Abstract
Background
Pemetrexed is approved in the treatment of advanced stage non-squamous non-small-cell lung cancer (NSCLC). The length of response is variable, and we thus sought to identify which clinicopathologic characteristics are associated with long term disease control with pemetrexed.
Methods
Patients with metastatic NSCLC were identified who received pemetrexed (with or without bevacizumab) for 12 months or longer, either as maintenance treatment after first-line platinum-based chemotherapy or as subsequent treatment. Clinical and pathological characteristics were collected.
Results
Of a total of 196 patients who received pemetrexed starting in 2007, 25 patients were identified who received pemetrexed for over one year. Of these, 15 patients received pemetrexed with or without bevacizumab as maintenance treatment and 10 patients received pemetrexed as subsequent treatment. Fifteen of the 25 patients (60%) had an oncogenic driver mutation as follows: five (20%) had ROS1 gene rearrangements, four (16%) had ALK gene rearrangements, three (12%) had KRAS mutations, two (8%) had epidermal growth factor receptor (EGFR) mutations, and one (4%) had an NRAS mutation. The median overall survival (OS) was 42.2 months (95% confidence interval [CI]: 37.4–61.3) and median progression free survival (PFS) was 22.1 months (95% CI: 15.1–29.1). Patients with an oncogenic driver mutation had significantly better PFS (p=0.006) and OS (p=0.001).
Conclusions
Among patients with NSCLC who received pemetrexed for an extended time, those with ALK and ROS1 gene rearrangements are proportionally overrepresented compared with that anticipated in a general non-squamous NSCLC population, and patients with oncogenic driver mutations had improved outcomes.
Keywords: Non-small cell lung cancer, Pemetrexed, Driver oncogene, Anaplastic lymphoma kinase (ALK), ROS1, KRAS, NRAS, EGFR(Epidermal growth factor receptor)
INTRODUCTION
In the treatment of metastatic non–small-cell lung cancer (NSCLC), palliative chemotherapy has 1-year survival rates of 30% to 40%1,2. Historically, first line chemotherapy was administered for 3–4 months, followed by a period of observation given the limitations of cumulative drug toxicity. Pemetrexed is approved by the United States Food and Drug Administration (FDA) for treatment of patients with non-squamous NSCLC as single agent second-line treatment3, first-line treatment in combination with platinum2, and for maintenance therapy after first-line platinum-based chemotherapy4,5. Unlike most other cytotoxic chemotherapeutic agents used in NSCLC, pemetrexed is relatively well tolerated at full doses despite long term administration without a drug holiday.
Continuing pemetrexed as maintenance therapy either after first-line platinum or as monotherapy in subsequent treatment lines is increasingly common clinical practice. An overall survival (OS) and progression free survival benefit was established for maintenance pemetrexed after cisplatin therapy in the PARAMOUNT5 study, and the AVAPERL6 study demonstrated that maintenance pemetrexed and bevacizumab was superior to maintenance bevacizumab alone following cisplatin-based first line therapy.
In these trials, plus the JMEN4, PointBreak7 and JMEI3 trials of pemetrexed, some patients remained on pemetrexed based therapy without progression for more than 12 months, but the molecular characteristics of their tumors were not described. Since tumors are now routinely tested at least for epidermal growth factor receptor (EGFR) mutations and anaplastic lymphoma kinase (ALK) gene rearrangements, the interaction between these favorable driver oncogenes and duration of pemetrexed benefit is of clinical interest. Initial reports suggested that the progression free survival (PFS) on pemetrexed in metastatic NSCLC patients is significantly longer among those harboring ALK gene rearrangements than those without, with median PFS of about 9 months8, 9. In a subsequent modestly larger retrospective study10, the median PFS of patients with ALK positive tumors was more modest at 8.5 months when administered as a platinum-based doublet and 4.4 months as a single agent in the second and third line setting, as compared with KRAS which showed a relatively shorter median PFS of only 4.2 months as first line combination therapy, but longer 7.8 month PFS in the second and third line monotherapy setting. In phase III trials of 1st line11 and 2nd line12 crizotinib studies versus chemotherapy in ALK arrangement NSCLC patients, pemetrexed had an intermediate PFS of 7.0 and 7.7 months, respectively. A recent case series from our institution suggested that some lung adenocarcinoma patients whose tumors harbored the ROS1 gene rearrangements also had a prolonged PFS when treated with pemetrexed.13 Interestingly, the outcomes of EGFR mutant patients have not been reported as an independent subgroup with regard to long-term pemetrexed therapy. Together, these prior studies suggested a potential interaction between pemetrexed response and molecular features of NSCLC. In the current retrospective study, patients were selected who were treated with pemetrexed for more than 12 months sequentially, with or without bevacizumab, to determine which clinicopathologic characteristics were associated with long term disease control.
PATIENTS AND METHODS
Patients
We identified patients with metastatic non-squamous NSCLC who received pemetrexed for 12 months or more either as maintenance treatment after first-line platinum-based chemotherapy or as subsequent treatment at Stanford between 10/1/2007 to 05/30/2012 with the assistance of the Stanford Cancer Institute Research Database (SCIRDB) group. Stage was adjusted to conform to the 7th edition American Joint Committee on Cancer (AJCC)/International Union Against Cancer (IUCC) staging system (the 2009 TNM Classification of Malignant Tumors)14. Clinical and pathological characteristics were collected using retrospective chart review. Adverse event (AE) information was retrospectively collected from the chart and classified according to the National Cancer Institute Common Terminology Criteria version 3.0. Patients were defined as “never-smoker” if they smoked ≤100 cigarettes in their lifetime. This chart review protocol was approved by the Stanford Institutional Review Board.
Statistical Analyses
All statistical analyses were performed using SPSS (Solutions Statistical Package for the Social Sciences software), version 19.0 (IBM SPSS, Chicago, IL). To enrich for patients who had benefit from pemetrexed, the start date of pemetrexed was defined as the date of continuation or switch maintenance pemetrexed start (with or without bevacizumab) following completion of first-line platinum-based chemotherapy or from the initial administration date when given as a second-line or beyond treatment. PFS was taken as the interval from the date of pemetrexed initiation as maintenance therapy after first-line platinum-based chemotherapy or as a second-line or beyond treatment until first documented clinical or radiographic progression, escalation or change in therapy (“systemic progression”), or death from any cause, as described in Camidge et al8. OS was measured from the date of pemetrexed initiation as maintenance therapy after first-line platinum-based chemotherapy or as a second-line or beyond treatment to the date of death from any cause or was censored at the date of data cutoff (Jun. 30, 2014). Survival functions were estimated by Kaplan-Meier method and the log-rank test was used to compare the difference between two groups. Significance levels and estimates of hazard ratios (HRs) and their 95% confidence intervals (CIs) were calculated with a Cox proportional hazard model. Two-sided significance level was defined as P < 0.05.
RESULTS
Patient Characteristics
From 10/1/2007 to 5/30/2012, a total of 196 advanced NSCLC patients received pemetrexed (either as a monotherapy or combined with bevacizumab) in maintenance therapy after first-line platinum-based chemotherapy or as monotherapy in a second-line or beyond treatment. Among these 196 patients, 25 (12.8%) patients were identified for further description whose PFS of pemetrexed treatment was more than 12 months. Characteristics of the study patients were shown in Table 1, and notable for a predominance of women and never-smokers.
Table 1.
Patient and Tumor Characteristics
| Variable | Patient number |
% | |
|---|---|---|---|
| Gender | |||
| Male | 7 | 28% | |
| Female | 18 | 72% | |
| Age(year) | |||
| Median | 60 | ||
| range | 19–82 | ||
| <60 years | 12 | 48% | |
| ≥60 years | 13 | 52% | |
| Smoking status | |||
| Former or current smoker | 12 | 48% | |
| Never-smoker | 13 | 52% | |
| WHO performance status | |||
| 0 | 3 | 12% | |
| 1 | 20 | 80% | |
| 2 | 2 | 8% | |
| Stage | |||
| Stage IV | 20 | 80% | |
| Recurrent/Metastatic | 5 | 20% | |
| Histology | |||
| Adenocarcinoma | 23 | 92% | |
| NSCLC, NOS | 2 | 8% | |
| Ethnics | |||
| Asian | 7 | 28% | |
| Non-Asian | 18 | 72% | |
| Site of metastasis | |||
| Pleural effusion | 5 | 20% | |
| Lung metastasis | 14 | 56% | |
| Adrenal metastasis | 4 | 16% | |
| Liver metastasis | 4 | 15% | |
| Bone metastasis | 12 | 48% | |
| Brain Metastasis | 10 | 40% | |
Abbreviations: NSCLC, non–small-cell lung cancer; NOS, not otherwise specified;
Treatment
Of the entire group of 25 identified patients, fifteen patients (60%) received pemetrexed with or without bevacizumab as maintenance treatment after first-line chemotherapy consisting of pemetrexed/platinum/bevacizumab in 8/25 patients (32%), paclitaxel/carboplatin/bevacizumab in one patient (8%), and pemetrexed/platinum in 6/25 patients (24%). Of this group, 10/25 (40%) received pemetrexed and bevacizumab and 5/25 (20%) patients received pemetrexed alone. Nine of the initial 25 patients (36%) received pemetrexed monotherapy and one patient (4%) received pemetrexed and bevacizumab as second-line or beyond treatment. At the time of data cutoff, there were 7 and 2 patients in the maintenance therapy and second-line or beyond treatment groups, respectively, who were still continuing therapy. Six of the 25 patients (24%) developed brain metastases during treatment, and all continued to receive pemetrexed after local radiosurgical brain treatment of limited brain-only progression. These brain metastases developed at a median of 10.9 months into treatment (range: 2.6–25.4 months), then patients went on to continue pemetrexed for an additional median of 4.0 months (range 1.7–15.8) after CNS-only progression. All systemic progression events occurred while patients were still on pemetrexed. As of last follow-up date, 25 patients received a total of 755 cycles of treatment with a median number of cycles of 25 (range: 15–62). The 10 patients who had pemetrexed with bevacizumab as maintenance treatment after first-line chemotherapy received a median of 34 cycles of therapy [bevacizumab: median 23 (range, 3–27) cycles, pemetrexed: 34 (range, 15–62) cycles]. Five patients discontinued bevacizumab (4 because of AE) and 3 patients were still continuing pemetrexed and bevacizumab treatment at the cutoff date. Subsequent post-progression (PD) treatment included docetaxel, gemcitabine, erlotinib, crizotinib, other ALK inhibitors, and palliative radiotherapy.
Immunohistochemical Results and Molecular Analysis
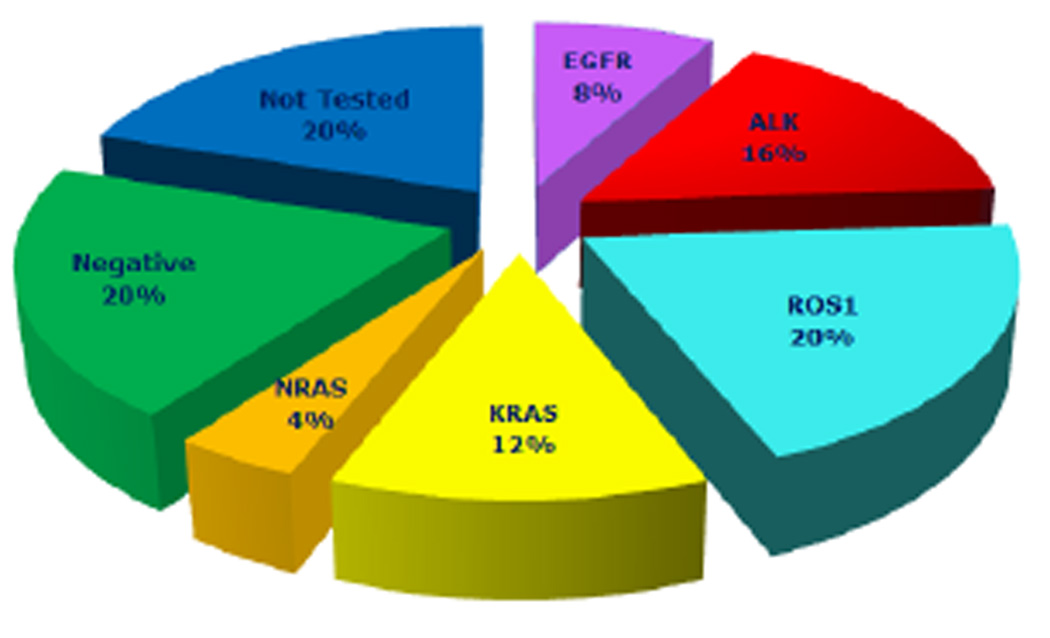
Immunohistochemical testing performed by standard methodology on most tumors revealed positive results as follows: cytokeratin 7 (CK7) in 16/16, CK20 in 0/13, thyroid transcription factor 1(TTF-1) in 21/21. Molecular testing was also performed in most patients as follows: ALK status was determined using the standard break-apart ALK fluorescent in situ hybridization (FISH) assay15, ROS1 status was detected with break-apart FISH16, EGFR, KRAS, and other cancer-related genes using DNA sequencing (2007–2011) or SNaPshot (2011–2013)17. These results are shown in table 2. Twenty of twenty-five (80%) patients had at least one molecular test performed and 15/25 (60%) patients had an oncogenic driver mutation (Table 2 and Figure 1). Two of twenty-five (8%) patients who received EGFR testing had L858R mutations. KRAS and NRAS mutation were found in 3/25(12%) and 1/25 (4%) patients, respectively. ALK and ROS1 gene rearrangements were identified with FISH in 4/25 (16%) and 5/25(20%) patients, respectively. No other molecular alterations including BRAF, APC, CTNNB1, IDH1, IDH2, NOTCH1, PIK3CA, PTEN, P53 were found among patients. Five patients’ tumors were negative for molecular alterations following at least EGFR, KRAS, and ALK testing.
Table 2.
Clinical and Molecular Characteristics of Individual Patients
| Pt | Race/Ethnicity | Age | Gender | Smoking status (Pack years) |
ECOG Performance Status |
Histology | Oncogenic driver mutation |
Line of Therapy |
Regimen | Number of Pem cycles |
PFS (m) |
OS (m) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Caucasian | 62 | F | 4 | 1 | Adeno | EGFR | 2nd-line | Pem+Bev | 37 | 33.4 | 70.6+ |
| 2 | Hispanic | 42 | F | 0 | 0 | Adeno | EGFR | Maintenance | Pem+Bev | 50 | 35.4+ | 35.4+ |
| 3 | Hispanic | 47 | F | 0 | 1 | Adeno | ALK | 2nd-line | Pem | 39 | 30.8+ | 30.8+ |
| 4 | Asian | 35 | M | 0 | 1 | Adeno | ALK | 3nd-line | Pem | 58 | 48.2+ | 48.2+ |
| 5 | Asian | 55 | M | 0 | 1 | Adeno | ALK | Maintenance | Pem | 17 | 12.6 | 29.2 |
| 6 | Asian | 54 | M | 120 | 1 | Adeno | ALK | Maintenance | Pem+Bev | 15 | 13.8 | 27.7+ |
| 7 | Caucasian | 64 | F | 3 | 2 | Adeno | ROS1 | 2nd-line | Pem | 24 | 18.4 | 23.0 |
| 8 | Asian | 56 | F | 0 | 1 | Adeno | ROS1 | Maintenance | Pem | 22 | 15.4 | 25.2+ |
| 9 | Caucasian | 60 | M | 5 | 0 | Adeno | ROS1 | Maintenance | Pem+Bev | 62 | 48.7+ | 48.7+ |
| 10 | Hispanic | 19 | F | 0 | 0 | Adeno | ROS1 | Maintenance | Pem+Bev | 34 | 23.9+ | 23.9+ |
| 11 | Caucasian | 33 | F | 0 | 1 | Adeno | ROS1 | Maintenance | Pem+Bev | 42 | 36.4+ | 36.4+ |
| 12 | Caucasian | 82 | M | 80 | 1 | Adeno | KRAS | 2nd-line | Pem | 20 | 19.6 | 26.7+ |
| 13 | Caucasian | 80 | F | 9 | 1 | Adeno | KRAS | Maintenance | Pem | 17 | 18.6+ | 24.6+ |
| 14 | Caucasian | 67 | F | 40 | 1 | Adeno | KRAS | Maintenance | Pem+Bev | 22 | 28.1 | 28.8+ |
| 15 | Caucasian | 48 | F | 7 | 1 | Adeno | NRAS | Maintenance | Pem+Bev | 40 | 40.3+ | 40.3+ |
| 16 | Hispanic | 68 | F | 0 | 1 | Adeno | None1 | Maintenance | Pem | 22 | 15.2 | 20.1 |
| 17 | Caucasian | 69 | F | 64 | 1 | NSCLC, NOS | None2 | Maintenance | Pem | 31 | 20.6+ | 20.6+ |
| 18 | Caucasian | 60 | F | 40 | 1 | Adeno | None3 | Maintenance | Pem+Bev | 16 | 13.0 | 15.5+ |
| 19 | Caucasian | 43 | F | 0 | 1 | Adeno | None4 | Maintenance | Pem+Bev | 28 | 22.1 | 26.4+ |
| 20 | Caucasian | 65 | F | 120 | 1 | Adeno | None5 | Maintenance | Pem+Bev | 25 | 15.8 | 20.8+ |
| 21 | Caucasian | 69 | M | 0 | 1 | NSCLC, NOS | Not Tested | 2nd-line | Pem | 18 | 14.4 | 17.7 |
| 22 | Caucasian | 51 | F | 0 | 1 | Adeno | Not Tested | 3nd-line | Pem | 30 | 21.7 | 22.2 |
| 23 | Asian | 74 | F | 0 | 2 | Adeno | Not Tested | 3nd-line | Pem | 21 | 12.9 | 14.5 |
| 24 | Asian | 46 | F | 0 | 1 | Adeno | Not Tested | 3nd-line | Pem | 25 | 17.1 | 18.5 |
| 25 | Asian | 69 | M | 23 | 1 | Adeno | Not Tested | 4th-line | Pem | 40 | 24.6 | 42.2 |
Abbreviations: Pt, Patient; Adeno, Adenocarcinoma; ALK, Anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; Pem, Pemetrexed; M, Months, Bev, Bevacizumab; OS, overall survival; F, female; M, male. NSCLC, non–small-cell lung cancer; NOS, not otherwise specified;
No oncogenic driver mutations for: EGFR, ALK, KRAS;
No oncogenic driver mutations for: EGFR, ROS1, KRAS, BRAF, APC, CTNNB1, IDH1, IDH2, NOTCH1, NRAS, PIK3CA, PTEN, P53, ALK unsuccessful;
No oncogenic driver mutations for: EGFR, ALK, KRAS, BRAF;
No oncogenic driver mutations for: EGFR, ALK, KRAS;
No oncogenic driver mutations for: EGFR, ALK, KRAS, BRAF, APC, CTNNB1, IDH1, IDH2, NOTCH1, NRAS, PIK3CA, PTEN, P53. No enough tissue to detect ROS1.
Fig 1.
Incidence of driver oncogene in the patients receiving pemetrexed with or without bevacizumab for 12 months or more as maintenance or second line/ beyond treatment.
Efficacy
In the fifteen patients who received maintenance treatment following first-line chemotherapy, 6/15 (40%) patients achieved a partial response (PR) from the first-line platinum-base chemotherapy: among these 6 patients there were 4 patients who received pemetrexed/ platinum chemotherapy (2 patients received additional bevacizumab). There are 1/10(10%) patients who achieved PR and 9/10 (90%) patients who achieved stable disease (SD) as best response during pemetrexed in second-line chemotherapy with no complete response (CR).
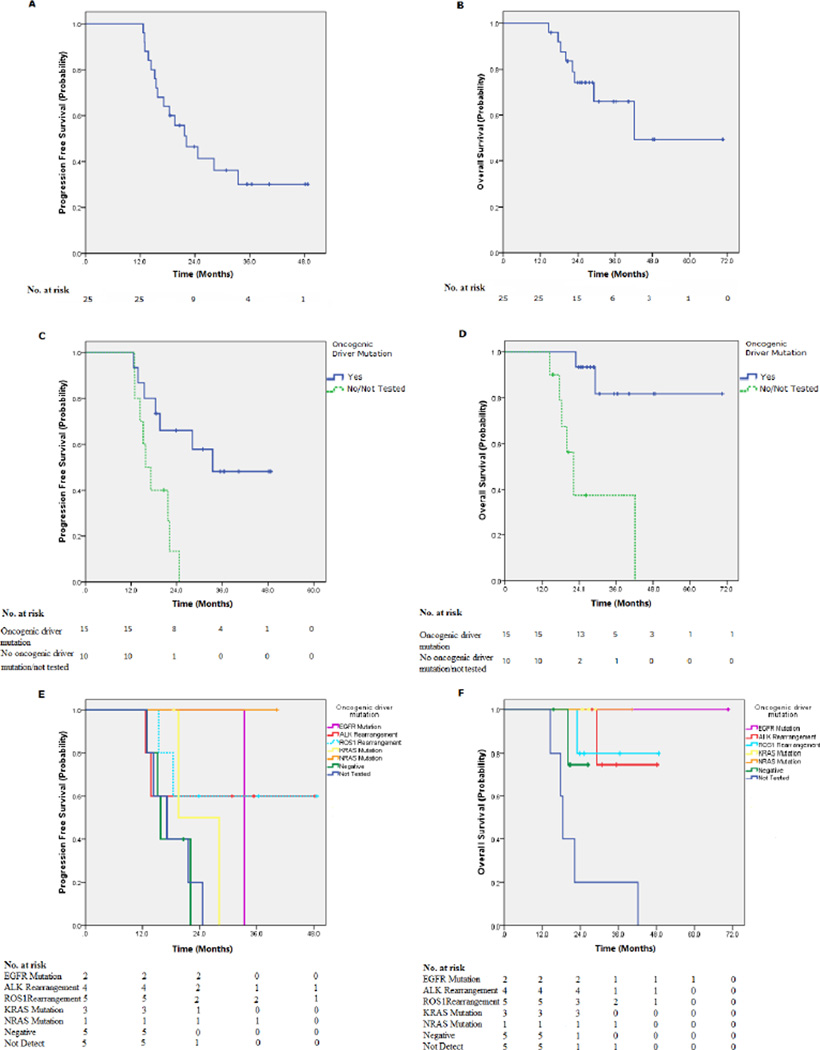
At the time of data cutoff, survival of all 25 patients was evaluated. After median follow-up time of 40.1 months (range, 38.2–62.5 months), the median PFS was 22.1 months (95% confidence interval [CI]: 15.1–29.1), the median overall survival time was 42.2 months (95% CI: 37.4–61.3) and 2-year and 3-year OS rates were 66.0% and 49.5%, respectively. (Figure 2A–B). The median survival time of first-line continuation or switch maintenance treatment and second-line/beyond chemotherapy was not reached vs. 23.0 months, respectively (p= 0.057). The PFS was not different between these two groups, with median PFS of 28.1 vs. 19.6 months (p=0.47). With respect to bevacizumab treatment in the maintenance setting, patients receiving pemetrexed and bevacizumab had improved OS (p=0.021) compared with patients receiving pemetrexed maintenance alone, but no difference was observed in PFS (p=0.251).
Fig 2.
Overall survival (OS) and progression free survival (PFS) of patients receiving pemetrexed with or without bevacizumab for 12 months or more as maintenance or second line/ beyond treatment. (A) PFS of whole group; (B) OS of whole group;(C) Improved PFS of patients with tumors harboring identified oncogenic driver mutation (p=0.006); (D) Improved OS of patients with tumors harboring identified oncogenic driver mutation PFS (p=0.001); (E) PFS of patients with different specific driver oncogenic mutations; (F) OS of patients with different specific driver oncogenic mutations.
For the whole group, OS and PFS were not associated with sex, age, or smoking status (Table 3). However, patients with any identified oncogenic driver mutation had significantly better OS (p=0.001) and PFS (p=0.006) (Table 3 and Figure 2C–D). The OS and PFS of patients with different oncogenic driver mutation did not demonstrate a significant difference between the groups, though the numbers compared were small (Figure 2E–F).
Table 3.
Subgroup analysis of overall survival and progression-free survival
| Variable | OS | PFS | ||
|---|---|---|---|---|
| HR (95%CI) |
P value | HR (95%CI) |
P value | |
| Sex | ||||
| M | 1.014 (0.225–4.569) | 0.986 | 0.768 (0.266–2.218) | 0.624 |
| F | ||||
| Age(year) | ||||
| <60 years | 1.889 (0.437–8.163) | 0.176 | 1.998 (0.719–5.551) | 0.387 |
| ≥60 years | ||||
| Smoking status | ||||
| Former/current smoker | 3.477 (0.681–17.749) | 0.113 | 1.020 (0.382–2.757) | 0.969 |
| Non-smoker | ||||
| Oncogenic driver mutation | ||||
| Yes | 10.743 (2.050–56.306) | 0.001 | 4.296 (1.399–13.193) | 0.006 |
| No | ||||
OS, overall survival; PFS, progression-free survival; HR, hazard Ratio; CI, confidence interval
Tolerability of long term pemetrexed administration
During treatment, most AEs were grade 1 or 2 and non-hematologic, with the most common being fatigue, nausea, and constipation (Table 4). There were 5 (20%) patients who experienced a grade 3 or 4 AE. All grade ≥3 toxicities were non-hematologic and occurred among patients receiving concurrent bevacizumab: one patient with grade 4 proteinuria and nephrotic syndrome, one patient with grade 3 left ventricular systolic dysfunction, and one patient with grade 3 pulmonary embolisms. There were 8 deaths at last follow-up and all of them attributed to tumor PD. No deaths appeared related to pemetrexed treatment.
Table 4.
Drug-related toxicity
| All grades | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
|---|---|---|---|---|---|
| Hematological toxicities | |||||
| Leukopenia | 6(24%) | 2(8%) | 2(8%) | 2(8%) | 0 |
| Neutropenia | 4(16%) | 1(4%) | 1(4%) | 2(8%) | 1(4%) |
| Anemia | 7(28%) | 4(16%) | 1(4%) | 2(8%) | 1(4%) |
| Thrombocytopenia | 2(8%) | 2(8%) | 0 | 0 | 0 |
| Non-Hematological toxicities | |||||
| Nausea | 17(68%) | 16(64%) | 1(4%) | 0 | 0 |
| Vomiting | 5(20%) | 5(20%) | 0 | 0 | 0 |
| Anorexia | 4(16%) | 4(16%) | 0 | 0 | 0 |
| Rash | 6(24%) | 6(24%) | 0 | 0 | 0 |
| Edema | 11(44%) | 7(28%) | 4(16%) | 0 | 0 |
| Fatigue | 17(68%) | 16(64%) | 1(4%) | 0 | 0 |
| Neuropathy | 6(24%) | 6(24%) | 0 | 0 | 0 |
| Diarrhea | 4(16%) | 4(16%) | 0 | 0 | 0 |
| Constipation | 12(48%) | 12(48%) | 0 | 0 | 0 |
| ALT | 2(8%) | 2(8%) | 0 | 0 | 0 |
| Hyponatremia | 5(20%) | 4(16%) | 1(4%) | 0 | 0 |
| Hypokalemia | 3(12%) | 2(8%) | 1(4%) | 0 | 0 |
| Creatinine | 1(4%) | 1(4%) | 0 | 0 | 0 |
| Proteinuria | 5(20%) | 3(12%) | 1(4%) | 0 | 1(4%) |
| Thrombus | 4(16%) | 0 | 3(12%) | 1(4%) | 0 |
| Hypertension | 2(8%) | 0 | 1(4%) | 1(4%) | 0 |
| rhinitis | 1(4%) | 1(4%) | 0 | 0 | 0 |
| Epistaxis | 5(20%) | 5(20%) | 0 | 0 | 0 |
| Left ventricular systolic dysfunction | 1(4%) | 0 | 0 | 1(4%) | 0 |
Discussion
In this landmark analysis, we selected patients who tolerated long term pemetrexed administration to evaluate their characteristics and tolerability of treatment. In our study, 25 patients were selected from a population of 196 patients (12.8%) who received pemetrexed for more than 12 months (either as maintenance, or as second line therapy or beyond). This percentage was comparable to that identified in the PARAMOUNT trial5, on which 67 of 359 (17.0%) patients were still on pemetrexed maintenance without progression at 12 months, and the JMEN4 trial in which 27/326 (8.3%) of non-squamous patients remained on pemetrexed switch maintenance therapy for 12 months. However, in the JMEI second-line treatment trial3, there were only 2/283 (0.7%) patients still on pemetrexed second-line treatment without progression at 15 months.
We found that long term pemetrexed use was quite tolerable, with chronic side effects of edema and fatigue, which did not preclude continuation of therapy. Patients who received pemetrexed with bevacizumab as maintenance treatment had significantly better OS than those receiving pemetrexed monotherapy alone. While virtually all of the bevacizumab patients were continuing first line maintenance treatment, a large potential confounder, our data, as well as the conclusion from AVAPERL6 that pemetrexed plus bevacizumab maintenance is superior to bevacizumab alone, raises the question of whether maintenance pemetrexed plus bevacizumab is superior to pemetrexed alone. This is being addressed by the ongoing ECOG 5508 trial (NCT01107626) comparing maintenance therapy with bevacizumab, pemetrexed, or a combination of bevacizumab and pemetrexed following 4 cycles of first line carboplatin/paclitaxel/bevacizumab chemotherapy, but it is unlikely that many patients with known EGFR, ALK, or ROS1 oncogenic driver mutations will participate in this trial.
Rationalizing that progression in the central nervous system (CNS) alone may reflects the failure of CNS penetration due to blood-brain barrier and that systemic disease may maintain sensitivity, we observed that a group of patients that developed brain metastatses had radiosurgical brain treatment then resumed pemetrexed for an additional median of 4.0 months PFS. This strategy has been previously described in other studies of the efficacy of pemetrexed in ALK-positive patients8, 18, and is also a recommended practice guidelines option for patients with EGFR or ALK positive lung cancer receiving treatment with tyrosine kinase inhibitors. The prospective ASPIRATION trial20 also showed that continuing erlotinib beyond RECIST PD is feasible, with additional median PFS of 3.1 months in post-PD erlotinib patients. In the present report, the additional PFS gained was only 4.0 months using this strategy in a selected population who had already received pemetrexed for more than 12 months, suggesting that the development of brain metastases often heralds the development of systemic resistance.
Limitations of our retrospective study included that survival numbers have little population meaning when selecting patients with a more favorable response, and bias related to single institution practice patterns. Additionally, there is bias in the molecular testing itself – our 20% overall ROS1 positive rate (and greater than 70% of those tested) reflects that long term responders with no known oncogenic driver mutation were subjected to additional testing as testing for new “actionable” drivers was performed to identify future effective treatments. Despite these limitations, we found that patients with any known oncogenic driver mutation did particularly well with maintenance pemetrexed. There were a disproportionately high number of patients with ALK and ROS1 rearrangements in our cohort, as well as some with KRAS mutations who did quite well over time. Interestingly, one patient with an NRAS-mutant tumor received first line continuation pemetrexed and bevacizumab for over 40 months. Of note, only two patients with EGFR mutant tumors were in the selected cohort, perhaps an underrepresentation of this population of patients related to use of EGFR targeted agents preferentially, or more interestingly suggesting less inherent sensitivity of these tumors to pemetrexed. Overall, our cohort of patients with any oncogenic driver mutation had significantly better PFS and OS than molecular wild type or undetected patients, consistent with the recent Lung Cancer Mutational Consortium21 results. Interestingly, our PFS findings in particular did not depend on receipt of a tyrosine kinase inhibitor therapy for an actionable driver. Since most patients with targeted alterations still receive chemotherapy at some point, patients with known ALK or ROS1 alterations could be prioritized for a pemetrexed containing regimen, and patients with KRAS and NRAS alterations without targeted options could reasonably receive first line pemetrexed based therapy. This work also demonstrates an apparent sensitivity of ROS1 NSCLC to pemetrexed treatment, as previously suggested by our group13.
In this era of molecular targeted therapies, conventional chemotherapy is still a standard treatment before or after the failure of targeted agents. By maintaining tolerable treatments, a subset of patients can achieve long term disease control even with conventional cytotoxic chemotherapy, suggesting that chemotherapy and targeted therapies are indeed complementary and work in concert to prolong both overall survival and quality of life.
Clinical Practice Points.
-
-
Previous studies have shown that some patients may remain on maintenance pemetrexed therapy without progression or undue toxicity for extended durations.
-
-
ALK and ROS1 rearranged NSCLC appear particularly sensitive to pemetrexed based treatment.
-
-
Among patients who received more than 12 months of pemetrexed therapy, the majority had defined oncogenic driver mutations including EGFR, ALK, ROS1, and KRAS
-
-
As a group, patients with oncogenic driver mutation positive NSCLC had significantly better progression free and overall survival than wild type and untested patients.
-
-
Patients with known molecular driver positive non-squamous NSCLC should be prioritized to receive a pemetrexed based regimen, with maintenance pemetrexed therapy, in their course of treatment.
Acknowledgments
We thank Dr. Solomon Henry from the Stanford Cancer Institute Research Database (SCIRDB) group for cohort generation support. The work was funded in part by Stanford University Asia Medical Fund and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Sun Yat-Sen University and State Education Ministry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors report no related conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Reference
- 1.Network NCC. NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer. version 1. 2015 www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 2.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- 3.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 4.Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374:1432–1440. doi: 10.1016/S0140-6736(09)61497-5. [DOI] [PubMed] [Google Scholar]
- 5.Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:2895–2902. doi: 10.1200/JCO.2012.47.1102. [DOI] [PubMed] [Google Scholar]
- 6.Barlesi F, Scherpereel A, Rittmeyer A, et al. Randomized Phase III Trial of Maintenance Bevacizumab With or Without Pemetrexed After First-Line Induction With Bevacizumab, Cisplatin, and Pemetrexed in Advanced Nonsquamous Non-Small-Cell Lung Cancer: AVAPERL (MO22089) Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31:3004–3011. doi: 10.1200/JCO.2012.42.3749. [DOI] [PubMed] [Google Scholar]
- 7.Patel JD, Socinski MA, Garon EB, et al. PointBreak: A Randomized Phase III Study of Pemetrexed Plus Carboplatin and Bevacizumab Followed by Maintenance Pemetrexed and Bevacizumab Versus Paclitaxel Plus Carboplatin and Bevacizumab Followed by Maintenance Bevacizumab in Patients With Stage IIIB or IV Nonsquamous Non-Small-Cell Lung Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013 doi: 10.1200/JCO.2012.47.9626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camidge DR, Kono SA, Lu X, et al. Anaplastic lymphoma kinase gene rearrangements in non-small cell lung cancer are associated with prolonged progression-free survival on pemetrexed. J Thorac Oncol. 2011;6:774–780. doi: 10.1097/JTO.0b013e31820cf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JO, Kim TM, Lee SH, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011;6:1474–1480. doi: 10.1097/JTO.0b013e3182208fc2. [DOI] [PubMed] [Google Scholar]
- 10.Shaw AT, Varghese AM, Solomon BJ, et al. Pemetrexed-based chemotherapy in patients with advanced, ALK-positive non-small cell lung cancer. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2013;24:59–66. doi: 10.1093/annonc/mds242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon BJ, Mok T, Kim DW, et al. First-Line Crizotinib versus Chemotherapy in ALK-Positive Lung Cancer. The New England journal of medicine. 2014;371:2167–2177. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. The New England journal of medicine. 2013;368:2385–2394. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 13.Riess JW, Padda SK, Bangs CD, et al. A case series of lengthy progression-free survival with pemetrexed-containing therapy in metastatic non--small-cell lung cancer patients harboring ROS1 gene rearrangements. Clinical lung cancer. 2013;14:592–595. doi: 10.1016/j.cllc.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstraw PCJ, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumour. J Thorac Oncol. 2007;2:706–714. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 15.Shaw AT, Yeap BY, Mino-Kenudson M, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4247–4253. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergethon K, Shaw AT, Ou SH, et al. ROS1 rearrangements define a unique molecular class of lung cancers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:863–870. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dias-Santagata D, Akhavanfard S, David SS, et al. Rapid targeted mutational analysis of human tumours: a clinical platform to guide personalized cancer medicine. EMBO molecular medicine. 2010;2:146–158. doi: 10.1002/emmm.201000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berge EM, Lu X, Maxson D, et al. Clinical Benefit From Pemetrexed Before and After Crizotinib Exposure and From Crizotinib Before and After Pemetrexed Exposure in Patients With Anaplastic Lymphoma Kinase-Positive Non-Small-Cell Lung Cancer. Clinical lung cancer. 2013 doi: 10.1016/j.cllc.2013.06.005. 2013 Aug 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang JJ, Chen HJ, Yan HH, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung cancer. 2013;79:33–39. doi: 10.1016/j.lungcan.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 20.Park K, Ahn M, Yu C, et al. ASPIRATION: First-line Erlotinib (E) until and beyond RECIST progression (PD) in Asian patients with EGFR mutation-positive NSCLC. Annals of Oncology. 2014;25:iv426–iv470. [Google Scholar]
- 21.Kris MG, Johnson BE, Berry LD, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA : the journal of the American Medical Association. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]