Abstract
Gastrodia elata (GE) is traditionally used for treatment of various disorders including neurodegenerative diseases such as Alzheimer's disease. To investigate the neuroprotective effect of GE, amyloid-β peptide (Aβ)-treated PC12 cells were cultured with GE aqueous extract. In vitro assay demonstrated that 50 µM of pre-aggregated Aβ was lethal to about a half portion of PC12 cells and that Aβ aggregate-induced cell death was significantly decreased with GE treatment at ≤10 mg/mL in a dose-dependent manner. To further examine in vivo cognitive-improving effects, an artificial amnesic animal model, scopolamine-injected Sprague-Dawley rats, were orally administered the extract for 6 weeks followed by behavioral tests (the passive avoidance test and Morris water maze test). The results showed that an acute treatment with scopolamine (1 mg/kg of body weight) effectively induced memory impairment in normal rats and that the learning and memory capability of scopolamine-treated rats improved after prolonged administration of GE extract (50, 250 and 500 mg/kg of body weight for 6 weeks). These findings suggest that a GE regimen may potentially ameliorate learning and memory deficits and/or cognitive impairments caused by neuronal cell death.
Keywords: Gastrodia elata, scopolamine-induced memory impairment, amyloid-β peptide, neuroprotective effect, cognitive-enhancing effect
Alzheimer's disease (AD) is the most common form of dementia in the elderly and is characterized as a neurodegenerative disorder with relentless progression that affects more than 35 million patients worldwide (about 5 million patients aged 64 and older in the United States, 2013) [1,2]. A prominent pathological feature of AD is the structural abnormality which is caused by the formation of cerebral plaques resulting in neuritic dystrophy and neurofibrillary tangles in the brain. Numerous studies suggest that AD pathogenesis may be triggered by accumulation of amyloid-β peptide (Aβ) due to overproduction of the peptide and/or dysfunctional clearance machinery for misfolded proteins [3]. Aβ is generated by cleavage of the Aβ precursor protein and has the ability to self-aggregate into various sizes of oligomers, which form neuritic plaques. Aβ aggregates inhibit mitochondrial activity, alter intracellular calcium levels and stimulate oxidative and inflammatory processes and apoptotic cell death. Thus the accumulation of Aβ oligomers contributes to the failure of excitatory synaptic transmission and further cognitive impairment by neurodegeneration in AD. Despite intensive efforts toward alleviation of AD progress (such as treatment of anti-oxidative agents, cell-based replacement and surgical trials), there has been no effective therapeutic breakthrough. Natural resources as functional food materials are emerging as an alternative treatment for protection from neuronal loss.
Gastrodia elata (GE) is traditionally used for the treatment of a variety of disorders. Recent reports demonstrated its efficacy on convulsions [4,5], the cardiovascular system [6,7], neuro-modulation [8,9] and cognitive functions [10]. A proteomic approach showed stress-related protein expression was decreased and neuroprotective genes were mobilized when neuronal cells were treated with GE, suggesting the neuroprotective or -regenerative effects of the herb [11]. The present study describes the in vitro neuroprotective effect of the aqueous extract from GE on Aβ aggregate-treated neuronal cells. In addition, an ameliorating influence of the extract on memory impairment (mimicking AD pathophysiology) is illustrated based on the passive avoidance test and the Morris water maze test in vivo using scopolamine-treated rats. Our findings have implications for evaluating GE as a functional food material to improve memory impairment or deficits, especially in the context of neurodegenerative diseases such as AD.
Materials and Methods
Preparation of Gastrodia elata extract
GE was cultivated at Muju, Jeollabuk-do, South Korea. Harvested GE was dried at 20-30℃ for 2 days followed by a steaming process (steaming at 90℃ for 12 hr followed by drying for 12 hr) that was repeated four times. Steamed GE was extracted in distilled water (150 kg of GE in 1,500 L of water) at 100℃ for 12 hr. After filtration, the extract was mixed with brown sugar at a 3:1 ratio by weight and ripened at 36-45℃ for 4-5 days. Ripened extract was freeze-dried at 50-55℃ for 7-8 hr. The concentrated GE extract was freshly prepared by diluting at the designated concentrations in distilled, sterilized water on the day of use and sample preps were filtered through a 0.22 µm filter and maintained in a refrigerator until used in experiments.
Cell culture
PC12 cells derived from rat pheochromocytoma were obtained from the Korean Cell Line Bank (Seoul, South Korea) and maintained in culture medium containing RPMI1640 supplemented with 10% fetal bovine serum and1% penicillin-streptomycin (All from Invitrogen, Carlsbad, CA) in culture incubator (37℃, 5% CO2, humidified)
Cell viability assay
To test cytotoxicity of β-amyloid (25-35) peptides (Aβ; American Peptide Company, Sunnyvale, CA) and cell protective effect of GE, PC12 cells were cultured with various concentrations of Aβ and/or GE sample. A WST-1 assay kit (EZ-Cytox; Daeil Lab service, Seoul, South Korea) was used according to the manufacturer instructions. Briefly, cells were plated in a 96-well plate at a cell number of 2×104 cells/well in 90 µL and pre-incubated for 24 hr. Then, Aβ alone (5, 10, 20, 40, 50, 70 and 100 µM; pre-aggregated in PBS for 1 week before used) for the Aβ-induced cytotoxicity test or GE extract (0.01-30 mg/mL, 1 hr before treatment with 50 µM of Aβ) for GE-mediated cell death protection test were applied to the cells. After a 48 hr-incubation under treatment conditions, 10 µL of WST-1 solution was added and a 1-hr reaction was permitted. The absorbance was measured at 490 nm (A260) using the VMAX microplate reader (Molecular devices, LLC, Sunnyvale, CA, USA). Cell viability was calculated by the following equation: Cell viability (%)=(A260 of sample-A260 of blank)/(A260 of control-A260 of blank)×100
Experimental animals
All animal studies were conducted according to the guidelines of the Committee on Care and Use of Laboratory Animals of the Wonkwang University (approval number: WKU13-27). Five-week old Sprague-Dawley rats weighing 120-150 g were purchased from Samtako Inc. (Gyunggi-do, South Korea). After adaptation for 1 week under a 12-hr light/12-hr dark regimen (temperature, 20-24℃; humidity, 45-55%; illumination, 150-300 lux) with access to food and water ad libitum, 6-week-old rats (160-200 g) were used in this study.
Sample treatment
Fifty Sprague-Dawley rats were randomly assigned to five groups (10 rats per group): No treated, only scopolamine-injected (no sample treated), GE-administered at three different concentrations [GElow (50mg of sample/kg of body weight), GEmid (250 mg/kg), and GEhigh (500 mg/kg)] with scopolamine-injected (1 mg/kg of body weight). The GE sample was orally administered using a gastric sonde attached to a syringe on a daily basis for up to 6 weeks. Scopolamine was intraperitoneally injected.
Passive avoidance test
To evaluate contextual fear-motivated learning and memory, the LeDoux method [12] was used with minor modifications in the fifth week. Briefly, the test chamber was sectioned into two compartments (light and dark; 25 cm×20 cm×30 cm each) by a guillotine door. For the training trial, animals were placed in the dark compartment with the door closed. After 10 sec, the door was open, and an electrical foot shock (moment maximum voltage, 500 V) was delivered for 2 sec in the dark compartment while the light compartment was illuminated. Animals learned to associate room properties with the foot shock. The latency for the animals to move to the light from the dark in order to avoid the shock was used as an indicator of learning and memory. Training was carried out 3 times a day for 5 days before the test. A test trial was implemented 1 day after the last training trial. Next, 90 min before the test trial animals were transferred to the behavioral test room and artificial hypomnesia was induced in the animals, with the exception of the normal group, by intraperitoneal injection of scopolamine (1 mg/kg of body weight). For the test trial, animals were placed in the dark compartment with no electrical shock. The latency to escape the dark compartment by passing through the door was measured. Shorter times indicated improvement in learning and memory. The test proceeded for 180 sec. After each session of the test, the chamber was cleaned with 70% ethanol to remove any scent from the previous session.
Morris water maze test
To evaluate spatial learning and memory, Morris' method was applied during experimental weeks three and four. The water maze was a white circular pool (diameter, 150 cm; height, 65 cm; featureless inner-surface) filled with water to a height of 50 cm. A white platform (diameter: 10 cm; height: 45 cm) was established and submerged 5 cm below the water surface. Styrofoam beads were added to make the platform invisible and the water temperature was adjusted to 23-27℃. For training before the test trials, the animal was permitted to stay for 10 sec once it successfully reached the platform. If the animal failed to locate the platform in 180 sec, it was placed on the platform for 10 sec to learn and memorize the location of the platform. Training was implemented twice a day and 3 days a week for 1 week. Test trials were repeated twice with a 20-min interval for each animal. The escape latency (time taken for the animal to swim to the platform) was measured and the values were averaged. As above, scopolamine (1 mg/kg body weight) was injected intraperitoneally 90 min before the test trial to induce AD-like features in the animals, with the exception of the animals in the normal group.
Statistical analysis
The data were analyzed by one-way ANOVA (analysis of variance) test. To determine statistical significance, Duncan's multiple range test was performed with adjustments for the P values less than an α of 0.05. Statistical differences were presented by different alphabetical letters.
Results
Protective effect against Aβ-induced neural cell death
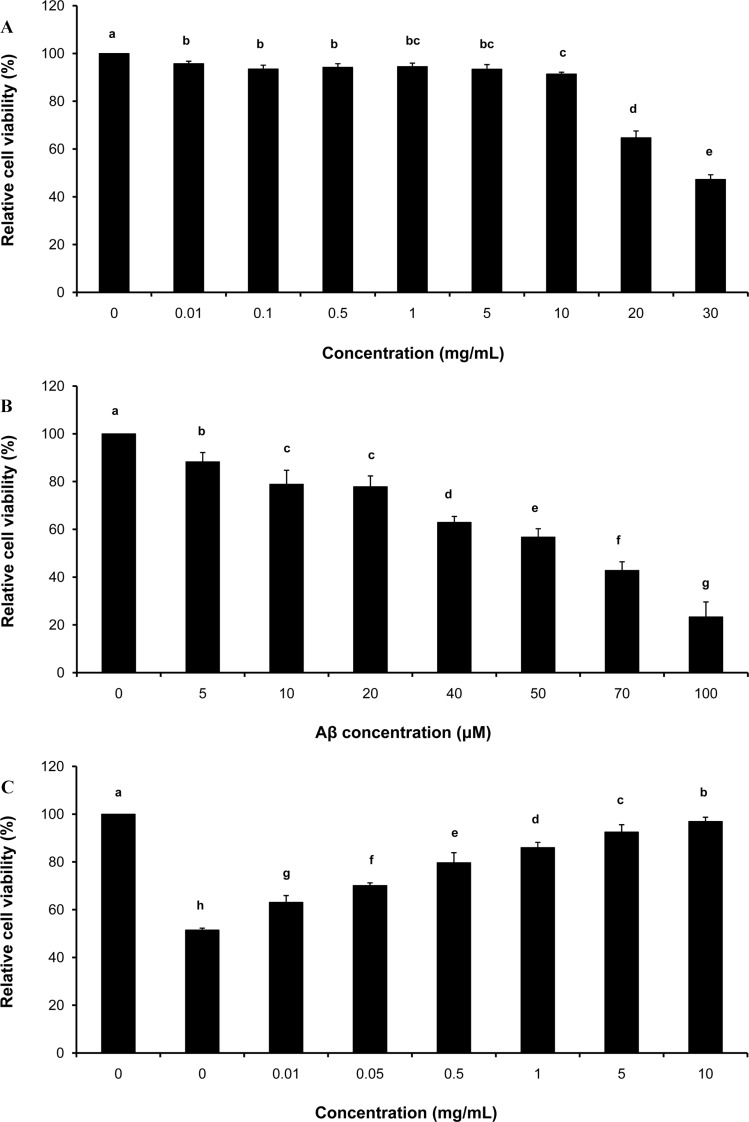
To test the cytotoxicity of GE, PC12 cells were treated with various concentrations of GE sample (0, 0.01, 0.05, 0.5, 1, 5, 10, 20 and 30 mg/mL) for 1 hr followed by cell viability assay. Results showed that ≥91% of the cells were viable at concentrations ≤10 mg/mL of GE (Figure 1A). However, average cell viabilities at 20 mg/mL and 30 mg/mL GE conditions were 65 and 47%, respectively. For further experiments, ≤10 mg/mL of GE were applied to PC12 cells.
Figure 1. Protective effect of GE against Aβ-induced neural cell death. (A) Cytotoxicity of GE sample at 0, 0.01, 0.05, 0.5, 1, 5, 10, 20 and 30 mg/mL applied to PC12 cells was examined. Relative cell viability normalized to the control (100, 96, 93, 94, 95, 93, 91, 65, and 47% at each concentration) decreased in a dose-dependent manner. N=5; error bars, mean±SEM. (B) Cytotoxicity of PC12 cells treated with pre-aggregated Aβ at 0, 5, 10, 20, 40, 50, 70 and 100 µM was assayed. Relative cell viability (normalized to the control; 100% at the 0-µM-treated condition) was decreased in a dose-dependent manner, 88.3, 78.9, 77.8, 62.9, 56.8, 42.8, and 23.4% on average at each respective concentration. N=5; error bars, mean±SEM. (C) The neural cell protective effect of GE against Aβ-induced cytotoxicity was evaluated. PC12 cells were pretreated with GE at various concentrations (0-10 mg/mL) for 1 hr and cultured in the presence of 50 µM of Aβ for 48 hr. Cell viability under GE-treated conditions were significantly higher compared to controls (no GE-treated). N=7; error bars, mean±SEM.
To determine the concentration of Aβ toxic to neural cells, PC12 cells were treated with pre-aggregated Aβ at various concentrations (0, 5, 10, 20, 40, 50, 70 and 100 µM) for 48 hr. After cell viability testing, cytotoxicity was computed based on absorbance and normalized to control conditions (no Aβ treatment). Our data showed that PC12 cell viability was significantly deceased in Aβ-treated conditions compared to the control group (Figure 1B); 50 µM of Aβ treatment resulted in an approximately 50% reduction in viable PC12 cells.
To examine the neuroprotective effects of GE, PC12 cells were pre-cultured with various concentrations of GE sample (0, 0.01, 0.05, 0.5, 1, 5 and 10 mg/mL) for 1 hr and then treated with Aβ (50 µM) for 48 hr. Results from the cell viability test showed that the susceptibility of PC12 cells to Aβ treatment at the cytotoxic level significantly decreased with GE treatment compared to none-GE treatment conditions (Figure 1C). This finding indicated that GE had a protective effect on Aβ-induced cell death in vitro.
Changes in body weight, food intake and water intake of rats after administration of GE extract
To evaluate the effects of a daily regimen of GE extract on feeding in vivo, 6-week-old rats were orally administered three different concentrations of GE extract (50, 250 and 500 mg/kg of body weight) for 6 weeks on a daily basis. Weekly changes in body weight, food intake and water intake were monitored for each animal and compared among the groups (Figure 2). As shown in the graphs in Figure 2, there were no significant differences among the five groups (no treatment vs only scopolamine-injected vs GE-administered at three different concentrations and scopolamine-injected) in each discipline (Figure 2A for body weight; 2B for food intake; 2C for water intake).
Figure 2. Weekly changes in the body weight, food intake and water intake. Sprague-Dawley rats were randomly assigned to five different groups (10 rats per group): No treatment, only scopolamine-treated, and GE extract and scopolamine-treated groups [GElow (50 mg of sample/kg of body weight), GEmid (250 mg/kg) and GEhigh (500 mg/kg)]. Body weight (A), food intake (B) and water intake (C) were monitored on a weekly basis for 7 weeks and within-group measurements for each week were averaged. There were no significant differences observed over the study period.
Improvement of learning and memory capability in scopolamine-treated rats
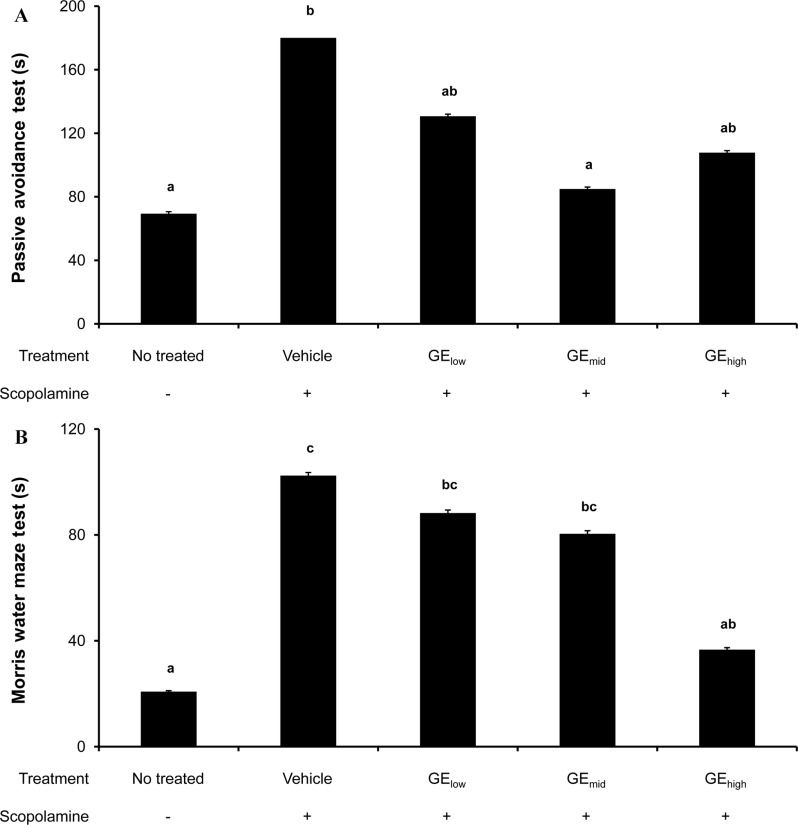
To investigate the influence of the GE regimen on learning and memory in vivo, the passive avoidance and Morris water maze tests were performed using an acute hypomnesic animal model after prolonged oral administration of GE extract. Intraperitoneal injection of scopolamine is known to induce memory impairment. Thus, rats received GE extract at three different concentrations and were trained to escape from a given circumstance, as described above in the method section. On the day of testing, acute memory impairment was induced by scopolamine treatment and the behavioral test was carried out. Data from passive avoidance testing demonstrated that (i) escape latency time for scopolamine-injected rats (control) was significantly longer than that for normal rats (180 sec vs 69 sec) and (ii) GE extract-administered rats showed significantly shorter latency time compared to the control group (Figure 3A). In the Morris water maze test, we observed that (i) the average escape latency time of the control group (scopolamine-injected) was significantly higher than it was in the normal group (102 sec vs 21 sec) and (ii) the latency time of the GE extract-treated rats was significantly shorter than seen in the non-treated rats (Figure 3B). These results based on the two behavioral tests suggest that oral administration of GE extract for a prolonged period alleviates scopolamine-induced memory impairment.
Figure 3. Ameliorative efficacy of oral administration of GE extract on memory impairment. Sprague-Dawley rats were randomly assigned to five different groups (10 rats per group): No treatment, only scopolamine-treated, and GE extract-administered at three different concentrations (50, 250 and 500 mg/kg body weight) with scopolamine-treated. Before learning and memory tests (A, passive avoidance test; B, Morris water maze test), rats were treated according to the experimental group and trained multiple times for a week to escape from the given circumstances. After training, acute memory impairment was induced by an intraperitoneal injection of scopolamine (1 mg/kg of body weight) on the day of the test. (A) Results from the passive avoidance test showed that the escape latency time was maximized to 180 sec for the scopolamine-injected group (control) compared to that for the normal group (69 sec). The latency time was significantly lowered in the groups that received GE extract treatment compared to controls. (B) Data from the Morris water maze test showed that prolonged administration of GE extract to the scopolamine-treated rat hypomnesic model decreased the escape latency time compared to the control group (no GE extract treatment).
Discussion
GE is a medicinal herb that has shown promising results in the treatment of neurodegenerative disorders such as AD. In this study, we evaluated the protective effect of aqueous GE extract on Aβ-induced neuronal cell death as well as memory impairment. Our in vitro and in vivo results demonstrated that (i) Aβ-induced PC12 cell death was decreased with GE treatment and (ii) acute artificial hypomnesia in rats was mitigated by prolonged oral administration of GE extract.
In vitro results from the present study imply that GE treatment can protect against neuronal cell death which may be induced by the accumulation of Aβ aggregates. AD is a progressive neurodegenerative disorder that is characterized by cognitive decline affecting memory, judgment, and reasoning. According to the 'amyloid hypothesis' established on the basis of genetic studies [2,13], an imbalance between generation and clearance resulting in aggregation of Aβ in the brain is believed to be the primary cause of AD-related pathogenic development involving the formation of neurofibrillary tangles, synaptic dysfunction and neuronal cell death [1,14]. In particular, Aβ (25-35) is known as the neurotoxic domain of the full-length Aβ, which affects neuronal function and causes learning and memory impairments [14].
Our data showed that pre-aggregated Aβ has induced cytotoxicity to PC12 cells and Aβ-induced cell death was prominently attenuated by GE treatment. These results are consistent with previous reports demonstrating that aqueous GE extract treatment augmented levels of neuroprotection-related proteins in neuronal N2a cells [11] or reduced Aβ-induced neurodegeneration in Drosophila [15]. Based on these biochemical studies, it is speculated that the neuroprotective effect of GE may be through reduction of oxidative stress and/or inhibition of apoptosis [11,15].
In addition, our data from the rat behavioral tests illustrate that GE administration can ameliorate memory impairment in the context of amnesia. Disruption of cholinergic neurotransmitter system is a pathological feature of the early stage of AD [16,17,18]. The cholinergic system is involved in memory storage and retrieval of information during learning [17]. The blockage of muscarinic receptors induces memory dysfunction, which is generally displayed in AD patients [19]. Scopolamine is a nonselective antagonist with activity against muscarinic receptors, which inhibits cholinergic neurotransmission with no alteration in concentrations of acetylcholinesterase or muscarinic receptors and induces memory impairment and cognitive deficits [18,20]. Therefore, scopolamine is widely used to artificially produce amnesic or AD models.
In this study, our in vivo behavioral tests revealed that scopolamine injection in normal rats induced impairments in learning and memory and that GE extract reversed scopolamine-induced memory defects. The findings suggest that GE could be a preventive or therapeutic candidate for AD treatment. Further intensive study may be required to identify molecular mechanisms to explain the efficacy of GE extract in alleviating AD pathogenesis.
Among the numerous compounds identified from GE, the primarily active constituents are gastrodin and ρ-hydroxybenzyl alcohol [21,22]. Gastrodin (GAS) is a phenolic glucoside composed of glucose and ρ-hydroxybenzyl alcohol (HBA); HBA is a metabolite of GAS [4]. GAS is known to have mitigative, anticonvulsive and neuroprotective effects [4,23,24,25] due to its strong anti-inflammatory and antioxidant activity [26,27,28]. Previous studies described the quantification of GAS and HBA in various biological samples. For example, after intravenous administration [29,30,31,32], (a) immediately after the injection of GAS, GAS was rapidly decomposed to HBA, (b) GAS and HBA were found in the brain, especially the cerebellum, even though their concentrations were very low, suggesting both GAS and HBA might be able to pass through the blood-brain barrier, and (c) both GAS and HBA levels in the blood, brain and bile peaked earlier than 30 min and rapidly declined within 240 min and they were excreted in the urine. In addition, the bioavailability of orally-administered GAS was influenced by the co-administration of other substances [33,34]. Our in vitro and in vivo observations in the present study on the efficacy of GE are consistent with these previous results demonstrating neuroprotective and impaired memory-ameliorating effects. These effects of GE extract may be caused by the bioactive compounds contained in the extract. To confirm the functionality of GE and maximize its biological activities, further physiological research in combination with advanced food processing technologies is required.
In conclusion, to examine the neuroprotective effect of GE extract, Aβ-treated PC12 cells were cultured in the presence of various concentrations of the extract. At concentrations of ≤10 mg/mL, greater than 97% of PC12 cells were viable. In the presence of cytotoxic Aβ, GE extract treatment significantly enhanced cell viability in a dose-dependent manner. To investigate the effect of GE extract on learning and memory in vivo, the passive avoidance test and the Morris water maze test were performed using scopolamine-treated Sprague-Dawley rats. Our data indicated that (i) scopolamine-treated rats in both tests showed significantly longer escape latency than non-treated normal rats and (ii) the escape latency period for scopolamine-treated rats was significantly decreased with administration of GE extract. Therefore, these results demonstrated that GE extract may have a protective effect against Aβ-mediated neuronal cell death and improve learning and memory in a hypomnesic animal model with prolonged treatment. The findings in this study suggest that GE might serve as a functional food material for amelioration of memory loss, improvement of learning and prevention of neuronal cell loss related to AD.
Acknowledgments
This study was supported by the Core Technology Development and Commercialization Support in Industry-Academia-Research Collaboration (project no. 2013C03) funded by the province of Jeonbuk, Korea.
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362(4):329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 2.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120(4):545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Green KN, Billings LM, Roozendaal B, McGaugh JL, LaFerla FM. Glucocorticoids increase amyloid-beta and tau pathology in a mouse model of Alzheimer's disease. J Neurosci. 2006;26(35):9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojemann LM, Nelson WL, Shin DS, Rowe AO, Buchanan RA. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006;8(2):376–383. doi: 10.1016/j.yebeh.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh CL, Tang NY, Chiang SY, Hsieh CT, Lin JG. Anticonvulsive and free radical scavenging actions of two herbs, Uncaria rhynchophylla (MIQ) Jack and Gastrodia elata Bl., in kainic acid-treated rats. Life Sci. 1999;65(20):2071–2082. doi: 10.1016/s0024-3205(99)00473-7. [DOI] [PubMed] [Google Scholar]
- 6.Zhu L, Guan H, Cui C, Tian S, Yang D, Wang X, Zhang S, Wang L, Jiang H. Gastrodin inhibits cell proliferation in vascular smooth muscle cells and attenuates neointima formation in vivo. Int J Mol Med. 2012;30(5):1034–1040. doi: 10.3892/ijmm.2012.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shu C, Chen C, Zhang DP, Guo H, Zhou H, Zong J, Bian Z, Dong X, Dai J, Zhang Y, Tang Q. Gastrodin protects against cardiac hypertrophy and fibrosis. Mol Cell Biochem. 2012;359(1-2):9–16. doi: 10.1007/s11010-011-0992-1. [DOI] [PubMed] [Google Scholar]
- 8.Ha JH, Shin SM, Lee SK, Kim JS, Shin US, Huh K, Kim JA, Yong CS, Lee NJ, Lee DU. In vitro effects of hydroxybenzaldehydes from Gastrodia elata and their analogues on GABAergic neurotransmission, and a structure-activity correlation. Planta Med. 2001;67(9):877–880. doi: 10.1055/s-2001-18844. [DOI] [PubMed] [Google Scholar]
- 9.Ha JH, Lee DU, Lee JT, Kim JS, Yong CS, Kim JA, Ha JS, Huh K. 4-Hydroxybenzaldehyde from Gastrodia elata B1. is active in the antioxidation and GABAergic neuromodulation of the rat brain. J Ethnopharmacol. 2000;73(1-2):329–333. doi: 10.1016/s0378-8741(00)00313-5. [DOI] [PubMed] [Google Scholar]
- 10.Mishra M, Huang J, Lee YY, Chua DS, Lin X, Hu JM, Heese K. Gastrodia elata modulates amyloid precursor protein cleavage and cognitive functions in mice. Biosci Trends. 2011;5(3):129–138. doi: 10.5582/bst.2011.v5.3.129. [DOI] [PubMed] [Google Scholar]
- 11.Manavalan A, Ramachandran U, Sundaramurthi H, Mishra M, Sze SK, Hu JM, Feng ZW, Heese K. Gastrodia elata Blume (tianma) mobilizes neuro-protective capacities. Int J Biochem Mol Biol. 2012;3(2):219–241. [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips RG, LeDoux JE. Lesions of the fornix but not the entorhinal or perirhinal cortex interfere with contextual fear conditioning. J Neurosci. 1995;15(7 Pt 2):5308–5315. doi: 10.1523/JNEUROSCI.15-07-05308.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayer TA, Wirths O, Majtényi K, Hartmann T, Multhaup G, Beyreuther K, Czech C. Key factors in Alzheimer's disease: beta-amyloid precursor protein processing, metabolism and intraneuronal transport. Brain Pathol. 2001;11(1):1–11. doi: 10.1111/j.1750-3639.2001.tb00376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu P, Jing Y, Collie ND, Campbell SA, Zhang H. Pre-aggregated Aβ(25-35) alters arginine metabolism in the rat hippocampus and prefrontal cortex. Neuroscience. 2011;193:269–282. doi: 10.1016/j.neuroscience.2011.07.054. [DOI] [PubMed] [Google Scholar]
- 15.Ng CF, Ko CH, Koon CM, Xian JW, Leung PC, Fung KP, Chan HY, Lau CB. The Aqueous Extract of Rhizome of Gastrodia elata Protected Drosophila and PC12 Cells against Beta-Amyloid-Induced Neurotoxicity. Evid Based Complement Alternat Med. 2013;2013:516741. doi: 10.1155/2013/516741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wesnes KA, Simpson PM, White L, Pinker S, Jertz G, Murphy M, Siegfried K. Cholinesterase inhibition in the scopolamine model of dementia. Ann N Y Acad Sci. 1991;640:268–271. doi: 10.1111/j.1749-6632.1991.tb00231.x. [DOI] [PubMed] [Google Scholar]
- 17.Deutsch JA. The cholinergic synapse and the site of memory. Science. 1971;174(4011):788–794. doi: 10.1126/science.174.4011.788. [DOI] [PubMed] [Google Scholar]
- 18.Ebert U, Kirch W. Scopolamine model of dementia: electro-encephalogram findings and cognitive performance. Eur J Clin Invest. 1998;28(11):944–949. doi: 10.1046/j.1365-2362.1998.00393.x. [DOI] [PubMed] [Google Scholar]
- 19.Bartus RT, Dean RL, 3rd, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–414. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 20.Izquierdo I. Mechanism of action of scopolamine as an amnestic. Trends Pharmacol Sci. 1989;10(5):175–177. doi: 10.1016/0165-6147(89)90231-9. [DOI] [PubMed] [Google Scholar]
- 21.Wu CR, Hsieh MT, Huang SC, Peng WH, Chang YS, Chen CF. Effects of Gastrodia elata and its active constituents on scopolamine-induced amnesia in rats. Planta Med. 1996;62(4):317–321. doi: 10.1055/s-2006-957892. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh MT, Wu CR, Chen CF. Gastrodin and p-hydroxybenzyl alcohol facilitate memory consolidation and retrieval, but not acquisition, on the passive avoidance task in rats. J Ethnopharmacol. 1997;56(1):45–54. doi: 10.1016/s0378-8741(96)01501-2. [DOI] [PubMed] [Google Scholar]
- 23.Kim HJ, Moon KD, Oh SY, Kim SP, Lee SR. Ether fraction of methanol extracts of Gastrodia elata, a traditional medicinal herb, protects against kainic acid-induced neuronal damage in the mouse hippocampus. Neurosci Lett. 2001;314(1-2):65–68. doi: 10.1016/s0304-3940(01)02296-0. [DOI] [PubMed] [Google Scholar]
- 24.An SJ, Park SK, Hwang IK, Choi SY, Kim SK, Kwon OS, Jung SJ, Baek NI, Lee HY, Won MH, Kang TC. Gastrodin decreases immunoreactivities of gamma-aminobutyric acid shunt enzymes in the hippocampus of seizure-sensitive gerbils. J Neurosci Res. 2003;71(4):534–543. doi: 10.1002/jnr.10502. [DOI] [PubMed] [Google Scholar]
- 25.Zeng X, Zhang S, Zhang L, Zhang K, Zheng X. A study of the neuroprotective effect of the phenolic glucoside gastrodin during cerebral ischemia in vivo and in vitro. Planta Med. 2006;72(15):1359–1365. doi: 10.1055/s-2006-951709. [DOI] [PubMed] [Google Scholar]
- 26.Dai JN, Zong Y, Zhong LM, Li YM, Zhang W, Bian LG, Ai QL, Liu YD, Sun J, Lu D. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS One. 2011;6(7):e21891. doi: 10.1371/journal.pone.0021891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao X, Zou Y, Xu H, Fan L, Guo H, Li X, Li G, Zhang X, Dong M. Gastrodin protect primary cultured rat hippocampal neurons against amyloid-beta peptide-induced neurotoxicity via ERK1/2-Nrf2 pathway. Brain Res. 2012;1482:13–21. doi: 10.1016/j.brainres.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Kumar H, Kim IS, More SV, Kim BW, Bahk YY, Choi DK. Gastrodin protects apoptotic dopaminergic neurons in a toxininduced Parkinson's disease model. Evid Based Complement Alternat Med. 2013;2013:514095. doi: 10.1155/2013/514095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Q, Chen G, Zeng S. Distribution and metabolism of gastrodin in rat brain. J Pharm Biomed Anal. 2008;46(2):399–404. doi: 10.1016/j.jpba.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Chen G, Zeng S. Pharmacokinetics of Gastrodin in rat plasma and CSF after i.n. and i.v. Int J Pharm. 2007;341(1-2):20–25. doi: 10.1016/j.ijpharm.2007.03.041. [DOI] [PubMed] [Google Scholar]
- 31.Lin LC, Chen YF, Tsai TR, Tsai TH. Analysis of brain distribution and biliary excretion of a nutrient supplement, gastrodin, in rat. Anal Chim Acta. 2007;590(2):173–179. doi: 10.1016/j.aca.2007.03.035. [DOI] [PubMed] [Google Scholar]
- 32.Lin LC, Chen YF, Lee WC, Wu YT, Tsai TH. Pharmacokinetics of gastrodin and its metabolite p-hydroxybenzyl alcohol in rat blood, brain and bile by microdialysis coupled to LC-MS/MS. J Pharm Biomed Anal. 2008;48(3):909–917. doi: 10.1016/j.jpba.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Zheng Q, Yue PF, Wu B, Hu PY, Wu ZF, Yang M. Pharmacokinetics comparative study of a novel Chinese traditional herbal formula and its compatibility. J Ethnopharmacol. 2011;137(1):221–225. doi: 10.1016/j.jep.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 34.Jiang L, Dai J, Huang Z, Du Q, Lin J, Wang Y. Simultaneous determination of gastrodin and puerarin in rat plasma by HPLC and the application to their interaction on pharmacokinetics. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;915-916:8–12. doi: 10.1016/j.jchromb.2012.12.011. [DOI] [PubMed] [Google Scholar]