Abstract
BACKGROUND
Dual-energy CT (DECT) has potential to improve myocardial perfusion for physiologic assessment of coronary artery disease (CAD). Diagnostic performance of rest-stress DECT perfusion (DECTP) is unknown.
OBJECTIVE
DECIDE-Gold is a prospective multicenter study to evaluate the accuracy of DECT to detect hemodynamic (HD) significant CAD, as compared to fractional flow reserve (FFR) as a reference standard.
METHODS
Eligible participants are subjects with symptoms of CAD referred for invasive coronary angiography (ICA). Participants will undergo DECTP, which will be performed by pharmacological stress, and participants will subsequently proceed to ICA and FFR. HD-significant CAD will be defined as FFR ≥ 0.80. In those undergoing myocardial stress imaging (MPI) by positron emission tomography (PET), single photon emission computed tomography (SPECT) or cardiac magnetic resonance (CMR) imaging, ischemia will be graded by % ischemic myocardium. Blinded core laboratory interpretation will be performed for CCTA, DECTP, MPI, ICA and FFR.
RESULTS
Primary endpoint is accuracy of DECTP to detect ≥ 1 HD-significant stenosis at the subject-level when compared to FFR. Secondary and tertiary endpoints are accuracies of combinations of DECTP at the subject and vessel levels compared to FFR and MPI.
CONCLUSION
DECIDE-Gold will determine the performance of DECTP for diagnosing ischemia.
Keywords: Computed tomography, myocardial perfusion, coronary artery disease
INTRODUCTION
Multicenter randomized trial data examining invasive methods have demonstrated that a combined anatomic-physiologic approach by invasive coronary angiography (ICA) and fractional flow reserve (FFR) improves identification of patients who may benefit from revascularization, by restricting revascularization to those with high-grade stenoses that specifically cause ischemia. In the Fractional Flow Reserve versus Angiography for Multivessel Evaluation (FAME) trial, of 1005 patients with high-grade stenoses randomized to angiographic stenosis-guided versus FFR ischemia-guided revascularization, event-free survival was improved in those undergoing FFR-guided revascularization.1, 2 The findings that only 35% and 80% of lesions with 50–70% and 71–90% diameter stenosis, respectively, caused ischemia as determined by FFR highlights the inability of angiographic methods alone to accurately identify stenoses that cause ischemia.3 Nevertheless, the combination of ICA plus FFR is invasive, is not widely adopted in clinical practice, and is costly.4 To date, an integrated anatomic-physiologic approach by non-invasive methods has been largely lacking, due in part to the lack of a test that is capable of providing both accurate anatomic and physiologic data in a single setting.
Computed tomography perfusion (CTP) is a novel non-invasive technique that can evaluate the physiologic significance of coronary artery disease (CAD), and is performed by adding a single image acquisition to cardiac computed tomography angiography (CCTA) in the same setting.5–7 The combination of CTP to CCTA may represent an ideal “one-stop shop” that may allow for both anatomic and physiologic evaluation of CAD, serve as a more effective gatekeeper to ICA, and better identify patients that would benefit from revascularization. CTP has generally been performed using single energy CT (SECT), a technique that relies upon comparisons of the relative differences of Hounsfield unit densities between myocardial regions;8 and has been correlated to myocardial blood flow (MBF) using microspheres in animal models.9 In a prospective multicenter study of chest pain patients entitled the Combined Coronary Atherosclerosis and Myocardial Perfusion Evaluation Using 320 Detector Row Computed Tomography (CORE320), CCTA with concomitant CTP was as effective as sequential single photon emission computed tomography-myocardial perfusion imaging (SPECT-MPI) and ICA for identifying flow-limiting atherosclerotic lesions. While early studies such as this are generally promising with sensitivity and specificity for high-grade stenosis ranging from 70–92% and 51–99%, respectively,10–23 they are subject to several limitations: 1) small populations precluding statistical power; 2) use of convenience patient samples, 3) ill-defined inclusion/exclusion criteria, 4) mixed CTP protocols, 5) nonstandardized interpretation methods; and 6) qualitative perfusion assessment. While these studies are highly innovative, they nevertheless emphasize the need for further study by a comprehensive, stepwise approach.
Recently, the emergence of dual-energy CT (DECT) techniques now enable potentially improved perfusion assessment. In particular, projection-based DECT is a novel CT method that incorporates energy-dependent models for basis material decomposition (MD) within tissue, and may allow for absolute quantification of specific materials of interest (e.g., myocardial blood [iodine] volume) with high accuracy. Further, it allows for single-energy monochromatic imaging that retains image stability while reducing common CT artifacts (e.g., shading or beam hardening artifacts). Both of these measures by projection-based DECT enable quantitative assessment of myocardial iodine uptake, and may improve the diagnostic performance of CTP.
The overall objective of the DECIDE-Gold trial is to determine the diagnostic accuracy of dual-energy CT (anatomic-based CCTA plus physiologic-based perfusion [DECTP]) for non-invasive assessment of the hemodynamic (HD) significance of CAD, as compared to direct measurement of FFR during ICA as a reference standard.
OVERALL STUDY DESIGN
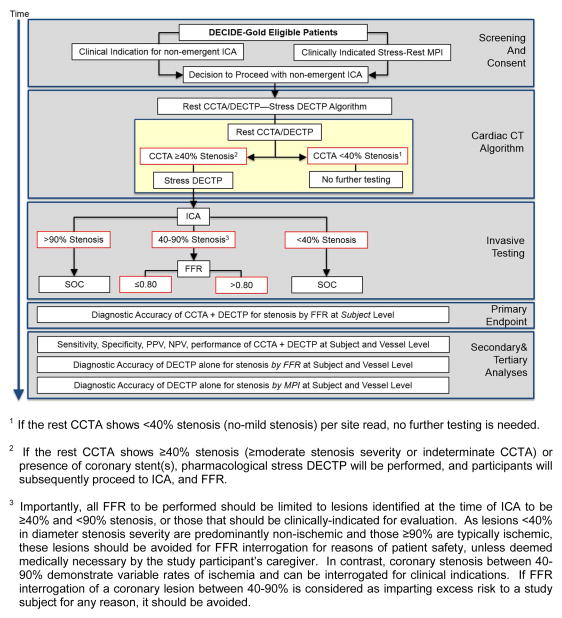
DECIDE-Gold (Dual Energy Computed Tomography for Ischemia Determination Compared to “Gold Standard” Non-Invasive and Invasive Techniques) is a multicenter prospective observational efficacy diagnostic trial wherein eligible participants will undergo CCTA with DECTP using a rest-stress protocol for myocardial perfusion assessment, as compared to an FFR reference standard (clinicaltrials.gov NCT02178904 and www.decidegold.org). The schematic summary of the trial design is depicted in Figure 1.
Figure 1.
DECIDE-Gold Study Design
ICA = Invasive coronary angiography; DECTP = Dual energy CT perfusion; FFR = fractional flow reserve; PPV = positive predictive value; NPV = Negative predictive value; MPI = Myocardial perfusion imaging
Briefly, participants will undergo DECT with a rest then selective stress protocol. A dual-energy rest CCTA/DECTP will be performed first with on-site live interpretation of the CCTA for coronary stenosis, followed by selective stress pharmacological stress DECTP. Rest CCTA result will be part of clinical care; while the rest and stress DECTP portion will be research, blinded, and will not influence patient care. If the rest CCTA shows <40% stenosis24 (no to mild stenosis) as deemed by site investigators, no further testing is needed. This is for reasons of patient safety and the need to curb unnecessary radiation. If the rest CCTA shows ≥40% stenosis (moderate or greater stenosis severity or indeterminate) or presence of intracoronary stent(s), pharmacological stress DECTP will be performed, and participants will subsequently proceed to ICA, and/or FFR. FFR will be performed for lesions with 40–90% stenosis at the time of ICA, or those that should be clinically-indicated for evaluation. The targeted population is adult individuals with symptoms suspicious of obstructive CAD who are referred for non-emergent ICA. The DECIDE-Gold study will be performed in up to 15 investigative sites in the United States, Canada, The Netherlands, Italy, Argentina, and China, with approximately 156 subjects entered into the study.
STUDY OBJECTIVES
Primary Objective
The primary objective of this study is determine the diagnostic accuracy of dual-energy CCTA plus DECTP for non-invasive assessment of the hemodynamic significance of CAD, as compared to direct measurement of FFR during ICA as a reference standard. Hemodynamically-significant CAD will be at the subject level using binary outcomes when compared to FFR as the reference standard. Hemodynamically-significant stenosis will be defined as a FFR ≤0.80 during adenosine-mediated hyperemia.
Secondary Objectives
The secondary objectives of the DECIDE-Gold study will include: Sensitivity, specificity, positive predictive value, and negative predictive value of CCTA plus DECTP for HD-significant stenosis at the subject-level when compared to FFR. Another key subjective objective will be the diagnostic performance (accuracy, sensitivity, specificity, PPV, and NPV) of CCTA plus DECTP for HD-significant stenosis at the vessel-level when compared to FFR.
Tertiary Objectives
The tertiary objectives of the DECIDE-Gold are manifold, and include the following: 1) Diagnostic accuracy of DECTP alone for HD-significant stenosis at the subject-level and vessel-level when compared to FFR; 2) Diagnostic accuracy of CCTA plus DECTP for HD-significant stenosis at the subject-level and vessel-level when compared to MPI; 3) Diagnostic accuracy of DECTP alone for HD-significant stenosis at the subject-level and vessel-level when compared to MPI.
TARGETED POPULATION
The DECIDE-Gold target population will comprise a large and representative sample of individuals with suspected CAD. In this regard, DECIDE-Gold will enroll individuals meeting the following inclusion criteria and none of the exclusion criteria, as detailed in Table 1. Notable inclusion is that patients with coronary stents will be allowed to participate in the trial, since coronary stents assessment for in-stent restenosis with CCTA remains a challenge with respect to interpretability and diagnostic accuracy.25, 26
Table 1.
Inclusion and Exclusion Criteria
Inclusion criteria
|
Exclusion criteria
|
EFFICACY ANALYSES
Primary Efficacy Analysis
The primary statistical measures will be diagnostic accuracy of rest CCTA plus rest-stress DECTP based on the primary endpoint of subject level diagnostic accuracy. Subject level diagnostic accuracy will be a function of the identification of the presence or absence of a HD-significant obstruction on the subject level by CCTA plus DECTP, as compared to measured FFR as a reference standard. Hemodynamically-significant obstruction of the coronary artery is defined as an FFR≤0.80 in any major epicardial coronary artery segments during adenosine-mediated hyperemia.
Definition of subject level diagnostic accuracy (per-patient analysis)
For CCTA plus DECTP as well as FFR, each coronary vessel segment will have one of three possible outcomes: HD-significant coronary obstruction (referred to as ‘Diseased’), non-HD-significant coronary obstruction or no obstruction (referred to as ‘Not Diseased’), and non-diagnostic or not visible (‘No Diagnosis’). CCTA plus DECTP will result in an outcome of “diseased” or “not diseased” for HD-significant obstruction using the measurement cut off of ≤0.80 (diseased/positive) and >0.80 (not diseased/negative). For study participants who do not undergo further testing due to site reads of CCTA <40% stenosis, an independent CCTA core lab will perform the CCTA interpretation and the core lab read will be deemed as the gold standard, with <40% stenosis as a negative result. On the subject level, the outcome is positive if any vessel yields a positive result and negative when all vessels yield a negative result.
Subject level diagnostic accuracy is defined as the proportion of correctly classified subjects (true positive + true negative) amongst all subjects within the efficacy population (true positive + true negative + false positive + false negative + no diagnosis). Hence, accurate results include subjects with at least one diseased segment by FFR who also have at least one of these segments diseased by CCTA plus DECTP and subjects with no diseased segments by FFR who also have no diseased segments by CCTA plus DECTP, expressed as a proportion of the entire study cohort of the entire efficacy population.
Statistical hypothesis and tests of primary statistical measures
Null and alternative hypotheses to be tested for are:
Ho: Diagnostic Accuracy ≤0.80
H1: Diagnostic Accuracy >0.80
The above hypotheses will be tested at the one-sided 0.05 Type I error rate.
Definition of successful demonstration of primary objective
The primary objective will be successfully demonstrated if the lower bound of the exact one-sided 95% confidence interval for diagnostic accuracy is greater than 80%.
SECONDARY AND TERTIARY ANALYSES
For CCTA plus DECTP and FFR, each coronary vessel segment will have one of three possible outcomes: HD-significant coronary obstruction (referred to as ‘Diseased’), non-HD-significant coronary obstruction or no obstruction (referred to as ‘Not Diseased’), and non-diagnostic or not visible (‘No Diagnosis’). Subject level sensitivity will be defined as the proportion of subjects with at least one diseased segment by FFR who also have at least one of these segments diseased by CCTA plus DECTP. Subject level specificity will be defined as the proportion of subjects with no diseased segments by FFR who also have no diseased segments by CCTA plus DECTP. Subject PPV is defined as the proportion of subjects with at least one diseased segment by CCTA plus DECTP who also have at least one of those segments diseased by FFR. Subject NPV is defined as the proportion of subjects with no diseased segments by CCTA plus DECTP who also have no diseased segments by FFR. Tertiary exploratory analyses will be performed in similar fashion. Positive predictive value (PPV), negative predictive value (NPV), sensitivity and specificity will be estimated with exact two-sided 95% confidence intervals.
EFFICACY POPULATION
All efficacy analyses will be performed on the evaluable population. The evaluable population will be comprised of individuals with independent blinded core lab-verified interpretable CT, MPI, and ICA/FFR images and/or tracings. Non-evaluable images will include those lost due to corrupted media or inability of site to transport to the image core lab or subjects where no images are acquired. Where images are evaluable for only specific vessels within a subject, the subject and evaluable vessels will be included in the efficacy population. In an intention-to-diagnosis fashion, subjects with uninterpretable DECTP images will not be excluded from efficacy analysis except for the estimation of positive and negative predictive values. Vessel and vessel segment diagnoses for these subjects will be set to “No Diagnosis”.
SAMPLE SIZE DETERMINATION AND STATISTICAL POWER
An a priori power analysis was performed to test the hypothesis that the combination of rest-stress DECTP will demonstrate a minimum of 80% diagnostic accuracy—which represents an absolute improvement of CTP by DECT of more than 30% over current MPI and current CTP methods—as compared to invasive FFR for the diagnosis of vessel-specific ischemia. Should the combination of CTP by DECT plus CCTA rate of diagnostic accuracy be at least 80%, then 156 evaluable subjects (468 vessels) would provide 85% power to test the study hypothesis at the one-sided 5% significance level. To ensure validity, we will also run a post hoc power analysis and report the power actually achieved by the analysis.
For purposes of comparison to FFR, only the combination of a CCTA stenosis >50% and reduced myocardial perfusion by DECTP in the territory subtended by the vessel accommodating the stenosis will be considered diagnostic of lesion-specific ischemia (Table 2). If stenosis or reduced perfusion exists in isolation, coronary lesion-specific ischemia by CT will be considered not present.
TABLE 2.
Defining Lesion-Specific Ischemia
| CCTA Stenosis | CTP by DECT* | FFR | Outcome |
|---|---|---|---|
| ≥50% | Low | Positive | True Positive |
| ≥50% | High | Positive | False negative |
| <50% | Low | Positive | False positive |
| <50% | High | Positive | False negative |
| ≥50% | Low | Negative | False positive |
| ≥50% | High | Negative | True negative |
| <50% | Low | Negative | True negative |
| <50% | High | Negative | True negative |
CCTA = coronary CT angiography; CTP = CT perfusion; DECT = dual energy CT; FFR = fractional flow reserve
CTP by DECT will be tested for (1) quantitative measures of iodine density by material decomposition, and explored for (2) relative measures of perfusion by monochromatic energy imaging
POTENTIAL BENEFITS TO DECIDE-Gold STUDY PARTICIPANTS
This prospective multicenter diagnostic accuracy study trial will assess the diagnostic performance of DECTP versus FFR and in a subset DECTP versus MPI. MPI will be performed as part clinical care in this study prior to patient enrollment. MPI can be performed by a variety of methods, including SPECT, PET, or CMR. These test are routinely and commonly employed for evaluation of patients with suspected CAD in the clinical setting. However, these tests are associated with a nonnegligible rate of false positives. For this study, we will include patients with normal MPI but still warranting ICA by their caregivers, since balanced ischemia may exist and this is an important factor that will be evaluated in the DECIDE-Gold study.
Indeed, prior studies have revealed that approximately 2/3 of patients referred for cardiac catheterizations have very mild or no angiographic CAD and thus, do not need to undergo invasive coronary angiography.27 Even amongst those who undergo ICA and are found to have obstructive CAD, approximately 50% are found not to be ischemia-causing.27 Thus, improved accuracy of non-invasive diagnostic procedures is critical.
The DECIDE-Gold study design includes a rest-selective stress dual energy CT algorithm that encourages ethical and safety benefits to study participants. Since CCTA is widely accepted for its negative predictive value (NPV) for excluding obstructive CAD,28 the local site can provide image interpretation of the rest portion of the DECTP (that is, rest CCTA) and non-cardiac findings, which will be part of clinical care. Since it remains unclear the diagnostic performance of DECTP, the rest and stress DECTP portions will be research, blinded, and will not influence patient care.
At the local site level, if the rest CCTA shows <40% stenosis (no-mild stenosis), no further testing is needed (including the stress DECTP or ICA). From both an ethical and safety standpoint, this algorithm will save study participants from unnecessary additional testing while offering a low radiation dose test to either confirm or refute the stress MPI findings. If the rest CCTA shows ≥40% stenosis (moderate or greater stenosis severity or indeterminate) or presence of coronary stent(s), pharmacological stress DECTP will be performed, and participants will subsequently proceed to ICA, and FFR for lesions with 40–90% stenosis at the time of ICA, i.e., those that should be clinically-indicated for evaluation.
The incremental potential risks to patients enrolled in the DECIDE-Gold study are solely and directly related to the performance of the study procedures after the patient is enrolled.
QUALITY ASSURANCE
CT Image Acquisition and Interpretation
All CTs will be performed by a scanner capable of projection-based DECT with rapid kVp switch between 140 and 80 kVp. CT interpretation has also been detailed, and will conform to Society of Cardiovascular Computed Tomography (SCCT) guidelines.24 CT image acquisition will be performed by protocols that enable the lowest radiation dose while optimizing diagnostic accuracy. CT images will be acquired by prospective ECG gating. The estimated radiation dose for rest-stress DECTP is between 5–10 mSv.
CT Core Laboratory readers will perform interpretation of coronary artery stenosis severity using an SCCT 18-segment coronary model, and myocardial perfusion deficits using a AHA 17-segment myocardial model from patient- and scan-specific parameters by adjusting thresholds based upon noise and signal.24 For coronary stenosis severity, lesions will be judged as normal (0%), very mild (1–24% diameter stenosis), mild (25–49%), moderate (50–69%) and severe (≥70%), in accordance with SCCT guidelines, with atherosclerosis will be defined as any tissue >1mm2 within or adjacent to the lumen that can be discriminated from surrounding pericardial tissue, epicardial fat, or lumen; and identified in ≥2 planes. All CTs will be interpreted by a minimum of 2 readers, with 20% randomly repeated for inter- and intraobserver variability.
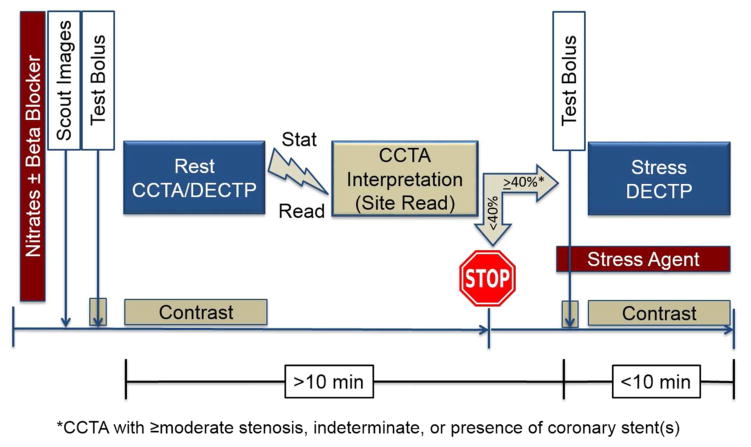
Stress portions of the DECTP examinations will be performed during maximal hyperemia as evoked by adenosine or regadenoson. Adenosine dosing will be through a continuous intravenous infusion at a rate of 140 mcg/kg/min, while regadenoson will be administered as a single slow bolus of 400 mcg. Perfusion deficits will be judged as none, mild, moderate or severe by myocardial segment, and will be summated by a summed rest score, summed stress score and summed difference score to examine extent, severity and reversibility of ischemia. Summed stress scores will be utilized to grade % ischemic myocardium, as has been previously described. The DECT image acquisition protocol is described in Figure 2.
Figure 2.
Rest with Selective Stress CT Image Acquisition Protocol.
CCTA = Coronary CT angiography; DECTP = Dual energy CT perfusion
ICA Performance
Patients will undergo diagnostic ICA by board-certified interventional cardiologists or country-equivalent in accord with usual clinical indications and by imaging standards set forth by the American College of Cardiology/ Society for Cardiac Angiography and Interventions. 100–200 micrograms intracoronary nitroglycerin will be sequentially administered in the left and right coronary arteries before initial cineangiograms, unless contraindicated. Coronary arterial images must be obtained with selective catheterization of left and right coronary arteries. ICA images will be transmitted to independent blinded readers at the Angiographic Core Laboratory.
Similar to CT images, an 18-segmental model of the coronary tree will be used for the coronary evaluation. Invasive angiograms will be analyzed by dedicated and validated quantitative coronary angiography software, employing the use of automated edge-detection algorithms. Employing the outer diameter of the coronary injection catheter as a standard for calibration, a minimum lumen diameter and percentage stenosis will be measured from the view that demonstrates the greatest reduction in luminal diameter with the least amount of foreshortening of the segment at a motion-free state during the cardiac cycle (typically during diastole). The stenosis will represent a relative reduction in comparison to the most normal appearing region proximal and distal to the stenosis. All vessels ≥1.5 mm in diameter will be measured. If a total occlusion is observed, all segments distal to that occlusion will not be assessed.
FFR Performance
The FFR procedure will be performed as clinically indicated by experienced interventional cardiologists. FFR will be obtained in up to 3 major coronary artery territories (left anterior descending artery, left circumflex artery, and right coronary artery) using a sensor-tipped 0.014-inch guidewire [PressureWire, St. Jude Medical, St. Paul, MN or PrimeWire, Volcano, San Diego, CA]. If left coronary dominance is noted, a maximum of 2 major coronary arteries will be considered (left anterior descending artery and left circumflex artery).
After positioning the pressure sensor in the distal third of the major epicardial vessel beyond the stenosis, maximal myocardial hyperemia will be induced by continuous intravenous infusion of adenosine in a central vein at an infusion rate of 140Wg/kg/minute for a minimum of 2 minutes or by regadenson bolus at 400 mcg. During maximum hyperemia, FFR will be calculated as the ratio of distal mean pressure measured (Pd) by the pressure wire divided by the mean proximal pressure measured by the guiding catheter (Pa). For subtotal or chronically occluded arteries, a default FFR value of 0.50 will be assigned to the vessel.
PATIENT FOLLOW-UP
After the completion of CCTA, DECTP, ICA and FFR, subject participation is complete for the DECIDE-Gold study. No post-CCTA, -DECTP, -ICA or –FFR follow-up is planned.
ORGANIZATION AND QUALITY ASSURANCE OF TESTING
This study will be coordinated by the Dalio Institute of Cardiovascular Imaging Clinical and Data Coordinating Center (DICI CDCC). The study protocol will be approved at each participating center by the local institutional review board (IRB). All patients will provide written informed consent prior to trial participation. Completed electronic case report forms (eCRFs) are to be entered by sites, and will be checked locally for possible errors or omissions. eCRFs will be transmitted to a central data repository at the DCC. Prior to completion of the trial, the primary sponsor will not be granted access to the data, nor will their advice be requested prior to publication of the primary study results.
Site qualification will be issued by the DICI CDCC for each modality before subject enrollment. Before beginning enrollment, eligible sites will be qualified by the imaging Core Laboratories based on site surveys and on successful transfer of 1 or more complete data sets with sufficient image quality and completeness for each modality. During the study, technical quality assessment of image and test acquisition will be accomplished on all studies by central repository research technicians trained by the CDCC. This ongoing review will ensure the adequate quality and completeness of data sets and will monitor radiation exposure throughout the trial.
CLINICAL EVENT ASCERTAINMENT AND PATIENT SAFETY
For patient safety, a data safety monitoring board (DSMB) has been organized as independent group advisory to the sponsor, NHLBI, and is required to provide recommendations about starting, continuing, and stopping the study. The DSMB is responsible for safeguarding the interests of study participants, assessing the safety and efficacy of study procedures, and for monitoring the overall conduct of the study. The DSMB is composed of three physicians who are not be directly involved in the conduct of the trial. DSMB members will have no affiliation with the Sponsor, nor will they participate as investigators in the study.
The DSMB has been asked to make recommendations, as appropriate, to the NHLBI about: 1) Patient safety; 2) Efficacy of the study intervention for DSMB purposes only; 3) Benefit/risk ratio of procedures and participant burden; 4) Selection, recruitment, and retention of participants; 5) Adherence to protocol requirements; 6) Completeness, quality, and analysis of measurements; 7) Amendments to the study protocol and consent forms; 8) Performance of individual centers and core labs; and 9) Notification of and referral for abnormal findings.
Further, a clinical events committee (CEC) has been organized and is comprised of three independent cardiologists with expertise in general cardiology, noninvasive cardiology, and interventional cardiology. They will review all serious adverse events and clinical endpoints reported by the sites, including initial cardiovascular diagnosis, laboratory values, and electrocardiograms (ECGs), etc. The CEC’s assessment of each potential endpoint will be documented in the clinical database and will be used in the safety endpoint analysis. Each event will be adjudicated by a panel consisting of two cardiologists based on review of the medical record, with disagreement resolved by consensus with a third cardiologist. The process will be coordinated by the DICI CDCC.
SUMMARY
The DECIDE-Gold study objectives and methods aim to evaluate the diagnostic performance of DECT using a rest CCTA-selective stress DECTP protocol for the detection and exclusion of hemodynamically significant CAD, as defined by FFR, the reference standard. The inclusion and exclusion criteria for DECIDE-Gold have been designed to be inclusive of a general cohort of individuals with suspected CAD undergoing non-emergent invasive coronary angiography. The multicenter prospective nature of this study mitigates biases related to selection and referral, and maximizes the generalizability of study results. The goal of this study will allow determination of whether DECTP findings can improve diagnostic performance incremental to CCTA alone for the detection and exclusion of hemodynamically-significant coronary artery lesions, when using measured FFR as a reference standard.
NEW KNOWLEDGE GAINED
The reader will gain knowledge on myocardial perfusion by computed tomography, and the potential added benefits from dual energy computed tomography.
ABBREVIATIONS
- ICA
invasive coronary angiography
- FFR
fractional flow reserve
- CTP
Computed tomography perfusion
- CAD
coronary artery disease
- CCTA
cardiac computed tomography angiography
- SECT
single energy CT
- MBF
myocardial blood flow
- SPECT-MPI
single photon emission computed tomography-myocardial perfusion imaging
- DECT
dual-energy CT
- MD
material decomposition
- HD
hemodynamic
Footnotes
Disclosures
This study was sponsored by the National Heart, Lung, and Blood Institute of the National Institutes of Health (Bethesda, MD) under Award R01 HL111141 and R01 HL118019 and by a generous gift from the Dalio Foundation and the Michael Wolk Foundation (New York, NY).
References
- 1.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 2.Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–184. doi: 10.1016/j.jacc.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 3.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 4.De Bruyne B, Sarma J. Fractional flow reserve: a review: invasive imaging. Heart. 2008;94:949–959. doi: 10.1136/hrt.2007.122838. [DOI] [PubMed] [Google Scholar]
- 5.Ambrose MS, Valdiviezo C, Mehra V, Lardo AC, Lima JA, George RT. CT perfusion: ready for prime time. Curr Cardiol Rep. 2011;13:57–66. doi: 10.1007/s11886-010-0152-3. [DOI] [PubMed] [Google Scholar]
- 6.Ko BS, Cameron JD, Defrance T, Seneviratne SK. CT stress myocardial perfusion imaging using multidetector CT--A review. J Cardiovasc Comput Tomogr. 2011;5:345–356. doi: 10.1016/j.jcct.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 7.So A, Lee TY. Quantitative myocardial CT perfusion: a pictorial review and the current state of technology development. J Cardiovasc Comput Tomogr. 2011;5:467–481. doi: 10.1016/j.jcct.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Mehra VC, Valdiviezo C, Arbab-Zadeh A, Ko BS, Seneviratne SK, Cerci R, et al. A stepwise approach to the visual interpretation of CT-based myocardial perfusion. J Cardiovasc Comput Tomogr. 2011;5:357–369. doi: 10.1016/j.jcct.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 9.George RT, Silva C, Cordeiro MA, DiPaula A, Thompson DR, McCarthy WF, et al. Multidetector computed tomography myocardial perfusion imaging during adenosine stress. J Am Coll Cardiol. 2006;48:153–160. doi: 10.1016/j.jacc.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 10.Bastarrika G, Ramos-Duran L, Schoepf UJ, Rosenblum MA, Abro JA, Brothers RL, et al. Adenosine-stress dynamic myocardial volume perfusion imaging with second generation dual-source computed tomography: Concepts and first experiences. J Cardiovasc Comput Tomogr. 2010;4:127–135. doi: 10.1016/j.jcct.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Bettencourt N, Rocha J, Ferreira N, Pires-Morais G, Carvalho M, Leite D, et al. Incremental value of an integrated adenosine stress-rest MDCT perfusion protocol for detection of obstructive coronary artery disease. J Cardiovasc Comput Tomogr. 2011;5:392–405. doi: 10.1016/j.jcct.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Blankstein R, Shturman LD, Rogers IS, Rocha-Filho JA, Okada DR, Sarwar A, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. J Am Coll Cardiol. 2009;54:1072–1084. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 13.Cury RC, Magalhaes TA, Borges AC, Shiozaki AA, Lemos PA, Junior JS, et al. Dipyridamole stress and rest myocardial perfusion by 64-detector row computed tomography in patients with suspected coronary artery disease. Am J Cardiol. 2010;106:310–315. doi: 10.1016/j.amjcard.2010.03.025. [DOI] [PubMed] [Google Scholar]
- 14.Cury RC, Magalhaes TA, Paladino AT, Shiozaki AA, Perini M, Senra T, et al. Dipyridamole stress and rest transmural myocardial perfusion ratio evaluation by 64 detector-row computed tomography. J Cardiovasc Comput Tomogr. 2011;5:443–448. doi: 10.1016/j.jcct.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Feuchtner G, Goetti R, Plass A, Wieser M, Scheffel H, Wyss C, et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ Cardiovasc Imaging. 2011;4:540–549. doi: 10.1161/CIRCIMAGING.110.961250. [DOI] [PubMed] [Google Scholar]
- 16.George RT, Arbab-Zadeh A, Miller JM, Kitagawa K, Chang HJ, Bluemke DA, et al. Adenosine stress 64- and 256-row detector computed tomography angiography and perfusion imaging: a pilot study evaluating the transmural extent of perfusion abnormalities to predict atherosclerosis causing myocardial ischemia. Circ Cardiovasc Imaging. 2009;2:174–182. doi: 10.1161/CIRCIMAGING.108.813766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho KT, Chua KC, Klotz E, Panknin C. Stress and rest dynamic myocardial perfusion imaging by evaluation of complete time-attenuation curves with dual-source CT. JACC Cardiovasc Imaging. 2010;3:811–820. doi: 10.1016/j.jcmg.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Ko SM, Choi JW, Song MG, Shin JK, Chee HK, Chung HW, et al. Myocardial perfusion imaging using adenosine-induced stress dual-energy computed tomography of the heart: comparison with cardiac magnetic resonance imaging and conventional coronary angiography. Eur Radiol. 2011;21:26–35. doi: 10.1007/s00330-010-1897-1. [DOI] [PubMed] [Google Scholar]
- 19.Magalhaes TA, Cury RC, Pereira AC, de Moreira VM, Lemos PA, Kalil-Filho R, et al. Additional value of dipyridamole stress myocardial perfusion by 64-row computed tomography in patients with coronary stents. J Cardiovasc Comput Tomogr. 2011;5:449–458. doi: 10.1016/j.jcct.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 20.Okada DR, Ghoshhajra BB, Blankstein R, Rocha-Filho JA, Shturman LD, Rogers IS, et al. Direct comparison of rest and adenosine stress myocardial perfusion CT with rest and stress SPECT. J Nucl Cardiol. 2010;17:27–37. doi: 10.1007/s12350-009-9156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocha-Filho JA, Blankstein R, Shturman LD, Bezerra HG, Okada DR, Rogers IS, et al. Incremental value of adenosine-induced stress myocardial perfusion imaging with dual-source CT at cardiac CT angiography. Radiology. 2010;254:410–419. doi: 10.1148/radiol.09091014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamarappoo BK, Dey D, Nakazato R, Shmilovich H, Smith T, Cheng VY, et al. Comparison of the extent and severity of myocardial perfusion defects measured by CT coronary angiography and SPECT myocardial perfusion imaging. JACC Cardiovasc Imaging. 2010;3:1010–1019. doi: 10.1016/j.jcmg.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 23.Weininger M, Schoepf UJ, Ramachandra A, Fink C, Rowe GW, Costello P, et al. Adenosine-stress dynamic real-time myocardial perfusion CT and adenosine-stress first-pass dual-energy myocardial perfusion CT for the assessment of acute chest pain: initial results. Eur J Radiol. 2012;81:3703–3710. doi: 10.1016/j.ejrad.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, et al. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O’Gara P, et al. ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 Appropriate Use Criteria for Cardiac Computed Tomography. A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Cardiovasc Comput Tomogr. 2010;4:407, e401–433. doi: 10.1016/j.jcct.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Haraldsdottir S, Gudnason T, Sigurdsson AF, Gudjonsdottir J, Lehman SJ, Eyjolfsson K, et al. Diagnostic accuracy of 64-slice multidetector CT for detection of instent restenosis in an unselected, consecutive patient population. Eur J Radiol. 2010;76:188–194. doi: 10.1016/j.ejrad.2009.05.030. [DOI] [PubMed] [Google Scholar]
- 27.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. doi: 10.1056/NEJMoa0907272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]