4: 6.5%). Disruption of the meniscus was noted in 35% of patients on the medial side and 16% in the lateral side. The average (SD) WBL was measured to be 0.43 (0.13). Medial compartment chondrosis was associated with BMI (p=0.025), alignment (p=0.002), and medial meniscus status (p=0.001). None of the knees with the WBL lateral to 0.625 had Grade 4 chondrosis in the medial compartment. Lateral compartment chondrosis was significantly associated with age (p=0.013) and lateral meniscus status (p<0.001). Subjects with ‘intact’ menisci were found to decrease their odds of having chondrosis by 64–84%.
Conclusions
The status of articular cartilage in the tibiofemoral compartments at the time of rACLR is related to meniscal status. Lower extremity alignment and BMI are associated with medial compartment chondrosis.
Clinical relevance
Providers and patients should be aware of the association of meniscal integrity, alignment and BMI with chondrosis at the time of rACLR. Further research into the potential benefits of interventions to optimize these parameters is warranted.
Keywords: meniscus, meniscectomy, varus, valgus, osteoarthritis, ACL reconstruction
INTRODUCTION
Outcomes are known to be less favorable in revision anterior cruciate ligament reconstructions (rACLR) than after primary anterior cruciate ligament (ACL) reconstructions21,29,43,46,47. These outcomes are likely to be influenced by the status of the menisci and articular cartilage2. Knees undergoing rACLRs have more intra-articular injuries than knees undergoing primary reconstruction12,20, as 90% of knees undergoing rACLR have been found to have meniscal or chondral injury while 57% had both at the time of rACLR24. Meniscal injury28,39,48 and the amount of meniscus removed at ACL reconstruction17 have been shown to be associated with the subsequent development of arthrosis. In a previous study of the MARS cohort, partial meniscectomies occurring prior to rACLR were shown to be associated with a higher rate of chondrosis at the time of rACLR compared to previous meniscal repair or no previous meniscus surgery6. There was no difference in the prevalence of chondrosis in the knee between patients who had a previous meniscal repair and patients who had no previous meniscal surgery. However, the status of the meniscus at the time of rACLR was not assessed in that study.
Another variable likely to influence the incidence of chondrosis is lower extremity alignment, specifically the location of the weight bearing axis in the knee. Varus malalignment has been shown to predict the development of medial compartment osteoarthritis1,8,42. The integrity of the menisci and the alignment of the lower extremity are likely to influence the prevalence of chondrosis in the tibiofemoral compartments of the knee at the time of rACLR. Since obesity has been shown to be associated with a greater risk of meniscus tears, partial meniscectomy11,15,18 and osteoarthritis in the knee4,7,16, body mass index (BMI) is another variable that may be important in this population.
The current study was designed to advance our understanding of factors influencing the prevalence of chondrosis in the tibiofemoral compartments of the knee at the time of rACLR. The purpose of this study was to test the following hypotheses: (i) meniscal loss is associated with the prevalence of chondral lesions in the same compartment of the knee at the time of rACLR, and (ii) lower extremity alignment is associated with the prevalence of chondral lesions in the tibiofemoral compartments at the time of rACLR.
METHODS
Setting and Study Population
The patients in this study were enrolled in the MARS Study, an NIH funded, AOSSM sponsored, academic and private practice multicenter consortium (83 surgeons over 52 sites) conducting an ongoing prospective cohort study of patients undergoing rACLR24. All participating sites obtained local Institutional Review Board (IRB) approval prior to enrolling subjects, and complied with a standardized manual of operations. Participating surgeons were required to complete a training session which integrated intra-articular agreement studies, review of the study design and inclusion criteria. They also completed a practice intra-articular grading sheet and a trial surgeon questionnaire.
The recruitment period for the current study was between 2006 and 2011. Subject inclusion criteria incorporated any patients undergoing revision of a previously failed ACL reconstruction that agreed to participate, signed an informed consent, and completed a series of patient-reported validated outcome instruments. Indications for rACLR included functional instability, abnormal laxity testing or an MRI indicating graft tear.
Data Sources and Measurement
Patients with bilateral weight bearing, long-leg alignment films taken just prior to their revision surgery were eligible to be included in this study. While these films had been recommended for all patients enrolled in the MARS study, they were not required and were only collected if surgeons used them as standard of care. A total of 246 patients out of 1,200 had lower extremity alignment films available (20.5%). There was little variability in the indications for obtaining films, as participating surgeons typically either obtained these films on nearly all of their patients (as standard of care), or almost none.
Limb alignment was measured on a bilateral long leg standing x-ray23. It was recommended that all centers use a similar technique with full extension long leg standing films in neutral rotation. The line from the center of the femoral head to the center of the ankle tibial plafond was drawn. The point where it intersected the tibial plateau was noted. The distance from this point to the medial border of the tibial plateau divided by the total width of the tibial plateau was expressed as a percentage for both extremities.
At the time of each surgery, surgeons documented all meniscal and chondral injuries and treatment utilizing a standardized form. The chondral status of each weight bearing compartment was defined as normal (none/grade 1 chondrosis), intermediate (grades 2/3 chondrosis on at least 1 surface) or advanced (grade 4 on at least 1 surface), using the modified Outerbridge classification system30,33. Each meniscus was defined as intact or disrupted (torn or previously debrided) at the time of rACLR. Previous studies have demonstrated the ability of fellowship trained sports surgeons to have agreement on meniscal14 and chondral lesions30.
Quantitative Variables and Statistical Methods
The purpose of the study was to determine the association between chondrosis and meniscal status and lower extremity alignment at the time of rACLR. To that end, medial and lateral compartment chondrosis were the two dependent outcome variables, and the associations of factors at the time of rACLR were examined using proportional odds ordinal logistic models. Independent covariates controlled for in the model included age at the time of surgery, gender, BMI, activity level, revision number, previous graft type, lower extremity alignment, and compartmental meniscus status (Table 1). Parameter estimates were exponentiated to obtain odds ratios along with their corresponding 95% confidence intervals. All continuous covariates were modeled using a 3 knot restricted cubic spline to allow for a nonlinear relationship with the outcomes measures. To avoid case wise deletion of records with missing covariates we employed multiple imputations via prediction mean matching. P-values are reported for each statistical contrast, utilizing either the Kruskal-Wallis test (for continuous variables) or the Pearson test (for categorical variables). Statistical analysis was performed with the free open source R statistical software using the Hmisc and rms packages (http://www.r-project.org)27.
Table 1.
List of Modeling Variables
| Category | Variable | Degrees of Freedom (df) | Levels |
|---|---|---|---|
|
| |||
| Patient Demographics | Age (years) | 1 | continuous |
| Gender | 1 | male, female | |
| Body Mass Index (BMI) | 1 | continuous | |
| Baseline activity level (Marx) | 1 | continuous | |
|
| |||
| Surgical Information | Revision number | 1 | 1, 2 or more |
| Prior graft type | 3 | autograft (BTB), autograft (soft tissue), allograft, other/unknown | |
| Alignment | 1 | continuous | |
| Meniscal pathology | |||
| * medial | 1 | intact, disrupted | |
| * lateral | 1 | intact, disrupted | |
| Articular cartilage pathology | 2 | grades 0/1 (“normal”), grades 2/3 (“intermediate”), grade 4 (“advanced”) on at least one surface | |
RESULTS
Study Population
The cohort included all patients with lower extremity alignment films at the time of rACLR (246). Mean (SD) patient age was 26.9 (9.5) years with a mean BMI of 26.4 (4.6) (Table 2). One hundred forty-three patients (58%) were male and 213 (87%) were first-time revisions. The medial compartment had more chondrosis (intermediate 42%, advanced 6.5%) than the lateral compartment (intermediate 26%, advanced 6.5%). Disruption of the meniscus was noted in 35% of patients on the medial side and 16% in the lateral side. The mean (SD) weight-bearing alignment axis was measured to be 0.43 (0.13), compared with 0.41 (0.13) in the contralateral limbs23.
Table 2.
Baseline Demographic and Clinical Characteristics at the time or rACLR
| Characteristic | % (n) |
|---|---|
| Age (years) | 19 : 25 : 33 |
|
| |
| Gender | |
| males | 58% (143) |
| females | 42% (103) |
|
| |
| Body Mass Index (BMI) | 22.7 : 25.2 : 28.7 |
|
| |
| Baseline activity level (Marx) | 4 : 11 : 16 |
|
| |
| Revision number | |
| 1st | 87% (213) |
| 2 or more | 13% (33) |
|
| |
| Previous graft type | |
| Autograft – BTB | 38% (93) |
| Autograft – soft tissue | 32% (78) |
| Allograft | 26% (65) |
| Other / unknown | 4% (10) |
|
| |
| Alignment | 0.35 : 0.43 : 0.50 |
|
| |
| Medial Meniscus Status | |
| intact | 65% (161) |
| disrupted | 35% (85) |
|
| |
| Lateral Meniscus Status | |
| intact | 84% (207) |
| disrupted | 16% (39) |
|
| |
| Medial Compartment Chondrosis | |
| grades 0/1 (“normal”) | 51% (126) |
| grades 2/3 (“intermediate”) | 42% (104) |
| grade 4 (“advanced”) | 7% (16) |
|
| |
| Lateral Compartment Chondrosis | |
| grades 0/1 (“normal”) | 67% (166) |
| grades 2/3 (“intermediate”) | 26% (64) |
| grade 4 (“advanced”) | 7% (16) |
|
| |
| Previous High Tibial Osteotomy (HTO) | |
| no | 98% (242) |
| yes | 2% (4) |
|
| |
| Previous Medial Meniscus Transplant | |
| no | 100% (245) |
| yes | 0% (1) |
|
| |
| Previous Lateral Meniscus Transplant | |
| no | 100% (246) |
| yes | 0% (0) |
Numbers after percentages are frequencies.
Values: a : b : c represent the lower quartile a, the median b, and the upper quartile c for continuous variables.
Medial Compartment Chondrosis
Medial compartment chondrosis was associated with BMI (odds ratio=1.08; 95% CI=1.01–1.15; p=0.025), alignment (OR=0.03; 95% CI=0.0–0.3; p=0.002), and medial meniscus status (OR=0.36; 95% CI=0.20–0.66; p=0.001) (Table 3). The risk of medial compartment chondrosis at the time of rACLR increased by 8% with each unit of BMI. An intact medial meniscus decreased the risk of medial compartment chondrosis by 64%. For every ten percent shift in the WBL lateral on the tibial plateau, the risk of medial compartment chondrosis decreased by 9.7%. None of the knees with the WBL lateral to 0.625 had Grade 4 chondrosis in the medial compartment (Figure 1).
Table 3.
Medial Compartment Chondrosis (logistic model results)
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
|
| |||
| Age (years) | 1.02 | 0.99 – 1.06 | 0.149 |
| Gender (males vs. females) | 0.70 | 0.39 – 1.26 | 0.234 |
| Body Mass Index (BMI) | 1.08 | 1.01 – 1.15 | 0.025 |
| Baseline activity level (Marx) | 0.97 | 0.92 – 1.02 | 0.198 |
| Revision number (2 or more vs. 1) | 0.72 | 0.30 – 1.73 | 0.464 |
| Previous Graft Type | |||
| * autograft (BTB) vs. allograft | 0.83 | 0.41 – 1.70 | 0.613 |
| * autograft (soft tissue) vs. allograft | 0.88 | 0.43 – 1.80 | 0.720 |
| * other / unknown vs. allograft | 1.21 | 0.29 – 5.05 | 0.793 |
| Alignment | 0.03 | 0.00 – 0.30 | 0.002 |
| Medial Meniscus Status (intact vs. disrupted) | 0.36 | 0.20 – 0.66 | 0.001 |
CI: confidence interval
Figure 1.
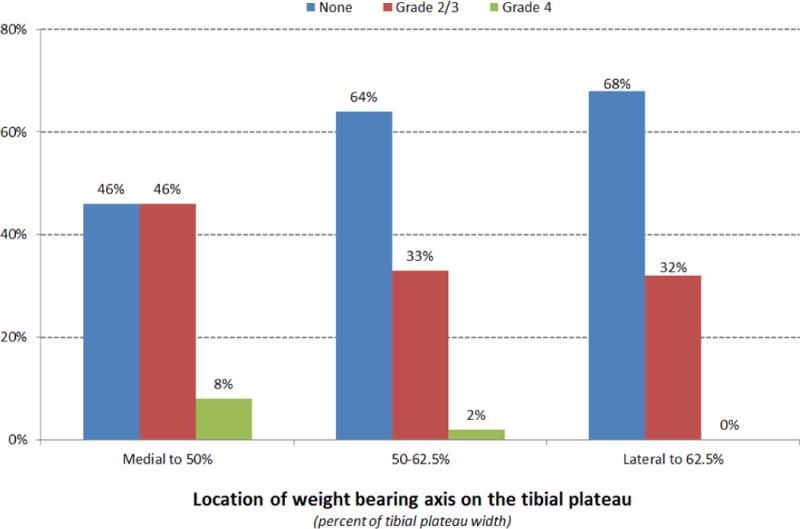
Distribution of chondrosis in medial compartment by lower extremityalignment
Lateral Compartment Chondrosis
Lateral compartment chondrosis was significantly associated with age (OR=1.04; 95% CI=1.01–1.08; p=0.013) and lateral meniscus status (OR=0.16; 95% CI=0.08–0.32; p<0.001) (Table 4). The risk of lateral compartment chondrosis increases by 4% with each year of aging while an intact lateral meniscus decreases the risk of lateral compartment chondrosis by 84%. BMI and alignment were not associated with chondrosis in the lateral compartment.
Table 4.
Lateral Compartment Chondrosis (logistic model results)
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
|
| |||
| Age (years) | 1.04 | 1.01 – 1.08 | 0.013 |
| Gender (males vs. females) | 0.79 | 0.42 – 1.50 | 0.475 |
| Body Mass Index (BMI) | 1.02 | 0.96 – 1.09 | 0.500 |
| Baseline activity level (Marx) | 1.04 | 0.99 – 1.10 | 0.137 |
| Revision number (2 or more vs. 1) | 1.20 | 0.49 – 2.99 | 0.688 |
| Previous Graft Type | |||
| * autograft (BTB) vs. allograft | 0.93 | 0.43 – 2.00 | 0.855 |
| * autograft (soft tissue) vs. allograft | 1.54 | 0.72 – 3.29 | 0.264 |
| * other / unknown vs. allograft | 1.38 | 0.31 – 6.04 | 0.671 |
| Alignment | 0.80 | 0.08 – 7.78 | 0.845 |
| Lateral Meniscus Status (intact vs. disrupted) | 0.16 | 0.08 – 0.32 | <0.001 |
CI: confidence interval
Patient age, sex, activity level, revision number, and previous ACL graft type were not associated with chondrosis.
DISCUSSION
Chondrosis in the tibiofemoral compartments at the time of rACLR relates to the status of the meniscus in that compartment. Alignment and BMI are significantly associated with articular cartilage status in the medial compartment but not in the lateral compartment. Activity level, number of revisions and previous ACL graft type were not associated with cartilage changes in the tibiofemoral compartments at the time of rACLR.
In a prior study from this cohort, the status of the articular cartilage at the time of rACLR was shown to relate to previous meniscal surgery6. Patients with previous partial meniscectomy had higher rates of chondrosis than patients with previous meniscal repair or no previous meniscal surgery. However, that study did not look at the status of the meniscus at the time of rACLR. The current study confirms that an intact meniscus at the time of rACLR reduces the risk of articular cartilage damage which is not surprising given that meniscus tears are associated with chondrosis and osteoarthritis in the knee5,25,31,36,38. While one previous study reported six times more arthrosis in knees with concomitant partial meniscectomy at the time of ACL reconstruction28, another study did not find any association between the development of arthrosis and meniscal injury at the time of ACL reconstruction12.
More varus alignment increased the incidence of chondral damage in the medial compartment but alignment was not associated with the rate of chondrosis in the lateral compartment. High tibial osteotomies are commonly accepted as an effective treatment for painful arthritis in the varus knee19. A recent biomechanical study showed that correction to between 6° and 10° of anatomic valgus completely unloads the medial compartment32. While many would agree that transferring the weight bearing axis to 62.5% of the tibial width (from medial to lateral) is the target for high tibial osteotomy in the varus knee19, there is limited data on the dose response to, and optimal target for, correction of alignment. Our data demonstrates that the medial compartment is at risk for chondral damage in varus knees that have undergone ACL reconstruction, but that knees with more valgus alignment are less likely to have chondrosis in the medial compartment. This association suggests that a high tibial osteotomy is an intervention with potential for chondroprotection in these patients, particularly when the weight bearing axis is medial to 50% of the tibial width, and possibly when the weight bearing axis is medial to 62.5% of the tibial width. However, the current study did not evaluate the effect of realignment surgery, and more research is necessary to assess this possible relationship. Our findings are consistent with a recent study reporting that many rACLR patients are good candidates for high tibial osteotomy45.
Elevated BMI is a potentially modifiable, and even preventable, risk factor for knee osteoarthritis9,16,37. Obesity is likely to have both biomechanical and biochemical links to osteoarthritis35,44. A recent study demonstrated obesity modulated changes in the gene expression of meniscus tears, which may be particularly pertinent in rACLR patients with meniscus tears34. The elevated risk for medial compartment chondrosis at the time of rACLR with higher BMI may partly explain why BMI at the time of ACL reconstruction has been shown to predict lower activity level at 2 and 6 years after surgery13,40. Patients, who have undergone ACLR, and especially those undergoing rACLR, should be counseled on the potential benefits to their knee cartilage from maintaining a normal BMI.
Meniscal and articular cartilage damage is associated with worse outcomes following ACL reconstruction26,39,48 and the presence of chondral injury at the time of rACLR has been associated with worse outcomes in these patients3,10,22,41. Considering the increased incidence of medial compartment chondrosis at the time of rACLR in patients with varus malalignment and deficient medial meniscus, knees in varus malalignment undergoing partial meniscectomy at the time of ACL reconstruction have the potential to benefit from a high tibial osteotomy to reduce the risk of developing chondrosis. The exact alignment at which such a procedure should be considered, i.e. medial to 50% or 62.5% of the tibial width, is not clear from our data. Again, much more research is needed before such intervention could be recommended.
Lateral compartment chondrosis was not associated with BMI and alignment whereas medial compartment chondrosis was associated with these variables. The differential association of alignment may be at least partly explained by the preponderance of varus alignment in this population. However, the lack of association with BMI in the lateral compartment is less easily explained. Perhaps, if the influence of BMI on chondrosis is magnified by meniscal deficiency, the greater prevalence of medial meniscus tears (35% versus 16%) is important.
It is also not clear why the medial compartment had a higher prevalence of meniscus tears and chondrosis in this population. The role of the medial meniscus as a secondary stabilizer of the knee may be relevant, especially in these knees which have experienced at least 2 episodes of ACL deficiency (following the tear of the native ACL and at least one tear of a reconstructed ACL). As mentioned above, the bias towards varus alignment, and the association of alignment and BMI with medial compartment chondrosis but not lateral compartment chondrosis, may contribute to this discrepancy.
Limitations of the present study include the potential inter-observer variance in reported chondrosis and meniscal status data and we did not quantify the extent of the meniscal or cartilage loss. A number of potential confounding variables, including previous articular cartilage injury, the status of the articular cartilage injury at the time of previous knee surgery, and the presence/absence of subchondral bone bruise at the time of initial/secondary ACL injury, were not consistently available for this cohort and were therefore not analyzed. Association does not necessarily imply causation, for example, it is possible that chondrosis leads to meniscus tears rather than the converse relationship. There was no data on time intervals between initial knee injury and primary ACLR, previous knee surgery and current rACLR, or the most recent graft failure and current rACLR, all of which could impact findings. The association between cartilage loss and varus alignment does not support conclusions about causation. While varus malalignment may predispose to cartilage loss in the medial compartment, it is also likely that cartilage loss and/or meniscal deficiency in the medial compartment contributes to varus malalignment. We cannot differentiate between patients with such a secondary varus malalignment and those with a primary varus malalignment. Furthermore, it is possible that a selection bias exists with regards to which patients had alignment films, although they were collected as standard of care based on surgeon practice. Finally, this study includes patients who have undergone surgery by a wide variety of surgeons, which may be a limitation, or a strength, in terms of the generalizability of the findings.
Despite these limitations, this is a large prospective study correlating meniscal status and alignment with intra-articular findings. An intact meniscus is associated with less articular cartilage damage in the associated tibiofemoral compartment at the time of rACLR. More varus alignment and elevated BMI is associated with worse chondrosis in the medial compartment. These findings emphasize the importance of the meniscus and relevance of alignment and BMI to the articular cartilage in these patients. Further research is needed to understand the potential of surgical interventions (i.e. meniscal repair or replacement, realignment osteotomy) as well as weight maintenance and/or loss, to reduce or delay this cartilage damage.
What is known about the subject
A history of partial meniscectomy is associated with an increased risk for the development of osteoarthritis in the knee. Previous partial meniscectomy and age increase the risk of cartilage degeneration at the time of rACLR.
Adds to existing knowledge
The status of the meniscus at the time of rACLR is associated with the degree of chondrosis in the weight bearing compartments of the knee. Lower extremity alignment and BMI are associated with degenerative changes in the articular cartilage of the medial tibiofemoral compartment at the time of rACLR.
MARS Group
David C. Harris, BA, Washington University, St. Louis
Kushal Patel, MD, University of Illinois-Chicago
David Pearson, MD, Washington University, St. Louis
Jake Schutzman, Washington University, St. Louis
Majd Tarabichi, Royal College of Surgeons, Ireland-Bahrain
David Ying, MD, Washington University, St. Louis, MO
John P. Albright, MD, University of Iowa Hospitals and Clinics
Christina R. Allen, MD, University of California, San Francisco
Annunziato (Ned) Amendola, MD, University of Iowa Hospitals and Clinics
Allen F. Anderson, MD, Tennessee Orthopaedic Alliance
Jack T. Andrish, MD, Cleveland Clinic
Christopher C. Annunziata, MD, Commonwealth Orthopaedics & Rehab
Robert A. Arciero, MD, University of Connecticut Health Center
Bernard R. Bach Jr, MD, Rush University Medical Center
Champ L. Baker, III, MD, The Hughston Clinic
Arthur R. Bartolozzi, MD, 3B Orthopaedics, University of Pennsylvania Health System
Keith M. Baumgarten, MD, Orthopedic Institute
Jeffery R. Bechler, MD, University Orthopedic Associates, LLC
Jeffrey H. Berg, MD, Town Center Orthopaedic Associates
Geoffrey A. Bernas, MD, State University of New York at Buffalo
Stephen F. Brockmeier, MD, University of Virginia
Charles A. Bush-Joseph, MD, Rush University Medical Center
J. Brad Butler, V, MD, Orthopedic and Fracture Clinic
John D. Campbell, MD, Bridger Orthopaedic and Sports Medicine
James L. Carey, MD, MPH, University of Pennsylvania
James E. Carpenter, MD, University of Michigan
Brian J. Cole, MD, Rush University Medical Center
Daniel E. Cooper, MD, W.B. Carrell Memorial Clinic
Jonathan M. Cooper, DO, HealthPartners Specialty Clinic
Charles L. Cox, MD, MPH, Vanderbilt University
R. Alexander Creighton, MD, University of North Carolina Medical Center
Diane L. Dahm, MD, Mayo Clinic Rochester
Tal S. David, MD, Arthroscopic and Orthopedic Sports Medicine Associates
Thomas M. DeBerardino, MD, University of Connecticut Health Center
Warren R. Dunn, MD, MPH, University of Wisconsin
David C. Flanigan, MD, The Ohio State University
Robert W. Frederick, MD, The Rothman Institute/Thomas Jefferson University
Theodore J. Ganley, MD, Children’s Hospital of Philadelphia
Elizabeth A. Garofoli, Washington University, St. Louis
Charles J. Gatt, Jr., MD, University Orthopedic Associates, LLC
Steven R. Gecha, MD, Princeton Orthopaedic Associates
James Robert Giffin, MD, Fowler Kennedy Sports Medicine Clinic- University of Western Ontario
Sharon L. Hame, MD, David Geffen School of Medicine at UCLA
Jo A. Hannafin, MD, PhD, Hospital for Special Surgery
Christopher D. Harner, MD, University of Pittsburgh Medical Center
Norman Lindsay Harris, Jr., MD, Orthopaedic Associates of Aspen & Glenwood
Keith S. Hechtman, MD, UHZ Sports Medicine Institute
Elliott B. Hershman, MD, Lenox Hill Hospital
Rudolf G. Hoellrich, MD, Slocum Research and Education Foundation
Timothy M. Hosea, MD, University Orthopedic Associates, LLC
David C. Johnson, MD, National Sports Medicine Institute
Timothy S. Johnson, MD, National Sports Medicine Institute
Morgan H. Jones, MD, Cleveland Clinic
Christopher C. Kaeding, MD, The Ohio State University
Ganesh V. Kamath, MD, University of North Carolina Medical Center
Thomas E. Klootwyk, MD, Methodist Sports Medicine Center-The Orthopedic Specialists
Brett (Brick) A. Lantz, MD, Slocum Research and Education Foundation
Bruce A. Levy, MD, Mayo Clinic Rochester
C. Benjamin Ma, MD, University of California, San Francisco
G. Peter Maiers, II, MD, Methodist Sports Medicine Center-The Orthopedic Specialists
Robert G. Marx, MD, Hospital for Special Surgery
Matthew J. Matava, MD, Washington University, St. Louis
Gregory M. Mathien, MD, Knoxville Orthopedic Clinic
David R. McAllister, MD, David Geffen School of Medicine at UCLA
Eric C. McCarty, MD, University of Colorado Denver School of Medicine
Robert G. McCormack, MD, University of British Columbia
Bruce S. Miller, MD, MS, University of Michigan
Carl W. Nissen, MD, Connecticut Children’s Medical Center
Daniel F. O’Neill, MD, Ed.D, Littleton Regional Hospital
LTC Brett D. Owens, MD, Keller Army Community Hospital-United States Military Academy
Richard D. Parker, MD, Cleveland Clinic
Mark L. Purnell, MD, Orthopaedic Associates of Aspen & Glenwood
Arun J. Ramappa, MD, Beth Israel Deaconess Medical Center
Michael A. Rauh, MD, State University of New York at Buffalo
Arthur C. Rettig, MD, Methodist Sports Medicine Center-The Orthopedic Specialists
Jon K. Sekiya, MD, University of Michigan
Kevin G. Shea, MD, Intermountain Orthopedics
Orrin H. Sherman, MD, NYU Hospital for Joint Diseases
James R. Slauterbeck, MD, University of Vermont College of Medicine
Matthew V. Smith, MD, Washington University, St. Louis
Jeffrey T. Spang, MD, University of North Carolina Medical Center
Kurt P. Spindler, MD, Vanderbilt University
Michael J. Stuart, MD, Mayo Clinic Rochester
LTC. Steven J. Svoboda, MD, Keller Army Community Hospital-United States Military Academy
Timothy N. Taft, MD, University of North Carolina Medical Center
COL Joachim J. Tenuta, MD, Keller Army Community Hospital-United States Military Academy
Edwin M. Tingstad, MD, Inland Orthopaedics/ Washington State University
Armando F. Vidal, MD, University of Colorado Denver School of Medicine
Darius G. Viskontas, MD, Royal Columbian Hospital
Richard A. White, MD, University of Missouri-Columbia
James S. Williams Jr, MD, Cleveland Clinic
Michelle L. Wolcott, MD, University of Colorado Denver School of Medicine
Brian R. Wolf, MD, University of Iowa Hospitals and Clinics
James J. York, MD, Chesapeake Orthopaedics & Sports Medicine Center
Contributor Information
Robert H. Brophy, Washington University, St. Louis.
Amanda K. Haas, Washington University, St. Louis.
Laura J. Huston, Vanderbilt University.
Sam K. Nwosu, Vanderbilt University.
Rick W. Wright, Washington University, St. Louis.
References
- 1.Aglietti P, Rinonapoli E, Stringa G, Taviani A. Tibial osteotomy for the varus osteoarthritic knee. Clin Orthop Relat Res. 1983:239–251. [PubMed] [Google Scholar]
- 2.Ahn JH, Lee YS, Chang MJ, Yim HS. Analysis of Revision Anterior Cruciate Ligament Reconstruction according to the combined injury, degenerative change, and MRI findings. Knee. 2011;18:382–386. doi: 10.1016/j.knee.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Ahn JH, Lee YS, Ha HC. Comparison of revision surgery with primary anterior cruciate ligament reconstruction and outcome of revision surgery between different graft materials. Am J Sports Med. 2008;36:1889–1895. doi: 10.1177/0363546508317124. [DOI] [PubMed] [Google Scholar]
- 4.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 5.Biswal S, Hastie T, Andriacchi TP, Bergman GA, Dillingham MF, Lang P. Risk factors for progressive cartilage loss in the knee: a longitudinal magnetic resonance imaging study in forty-three patients. Arthritis Rheum. 2002;46:2884–2892. doi: 10.1002/art.10573. [DOI] [PubMed] [Google Scholar]
- 6.Brophy RH, Wright RW, David TS, et al. Association between previous meniscal surgery and the incidence of chondral lesions at revision anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40:808–814. doi: 10.1177/0363546512437722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cicuttini FM, Baker JR, Spector TD. The association of obesity with osteoarthritis of the hand and knee in women: a twin study. J Rheumatol. 1996;23:1221–1226. [PubMed] [Google Scholar]
- 8.Coventry MB. Osteotomy of the upper portion of the tibia for degenerative arthritis of the knee. A preliminary report. 1965. Clin Orthop Relat Res. 1989:4–8. [PubMed] [Google Scholar]
- 9.Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am J Epidemiol. 1989;130:278–288. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- 10.Diamantopoulos AP, Lorbach O, Paessler HH. Anterior cruciate ligament revision reconstruction: results in 107 patients. Am J Sports Med. 2008;36:851–860. doi: 10.1177/0363546507312381. [DOI] [PubMed] [Google Scholar]
- 11.Ding C, Martel-Pelletier J, Pelletier JP, et al. Meniscal tear as an osteoarthritis risk factor in a largely non-osteoarthritic cohort: a cross-sectional study. J Rheumatol. 2007;34:776–784. [PubMed] [Google Scholar]
- 12.Drogset JO, Grontvedt T. Anterior cruciate ligament reconstruction with and without a ligament augmentation device : results at 8-Year follow-up. Am J Sports Med. 2002;30:851–856. doi: 10.1177/03635465020300061601. [DOI] [PubMed] [Google Scholar]
- 13.Dunn WR, Spindler KP, Consortium M. Predictors of activity level 2 years after anterior cruciate ligament reconstruction (ACLR): a Multicenter Orthopaedic Outcomes Network (MOON) ACLR cohort study. Am J Sports Med. 2010;38:2040–2050. doi: 10.1177/0363546510370280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunn WR, Wolf BR, Amendola A, et al. Multirater agreement of arthroscopic meniscal lesions. Am J Sports Med. 2004;32:1937–1940. doi: 10.1177/0363546504264586. [DOI] [PubMed] [Google Scholar]
- 15.Englund M, Guermazi A, Gale D, et al. Incidental meniscal findings on knee MRI in middle-aged and elderly persons. N Engl J Med. 2008;359:1108–1115. doi: 10.1056/NEJMoa0800777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felson DT. Does excess weight cause osteoarthritis and, if so, why? Ann Rheum Dis. 1996;55:668–670. doi: 10.1136/ard.55.9.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fink C, Hoser C, Hackl W, Navarro RA, Benedetto KP. Long-term outcome of operative or nonoperative treatment of anterior cruciate ligament rupture–is sports activity a determining variable? Int J Sports Med. 2001;22:304–309. doi: 10.1055/s-2001-13823. [DOI] [PubMed] [Google Scholar]
- 18.Ford GM, Hegmann KT, White GL, Jr, Holmes EB. Associations of body mass index with meniscal tears. Am J Prev Med. 2005;28:364–368. doi: 10.1016/j.amepre.2005.01.013. [DOI] [PubMed] [Google Scholar]
- 19.Gardiner A, Gutierrez Sevilla GR, Steiner ME, Richmond JC. Osteotomies about the knee for tibiofemoral malalignment in the athletic patient. Am J Sports Med. 2010;38:1038–1047. doi: 10.1177/0363546509335193. [DOI] [PubMed] [Google Scholar]
- 20.George MS, Dunn WR, Spindler KP. Current concepts review: revision anterior cruciate ligament reconstruction. Am J Sports Med. 2006;34:2026–2037. doi: 10.1177/0363546506295026. [DOI] [PubMed] [Google Scholar]
- 21.Griffith TB, Allen BJ, Levy BA, Stuart MJ, Dahm DL. Outcomes of repeat revision anterior cruciate ligament reconstruction. Am J Sports Med. 2013;41:1296–1301. doi: 10.1177/0363546513482568. [DOI] [PubMed] [Google Scholar]
- 22.Grossman MG, ElAttrache NS, Shields CL, Glousman RE. Revision anterior cruciate ligament reconstruction: three- to nine-year follow-up. Arthroscopy. 2005;21:418–423. doi: 10.1016/j.arthro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 23.Group M. Radiographic findings in revision anterior cruciate ligament reconstructions from the Mars cohort. J Knee Surg. 2013;26:239–247. doi: 10.1055/s-0032-1329717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Group M, Wright RW, Huston LJ, et al. Descriptive epidemiology of the Multicenter ACL Revision Study (MARS) cohort. Am J Sports Med. 2010;38:1979–1986. doi: 10.1177/0363546510378645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hede A, Larsen E, Sandberg H. The long term outcome of open total and partial meniscectomy related to the quantity and site of the meniscus removed. Int Orthop. 1992;16:122–125. doi: 10.1007/BF00180200. [DOI] [PubMed] [Google Scholar]
- 26.Ichiba A, Kishimoto I. Effects of articular cartilage and meniscus injuries at the time of surgery on osteoarthritic changes after anterior cruciate ligament reconstruction in patients under 40 years old. Arch Orthop Trauma Surg. 2009;129:409–415. doi: 10.1007/s00402-008-0786-4. [DOI] [PubMed] [Google Scholar]
- 27.Ihaka R, GR R. A language for data analysis and graphics. J Computational Graphical Statistics. 1996;5:299–314. [Google Scholar]
- 28.Jomha NM, Borton DC, Clingeleffer AJ, Pinczewski LA. Long-term osteoarthritic changes in anterior cruciate ligament reconstructed knees. Clin Orthop Relat Res. 1999:188–193. [PubMed] [Google Scholar]
- 29.Lind M, Menhert F, Pedersen AB. Incidence and outcome after revision anterior cruciate ligament reconstruction: results from the Danish registry for knee ligament reconstructions. Am J Sports Med. 2012;40:1551–1557. doi: 10.1177/0363546512446000. [DOI] [PubMed] [Google Scholar]
- 30.Marx RG, Connor J, Lyman S, et al. Multirater agreement of arthroscopic grading of knee articular cartilage. Am J Sports Med. 2005;33:1654–1657. doi: 10.1177/0363546505275129. [DOI] [PubMed] [Google Scholar]
- 31.McDermott ID, Amis AA. The consequences of meniscectomy. J Bone Joint Surg Br. 2006;88:1549–1556. doi: 10.1302/0301-620X.88B12.18140. [DOI] [PubMed] [Google Scholar]
- 32.Mina C, Garrett WE, Jr, Pietrobon R, Glisson R, Higgins L. High tibial osteotomy for unloading osteochondral defects in the medial compartment of the knee. Am J Sports Med. 2008;36:949–955. doi: 10.1177/0363546508315471. [DOI] [PubMed] [Google Scholar]
- 33.Outerbridge RE. The etiology of chondromalacia patellae. J Bone Joint Surg Br. 1961;43-B:752–757. doi: 10.1302/0301-620X.43B4.752. [DOI] [PubMed] [Google Scholar]
- 34.Rai MF, Patra D, Sandell LJ, Brophy RH. Transcriptome analysis of injured human meniscus reveals a distinct phenotype of meniscus degeneration with aging. Arthritis Rheum. 2013;65:2090–2101. doi: 10.1002/art.37984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rai MF, Sandell LJ. Inflammatory mediators: tracing links between obesity and osteoarthritis. Crit Rev Eukaryot Gene Expr. 2011;21:131–142. doi: 10.1615/critreveukargeneexpr.v21.i2.30. [DOI] [PubMed] [Google Scholar]
- 36.Rangger C, Klestil T, Gloetzer W, Kemmler G, Benedetto KP. Osteoarthritis after arthroscopic partial meniscectomy. Am J Sports Med. 1995;23:240–244. doi: 10.1177/036354659502300219. [DOI] [PubMed] [Google Scholar]
- 37.Riddle DL, Stratford PW. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: a cohort study. Arthritis Care Res (Hoboken) 2013;65:15–22. doi: 10.1002/acr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scheller G, Sobau C, Bulow JU. Arthroscopic partial lateral meniscectomy in an otherwise normal knee: Clinical, functional, and radiographic results of a long-term follow-up study. Arthroscopy. 2001;17:946–952. doi: 10.1053/jars.2001.28952. [DOI] [PubMed] [Google Scholar]
- 39.Shelbourne KD, Gray T. Results of anterior cruciate ligament reconstruction based on meniscus and articular cartilage status at the time of surgery. Five- to fifteen-year evaluations. Am J Sports Med. 2000;28:446–452. doi: 10.1177/03635465000280040201. [DOI] [PubMed] [Google Scholar]
- 40.Spindler KP, Huston LJ, Wright RW, et al. The prognosis and predictors of sports function and activity at minimum 6 years after anterior cruciate ligament reconstruction: a population cohort study. Am J Sports Med. 2011;39:348–359. doi: 10.1177/0363546510383481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas NP, Kankate R, Wandless F, Pandit H. Revision anterior cruciate ligament reconstruction using a 2-stage technique with bone grafting of the tibial tunnel. Am J Sports Med. 2005;33:1701–1709. doi: 10.1177/0363546505276759. [DOI] [PubMed] [Google Scholar]
- 42.Tjornstrand B, Egund N, Hagstedt B, Lindstrand A. Tibial osteotomy in medial gonarthrosis. The importance of over-correction of varus deformity. Arch Orthop Trauma Surg. 1981;99:83–89. doi: 10.1007/BF00389742. [DOI] [PubMed] [Google Scholar]
- 43.Wegrzyn J, Chouteau J, Philippot R, Fessy MH, Moyen B. Repeat revision of anterior cruciate ligament reconstruction: a retrospective review of management and outcome of 10 patients with an average 3-year follow-up. Am J Sports Med. 2009;37:776–785. doi: 10.1177/0363546508330141. [DOI] [PubMed] [Google Scholar]
- 44.Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol. 2013;9:225–235. doi: 10.1038/nrrheum.2012.224. [DOI] [PubMed] [Google Scholar]
- 45.Won HH, Chang CB, Je MS, Chang MJ, Kim TK. Coronal limb alignment and indications for high tibial osteotomy in patients undergoing revision ACL reconstruction. Clin Orthop Relat Res. 2013;471:3504–3511. doi: 10.1007/s11999-013-3185-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wright R, Spindler K, Huston L, et al. Revision ACL reconstruction outcomes: MOON cohort. J Knee Surg. 2011;24:289–294. doi: 10.1055/s-0031-1292650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright RW, Gill CS, Chen L, et al. Outcome of revision anterior cruciate ligament reconstruction: a systematic review. J Bone Joint Surg Am. 2012;94:531–536. doi: 10.2106/JBJS.K.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu WH, Hackett T, Richmond JC. Effects of meniscal and articular surface status on knee stability, function, and symptoms after anterior cruciate ligament reconstruction: a long-term prospective study. Am J Sports Med. 2002;30:845–850. doi: 10.1177/03635465020300061501. [DOI] [PubMed] [Google Scholar]


