Abstract
We developed pancreatic and duodenal homeobox1 (Pdx1) knockout mice to improve a compensatory hyperinsulinemia, which was induced by hyperplasia in the β cells or Langerhans' islands, as the diabetic model mice. For targeting of Pdx1 gene by homologous recombination, ES cells derived from a 129+Ter/SvJcl×C57BL/6JJcl hybrid mouse were electroporated and subjected to positive-negative selection with hygromycin B and ganciclovir. As these results, one of the three chimeric mice succeeded to produce the next or F1 generation. Then, the mouse fetuses were extracted from the mother's uterus and analyzed immunohistologically for the existence of a pancreas. The fetuses were analyzed at embryonic day 14.5 (E14.5) because Pdx1 knockout could not alive after birth in this study. Immunohistochemical staining revealed that 10 fetuses out of 26 did not have any PDX1 positive primordium of the pancreas and that the PDX1 expresses in both the interior and exterior regions of intestine. In particular, one the exterior of the intestine PDX1 was expressed in glands that would be expected to form the pancreas. The result of PCR genotyping with extracted DNA from the paraffin sections showed existence of 10 Pdx1-knockout mice and corresponded to results of immunostaining. Thus, we succeeded to establish a Pdx1-knockout (Pdx1-/-) mice.
Keywords: Pdx1 gene, knockout mice, pancreas
The Xenopus homeobox gene XlHbox8 plays an important role in regionalization of the posterior foregut, specifically in pancreatic and duodenal development [1]. Mammalian homologs of XlHbox8 were cloned as putative regulators of insulin and somatostatin gene transcription. In mouse, the protein product was called insulin promoter factor-1 (IPF-1) [2], and in rat, somatostatin transcription factor-1 (STF-1) [3] or islet duodenum homeobox gene-1 (IDX-1) [4]. The gene has since been renamed "Pdx1" by the International Committee on Standardized Genetic Nomenclature for mice. PDX1 expression is maintained in the duodenal epithelium [4,5] and in the insulin-secreting islet β-cells, where it transactivates the insulin gene [2,6]. The key characteristic of type 2 diabetes in the Japanese and Asians generally is insufficient insulin secretion from β-cells. We previously established insulin receptor substrate-2 (Irs2) knockout mice with a C57BL/6JJcl genetic background with hyperinsulinemia [7,8,9] similar to the report by Terauchi et al. [10]. However, the type 2 diabetes indicated by these Irs2 knockout mice was different from that of human, especially Japanese [11], because the atrophy of the Langearhans' islands and β-cells was not induced by insulin-oversecretions in mice [9,12]. On the other hands, the expression of Pdx1 gene in heterozygous Pdx1 gene knockout (Pdx1+/-) mice was reduced to 68% compared with wild type (Pdx1+/+) mice [13]. Therefore, we expect that Irs2-knockout mice with introduction of the heterozygous Pdx1 gene mutant allele could suppress the hyperplasia of β-cells or Langerhans' islands, similar to type 2 diabetes in the Japanese and Asians. Some research institutes have developed Pdx1-knockout mice [14,15], but use of these mice is restricted and several unnecessary genes are also introduced into these mice in addition to Pdx1 gene. Therefore, the authors developed Pdx1-/- mice at the CIEA.
Mouse Pdx1 genomic clones were isolated from C57BL/6JJcl strain mouse genomic clone library. Gene targeting constructs were produced in the pSINTK vector (accession No. AB242435), which contains the SV40 promoter-hygromycin-SV40 poly(A) tail and HSV promoter-thymidine kinase (Figure 1A). For targeting of Pdx1 gene by homologous recombination, 5×106 ES cells derived from a 129+Ter/SvJcl×C57BL/6JJcl hybrid mouse were electroporated with 15 µg of linearized targeting construct in 100-µL solution V (Nucleofector Kit, Lonza, Japan) with one pulse of 800 V/3 µF with an Amaxa Nucleofector I (Lonza Japan, Tokyo, Japan Co. Ltd.). ES cells were then subjected to positive-negative selection with 100 µg/mL hygromycin B (Wako Pure Chemical Industries, Ltd., Osaka, Japan) and 2 µM ganciclovir (Mitsubishi Tanabe Pharma Corporation, Osaka, Japan). After 7-10 days, 216 clones were isolated and screened for the presence of the targeted allele.
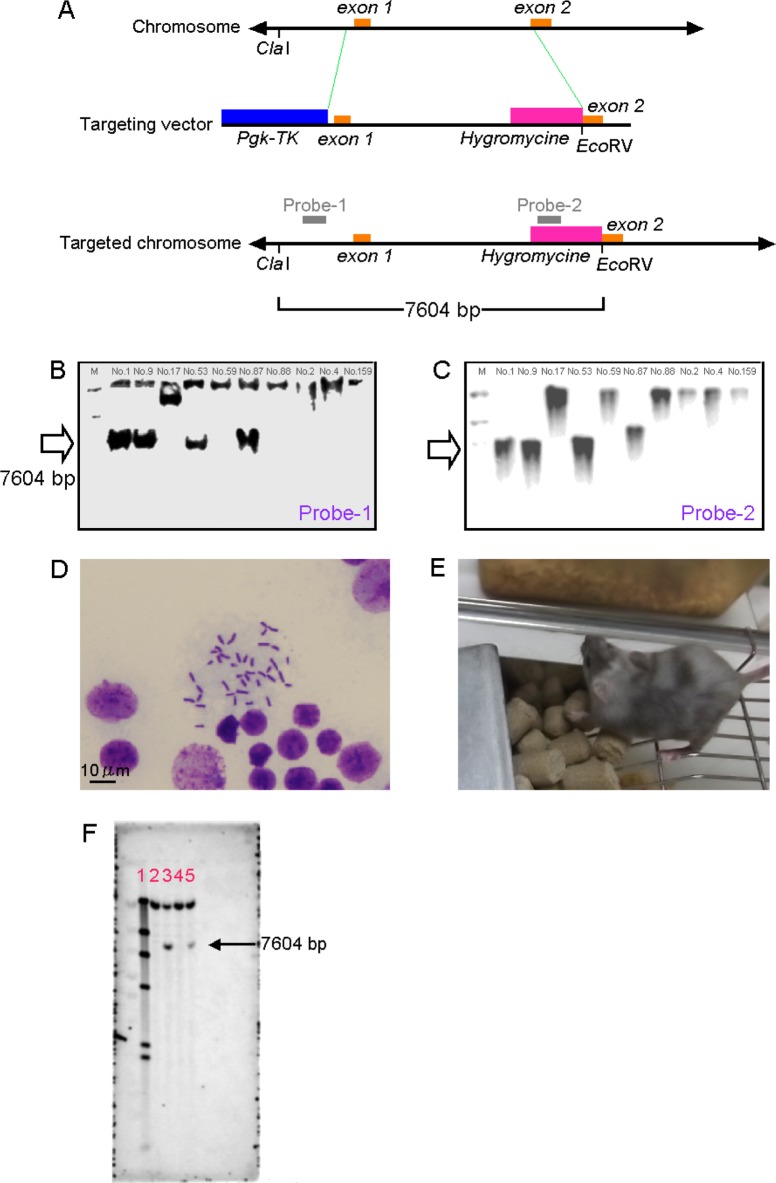
Figure 1. Targeting of Pdx1 gene. (A) The Pdx1 gene and targeting starategy. The gray bold bars indicate the position of the probe for genomic Southern blot analysis using ClaI- and EcoRV-digested samples (mutated allele, 7,604 bp; wild-type allele, uncut (Probe 1) or none (Probe 2)). Genomic DNA from ES cells was digested with Cla I and Eco RV, and subjected to hybridization with the probe-1 (B) and probe-2 (C). Lane 1: DIG size marker, lane 2: ES-No.1, lane 3: ES-No. 9, lane 4: ES-No.17, lane 5: ES-No.53, lane 6: ES-No.59, lane 7: ES-No.87, lane 8: ES-No.88, lane 9: ES-No.2, lane 10: ES-No.4, lane 11: ES-No.159. Each number indicates ES clone No. M: size marker. (D) Chromosome banding of ES-No. 53. (E) Chimeric mice generated from ES-No. 53. (F) Genomic DNA from F1 mice was digested with Cla I and Eco RV, and subjected to hybridization with the probe-1. Lane 1: DIG size marker, lane 2: female Pdx1+/+ mouse , lane 3: female Pdx1+/- mouse, lane 4: male Pdx1+/+ mouse, lane 5: male Pdx1+/- mouse.
Genomic DNA was extracted from doubly resistant ES cell clones using phenol and chloroform. As a first screening, the presence of the targeted allele was analyzed by PCR using oligonucleotide primers (5'-CCAGCCA GGCTACAAAATTA-3' and GTATACCTCTAGAATAA GCTT-3') under PCR conditions of 35 cycles consisting of 94℃ for 30 s, 54℃ for 30 s, and 72℃ for 40 s in a reaction mixture containing Taq polymerase (Takara Bio Inc. Shiga, Japan). As a secondary screening, correct homologous recombination was confirmed by Southern blotting using a 5' external probe and a hygromycin inner probe (Figure 1A). Chromosome banding of the targeted ES cell clones were investigated according to the protocol of Sugawara et al. (2006) [16]. Chromosome number was counted in 50 cells for each ES clone. ES cell clones, which have 40 chromosomes in over 70% of the cells, were established as normal ES cell clone. C57BL/6JJcl blastocysts that were injected into ES cells were transferred into pseudo-pregnant ICR females. Male chimeras were bred with IQI females (CLEA Japan, Inc., Tokyo, Japan) and agouti offspring (F1) were genotyped by PCR. Pdx1+/- offspring were intercrossed to produce Pdx1-/- mice. This study was performed with the animal experiment guideline defined by the Animal Committee of the Central Institute for Experimental Animals. E14.5 embryos were removed and fixed in 10% buffered formalin and embedded in paraffin wax. Paraffin sections (3-4 µm) were treated with 0.03% H2O2 in methanol to block endogenous peroxidase activity. The sections were incubated at 4℃ overnight with goat-anti-mouse PDX-1/IPF1 (1:100; Cat. No. AF2517, R&D systems Inc., Minneapolis, MN, USA) and incubated for 60 min with simple stain mouse MAX-PO(G) (Cat. No. 414351, Nichirei Bioscience Inc., Tokyo, Japan, diluted twofold). 3.3'-Diamino benzidine (DAB) was used as the chromogen. All slides were counterstained with hematoxylin. Genomic DNAs were extracted from paraffin wax sections (10 µm) of murine fetuses using TaKaRa DEXPAT (Code No. 9091, Takara Bio Inc., Shiga, Japan).
The 216 ES cells were cultured for genetic analysis and subjected to primary screening with PCR. Results indicated that 17 ES cells were positive for homologous recombination. These 17 ES cells were analyzed using Southern blot analysis. The results indicated a band of 7,604 bp in size for numbers. 1, 9, 53 and 87 with probe 1 (Figure 1B). The results revealed a similar band for numbers 1, 9, 53, and 87 with probe 2 (Figure 1C), but the band for No. 87 was larger than 7,604 bp. The reason for the difference in the size of the band could not be determined, so No. 87 was excluded from the cells used to generate animals. ES cells numbers 1, 9, and 53 were considered to be homologous recombinants.
ES cells numbers 1, 9, and 53 were confirmed to be homologous recombinants based on Southern blot analysis. The number of chromosomes was determined in 50 cells from each of these original ES cells. Results indicated that 90% of the No. 1 cells had the normal number of chromosomes, 82% of the No. 9 cells had that number, and 86% of the No. 53 cells had that number (Figure 1D). The passing criterion for generating animals at the CIEA is 80% or higher, so these ES cells were considered to be normal and were used to generate chimeras.
Chimeric mice were generated from ES cells numbers 1, 9, and 53 via artificial reproduction. However, pups from No. 9 cells were cannibalized soon after fostering. Thus, rearing and generation of F1 mice were limited to chimeric mice derived from ES cells numbers 1 and 53. An attempt was made to obtain F1 chimeric mice derived from No. 1 and No. 53 cells via natural mating, but only chimeric mice derived from No. 53 cells (Figure 1E) yielded F1 mice. F1 mice derived from No. 53 cells were found to have a region in which homologous recombination had occurred, according to Southern blot analysis using probe 1. Both male and female heterozygous Pdx1-knockout mice were found to have a band of 7,604 bp, similar in size to that in the ES cells (Figure 1F).
Based on these results, an attempt was made to breed male F1 mice derived from No. 53 cells and female C57BL/6JJcl mice. An attempt was also made to generate Pdx1-/- mice, B6-Pdx1tm1Jic/Jic homozygote (Pdx1-/-) mice.
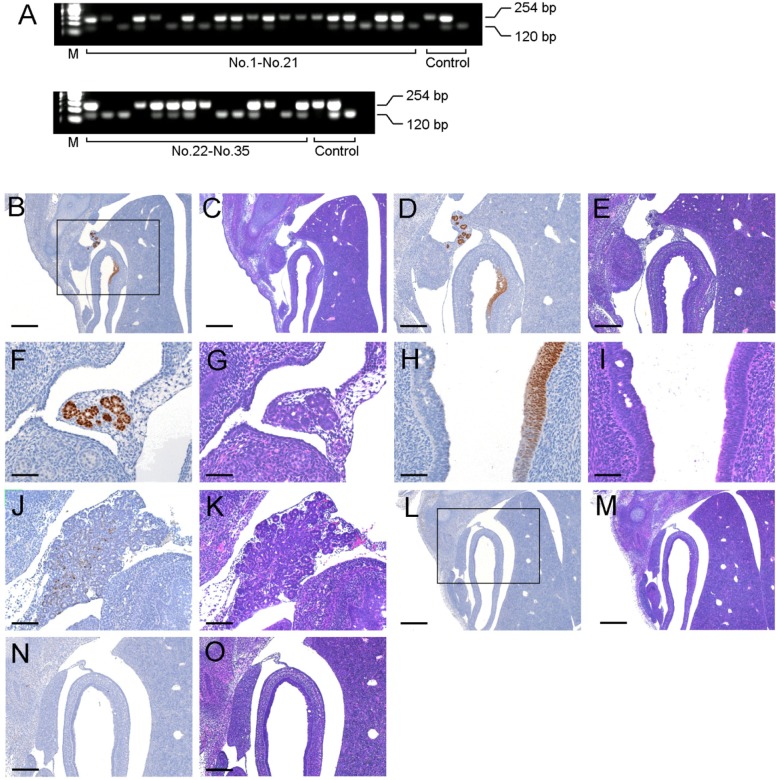
PDX1 protein is normally detected at E13.5 [16]. Additionally, Pdx1-/- mice could not alive after birth in this study although Pdx1-/- mice generated by Offield et al. (1996) lived until 7days after birth [17]. So Pdx1-/- mice were analyzed at E14.5 in this study. Out of 35 embryos analyzed by PCR, there were 10 Pdx1-/-, 16 Pdx1+/-, and 9 Pdx1+/+ embryos (Figure 2A). The expression of PDX1 protein was detected primordium of the pancreas in part of the foregut in 25 embryos including Pdx1+/- and Pdx1+/+. As shown in Figure 2B, C, D, and E, the PDX1 expressions of Pdx1+/- were shown in region in the interior and exterior regions of the intestine. The exterior of the intestine PDX1 (Figure 2F) showed the structures like exocrine glands (Figure 2G). Additionally, PDX1 expression observed in the interior region of the intestinal was weak or absent (Figure 2H, I). The PDX1 expression region (Figure 2J) observed in Pdx1+/+ developed to pancreatic formation with the structures like exocrine glands (Figure 2K) as compared with Pdx1+/-. In contrast, expression of PDX1 was not observed in the Pdx1-/- embryos (Figure 2L, M, N, O).
Figure 2. Analysis of Pdx1 gene and PDX1 protein expression at E14.5 embryos.(A) Genotyping of E14.5 embryos by PCR. DNA was extracted from paraffin-embedded tissues. (B-O) Immunohistochemical and HE staining of pancreatic primordium region at E14.5 embryos. Paraffin-embedded sections of Pdx1+/-, Pdx1+/+, and Pdx1-/- embryos were stained with anti-PDX1. D, E and N, O were expanded from B and L, respectively. Black bars represent 100 µm (B, C, L and M), 200 µm (D, E, N and O), and 400 µm(F, G, H, I, J, and K).
In the present study, we successfully developed Pdx1-/- mouse. Consistent with previous reports [14,15,18], the phenotype of Pdx1-/- embryos was deficit of a pancreas. The PDX1 protein is detected in the dorsal and ventral pancreatic buds at E9.5 embryos [17,19]. We showed that the PDX1 expresses in both the interior and exterior regions of intestine. One the exterior of the intestine PDX1 was expressed in glands that would be expected to form the pancreas. Additionally, the regions in which PDX1 expression was weak or absent were observed in the intestinal interior. These results suggest that PDX1 is expressed in the stomodeum from which the intestine originates during early development, and may promote the development of the pancreas from intestinal tissue, being absent, however, during later developmental stages. Various transgenic mice have been developed for research on diabetic therapy. However, the mice do not become diabetic as readily as do humans [20], because mice have a generally stronger compensatory hyperinsulinemia, induced by hyperplasia in the Langerhans' islands, than humans [12]. The difference in type 2 diabetes between humans and mice might be caused by differences in the insulin released from granules via glucokinase, mitochondrial processes, the mass-action ratio of ATP, and voltage-dependent Ca2+ influx into β-cells [21]. By the way, Pdx1 gene expression is essential for the maintenance of β-cells and is related to the hyperplasia in Langerhans' islands [22,23]. We also established Irs2-knockout mice with a C57BL/6J genetic background [7,8] and expected that Irs2-knockout mice with an introduced Pdx1 deficient allele would show suppressed hyperplasia in Langerhans' islands, and so resemble the human diabetic process.
Acknowledgments
We thank Masafumi Yamamoto, Central Institute for Experimental Animals, for assistance with PCR genotyping. This study was supported by a Grant-in-Aid for Challenging Exploratory Research (No. 15K14374) to Haruo Hashimoto from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Footnotes
Conflict of interests: The authors declare that there is no financial conflict of interests to publish these results.
References
- 1.Wright CV, Schnegelsberg P, De Robertis EM. XlHbox 8: a novel Xenopus homeo protein restricted to a narrow band of endoderm. Development. 1989;105(4):787–794. doi: 10.1242/dev.105.4.787. [DOI] [PubMed] [Google Scholar]
- 2.Ohlsson H, Karlsson K, Edlund T. IPF1, a homeodomain-containing transactivator of the insulin gene. EMBO J. 1993;12(11):4251–4259. doi: 10.1002/j.1460-2075.1993.tb06109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonard J, Peers B, Johnson T, Ferreri K, Lee S, Montminy MR. Characterization of somatostatin transactivating factor-1, a novel homeobox factor that stimulates somatostatin expression in pancreatic islet cells. Mol Endocrinol. 1993;7(10):1275–1283. doi: 10.1210/mend.7.10.7505393. [DOI] [PubMed] [Google Scholar]
- 4.Miller CP, McGehee RE, Jr, Habener JF. IDX-1: a new homeodomain transcription factor expressed in rat pancreatic islets and duodenum that transactivates the somatostatin gene. EMBO J. 1994;13(5):1145–1156. doi: 10.1002/j.1460-2075.1994.tb06363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121(1):11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 6.Peshavaria M, Gamer L, Henderson E, Teitelman G, Wright CV, Stein R. XIHbox 8, an endoderm-specific Xenopus homeodomain protein, is closely related to a mammalian insulin gene transcription factor. Mol Endocrinol. 1994;8(6):806–816. doi: 10.1210/mend.8.6.7935494. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto H, Arai T, Takeguchi A, Hioki K, Ohnishi Y, Kawai K, Ito M, Suzuki R, Yamauchi T, Ohsugi M, Saito M, Ueyama Y, Tobe K, Kadowaki T, Tamaoki N, Kosaka K. Ontogenetic characteristics of enzyme activities and plasma metabolites in C57BL/6J:Jcl mice deficient in insulin receptor substrate 2. Comp Med. 2006;56(3):176–187. [PubMed] [Google Scholar]
- 8.Hashimoto H. Study on establishment of congenic strains and screening of characteristics in IRS-2 deficient mice to support translational research on type 2 diabetes. Exp Anim. 2011;60(1):21–32. doi: 10.1538/expanim.60.21. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto H, Arai T, Mori A, Kawai K, Hikishima K, Ohnishi Y, Eto T, Ito M, Hioki K, Suzuki R, Ohsugi M, Saito M, Ueyama Y, Okano H, Yamauchi T, Kubota N, Ueki K, Tobe K, Tamaoki N, Kadowaki T, Kosaka K. Reconsideration of insulin signals induced by improved laboratory animal diets, Japanese and American diets, in IRS-2 deficient mice. Exp Clin Endocrinol Diabetes. 2009;117(10):577–586. doi: 10.1055/s-0029-1225352. [DOI] [PubMed] [Google Scholar]
- 10.Terauchi Y, Takamoto I, Kubota N, Matsui J, Suzuki R, Komeda K, Hara A, Toyoda Y, Miwa I, Aizawa S, Tsutsumi S, Tsubamoto Y, Hashimoto S, Eto K, Nakamura A, Noda M, Tobe K, Aburatani H, Nagai R, Kadowaki T. Glucokinase and IRS-2 are required for compensatory beta cell hyperplasia in response to high-fat diet-induced insulin resistance. J Clin Invest. 2007;117(1):246–257. doi: 10.1172/JCI17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66(Suppl 1):S37–S43. doi: 10.1016/j.diabres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 12.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 13.Brissova M, Shiota M, Nicholson WE, Gannon M, Knobel SM, Piston DW, Wright CV, Powers AC. Reduction in pancreatic transcription factor PDX-1 impairs glucose-stimulated insulin secretion. J Biol Chem. 2002;277(13):11225–11232. doi: 10.1074/jbc.M111272200. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi T, Yamaguchi T, Hamanaka S, Kato-Itoh M, Yamazaki Y, Ibata M, Sato H, Lee YS, Usui J, Knisely AS, Hirabayashi M, Nakauchi H. Generation of rat pancreas in mouse by interspecific blastocyst injection of pluripotent stem cells. Cell. 2010;142(5):787–799. doi: 10.1016/j.cell.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 15.Jonsson J, Carlsson L, Edlund T, Edlund H. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371(6498):606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara A, Goto K, Sotomaru Y, Sofuni T, Ito T. Current status of chromosomal abnormalities in mouse embryonic stem cell lines used in Japan. Comp Med. 2006;56(1):31–34. [PubMed] [Google Scholar]
- 17.Offield MF, Jetton TL, Labosky PA, Ray M, Stein RW, Magnuson MA, Hogan BL, Wright CV. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(3):983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 18.Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122(5):1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- 19.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PP, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129(10):2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi M, Ikemoto S, Ezaki O. Effect of the fat/carbohydrate ratio in the diet on obesity and oral glucose tolerance in C57BL/6J mice. J Nutr Sci Vitaminol (Tokyo) 1999;45(5):583–593. doi: 10.3177/jnsv.45.583. [DOI] [PubMed] [Google Scholar]
- 21.Matschinsky FM. Banting Lecture 1995. A lesson in metabolic regulation inspired by the glucokinase glucose sensor paradigm. Diabetes. 1996;45(2):223–241. doi: 10.2337/diab.45.2.223. [DOI] [PubMed] [Google Scholar]
- 22.Kulkarni RN, Jhala US, Winnay JN, Krajewski S, Montminy M, Kahn CR. PDX-1 haploinsufficiency limits the compensatory islet hyperplasia that occurs in response to insulin resistance. J Clin Invest. 2004;114(6):828–836. doi: 10.1172/JCI21845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okada T, Liew CW, Hu J, Hinault C, Michael MD, Krtzfeldt J, Yin C, Holzenberger M, Stoffel M, Kulkarni RN. Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc Natl Acad Sci U S A. 2007;104(21):8977–8982. doi: 10.1073/pnas.0608703104. [DOI] [PMC free article] [PubMed] [Google Scholar]