Abstract
Autism spectrum disorder (ASD) is over four times more prevalent in males compared to females. Increased understanding of sex differences in ASD endophenotypes could add insight into possible etiologies and the assessment and management of the disorder. Consequently, the purpose of this review is to describe current literature regarding sex differences in the developmental, psychiatric, and medical endophenotypes of ASD in order to illustrate current knowledge and areas in need of further research. Our review found that repetitive behaviors and restricted interests are more common in males than females with ASD. Intellectual disability is more common in females than males with ASD. Attention to detail may be more common in males than females with ASD and epilepsy may be more common in females than males with ASD, although limited research in these areas prevent definitive conclusions from being drawn. There does not appear to be a sex difference in other developmental, psychiatric, and medical symptoms associated with ASD, or the research was contradictory or too sparse to establish a sex difference. Our review is unique in that it offers detailed discussion of sex differences in three major endophenotypes of ASD. Further research is needed to better understand why sex differences exist in certain ASD traits and to evaluate whether phenotypic sex differences are related to different pathways of development, assessment, and treatment of the disorder.
Keywords: Autism spectrum disorder, Sex differences, Endophenotypes, Co-occurring conditions
In Leo Kanner’s original case series on “autistic disorder of affective contact,” eight males and three females presented with symptoms of what would be called autism (Kanner, 1943). Since then it has been well established that autism spectrum disorder (ASD) is more prevalent in males than in females. The ratio of males with ASD compared to females with ASD is generally around 4:1 (Autism and Developmental Disabilities Monitoring Network, 2012; Blumberg et al., 2013; Fombonne, 2003). This ratio is known to be lower in children of multiplex families (Zwaigenbaum et al., 2012) and in children with co-occurring intellectual disability (ID) (Hartley and Sikora, 2009). According to the results from the Autism and Developmental Disabilities Monitoring Network (ADDM), a multi-site population-based surveillance system conducted in the United States, the difference in estimated prevalence between eight-year-old males and females with ASD has increased in the past 10 years (Autism and Developmental Disabilities Monitoring Network, 2012). The sex difference in estimated prevalence per 1,000 children was 7.8 in the 1994 birth cohort, 11.3 in the 1998 birth cohort, and 14.6 in the 2000 birth cohort; this translates into an increase in the male to female sex ratios from 4.25:1 in children born in 1994, to 4.54:1 in children born in 1998, to 4.65:1 in children born in 2000 (Autism and Developmental Disabilities Monitoring Network, 2012). Differences in the male to female ASD sex ratio could highlight an under sampling of females, meaning a greater proportion of males than females are recognized with ASD (Dworzynski et al., 2012). Differences in the male to female ASD sex ratio could also indicate more biologic or environmental exposures (e.g. hormonal exposures) that make males more susceptible to the disorder (Baron-Cohen et al., 2011; Knickmeyer et al., 2005)
Previous research suggests that sex could represent a naturally occurring subgroup that differentiates ASD symptoms (Ben-Itzchak et al., 2013; Hartley and Sikora, 2009; Holtmann et al., 2007; Lai et al., 2013; Carter et al., 2007; Mandy et al., 2012; Kirkovski et al., 2013). Endophenotypes may also represent subgroups that differentiate ASD symptoms and lend clues to causes of the disorder. Endophenotypes are constructs that connect measurable phenotypic behaviors and underlying genetic influences (Gottesman and Gould, 2003). Endophenotypic approaches to understanding health outcomes are particularly useful when there is a great deal of variability in symptom severity and presentation associated with a disorder, such as the heterogeneity associated with ASD. For instance, attempting to link genetic influences to a complex set of behaviors is challenging whereas attempting to link genetic influences to a particular symptom or certain sets of symptoms may yield more useful results. Consequently, using an endophenotypic approach to better understand ASD and sex differences within ASD symptoms may help elucidate meaningful subgroups that can guide etiologic research and treatment programs (Viding and Blakemore, 2007).
Currently, the research on sex differences in ASD is sparse or contradictory. The research on ASD endophenotypes is emerging but holds considerable promise for the study of gene-behavior pathways (Viding and Blakemore, 2007). There are no reviews to date that focus on sex differences by ASD endophenotypes, although reviewing sex differences by endophenotype may highlight potential mechanisms of ASD development and areas in need of future research. The purpose of this review was to compile current literature on ASD sex differences, with a focus on three broad ASD endophenotypes – (1) developmental, (2) psychiatric, and (3) medical – to show the variety of ways ASD manifests in males versus females, illustrate areas in need of further research, and add insight into possible etiological mechanisms of the disorder.
Methods
Search Criteria
This review was based on published peer-reviewed journal articles listed in PsychInfo and PubMed. Articles with titles that contained ASD diagnostic descriptions, diagnostic criteria, sex descriptors, developmental issues, psychiatric comorbidities, and medical comorbidities were selected. The specific search criteria comprised the following key terms: all searches included one of (1) autism, (2) autism spectrum disorder, (3) ASD, (4) Asperger’s, or (5) pervasive developmental disorder and one of (1) gender, (2) male, (3) female, or (4) sex. In addition, one of (1) social communication, (2) social interaction, (3) repetitive behaviors, (4) restricted interests, (5) attention deficit and hyperactivity disorder, (6) attention deficit disorder, (7) hyperactivity, (8) challenging behavior, (9) aggression, (10) temper tantrums, (11) oppositional defiance, (12) cognitive skills, (13) intellectual disability, (14) mental retardation, (15) cognitive flexibility, (16) response inhibition, (17) working memory, (18) attention to detail, (19) developmental regression, (20) excess of fear, (21) absence of fear, (22) safety issues, (23) self-injurious behavior, (24) elopement, (25) wandering off, (26) anxiety, (27) mood disorder, (28) depression, (29) obsessive compulsive disorder, (30) schizophrenia, (31) birth defect, (32) chromosomal disorder, (33) genetic condition, (34) Down syndrome, (35) fragile X, (36) cerebral palsy, (37) head size, (38) head circumference, (39) brain size, (40) encephalopathy, (41) abnormal eating, (42) abnormal drinking, (43) seizures, (44) epilepsy, or (45) sleep disorders was included.
Inclusion / Exclusion Criteria
There were a total of 2,055 search results found that met search criteria. Results that were excluded were case reports, commentaries, editorials, dissertation abstracts, and books. Studies that did not compare males to females and studies that were not in English were also excluded. Articles that provided a population prevalence estimate for a given search criterion were cited but not included in the count of articles reviewed for said endophenotype. The final sample consisted of 69 articles used to review sex differences in ASD endophenotypes.
Characteristics of Articles Reviewed
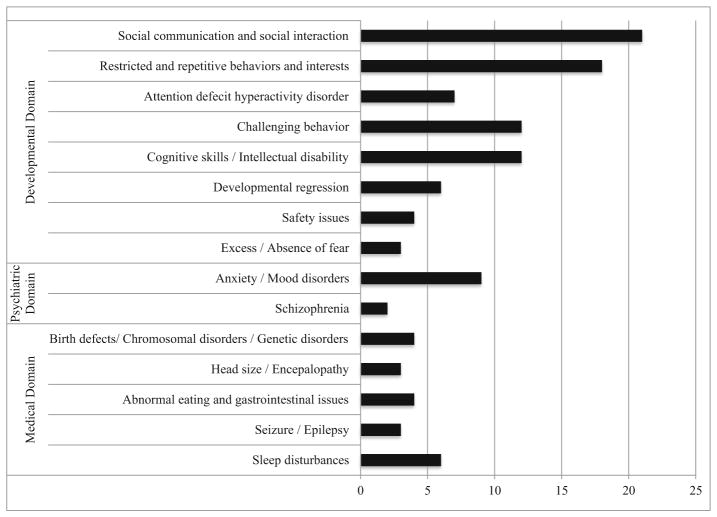
Figure 1 illustrates the 69 articles reviewed for this paper divided by topic area. These studies were published between 1983 and 2013 and total sample sizes ranged from 28 (Matson and Love, 1990) to 337,000 (Autism and Developmental Disabilities Monitoring Network, 2012). A range of 4 (Bernabei et al., 2007) to 5,522 (Beuker et al., 2013) females contributed to the study samples. There were 35 cross-sectional studies, 18 case-control studies, 5 cohort studies, 6 surveillance studies, 2 systematic reviews, 1 clinical trial, 1 meta-analysis, and 1 online survey. The two systematic reviews and one meta-analysis were all cited within the section on medical endophenotypes and did not include articles that were reviewed in this paper. Twenty-nine studies were conducted in the United States, 37 studies were conducted outside of the United States, and three were a meta-analysis or systematic review.
Fig. 1.
Number of articles cited for each ASD endophenotype in developmental, psychiatric, and medical domains
Categorization of Articles Reviewed
Articles were categorized by title and into developmental, psychiatric, and medical endophenotypes based on DSM 5 diagnostic criteria and associated features (American Psychiatric Association, 2013), as well as co-occurring conditions categorized by Levy et al. (2010). Criteria not included in the Levy study but included in this study were categorized first by the lead author and then reviewed and approved by the second author who is a doctorate level developmental psychologist familiar with the assessment and diagnosis of children with ASD.
Results
Developmental Domain: ASD Diagnostic Criteria
Social Communication/Social Interaction
Deficits in social communication and social interaction are core factors in the diagnostic criteria of ASD. Impairments in social communication may present as abnormalities in eye contact, poor integration of verbal and nonverbal behaviors, and difficulties understanding the nonverbal communication of others (American Psychiatric Association, 2013). In some cases, persons with ASD may not participate in conversation or struggle with pragmatic language (American Psychiatric Association, 2013). Impairments in social interaction include difficulties with social-emotional reciprocity, failure to develop peer relationships, and reduced empathetic understanding and/or response (American Psychiatric Association, 2013).
There were 21 articles reviewed on social communication and social interaction in persons with ASD published between 1993 and 2013: 13 case-control studies, 7 cross-sectional studies, and 1 surveillance study. Nine studies were conducted in the United States and 12 studies were conducted at outside of the US. Of these 21 articles, 14 address social communication or other language abilities. In samples of children with varying cognitive abilities, some studies showed no significant differences in communication, conversational deficits, and language levels between males and females with ASD (Andersson et al., 2013; Dawson et al., 2007; Nicholas et al., 2008; Pilowsky et al., 1998; Park et al., 2012a, 2012b; Mayes and Calhoun, 2011; Mandy et al., 2012; Sipes et al., 2011; Solomon et al., 2012; Amr et al., 2011). One study found that males with ASD had greater expressive and receptive language skills than females with ASD (Carter et al., 2007) and another study found that females with ASD had more impaired social communication skills than males with ASD (Hartley and Sikora, 2009). Conversely, Park et al. (2012b) found that females with ASD had stronger non-verbal communication abilities than males with ASD. The majority of the literature reviewed on social communication found no difference between males and females with ASD, but there is some inconsistency.
The literature on sex differences in social interaction among persons with ASD (13 of 21 articles reviewed) is also inconsistent but generally suggests no significant sex differences in social interaction skills (Andersson et al., 2013; Dawson et al., 2007; Nicholas et al., 2008; Pilowsky et al., 1998; Mayes and Calhoun, 2011; Park et al. 2012b; Mandy et al., 2012; Sipes et al., 2011; Solomon et al., 2012). In population-level surveillance of eight-year olds, 80 % of children with ASD had poor social-emotional reciprocity with no significant difference between males and females (Nicholas et al., 2008). Szatmari et al. (2012) also found no sex differences in social-emotional reciprocity for children with varying cognitive abilities in a study from the Autism Genome Project. Oppositely, in an age and IQ matched case–control study, female adults with ASD were found to have fewer socio-communication difficulties during interpersonal interaction than male adults with ASD (Lai et al., 2013). Lastly, no sex differences have been noted in emotional reactiveness or being withdrawn (Hartley and Sikora, 2009) and in empathetic understanding and responses (Auyeung et al., 2009).
Cognitive functioning is likely to play a role in these social processes. Lower intellectual abilities are often linked with greater social impairment regardless of sex (Dawson et al., 2007). Among children with high functioning autism (IQ≥70), social skills have been found to be more impaired in female children than male children (Holtmann et al., 2007) and more severe as children aged (McLennan et al., 1993). In contrast, other studies found adult females with high functioning autism to have less socio-communication issues compared to males (Lai et al., 2011) or there were no sex differences in socio-communication skills in older children and adolescents (Holtmann et al., 2007; Kopp and Gillberg, 2011).
Overall, the 21 articles reviewed suggest no difference in social interaction between males and females with ASD and inconsistent differences in social communication between males and females with ASD, although both social interaction and social communication may be influenced by intellectual ability and age.
Restricted, Repetitive Patterns of Behavior, Interests, or Activities
There were 18 articles reviewed on restricted and repetitive patterns of behaviors and interests (RRBI) published between 1993 and 2013: nine cross-sectional studies, seven case–control studies, one cohort study, and one surveillance study. Eleven studies were conducted in the United States and seven studies were conducted in other countries. Based on this review, the literature suggests that males with ASD have more RRBI than females with ASD (Hattier et al., 2011; Carter et al., 2007). When assessing individual facets of RRBI, restricted interests are seen more often in males with ASD than females with ASD independent of cognitive ability (Kohane et al., 2012; May et al., 2012; Mandy et al., 2012; Szatmari et al., 2012). Males with ASD are also more likely to have more routines, rituals, and fascination with parts of objects than females with ASD (Nicholas et al., 2008; Park, et al., 2012b; Beuker et al., 2013). The literature on repetitive motor movements is less consistent: adult males with high functioning autism or Asperger’s syndrome had more repetitive motor movements than adult females with high functioning autism or Asperger’s syndrome in one case-control study (May et al., 2012), but there were no sex differences in repetitive motor movements among persons with autism in two other case-control studies (McLennan et al., 1993; Worley and Matson, 2011), one cross-sectional study (Auyeung et al., 2009), and one population-based cross-sectional study (Nicholas et al., 2008).
Age may influence the presentation of RRBI in males and females with ASD. One study found no sex difference among RRBI in toddlers (Sipes et al., 2011), whereas a different study found significantly more RRBI among adult males with ASD compared to females with ASD (Hattier et al., 2011). In summation, most studies reviewed suggested that males with ASD are likely to have more RRBI than females with ASD across levels of cognitive ability, although RRBI may be influenced by age.
Sensory issues are prevalent among persons with ASD (Nicholas et al., 2008) and are included as a RRBI in the DSM 5 (American Psychiatric Association, 2013). Common issues are oversensitivity to touch, sound, smell, taste, and attraction to certain tactile stimuli (American Psychiatric Association, 2013; Baranek et al., 2006; Rogers et al., 2003). Abnormal sensory reactions have been reported to occur in up to 47 % of persons with ASD, which is a rate ten times higher than reported in the general population (Nicholas et al., 2008). Additionally, there is a significant correlation between sensory issues in each of the individual senses (Kern et al., 2007); therefore, impairment is compounded for persons with ASD and sensory abnormalities.
Cross-sectional studies found no observed differences between males and females in sensitivity to sound (Mandy et al., 2012) or sensory sensitivity in general (Louisa et al., 2012; Mayes and Calhoun, 2011; Baranek et al., 2006). Mandy et al. (2012) examined RRBI in children and adolescents 3 to 18 years of age and found that age did not influence the presentation of RRBI. However, Lai et al. (2011) found that adult females with high functioning autism had more lifetime sensory issues than males with ASD. Overall, the majority of articles reviewed that addressed sensory issues in ASD do not suggest a sex difference, although aging may be a factor and should be further explored.
Developmental Domain: other Developmental Endophenotypes
Attention Deficit Hyperactivity Disorder
Attention deficit hyperactivity disorder (ADHD) and corresponding symptoms are common in children with ASD (Bradley and Isaacs, 2006; Nicholas et al., 2008). Previous studies show that 50 % to 83 % of children and teenagers with ASD had hyperactivity and attention problems (Nicholas et al., 2008; Bradley and Isaacs, 2006). There were seven articles that met search criteria and addressed ADHD. These seven articles were published between 2008 and 2012 with five cross-sectional studies and two cohort studies. Two studies were conducted in the United States and five were conducted in other countries.
A study of 7 to 12 year-olds with varying cognitive abilities found that males with ASD had higher levels of hyperactivity and impulsivity than females with ASD and this difference was more pronounced at younger ages (May et al., 2012). Males with high functioning autism from middle childhood to adolescence had higher levels of hyper-activity and inattention in teacher reports as compared to female peers, but there was no difference in parental reports (Mandy et al., 2012). A study of children and young adults aged 5 to 20 with high functioning autism found females had more attention problems (Bryson et al., 2008). A majority of studies found no difference in ADHD co-occurrence between males and females with ASD (Simonoff et al., 2008; Sinzig et al., 2009; Mayes and Calhoun, 2011; Horovitz et al., 2011). In summary, the current literature leans toward no sex differences in the co-occurrence of ADHD and ASD, but there is still inconsistency in the literature and thus, the sex difference is largely inconclusive
Challenging Behavior (Aggressiveness/Temper Tantrums/Oppositional Tendencies)
Challenging behavior is a common associated feature of ASD and includes aggression expressed toward other people, temper tantrums, and oppositional and defiant tendencies. Aggression expressed toward other people and temper tantrums are found in 50 % and 54 % of children with ASD compared to only 28 % and 23 % of children without ASD (Nicholas et al., 2008). The 12 articles in this review that met search criteria and addressed challenging behaviors were published between 2005 and 2012 and included six cross-sectional studies, four case–control studies, one clinical trial, and one surveillance study. Six studies were conducted in the United States and six were conducted in other countries. In general, there were no differences in aggression, temper tantrums, or anger between child sexes, regardless of age or cognitive ability (Kozlowski et al., 2012; Worley and Matson, 2011; Carter et al., 2007; Murphy et al., 2009; Mandy et al., 2012; Quek et al., 2012; Mayes and Calhoun, 2011; Horovitz et al., 2011). One study found that females with ASD had more “challenging behaviors” than males with ASD, although challenging behaviors were not explicitly defined (Dworzynski et al., 2012). Delinquent behavior (Park et al., 2012b) and oppositional defiance (Gadow et al., 2005) were more prevalent in males than in females with ASD in two studies reviewed.
Cognitive Skills and Intellectual Disability
In a review from 1966 to 2001, Fombonne (2003) found that the median prevalence of intellectual impairment in persons with ASD was 70 % in the studies evaluated. More recent population-based studies have found lower rates of ID in persons with ASD, with a range from 18 % to 55 % (Charman et al., 2011). The National Health Interview Study found 0.71 % of all children aged 3 to 17 from 1998 to 2007 had an ID (Boyle et al., 2011). Our review found 12 articles that met the search criteria and addressed ID or specific cognitive skills. These 12 articles were published between 1983 and 2011, and included seven cross-sectional studies, four case–control studies, and one-surveillance study. Four studies were conducted in the United States and eight were conducted in other countries.
The 12 articles reviewed support a relationship between child sex and co-occurring ID in children with ASD. The sex ratio between males and females without ID is greater than the sex ratio for all levels of cognitive ability combined (Nicholas et al., 2008; Hartley and Sikora, 2009). Consequently, the male to female ratio is lower when there is co-occurring ID compared to when there is no co-occurring ID (Hartley and Sikora, 2009; Nicholas et al., 2008; Yeargin-Allsopp et al., 2003). The ratio of males to females with ASD and co-occurring ID has been seen to range from 1.3:1 (Tsai and Beisler, 1983) to 2.8:1 (Bryson et al., 2008) with a trend toward fewer sex differences as ID becomes more severe (Yeargin-Allsopp et al., 2003). This differential sex difference in ID results in females with ASD, on average, having lower intelligence test scores than males with ASD (Banach et al., 2009; Volkmar et al., 1993).
Specific cognitive skills posited to vary between males and females with ASD include cognitive flexibility, response inhibition, working memory, and attention to detail (Geurts et al., 2004). Female adolescents with high functioning autism were seen to have superior information processing, multiple conceptual tracking, divided attention, and cognitive flexibility compared to male adolescents with high functioning autism (Bolte et al., 2011). In contrast, studies show males with ASD have superior attention to detail, visuo-spatial skills (Auyeung et al., 2009), and inhibitory control (Lemon et al., 2011) compared to females with ASD. There were no sex differences between adults with ASD in the “eyes test” which measures ability to infer mental states through the eyes (Lai et al., 2011). In summary, the 12 journal articles reviewed in this section suggest that females with ASD generally have lower intelligence test scores than males with ASD and that specific cognitive skills may vary by sex.
Developmental Regression
Parents of some children with ASD report a period of typical development followed by a loss in language, social, motor, self-help, imaginative play, or other skills. This developmental regression is usually reported to occur between 15 and 24 months of age (Meilleur and Fombonne, 2009). Our review found six articles that met search criteria and addressed developmental regression. These six articles were published between 2007 and 2013 and comprised two cohort studies, three cross-sectional studies, and one surveillance study. In a population-based surveillance study of children with ASD, 17 % of children had documented developmental regression and that percentage rose if the child had a previous ASD diagnosis (Wiggins et al., 2009). Males had significantly more regression than females and were more likely to regress at a younger age (Wiggins et al., 2009). This higher risk of regression in males was also seen in smaller, non-population based studies (n=4, 8, and 17 female children) (Bernabei et al., 2007; Ekinci et al., 2012; Zhang et al., 2012). In contrast, a cross-sectional study found that females aged 18 months to 15 years had significantly higher occurrence of regression as compared to males (30 % vs. 19 %) (Ben-Itzchak et al., 2013). No difference in the presence of developmental regression between males and females with ASD was observed in a small clinical sample of 20 females (Meilleur and Fombonne, 2009). In sum, the review of sex differences of developmental regression is contradictory and thus inconclusive.
Excess/Absence of Fear
Excess or absence of fear is more common in children with ASD than other children (Evans et al. 2005; Nicholas et al., 2008). In a population-based surveillance of eight-year olds, Nicholas et al. (2008) found that 32 % of children with ASD had atypical fear noted in service records compared to 6 % of children with ASD symptoms but no ASD diagnosis. Three articles met search criteria and addressed excess or absence of fear. All three of these articles were published in the United States between 1990 and 2011 and were two cross-sectional studies and one case–control study. In these studies, females with ASD had more specific phobias than males with ASD (Gadow and DeVincent, 2012; Matson and Love, 1990) and more unusual fears (Mayes et al., 2013). One study conducted by Matson and Love (1990) found more fear in typically developing female children compared to male children and no significant difference in fear between typically developing female children and female children with ASD. Given the sparse amount of research on this topic, further exploration is warranted to understand sex differences in fear among persons with ASD.
Safety Issues (Self-Injury/Elopement)
About 50 % of children with ASD engage in self-injurious behavior (Richards et al., 2012; Baghdadli et al., 2003; Duerden et al., 2012). Four studies met search criteria and three pertained to self-injurious behavior. All three of these studies were cross-sectional designs with two being conducted in Europe and one in the United States. No difference in self-injurious behavior was found between males and females with ASD (Richards et al., 2012; Baghdadli et al., 2003; Duerden et al., 2012).
Elopement, also known as wandering off, is a rising concern among parents of children with ASD. One online survey addressing elopement met our search criteria. This survey was conducted in the United States in 2013 and found that 49 % of parents reported that their child with an ASD wandered off at least once after the age of four years (Anderson et al., 2012). Results also found that sex did not influence the prevalence of elopement, although children with more intellectual impairment were more likely to elope (Anderson et al., 2012). Few conclusions can be drawn since there is little research on elopement and other safety issues in children with ASD and associated sex differences.
Psychiatric Domain
Anxiety/Mood Disorders
Symptoms of anxiety and mood disorders are more prevalent in children with ASD than in typically developing children (Worley and Matson, 2011; Nicholas et al., 2008). Among children who met a surveillance definition for an ASD, 55 % had abnormal mood or affect compared to 26 % of children with at least one symptom of an ASD but no ASD diagnosis (Schendel et al., 2009). The Special Needs Autism Project in the UK found 44 cases of emotional disorder per 100 children with ASD (Simonoff et al., 2008). Moreover, among eight-year-old children who met a surveillance definition for an ASD, 3 % had anxiety, 2 % had emotional disorder, 2 % had mood disorder, and less than 2 % had obsessive-compulsive disorder (OCD), depression, bipolar, or oppositional defiant disorder (Levy et al., 2010). Nine studies were found that met search criteria and addressed anxiety or mood disorders. These nine studies were published between 2005 and 2012 and included five cross-sectional studies and four case–control studies. Four of the studies were conducted in the United States and five were conducted in other countries.
The literature on sex differences in co-occurring anxiety or mood disorders and ASD is mixed and dependent on cognitive abilities. In some studies, females with high functioning autism were at greater risk for internalizing psychopathology than both male children with ASD and typically developing female children (Solomon et al., 2012; Mandy et al., 2012). These studies are supported by a Finnish report that found female children with ASD had lower scores on a test associated with major depressive disorder compared to male children with ASD (Mattila et al., 2010). Other studies found no sex differences in the of co-occurrence of anxiety or depression in children with ASD and varying cognitive abilities (Quek et al., 2012; Gadow et al., 2005; Park et al., 2012b; Simonoff et al., 2008; Mayes and Calhoun, 2011; Lai et al., 2011). In the general population, females have more panic attacks, generalized anxiety disorders and males have more social anxiety (American Psychiatric Association, 2013). Based on this review, the current literature is inconclusive on whether a sex difference in children with ASD and co-occurring anxiety or mood disorders exists, although a few studies suggest more anxiety and mood disorders in females than males with ASD.
Schizophrenia
Schizophrenia is a mental disorder that involves delusions, disorganized behavior, disorganized speech, hallucinations, and restrictions in the range and intensity of emotions (American Psychiatric Association, 2013). Schizophrenia typically presents between 18 and 30 years of age with earlier onset associated with male sex. Lifetime prevalence of schizophrenia is near 0.2 % (American Psychiatric Association, 2013) The prevalence of schizophrenia in eight-year olds with ASD is less than 1 % (Levy et al., 2010). Two articles met search criteria and addressed schizophrenia. These two articles were published in 2005 and 2010 and were both case–control studies. Review of the two studies found conflicting results on sex differences and the co-occurrence of ASD and schizophrenia or schizophrenia spectrum traits. A parental survey of 6 to 12 year olds with ASD found schizophrenia spectrum traits to be twice as prevalent in females compared to males (57 %: 28 %) independent of ID (Gadow and DeVincent, 2012). Conversely, in a group of 6 to 12 year olds with ASD and ID, schizophrenia was more common in males than females (Tsakanikos et al., 2011). Again, the literature is relatively sparse due to the late onset of schizophrenia and the rarity of co-occurring schizophrenia: future research is warranted.
Medical Domain
Birth defects/Chromosomal Disorders /Genetic Disorders
Population-based surveillance data from the 2008 ADDM report found that among children with ASD, less than 1 % had a co-occurrence of fragile X syndrome, Down syndrome, chromosomal disorders, or other genetic and congenital diagnoses (Levy et al., 2010). It is likely that these rates are under-reported because investigation of ASD co-occurring conditions was not the focus of the ADDM surveillance effort. However, a cohort study of children in Georgia found a similar prevalence of chromosomal disorders and Down syndrome in persons with ASD (Schendel et al., 2009).
There were four studies reviewed that met search criteria and addressed ASD sex differences in birth defects, chromosomal disorders, and genetic disorders: two systematic reviews, one case–control study, and one surveillance study. Co-occurring birth defects, such as impairments to the central nervous system, cardiovascular system, genitourinary system, or musculoskeletal system, appear more often in males than females with ASD. Among children with ASD, the male to female ratio was 9:1 if a child had a co-occurring birth defect and 3.6:1 if the child did not have a co-occurring birth defect (Schendel et al., 2009).
A review conducted by Reilly (2009) found that males with ASD have more co-occurring Down syndrome than females with ASD and the male to female ratio among children with both ASD and Down syndrome may be near the overall ASD prevalence ratio of 4:1. A review conducted by Wiznitzer (2004) found no difference between males and females with ASD and the co-occurrence of tuberous sclerosis. Clifford et al. (2007) found that about 70 % of males aged 5 to 80 with fragile X syndrome had co-occurring ASD while 23 % of females in the same age range with fragile X had co-occurring ASD. The difference in co-occurrence of ASD between males and females may suggest a greater association between the two conditions in males as compared to females.
It is important to note that birth defects, chromosomal disorders, and genetic disorders are rare and seldom studied. Therefore, the results on sex differences for co-occurring ASD and chromosomal and genetic conditions are inconclusive.
Head Size / Encephalopathy
Head size, specifically an enlarged head circumference or macrocephaly, has been associated with ASD (Wallace and Treffert, 2004). Three articles were reviewed that examined differences in head size between males and females with ASD. Studies include two case–control studies and one cross-sectional study. Two studies were conducted in the US and one study was conducted in Italy. A cross-sectional study by Fombonne et al. (1999) found no difference in head size between males and females aged 2 to 16 with ASD. Sacco et al. (2007) also found no difference in head size between males and females aged 3 to 16 with ASD. In contrast, Aylward et al. (2002) found larger head sizes in male adults and children compared to female adults and children with ASD, but the female sample size was low (n=9).
Abnormal Eating and Gastrointestinal Issues
In a population-based study conducted by Nicholas et al. (2008), about 54 % of children with ASD had an abnormality in eating, drinking, or sleeping, which is nearly 40 % higher than that of children with at least one symptom of ASD but no diagnosis (Nicholas et al., 2008). Some studies have shown an increase in certain gastrointestinal symptoms among persons with ASD, including constipation (Ibrahim et al., 2009) and diarrhea (Wang et al., 2006), while other studies found no significant increase in overall gastrointestinal symptoms or symptoms such as esophageal reflux, vomiting, and abdominal discomfort (Ibrahim et al., 2009; Wang et al., 2006; Valicenti-McDermott et al., 2007). However, little research on gastrointestinal issues has been conducted at a population level and no studies were found that compared males to females on gastrointestinal response. More research is needed to determine if there is an association between gastrointestinal symptoms and ASD and whether the association differs between the sexes.
Four studies were found that compared the sexes and addressed food selectivity. These four studies were conducted in the United States between 2006 and 2010 and included one case–control and three cross-sectional designs. In general, review of these studies found food selectivity and feeding issues to be more frequent in children with ASD than children without ASD (Valicenti-McDermott et al., 2007; Ibrahim et al., 2009), although limited research is available in this area. There was no difference between child sexes in over or under-eating in a study of children with high functioning autism (Worley and Matson, 2011) and no differences between the sexes in eating abnormalities in two studies conducted in children with ASD and varying cognitive abilities (Mayes and Calhoun, 2011; Horovitz et al., 2011). Based on this limited review, it appears unlikely that there is a difference in eating habits between males and females with ASD.
Seizures/ Epilepsy
Epilepsy and other seizure disorders co-occur in 5 % to 40 % of children with ASD and there is differential prevalence based on ID (Baird et al., 2008; Nicholas et al., 2008). This review found three articles that met search criteria and addressed seizures or epilepsy. These three articles were published between 2008 and 2013 and consist of a cohort study, one cross-sectional study, and one meta-analysis. Females with ASD were found to have more epilepsy than males with ASD; the male to female ratio drops to near 2:1 in children with ASD and co-occurring epilepsy, but this may be partly due to differential ID (Amiet et al., 2008; Bolton et al., 2011; Ben-Itzchak et al., 2013). There may be an increased likeliness in females with ASD to have co-occurring epilepsy or seizure disorder; however, the literature is sparse so a conclusion cannot be drawn. Further research is needed to enhance current knowledge of sex differences in children with ASD and epilepsy or seizure disorder.
Sleep Disturbances
In a systematic review of parental sleep surveys, sleep problems were present in 50 % to 80 % of children with ASD compared to 9 % to 50 % in matched typically developing children (Kotagal and Broomall, 2012). There were six articles reviewed that met search criteria and addressed sleep disturbances. These six articles were published between 2004 and 2012 and included two case–control studies, two cohort studies, and two cross-sectional studies. Four were conducted in the United States and three were conducted in other countries. Based on this review, some studies found no sex differences in sleep problems among persons with ASD (Liu et al., 2006; Wiggs and Stores, 2004; Mayes and Calhoun, 2011; Horovitz et al., 2011), one study found that female children with ASD have less sleep problems than male children with ASD (Sivertsen et al., 2012), and one study found female children with ASD have more sleep problems than male children with ASD (Hartley and Sikora, 2009). The minimal amount of research in this area leads to inconsistent results and prevents definitive conclusions on whether a sex difference exists in sleep disturbance.
Discussion
Our review of sex differences in ASD endophenotypes is unique in that it provides detailed discussion on ways ASD manifests in male and females not covered in previous reports (Kirkovski et al., 2013; Van Wijngaarden-Cremers et al., 2013) and illustrates areas in need of further research. In general, the current literature suggests that males with ASD present with more RRBI than females with ASD and females with ASD are more likely to have ID than males with ASD. Some articles suggest more males than females with ASD show a preference for attention to detail and more females than males with ASD have epilepsy or seizure disorder, but there is not enough research in these areas to make firm conclusions. There does not appear to be a difference in social interaction and sensory abnormalities between the sexes; the research pertaining to challenging behaviors, developmental regression, excess or absence of fear, safety issues, anxiety, mood disorders, schizophrenia, birth defects/ chromosomal disorders/genetic disorders, eating or gastrointestinal issues, and sleep disturbances were sparse or had contradictory findings. Table 1 summarizes these results.
Table 1.
Summary of differences between males and females with ASD in regards to developmental, psychiatric, and medical endophenotypes
| ASD Developmental Domain, total number of articles | Result, number of articles that support claim |
|---|---|
| Social communication and social interaction, N=21 | The literature leans toward no sex differences in social communication (nine articles) or social interaction (nine articles), although findings are inconclusive and possibly influenced by cognitive ability (five articles)*. |
| Restricted and repetitive behaviors and interests (RRBI), N=18 | Males are more likely to have more RRBIs regardless of age or cognitive ability than females (eight articles). There are no established differences between the sexes in sensory issues (five articles). |
| Attention deficit hyperactivity disorder (ADHD), N=7 | The literature leans toward no sex differences in symptoms of ADHD among those with ASD (four articles) but is largely inconclusive. |
| Challenging behavior, N=12 | In general, there are no differences in aggression toward other people, temper tantrums, or anger between child sexes, regardless of age or cognitive ability (eight articles). Delinquent behavior (one article) and oppositional defiance (one article) were found to be more prevalent in males with ASD than females with ASD, but the literature in these areas was minimal. |
| Cognitive skills / Intellectual Ability, N=12 | Co-occurring intellectual disability is more prevalent in females than males with ASD (eight articles). Males and females had significant differences in specific cognitive skills, such as attention to detail (three articles), but no particular skill was assessed in more than one study. |
| Developmental regression, N=6 | Findings are inconsistent but most articles reviewed (four articles) found that males are more likely to lose previously acquired skills than females. |
| Excess/Absence of fear, N=3 | The minimal amount of research in this area (three articles) leads to inconsistent results and prevents definitive conclusions on whether a sex difference exists in co-occurring ASD and fear response. |
| Safety issues, N=4 | The literature is minimal but suggests no established sex differences in self-injurious behaviors (three articles) or elopement (one article) among those with ASD. |
| ASD Psychiatric Domain | |
| Anxiety/Mood disorders, N=9 | The literature is sparse but suggests more anxiety and mood disorders in female children with high functioning autism (three articles) and no difference in children with ASD and varying cognitive ability (six articles). |
| Schizophrenia, N=2 | The minimal amount of research in this area leads to inconsistent results and prevents definitive conclusions on whether a sex difference exists in co-occurring ASD and schizophrenia. |
| ASD Medical Domain | |
| Birth defects/Chromosomal disorders /Genetic disorders, N=4 | These disorders are very rare and seldom studied (four articles); results on sex differences in co-occurring ASD and birth defects, chromosomal disorders, or genetic disorders are thus inconclusive. |
| Head size / encephalopathy, N=3 | Literature is sparse but suggest no sex difference in head size (two articles) more than a sex difference in head size (one article). |
| Abnormal eating and gastrointestinal issues, N=4 | The literature in this area is minimal but suggests no established sex differences in eating habits and gastro-intestinal issues among those with ASD (four articles). |
| Seizure / epilepsy, N=3 | There was minimal amount of research comparing epilepsy between sexes, but the literature that exists finds more epilepsy and seizures in females than males with ASD (three articles). |
| Sleep disturbances, N=6 | The minimal amount of research (six articles) and inconsistent results prevents definitive conclusions on whether a sex difference exists in co-occurring ASD and sleep disturbance. |
One study contributed to all three of these claims and is included in all three counts
The importance of studying sex differences in the presentation of ASD cannot be understated. As previously mentioned, differences in the male to female ASD sex ratio could highlight the under-identification of females with ASD or different risk exposures that increase male susceptibility to the disorder. Consequently, understanding specific facets of ASD endophenotypes that elucidate these sex differences could help guide clinical assessment and treatment of ASD and the search for etiologies. For instance, since ASD occurs more often in males than females, some professionals may discount ASD symptoms in females unless they present during the assessment of co-occurring conditions, such as ID. In fact, in the absence of co-occurring conditions, females are less likely to be identified with ASD than their male counterparts (Dworzynski et al., 2012). These findings coupled with the results of our review suggest that healthcare professionals should be encouraged to screen all children – and particularly females –for ASD even when co-occurring conditions are not present. Of course, more research is needed to gain a better understanding of the female ASD phenotype in order to improve diagnostic precision.
The sex bias toward more females with ASD and ID than males with ASD and ID may highlight different causal pathways to the disorder. Our review found a few studies that suggest more females with ASD have co-occurring seizures or epilepsy than males with ASD, although findings were inconclusive due to limited research. However, the sex difference in ASD and ID could be explained by a possible sex difference in ASD and epilepsy since epilepsy is also associated with ID alone (Selassie et al., 2014; Helmstaedter et al., 2014). Previous research on epilepsy suggests that females experience more idiopathic generalized epilepsy (IGE) than males (Camfield et al., 2013; Camfield and Camfield, 2009; Valentin et al., 2007). IGE is familial related and some genetic causes of IGE are known (e.g., mutations on the sodium channel and GABA receptors) (Velisek et al., 2011; Amiet et al., 2008). It has been suggested that some of these same genetic causes of epilepsy – particular those that implicate genetic mutations on sodium channels –may represent ASD phenocopies that deserve further exploration (Amiet et al., 2008). Thus, further exploration of the association between epilepsy and females with ASD may uncover some genetic causes of ASD and illuminate ways to better assess (e.g., obtaining detailed medical and development family histories) and treat (e.g., treating seizures in addition to developmental concerns) ASD in females.
Our review found that males with ASD were more likely to have RRBI than females with ASD, particularly those that involve restricted interests, routines and rituals, and fascination with parts of objects. It may be possible that clinicians are not as likely to identify RRBI in females and this may account for the sex difference in this developmental domain. Ultimately, little is known about potential causes of these RRBI, or RRBI in general, although animal models have suggested that RORA (RAR-related orphan receptor A) is a candidate gene that could contribute to these types of pathologies. For instance, RORA deficiency has been associated with perseverance in the form of limited maze patrol (Goodall and Gheusi, 1987), reduced exploration (Lalonde, 1987), and spatial disorientation (Lalonde and Strazielle, 2003) in mouse models. RORA deficiency has also been associated with Purkinji cell degeneration, which is also implicated in ASD (Palmen et al., 2004). In sum, the known sex differences in ASD and ID and RRBI could highlight different genetic pathways to the disorder that are important to understanding its complex etiologies. Further research is needed to tease apart these genetic pathways and how they interact with environmental exposures that contribute to the development of ASD in both males and females. Further research is also needed in areas with sparse empirical exploration but some indication of ASD sex differences (e.g., anxiety/mood disorders, developmental regression, and seizures/ epilepsy) to better understand the etiologic pathways and methods for assessing and treating ASD.
Varying study designs, different source populations, different participant characteristics (e.g., cognitive abilities) and limited numbers of females in the study samples contribute to the inconsistent results found in this review. Since ASD is rarer in females than males (1 in 252 females compared to 1 in 52 males (Autism and Developmental Disabilities Monitoring Network, 2012)), many studies either exclude females or control for sex in statistical analyses. Additionally, there may be selection bias in previous reports since more females than males with ASD have co-occurring ID and this could skew the results of the studies we summarized. Yet our ability to explore sex differences in ASD improves as the population of persons with ASD increases and more females with ASD are identified. As such, females with ASD should be actively recruited into various research studies so further research on ASD sex differences can be conducted. Large population-based surveillance systems like the ADDM Network or case–control studies in multiple geographic areas with adequate female representation are needed to expand our knowledge of sex differences in ASD presentation and how these sex differences relate to etiology and assessment and management of the disorder.
Contributor Information
Eric Rubenstein, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe Street, Room E6032, Baltimore, MD 21205, USA.
Lisa D. Wiggins, National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, 1600 Clifton Rd. MS E-86, Atlanta 30333 GA, USA
Li-Ching Lee, Email: llee38@jhu.edu, Department of Epidemiology, Johns Hopkins Bloomberg School of Public Health, 615 N. Wolfe Street, Room E6032, Baltimore, MD 21205, USA.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington: American Psychiatric Publishing; 2013. [Google Scholar]
- Amiet C, Gourfinkel-An I, Bouzamondo A, Tordjman S, Baulac M, Lechat P, et al. Epilepsy in autism is associated with intellectual disability and gender: Evidence from a meta-analysis. Biological Psychiatry. 2008;64(7):577–582. doi: 10.1016/j.biopsych.2008.04.030. [DOI] [PubMed] [Google Scholar]
- Amr M, Raddad D, El-Mehesh F, Mahmoud EH, El-Gilany AH. Sex differences in Arab children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2011;5(4):1343–1350. doi: 10.1016/j.rasd.2011.01.015. [DOI] [Google Scholar]
- Anderson C, Law JK, Daniels A, Rice C, Mandell DS, Hagopian L, et al. Occurrence and family impact of elopement in children with autism spectrum disorders. Pediatrics. 2012;130(5):870–877. doi: 10.1542/peds.2012-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson GW, Gillberg C, Miniscalco C. Pre-school children with suspected autism spectrum disorders: Do girls and boys have the same profiles? Research in Developmental Disabilities. 2013;34(1):413–422. doi: 10.1016/j.ridd.2012.08.025. [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network. Prevalence of Autism Spectrum Disorders: Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008. Morbidity and Mortality Weekly Report. Surveillance Summaries. 2012;61(3) [PubMed] [Google Scholar]
- Auyeung B, Wheelwright S, Allison C, Atkinson M, Samarawickrema N, Baron-Cohen S. The children’s empathy quotient and systemizing quotient: sex differences in typical development and in autism spectrum conditions. Journal of Autism and Developmental Disorders. 2009;39(11):1509–1521. doi: 10.1007/s10803-009-0772-x. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Minshew NJ, Field K, Sparks BF, Singh N. Effects of age on brain volume and head circumference in autism. Neurology. 2002;59(2):175–183. doi: 10.1212/wnl.59.2.175. [DOI] [PubMed] [Google Scholar]
- Baghdadli A, Pascal C, Grisi S, Aussilloux C. Risk factors for self-injurious behaviours among 222 young children with autistic disorders. Journal of Intellectual Disability Research. 2003;47(8):622–627. doi: 10.1046/j.1365-2788.2003.00507.x. [DOI] [PubMed] [Google Scholar]
- Baird G, Charman T, Pickles A, Chandler S, Loucas T, Meldrum D, et al. Regression, developmental trajectory and associated problems in disorders in the autism spectrum: the SNAP study. Journal of Autism and Developmental Disorders. 2008;38(10):1827–1836. doi: 10.1007/s10803-008-0571-9. [DOI] [PubMed] [Google Scholar]
- Banach R, Thompson A, Szatmari P, Goldberg J, Tuff L, Zwaigenbaum L, et al. Brief report: Relationship between non-verbal IQ and gender in autism. Journal of Autism and Developmental Disorders. 2009;39(1):188–193. doi: 10.1007/s10803-008-0612-4. [DOI] [PubMed] [Google Scholar]
- Baranek GT, David FJ, Poe MD, Stone WL, Watson LR. Sensory experiences questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47(6):591–601. doi: 10.1111/j.1469-7610.2005.01546.x. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Lombardo MV, Auyeung B, Ashwin E, Chakrabarti B, Knickmeyer R. Why are autism spectrum conditions more prevalent in males? PLoS Biology. 2011;9(6):1. doi: 10.1371/journal.pbio.1001081.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Itzchak E, Ben-Shachar S, Zachor DA. Specific neurological phenotypes in autism spectrum disorders are associated with sex representation. Autism Research. 2013;6(6):596–604. doi: 10.1002/aur.1319. [DOI] [PubMed] [Google Scholar]
- Bernabei P, Cerquiglini A, Cortesi F, D’Ardia C. Regression versus no regression in the autistic disorder: Developmental trajectories. Journal of Autism and Developmental Disorders. 2007;37(3):580–588. doi: 10.1007/s10803-006-0201-3. [DOI] [PubMed] [Google Scholar]
- Beuker KT, Schjolberg S, Lie KK, Donders R, Lappenschaar M, Swinkels SH, et al. The structure of autism spectrum disorder symptoms in the general population at 18 months. Journal of Autism and Developmental Disorders. 2013;43(1):45–56. doi: 10.1007/s10803-012-1546-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg SJ, Bramlett MD, Kogan MD, Schieve LA, Jones JR, Lu MC. Changes in prevalence of parent-reported autism spectrum disorder in school-aged U.S. children: 2007 to 2011–2012. National Health Statistics Reports. 2013;65:1–12. [PubMed] [Google Scholar]
- Bolte S, Duketis E, Poustka F, Holtmann M. Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism. 2011;15(4):497–511. doi: 10.1177/1362361310391116. [DOI] [PubMed] [Google Scholar]
- Bolton PF, Carcani-Rathwell I, Hutton J, Goode S, Howlin P, Rutter M. Epilepsy in autism: Features and correlates. British Journal of Psychiatry. 2011;198(4):289–294. doi: 10.1192/bjp.bp.109.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in us children, 1997–2008. Pediatrics. 2011;127(6):1034–1042. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- Bradley EA, Isaacs BJ. Inattention, hyperactivity, and impulsivity in teenagers with intellectual disabilities, with and without autism. Canadian Journal of Psychiatry. 2006;51(9):598–606. doi: 10.1177/070674370605100908. [DOI] [PubMed] [Google Scholar]
- Bryson SE, Bradley EA, Thompson A, Wainwrightn A. Prevalence of autism among adolescents with intellectual disabilities. Canadian Journal of Psychiatry. 2008;53(7):449–459. doi: 10.1177/070674370805300710. [DOI] [PubMed] [Google Scholar]
- Camfield CS, Camfield PR. Juvenile myoclonic epilepsy 25 years after seizure onset: A population-based study. Neurology. 2009;73(13):1041–1045. doi: 10.1212/WNL.0b013e3181b9c86f. [DOI] [PubMed] [Google Scholar]
- Camfield CS, Striano P, Camfield PR. Epidemiology of juvenile myoclonic epilepsy. Epilepsy & Behavior, 28, Supplement. 2013;1(0):S15–S17. doi: 10.1016/j.yebeh.2012.06.024. [DOI] [PubMed] [Google Scholar]
- Carter AS, Black DO, Tewani S, Connolly CE, Kadlec MB, Tager-Flusberg H. Sex differences in toddlers with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2007;37(1):86–97. doi: 10.1007/s10803-006-0331-7. [DOI] [PubMed] [Google Scholar]
- Charman T, Pickles A, Simonoff E, Chandler S, Loucas T, Baird G. IQ in children with autism spectrum disorders: Data from the special needs and autism project (SNAP) Psychological Medicine. 2011;41(3):619–627. doi: 10.1017/S0033291710000991. [DOI] [PubMed] [Google Scholar]
- Clifford S, Dissanayake C, Bui QM, Huggins R, Taylor AK, Loesch DZ. Autism Spectrum Phenotype in Males and Females with Fragile X Full Mutation and Premutation. Journal of Autism and Developmental Disorders. 2007;37(4):738–747. doi: 10.1007/s10803-006-0205-z. [DOI] [PubMed] [Google Scholar]
- Dawson G, Estes A, Munson J, Schellenberg G, Bernier R, Abbott R. Quantitative assessment of autism symptom-related traits in probands and parents: Broader phenotype autism symptom scale. Journal of Autism and Developmental Disorders. 2007;37(3):523–536. doi: 10.1007/s10803-006-0182-2. [DOI] [PubMed] [Google Scholar]
- Duerden EG, Oatley HK, Mak-Fan KM, McGrath PA, Taylor MJ, Szatmari P, et al. Risk factors associated with self-injurious behaviors in children and adolescents with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(11):2460–2470. doi: 10.1007/s10803-012-1497-9. [DOI] [PubMed] [Google Scholar]
- Dworzynski K, Ronald A, Bolton P, Happe F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? Journal of the American Academy of Childhood Adolescent Psychiatry. 2012;51(8):788–797. doi: 10.1016/j.jaac.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Ekinci O, Arman AR, Melek I, Bez Y, Berkem M. The phenomenology of autistic regression: Subtypes and associated factors. European Child and Adolescent Psychiatry. 2012;21(1):23–29. doi: 10.1007/s00787-011-0228-7. [DOI] [PubMed] [Google Scholar]
- Evans DW, Canavera K, Kleinpeter FL, Maccubbin E, Taga K. The fears, phobias and anxieties of children with autism spectrum disorders and Down syndrome: comparisons with developmentally and chronologically age matched children. Child Psychiatry and Human Development. 2005;36(1):3–26. doi: 10.1007/s10578-004-3619-x. [DOI] [PubMed] [Google Scholar]
- Fombonne E. Epidemiological surveys of autism and other pervasive developmental disorders: An update. Journal of Autism and Developmental Disorders. 2003;33(4):365. doi: 10.1023/a:1025054610557. [DOI] [PubMed] [Google Scholar]
- Fombonne E, Rogé B, Claverie J, Courty S, Frémolle J. Microcephaly and macrocephaly in autism. Journal of Autism and Developmental Disorders. 1999;29(2):113–119. doi: 10.1023/a:1023036509476. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ. Comparison of children with autism spectrum disorder with and without schizophrenia spectrum traits: gender, season of birth, and mental health risk factors. Journal of Autism and Developmental Disorders. 2012;42(11):2285–2296. doi: 10.1007/s10803-012-1473-4. [DOI] [PubMed] [Google Scholar]
- Gadow KD, Devincent CJ, Pomeroy J, Azizian A. Comparison of DSM-IV symptoms in elementary school-age children with PDD versus clinic and community samples. Autism. 2005;9(4):392–415. doi: 10.1177/1362361305056079. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verté S, Oosterlaan J, Roeyers H, Sergeant JA. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? Journal of Child Psychology and Psychiatry. 2004;45(4):836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Goodall G, Gheusi G. Abnormal patterns of maze patrolling in the mutant mouse staggerer. Behavioral and Neural Biology. 1987;47(3):307–320. doi: 10.1016/S0163-1047(87)90422-5. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. The American Journal of Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Hartley SL, Sikora DM. Sex differences in autism spectrum disorder: an examination of developmental functioning, autistic symptoms, and coexisting behavior problems in toddlers. Journal of Autism and Developmental Disorders. 2009;39(12):1715–1722. doi: 10.1007/s10803-009-0810-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattier MA, Matson JL, Tureck K, Horovitz M. The effects of gender and age on repetitive and/or restricted behaviors and interests in adults with autism spectrum disorders and intellectual disability. Research in Developmental Disabilities. 2011;32(6):2346–2351. doi: 10.1016/j.ridd.2011.07.028. [DOI] [PubMed] [Google Scholar]
- Helmstaedter C, Aldenkamp AP, Baker GA, Mazarati A, Ryvlin P, Sankar R. Disentangling the relationship between epilepsy and its behavioral comorbidities — The need for prospective studies in new-onset epilepsies. Epilepsy & Behavior. 2014;31(0):43–47. doi: 10.1016/j.yebeh.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Holtmann M, Bolte S, Poustka F. Autism spectrum disorders: Sex differences in autistic behaviour domains and coexisting psychopathology. Developmentl. 2007;49(5):361–367. doi: 10.1111/j.1469-8749.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Horovitz M, Matson JL, Sipes M. Gender differences in symptoms of comorbidity in toddlers with ASD using the biscuit-part 2. Developmental Neurorehabilitation. 2011;14(2):94–100. doi: 10.3109/17518423.2010.546825. [DOI] [PubMed] [Google Scholar]
- Ibrahim SH, Voigt RG, Katusic SK, Weaver AL, Barbaresi WJ. Incidence of gastrointestinal symptoms in children with autism: A population-based study. Pediatrics. 2009;124(2):680–686. doi: 10.1542/peds.2008-2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner L. Autistic Disturbances of Affective Contact. Nervous Child. 1943;2:217–250. [PubMed] [Google Scholar]
- Kern JK, Trivedi MH, Grannemann BD, Garver CR, Johnson DG, Andrews AA, et al. Sensory correlations in autism. Autism. 2007;11(2):123–134. doi: 10.1177/1362361307075702. [DOI] [PubMed] [Google Scholar]
- Kirkovski M, Enticott PG, Fitzgerald PB. A review of the role of female gender in autism spectrum disorders. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1811-1. [DOI] [PubMed] [Google Scholar]
- Knickmeyer R, Baron-Cohen S, Raggatt P, Taylor K. Foetal testosterone, social relationships, and restricted interests in children. Journal of Child Psychology and Psychiatry. 2005;46(2):198–210. doi: 10.1111/j.1469-7610.2004.00349.x. [DOI] [PubMed] [Google Scholar]
- Kohane IS, McMurry A, Weber G, MacFadden D, Rappaport L, Kunkel L, et al. The co-morbidity burden of children and young adults with autism spectrum disorders. PloS One. 2012;7(4):e33224. doi: 10.1371/journal.pone.0033224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp S, Gillberg C. The autism spectrum screening questionnaire (ASSQ)-revised extended version (ASSQ-REV): an instrument for better capturing the autism phenotype in girls? a preliminary study involving 191 clinical cases and community controls. Research in Developmental Disabilities. 2011;32(6):2875–2888. doi: 10.1016/j.ridd.2011.05.017. [DOI] [PubMed] [Google Scholar]
- Kotagal S, Broomall E. Sleep in children with autism spectrum disorder. Pediatric Neurology. 2012;47(4):242–251. doi: 10.1016/j.pediatrneurol.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Kozlowski AM, Matson JL, Rieske RD. Gender effects on challenging behaviors in children with autism spectrum disorders. Research in Autism Spectrum Disorders. 2012;6(2):958–964. doi: 10.1016/j.rasd.2011.12.011. [DOI] [Google Scholar]
- Lai MC, Lombardo MV, Pasco G, Ruigrok AN, Wheelwright SJ, Sadek SA, et al. A behavioral comparison of male and female adults with high functioning autism spectrum conditions. PloS One. 2011;6(6):e20835. doi: 10.1371/journal.pone.0020835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Suckling J, Ruigrok ANV, Chakrabarti B, Ecker C, et al. Biological sex affects the neurobiology of autism. Brain. 2013;136(9):2799–2815. doi: 10.1093/brain/awt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde R. Exploration and spatial learning in staggerer mutant mice. Journal of Neurogenetics. 1987;4(1):285–292. doi: 10.3109/01677068709102349. [DOI] [PubMed] [Google Scholar]
- Lalonde R, Strazielle C. Neurobehavioral characteristics of mice with modified intermediate filament genes. Reviews in the Neurosciences. 2003;14:369. doi: 10.1515/revneuro.2003.14.4.369. [DOI] [PubMed] [Google Scholar]
- Lemon JM, Gargaro B, Enticott PG, Rinehart NJ. Executive functioning in autism spectrum disorders: A gender comparison of response inhibition. Journal of Autism and Developmental Disorders. 2011;41(3):352–356. doi: 10.1007/s10803-010-1039-2. [DOI] [PubMed] [Google Scholar]
- Levy SE, Giarelli E, Lee LC, Schieve LA, Kirby R, Cunniff C, et al. Autism spectrum disorder and co-occurring developmental, psychiatric, and medical conditions among children in multiple populations of the United States. Journal of Developmental and Behavioral Pediatrics. 2010;31(4):267–275. doi: 10.1097/DBP.0b013e3181d5d03b. [DOI] [PubMed] [Google Scholar]
- Liu X, Hubbard JA, Fabes RA, Adam JB. Sleep disturbances and correlates of children with autism spectrum disorders. Child Psychiatry and Human Development. 2006;37(2):179–191. doi: 10.1007/s10578-006-0028-3. [DOI] [PubMed] [Google Scholar]
- Louisa M, Silva T, Schalock M. Sense and self-regulation checklist, a measure of comorbid autism symptoms- initial psychometric evidence. The American Journal of Occupational Therapy. 2012;66(2):177–186. doi: 10.5014/ajot.2012.001578. [DOI] [PubMed] [Google Scholar]
- Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: Evidence from a large sample of children and adolescents. Journal of Autism and Developmental Disorders. 2012;42(7):1304–1313. doi: 10.1007/s10803-011-1356-0. [DOI] [PubMed] [Google Scholar]
- Matson JL, Love SR. A comparison of parent-reported fear for autistic and nonhandicapped age-matched children and youth. Australia and New Zealand Journal of Developmental Disabilities. 1990;16(4):349–357. [Google Scholar]
- Mattila ML, Hurtig T, Haapsamo H, Jussila K, Kuusikko-Gauffin S, Kielinen M, et al. Comorbid psychiatric disorders associated with asperger syndrome/high-functioning autism: A community- and clinic-based study. Journal of Autism and Developmental Disorders. 2010;40(9):1080–1093. doi: 10.1007/s10803-010-0958-2. [DOI] [PubMed] [Google Scholar]
- May T, Cornish K, Rinehart NJ. Gender Profiles of Behavioral Attention in Children With Autism Spectrum Disorder. Journal of Attention Disorders. 2012;18(6) doi: 10.1177/1087054712455502. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Impact of IQ, age, SES, gender, and race on autistic symptoms. Research in Autism Spectrum Disorders. 2011;5(2):749–757. doi: 10.1016/j.rasd.2010.09.002. [DOI] [Google Scholar]
- Mayes SD, Calhoun SL, Aggarwal R, Baker C, Mathapati S, Molitoris S, et al. Unusual fears in children with autism. Research in Autism Spectrum Disorders. 2013;7(1):151–158. doi: 10.1016/j.rasd.2012.08.002. [DOI] [Google Scholar]
- McLennan JD, Lord C, Schopler E. Sex differences in higher functioning people with autism. Journal of Autism and Developmental Disorders. 1993;23(2):217–227. doi: 10.1007/BF01046216. [DOI] [PubMed] [Google Scholar]
- Meilleur AA, Fombonne E. Regression of language and non-language skills in pervasive developmental disorders. Journal of Intellectual Disability Research. 2009;53(2):115–124. doi: 10.1111/j.1365-2788.2008.01134.x. [DOI] [PubMed] [Google Scholar]
- Murphy O, Healy O, Leader G. Risk factors for challenging behaviors among 157 children with autism spectrum disorder in Ireland. Research in Autism Spectrum Disorders. 2009;3(2):474–482. doi: 10.1016/j.rasd.2008.09.008. [DOI] [Google Scholar]
- Nicholas JS, Charles JM, Carpenter LA, King LB, Jenner W, Spratt EG. Prevalence and characteristics of children with autism-spectrum disorders. Annals of Epidemiology. 2008;18(2):130–136. doi: 10.1016/j.annepidem.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Palmen SJMC, van Engeland H, Hof PR, Schmitz C. Neuropathological findings in autism. Brain. 2004;127(12):2572–2583. doi: 10.1093/brain/awh287. [DOI] [PubMed] [Google Scholar]
- Park CJ, Yelland GW, Taffe JR, Gray KM. Brief report: The relationship between language skills, adaptive behavior, and emotional and behavior problems in pre-schoolers with autism. Journal of Autism and Developmental Disorders. 2012a;42(12):2761–2766. doi: 10.1007/s10803-012-1534-8. [DOI] [PubMed] [Google Scholar]
- Park S, Cho SC, Cho IH, Kim BN, Kim JW, Shin MS, et al. Sex differences in children with autism spectrum disorders compared with their unaffected siblings and typically developing children. Research in Autism Spectrum Disorders. 2012b;6(2):861–870. doi: 10.1016/j.rasd.2011.11.006. [DOI] [Google Scholar]
- Pilowsky T, Yirmiya N, Shulman C, Dover R. The autism diagnostic interview-revised and the childhood autism rating scale: Differences between diagnostic symtpoms and comparison between genders. Journal of Autism and Developmental Disorders. 1998;28(2):143–151. doi: 10.1023/a:1026092632466. [DOI] [PubMed] [Google Scholar]
- Quek LH, Sofronoff K, Sheffield J, White A, Kelly A. Co-occurring anger in young people with asperger’s syndrome. Journal of Clinical Psychology. 2012;68(10):1142–1148. doi: 10.1002/jclp.21888. [DOI] [PubMed] [Google Scholar]
- Reilly C. Autism spectrum disorders in down syndrome: A review. Research in Autism Spectrum Disorders. 2009;3(4):829–839. doi: 10.1016/j.rasd.2009.01.012. [DOI] [Google Scholar]
- Richards C, Oliver C, Nelson L, Moss J. Self-injurious behaviour in individuals with autism spectrum disorder and intellectual disability. Journal of Intellectual Disability Research. 2012;56(5):476–489. doi: 10.1111/j.1365-2788.2012.01537.x. [DOI] [PubMed] [Google Scholar]
- Rogers SJ, Hepburn S, Wehner E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. Journal of Autism and Developmental Disorders. 2003;33(6):631–642. doi: 10.1023/b:jadd.0000006000.38991.a7. [DOI] [PubMed] [Google Scholar]
- Schendel DE, Autry A, Wines R, Moore C. The co-occurrence of autism and birth defects prevalence and risk in a population-based cohort. Developmental Medicine and Child Neurology. 2009;10(51):779–786. doi: 10.1111/j.1469-8749.2009.03310.x. [DOI] [PubMed] [Google Scholar]
- Selassie AW, Wilson DA, Martz GU, Smith GG, Wagner JL, Wannamaker BB. Epilepsy beyond seizure: A population-based study of comorbidities. Epilepsy Research. 2014;108(2):305–315. doi: 10.1016/j.eplepsyres.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G. Psychiatric disorders in children with autism spectrum disorders: Prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(8):921–929. doi: 10.1097/CHI.0b013e318179964f. [DOI] [PubMed] [Google Scholar]
- Sinzig J, Walter D, Doepfner M. Attention deficit/hyperactivity disorder in children and adolescents with autism spectrum disorder: Symptom or syndrome? Journal of Attention Disorders. 2009;13(2):117–126. doi: 10.1177/1087054708326261. [DOI] [PubMed] [Google Scholar]
- Sipes M, Matson JL, Worley JA, Kozlowski AM. Gender differences in symptoms of autism spectrum disorders in toddlers. Research in Autism Spectrum Disorders. 2011;5(4):1465–1470. doi: 10.1016/j.rasd.2011.02.007. [DOI] [Google Scholar]
- Sivertsen B, Posserud MB, Gillberg C, Lundervold AJ, Hysing M. Sleep problems in children with autism spectrum problems: a longitudinal population-based study. Autism. 2012;16(2):139–150. doi: 10.1177/1362361311404255. [DOI] [PubMed] [Google Scholar]
- Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42(1):48–59. doi: 10.1007/s10803-011-1215-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szatmari P, Liu XQ, Goldberg J, Zwaigenbaum L, Paterson AD, Woodbury-Smith M, et al. Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. American Journal of Medical Genetics Part B, Neuropsychiatric Genetics. 2012;159B(1):5–12. doi: 10.1002/ajmg.b.31238. [DOI] [PubMed] [Google Scholar]
- Tsai LY, Beisler JM. The development of sex differences in infantile autism. The British Journal of Psychiatry. 1983;142(4):373–378. doi: 10.1192/bjp.142.4.373. [DOI] [PubMed] [Google Scholar]
- Tsakanikos E, Underwood L, Kravariti E, Bouras N, McCarthy J. Gender differences in co-morbid psychopathology and clinical management in adults with autism spectrum disorders. Research in Autism Spectrum Disorders. 2011;5(2):803–808. doi: 10.1016/j.rasd.2010.09.009. [DOI] [Google Scholar]
- Valentin A, Hindocha N, Osei-Lah A, Fisniku L, McCormick D, Asherson P, et al. Idiopathic generalized epilepsy with absences: Syndrome classification. Epilepsia (Series 4) 2007;48(11):2187–2190. doi: 10.1111/j.1528-1167.2007.01226.x. [DOI] [PubMed] [Google Scholar]
- Valicenti-McDermott M, McVicar K, Rapin I, Wershil BK, Cohen H, Shinnar S. Frequency of gastrointestinal symptoms in children with autistic spectrum disorders and association with family history of autoimmune disease. Developmental and Behavioral Pediatrics. 2007;27(2):128–136. doi: 10.1097/00004703-200604002-00011. [DOI] [PubMed] [Google Scholar]
- Van Wijngaarden-Cremers PJ, van Eeten E, Groen WB, Van Deurzen PA, Oosterling IJ, Van der Gaag RJ. Gender and Age Differences in the Core Triad of Impairments in Autism Spectrum Disorders: A Systematic Review and Meta-analysis. Journal of Autism and Developmental Disorders. 2013 doi: 10.1007/s10803-013-1913-9. [DOI] [PubMed] [Google Scholar]
- Velisek L, Shang E, Veliskova J, Chachua T, Macchiarulo S, Maglakelidze G, et al. GABAergic neuron deficit as an idiopathic generalized epilepsy mechanism: The role of BRD2 haploinsufficiency in juvenile myoclonic epilepsy. PloS One. 2011;6(8):e23656. doi: 10.1371/journal.pone.0023656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viding E, Blakemore SJ. Endophenotype approach to developmental psychopathology: implications for autism research. Behavior Genetics. 2007;37(1):51–60. doi: 10.1007/s10519-006-9105-4. [DOI] [PubMed] [Google Scholar]
- Volkmar F, Szatmari P, Sparrow S. Sex differences in pervasive developmental disorders. Journal of Autism and Developmental Disorders. 1993;23(4):579–591. doi: 10.1007/BF01046103. [DOI] [PubMed] [Google Scholar]
- Wallace GL, Treffert DA. Head size and autism. Lancet. 2004;363:1003–1004. doi: 10.1016/S0140-6736(04)15877-7. [DOI] [PubMed] [Google Scholar]
- Wang LW, Tancredi DJ, Thomas DW. The prevalence of gastrointestinal problems in children across the United States with autism spectrum disorders from families with multiple affected members. Journal of Developmental and Behavioral Pediatrics. 2006;32(5):351–360. doi: 10.1097/DBP.0b013e31821bd06a. [DOI] [PubMed] [Google Scholar]
- Wiggins LD, Rice CE, Baio J. Developmental regression in children with an autism spectrum disorder identified by a population-based surveillance system. Autism. 2009;13(4):357–374. doi: 10.1177/1362361309105662. [DOI] [PubMed] [Google Scholar]
- Wiggs L, Stores G. Sleep patterns and sleep disorders in children with autistic spectrum disorders-insights using parent report and actigraphy. Developmental Medicine and Child Neurology. 2004;46(6):372–380. doi: 10.1017/s0012162204000611. [DOI] [PubMed] [Google Scholar]
- Wiznitzer M. Autism and Tuberous Sclerosis. Journal of Child Neurology. 2004;19(9):675–679. doi: 10.1177/08830738040190090701. [DOI] [PubMed] [Google Scholar]
- Worley JA, Matson JL. Psychiatric symptoms in children diagnosed with an autism spectrum disorder: An examination of gender differences. Research in Autism Spectrum Disorders. 2011;5(3):1086–1091. doi: 10.1016/j.rasd.2010.12.002. [DOI] [Google Scholar]
- Yeargin-Allsopp M, Rice C, Karapurkar T, Doernberg N, Boyle C, Murphy C. Prevalence of autism in a us metropolitan area. JAMA, the Journal of the American Medical Association. 2003;289(1):49–55. doi: 10.1001/jama.289.1.49. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu Q, Liu J, Li SC, Xu X. Risk factors for autistic regression: Results of an ambispective cohort study. Journal of Child Neurology. 2012;27(8):975–981. doi: 10.1177/0883073811430163. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson SE, Szatmari P, Brian J, Smith IM, Roberts W, et al. Sex differences in children with autism spectrum disorder identified within a high-risk infant cohort. Journal of Autism and Developmental Disorders. 2012;42(12):2585–2596. doi: 10.1007/s10803-012-1515-y. [DOI] [PubMed] [Google Scholar]