Abstract
Fluid homeostasis in vertebrates critically relies on the lymphatic system forming a hierarchical network of lymphatic capillaries and collecting lymphatics, for the efficient drainage and transport of extravasated fluid back to the cardiovascular system. Blind–ended lymphatic capillaries employ specialized junctions and anchoring filaments to encourage a unidirectional flow of the interstitial fluid into the initial lymphatic vessels, whereas collecting lymphatics are responsible for the active propulsion of the lymph to the venous circulation via the combined action of lymphatic muscle cells and intraluminal valves. Here we describe recent findings on molecular and physical factors regulating the development and maturation of these two types of valves and examine their role in tissue-fluid homeostasis.
Keywords: valve, lymphatic vessel, endothelium, interstitial fluid, biomechanics
INTRODUCTION
Water, solutes and proteins escape from the arteriole end of the capillary network through filtration and are not entirely reabsorbed by the capillaries once in the interstitial space. Return of the extravasated fluid and macromolecules is crucial for maintaining plasma volume and content, as well as preventing increases in tissue fluid volume and pressure (Planas-Paz and Lammert, 2014). In vertebrates, the lymphatic system has the vital role of maintaining fluid homeostasis in the body by draining the extravasated fluid from most tissues (except for brain, retina and cartilage) and transporting it back to the blood circulation. In addition, lymphatics actively participate in the absorption of dietary fats and in immune surveillance by transporting antigens and white blood cells to lymph nodes (Card et al., 2014; Randolph and Miller, 2014). The failure of lymphatics to accommodate the reabsorption of fluid can lead to its accumulation in the tissue stroma, leading to edema (Bellini and Hennekam, 2014). If left untreated, this debilitating disease can lead to chronic inflammation and tissue fibrosis. Hereditary conditions causing dysfunction of the lymphatic system can result in primary lymphedema, while tissue trauma following surgical removal of lymph nodes for cancer treatment, radiotherapy or parasitic infection can result in secondary lymphedema (Mortimer and Rockson, 2014).
The lymphatic system starts forming during embryonic development. Initially, lymphatic sacs grow in different parts of the body and give rise to a primary plexus. The latter progressively remodels into two basic types of vessels: the lymphatic capillaries (or initial lymphatics) and the collecting lymphatics, which serve separate functions. Two distinct mechanisms are employed by these specialized vessels to ensure a net unidirectional drainage of the interstitial fluid (IF) and its transport back to the cardiovascular system. Peripheral blind-ended initial lymphatics contain specialized junctions that regulate the drainage of the IF and the formation of the lymph. Once lymph is formed, it is transported through a hierarchical network of collecting lymphatics, lymph nodes and larger ducts that connect to the veins of the neck. The flow of lymph can be influenced by extrinsic forces in the body, such as skeletal muscle contraction, respiration, arterial pulsations, movement of other surrounding tissues, skin compression and the rate of lymph formation in initial lymphatics (Schmid-Schönbein, 2003; Swartz and Skobe, 2001). Active propulsion of the lymph is achieved by the contraction of lymphatic muscle cells (LMCs) in the collecting lymphatics, while intraluminal valves spaced frequently along the collecting vessels limit backflow. In this review, we examine the role of pressure differences between the interstitial space and initial lymphatics, placing emphasis on how these affect physical and molecular mechanisms promoting unidirectional entry of fluid and cells into the lumen of vessels leading to the formation of lymph. We also discuss molecular mechanisms important for valve formation along with factors that regulate active pumping and efficient transport of lymph back to the cardiovascular system through a series of intraluminal valves facilitated by the contraction of LMCs.
COMPONENTS OF THE INTERSTITIAL SPACE AND LYMPH FORMATION
The interstitium is comprised of the space between vascular compartments and the cells of the surrounding tissues, and varies in hydration and stiffness across different parts of the body (Aukland and Nicolaysen, 1981). The space is taken up by different extracellular matrix (ECM) components consisting mainly of collagen, hyaluronan and different types of proteoglycans (PGs) (Aukland and Reed, 1993) as well as immune cells emanating from blood capillaries. The composition and density of the ECM plays a crucial role in the regulation of cell behaviors such as migration, differentiation, polarity, survival, and proliferation (Kruegel and Miosge, 2010; Wiig et al., 2010). In addition, it helps control local needs for fluid homeostasis in the different tissues (Wiig and Swartz, 2012) and regulates vascular stability (Chan et al., 2013). The main ECM component in the interstitial space, collagen, has a dominant role in providing the structural framework (Hynes, 2009), and although it appears to have little effect on the interstitial net charge, small local charges of collagen as well as its relative concentration have been shown to influence the composition of fluid in the interstitial space resulting in changes in the local fluid balance (Bank et al., 2000). A repertoire of negatively charged glycosaminoglycans (GAG), such as hyaluronan, affect the IF pressure and volume as well as the plasma protein and oxygen distribution by controlling the osmotic pressure and thus the hydration of the tissue stroma (Wiig and Swartz, 2012). IF is kept at fairly constant volume under normal conditions by orchestrating structural changes of the ECM and by adjusting the forces acting across the wall of blood and lymphatic vessels (Wiig and Swartz, 2012). The amount of fluid entering the interstitium from the arterial capillaries is governed by a number of factors including material composition of the capillary wall, protein concentration and hydrostatic pressures. The rate of fluid flow can be described by Starling’s Law of Filtration (Kedem and Katchalsky, 1958):
| Eqn. 1 |
where Jv is the fluid flux from the capillary into the interstitium, Lp is the hydraulic conductivity of the capillary wall, S is the surface area available for filtration, ΔP is the hydrostatic pressure difference between the capillaries and interstitium, σs is the oncotic reflection coefficient (ranging from 0 to 1 with higher values indicating decreased wall permeability to proteins), and Δπ is the oncotic pressure difference between the capillaries and interstitium. High hydrostatic pressure in arterial cappillaries leads to plasma filtration into the interstitium, whereas lower hydrostatic pressure in venous capillaries causes them to absorb some but not all extravasated fluid. Increases in oncotic pressure decrease the overall fluid flux (Taylor, 1990). As the IF needs to be kept under a dynamic balance, fluid homeostasis is achieved through the drainage capacity of the initial lymphatics, which take up the remaining fluid left in the tissue stroma.
PRIMARY VALVES IN INTIAL LYMPHATICS
Initial lymphatic vessels have a characteristic topology and morphology to facilitate the homeostatic functions they serve and adjust their permeability depending on IF pressure changes (Wiig and Swartz, 2012). Variability in the shape of the lymphatic networks occurs within the same tissue but is more profound in different parts of the body and is accompanied by molecular heterogeneity (Garrafa et al., 2005; Moute-Bellum et al., 2009; Normen et al., 2009; Yao et al., 2012). Network shape has been shown to be important for interstitial-to-lymph flow, and numerical descriptions of hexagonal-shaped lymphatic capillary networks were found to provide an efficient architecture for interstitial and capillary fluid transport in the mouse tail (Roose and Swartz, 2012). Other recent studies have associated lymphatic growth with increased IF pressure in vivo (Planas-Paz et al., 2012), demonstrating that higher IF pressure is linked to increases in surface area to accommodate the local tissue demands. Growth of lymphatic vessels, a process called lymphangiogenesis, is part of the normal development of the lymphatic system (Planas-Paz et al., 2012), however, it is also active in other conditions such as inflammation and cancer (Kim et al., 2014; Zheng et al., 2014).
Irrespective of the local environment, initial lymphatics share common features; they are blind-ended vessels that are composed of a single layer of lymphatic endothelial cells (LECs) and generally appear oak leaf-shaped (Baluk et al., 2007). LECs are interconnected by overlapping junctions and express high levels of the lymphatic vessel endothelial hyaluronan receptor-1 (LYVE-1) (Baluk et al., 2007), while they lack pericyte or LMC coverage and have discontinuous basement membrane (BM) (Lutter and Makinen, 2014). Importantly, their abluminal side is tethered to the ECM by fibrillin-rich fibrils called anchoring filaments (Figure 1) (Leak and Burke, 1968; Solito et al., 1997). The anchoring filaments have the dual role of preventing initial lymphatics from collapsing, while in response to increased IF pressure they stretch and pull apart the LECs junctions (Figure 1) (Paupert et al., 2011; Trzewik et al., 2001). This action allows for water, ions, solutes as well as larger molecules and cells to enter the lumen of the initial lymphatics with little (if any) molecular exclusion, while the newly formed lymph does not leak back to the tissue stroma, suggesting a one-way transport mechanism (Trzewik et al., 2001). Defective anchoring of LECs in the initial lymphatics with the ECM can lead to impaired lymphatic function. Loss of Emilin-1, a major component of the anchoring filaments (Solito et al., 1997), leads to development of hyperplastic vessels with inefficient lymph drainage (Danussi et al., 2008). Interestingly, the LECs in these animals are connected by multiple overlapping intercellular junctions (Danussi et al., 2008), which possibly create a more stringent barrier than normal. The specialized junctions in-between LECs in the initial lymphatics express a repertoire of adherens (AJ) and tight junction (TJ) proteins that are also present in collecting lymphatics, such as VE-cadherin, claudins, zona occludens-1 (ZO-1), junction adhesion molecule (JAM-A), CD-31 and the endothelial selective adhesion molecule (ESAM) (Baluk et al., 2007). In contrast to collecting lymphatics, these specialized junctions, called “buttons”, organize in a discontinuous arrangement around the cell membrane with VE-Cadherin and CD31 expressed in a complementary fashion, and create valve-like gaps of around three microns that seem to be the preferential sites of cell entry (Figure 1, right) (Baluk et al., 2007; Pflicke and Sixt, 2009; Tal et al., 2011). Buttons are enriched when the junctional proteins remodel during development or in pathological conditions and lead to increased permeability of the lymphatics (Baluk et al., 2007; Holopainen et al., 2012; Mirza et al., 2012; Yao et al., 2012). This dynamic regulation possibly relies, at least to a degree, on the mechanical sensitivity of the junctional proteins (Conway et al., 2013; Huveneers et al., 2012; Tzima et al., 2005) and suggests that the development of buttons is a result of an integrated response that involves exchange of information between the interstitial space, LEC membrane and the cytoskeleton. The importance of gaps in the overlapping intercellular LEC junctions flanked by buttons is highlighted by the fact that the latter can facilitate one-way entry of fluids and cells without challenging the integrity of the lumen. Such a behavior of LEC junctions acting as valves during fluid entry into initial lymphatics has been described in mathematical and computational models, where small deflections between the overlapping junctions of LECs were quantified upon application of transmural pressure (Galie and Spilker, 2009; Mendoza and Schmid-Schönbein, 2003). When fluid pressure inside the lymphatics was greater than IF pressure, the LEC extensions contacted each other sealing the junctions to prevent interstitial-to-lymph flow, which is in agreement with the one-way valve system observed experimentally. Smaller cell deflections leading to lower flow rates were observed when a porous fluid domain (representing ECM) was considered on the abluminal side of the LECs, however, a combination of decreased porosity and permeability of the ‘ECM’ in the interstitial space decreased the rate of lymph formation (Galie and Spilker, 2009). In addition, Causey et al. demonstrated experimentally that in skeletal muscle fibers, the expansion and contraction of arterioles and venules in the perimysial space results in contraction/expansion of initial lymphatics, facilitating lymph entry (Causey et al., 2012).
Figure 1.
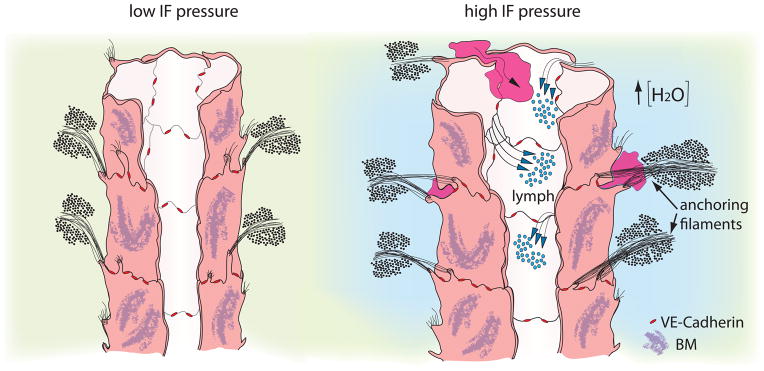
Primary valves in initial lymphatics respond to fluid pressure changes and allow drainage of IF and passage of cells.
When IF pressure is low (left), lymph flow is not promoted towards the lumen. Upon increase in IF pressure (right) anchoring filaments pull the overlapping junctions apart and allow entry of fluid (lymph) and dendritic cells (purple) towards the lumen. The integrity of the lumen is secured by VE-Cadherin located at the “buttons” (red) [Adapted from Leak and Burke, 1968, (Leak and Burke, 1968)].
LYMPH PROPULSION BETWEEN LYMPHANGIONS
Upon formation in the initial lymphatics, lymph is subsequently transported back to the cardiovascular system through a hierarchical network of prenodal collecting lymphatic vessels, high endothelial venules in lymph nodes and postnodal lymphatic vessels and ducts. Their main function is to secure the unidirectional transport of lymph: for this they employ LMCs and intraluminal valves that limit backflow by opening and closing their leaflets. The area between two valves, called a lymphangion, is overlaid with LMCs, whereas the valve site is almost devoid of LMCs (Bouvrée et al., 2012; Gnepp and Green, 1980; Jurisic et al., 2012). The flow of lymph is determined by an active propulsion mechanism but is also influenced by extrinsic forces. In reptiles and amphibians, lymph hearts, composed of an inner layer of LECs and an outer layer of muscle cells that are covered by fibroelastic tissue (Ny et al., 2005), provide an active pump that prompts lymph return to the venous circulation (Hedrick et al., 2013). In the absence of a lymph heart, collecting lymphatics in mammals use many vascular control mechanisms common to the arterioles, including myogenic contraction to neurogenic, purinergic, endothelial-dependent and -independent controls (von der Weid and Zawieja, 2004). Active propulsion of lymph involves activation of LMCs causing a contraction of the lymphatic vessel wall. Ideally, the upstream valve closes, and the downstream valve opens, resulting in net forward flow, although some retrograde flow can occur during valve closure (Figure 2) (Gashev, 1991; McHale and Roddie, 1976). Mathematical studies have indicated the optimum lymphangion length is between 13 and 14.5 times its diameter (Jamalian et al., 2013), suggesting that regional variations in lymphatic network dimensions can influence overall pumping efficiency. Inherent variability in contractions of collecting lymphatics has been found in different locations and could also be attributed to vessel diameter (Gashev et al., 2004; McHale and Meharg, 1992), however, there is currently no direct link between the amount of LMC distribution, contractility frequency and lymphatic vessel diameter. Interestingly, mouse mutants with lymphatic hyperplasia have aberrant deposition of LMCs and are associated with poorly developed or lack of intraluminal valves (Mäkinen et al., 2005; Petrova et al., 2004). Deposition of LMCs around the valve region is linked with a dysregulation of the Semaphorin3A/Neuropilin1/plexin-1 complex, which is expressed in lymphatic valves and normally prevents attachment of LMCs there (Bouvrée et al., 2012; Jurisic et al., 2012). Recruitment of LMCs to lymphatics occurs as part of a dynamic process that involves the secretion of the BM component Reelin upon contact with the LEC on the collecting lymphatics and the progressive down-regulation of LYVE-1 (Lutter et al., 2012). Loss of Reelin leads to lack of LMC coverage and abnormal morphology of collecting lymphatics with compromised lymph transport (Lutter et al., 2012).
Figure 2.
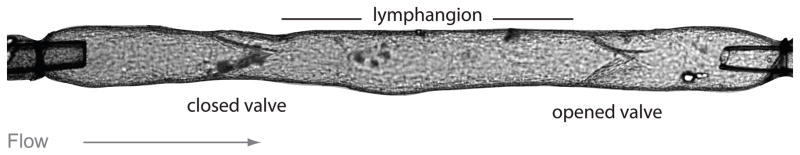
Lymphangion is the functional unit of collecting lymphatic vessels.
Image of a cannulated collecting lymphatic vessel containing two intraluminal valves, the first one is closed and the lymph is transported through the second open valve to the next lymphangion. Objects at the far left and right portions of the vessel are cannulating micropipettes. Calibration bar = 100 μm (Image kindly provided by Dr. Michael J. Davis, University of Missouri).
Nitric oxide (NO) plays an important role in modulating lymph transport (Ohhashi et al., 2005). In the vascular system NO causes vasodilation and increases the vascular permeability (Kim-Shapiro et al., 2006). In the lymphatic system, NO produced at the sinus contributes to the lowering of overall lymph flow by reducing the contraction frequency and stroke volume of individual lymhangions (Bohlen et al., 2011). Under basal conditions, LEC in the avalvular part of the lymphangion produce two-fold higher amounts of NO compared to the bulb region containing the lymphatic valve leaflets (Bohlen et al., 2009). Soluble guanylate cyclase (sGC), the only known physiological receptor for NO, promotes the conversion of GTP to cGMP and pyrophosphate upon binding (Lee et al., 2000). Increasing shear stress stimulates the production of NO by LECs (Bohlen et al., 2011; Bohlen et al., 2009) causing hyperpolarization of LMCs, which inhibits the pacemaker activity due to increased cGMP levels (von der Weid, 1998; von der Weid et al., 2001). Interestingly, computational modeling of NO transport in the valvular area of the lymphangion has demonstrated that the high NO concentration observed there is attributed to flow stagnation in these locations (Figure 3, left) (Wilson et al., 2013). Due to the highly viscous nature of the flow, fluid filling the space behind the valve leaflets is essentially stagnant (although it does move in slowly rotating vortex-like structures). Therefore, any NO that diffuses or is convected to this region becomes trapped, creating higher concentrations in these stagnant zones. Note that Wilson et al. prescribed that NO production increases with flow-induced shear stress, so the LECs in the stagnant zone are actually producing less NO than those exposed to the mainstream of the flow, especially on the inner surface of the valve leaflets (Figure 3, right). Thus, the flow phenomena end up being the major determinants of NO concentration distribution and could possibly affect other signaling pathways (Wilson et al., 2013). As this study was done with a stationary geometry, it remains to be determined how the motion of the leaflets towards the wall or back to the center of the lumen might influence these predicted NO distribution patterns.
Figure 3.
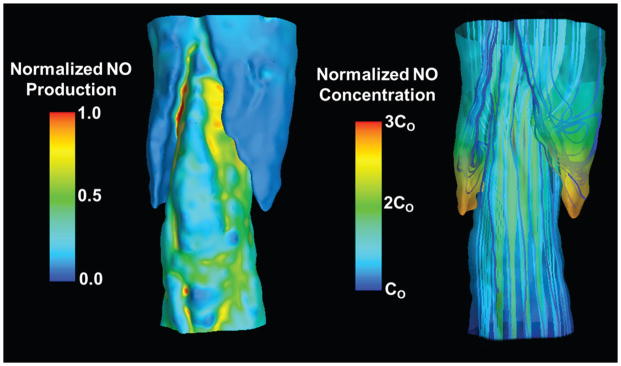
Flow and NO transport simulations performed using a computational geometry constructed from confocal images of a rat mesenteric lymphatic vessel.
The direction of flow is from bottom to top. Left) Distributions of normalized NO production (prescribed to increase with shear stress) at the wall. The areas of lowest NO production occur in the regions adjacent to the valve leaflets encapsulated by the sinus. Right) Distributions of normalized NO concentration at the wall of the vessel overlaid with representative lymph flow velocity streamlines. NO concentration values are normalized by a characteristic inlet concentration, Co. While streamlines adjacent to the valve leaflets appear vortex-like in nature, these are essentially regions of flow stagnation [Images based on data from Wilson et al. (Wilson et al., 2013)].
INFLUENCES OF PRESSURE AND FLOW ON LYMPHATIC VESSEL FUNCTION AND VALVE PERFORMANCE
As mentioned, within the collecting lymphatics, valves with semilunar bicuspid leaflets are frequent along the system; in humans they are generally spaced every 1 to 3 mm (Pan et al., 2010). To prevent retrograde flow, these valves must operate at very low flow rates and in vessels that may be of highly irregular geometry. The Reynolds number, Re, given as:
| Eqn. 2 |
where V is the velocity, D is diameter and ν is the kinematic viscosity of the fluid in this situation, represents the ratio of intertial to viscous forces. It is generally less than one in the lymphatic flow regime (Dixon et al., 2006; Wilson et al., 2013), indicating the valves must operate under highly viscous flow conditions. Turbulence typically does not begin to develop in straight tubes until Re approaches 2000. Thus, lymphatic valves operate in conditions that are very different from those encountered b yheart valves where the Re based on the average flow is usually >1000. Unsteady flow phenomena are also much more important in heart valves. The relevant dimensionless parameter in this case is the Womersley parameter, α, defined by the relation:
| Eqn. 3 |
where R is the tube radius and ω is the pulse frequency. Unsteady effects are important when this parameter has a value of greater than approximately 5. It is typically >15 for aortic valves, and ≪1 for lymphatics, again indicating the dominance of viscous effects in lymphatic flows. Venous valves also operate in flow environments where inertia is important, although unsteadiness effects can be less important depending on factors such as nearby muscle activity and respiration.
Experiments using contracting rat mesenteric lymphatics have often revealed dramatic changes in diameter of 50 % or greater (Dixon et al., 2006; Rahbar and Moore Jr, 2011), and it has been shown that for a contracting vessel containing two valves, a steady increase in the outlet pressure results in progressive decreases in opening times of both valves. Additionally, the closing pressure required for a one-valve segment varied greater than 20-fold (0.1–2.2 cmH2O) with increasing transmural pressure, while the opening pressure required was not strongly affected (Davis et al., 2011). The results from these experiments suggest the valves to be slightly biased in the open position, with the degree of bias depending on the transmural pressure, a finding that is subsequently used to refine computational models of the lymphatic network (Bertram et al., 2013a; Bertram et al., 2013b). Measurements of passive (non-contractile) pressure diameter relationships for rat mesenteric lymphatics showed a highly nonlinear, strain-stiffening response, with the maximum diameter reached at a transmural pressure of less than 5 cmH2O (Rahbar et al., 2012). No significant changes in pressure-diameter response have been observed between mid-lymphangion and valve regions (Rahbar et al., 2012).
MOLECULAR IDENTITY AND DEVELOPMENT OF INTRALUMINAL VALVES
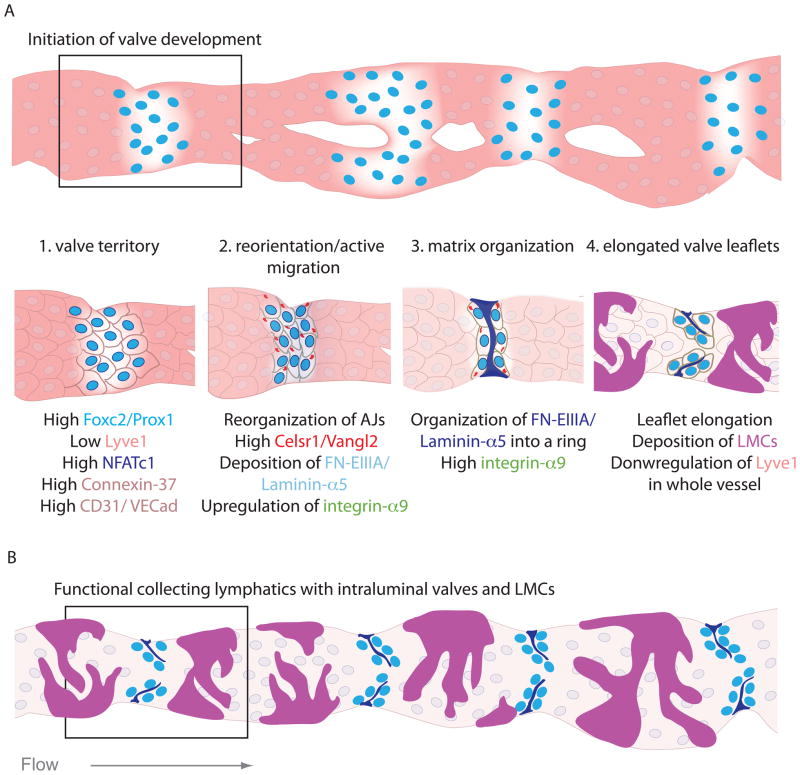
Intraluminal lymphatic valves develop during the remodeling of the primary lymphatic network at sites of vessel branching (Bazigou et al., 2009; Norrmén et al., 2009; Sabine et al., 2012), however mature functional valves in stable collecting lymphatics covered by LMCs are also present in unbranched regions (Figure 4 B) (Bazigou et al., 2009; Gashev et al., 2004). During the remodeling process, LECs, originally joined by continuous intercellular junctions (Yao et al., 2012), start depositing BM, reorganize and progressively recruit LMCs (Lutter et al., 2012; Norrmén et al., 2009). The first indication about the site of future valves is the upregulation of the transcription factors Prox1 and Foxc2 in a subset of LECs and their subsequent clustering in vessels that have not yet recruited LMCs (Figure 4 A) (Bazigou et al., 2009; Norrmén et al., 2009; Sabine et al., 2012). The AJ connecting the LECs in the clusters are enriched in CD-31 and VE-Cadherin (Bazigou et al., 2009; Sabine et al., 2012), while LYVE-1 and Neuropilin-2 expression in these cells is decreased (Mäkinen et al., 2005; Sabine et al., 2012).
Figure 4.
Development of intraluminal lymphatic valves.
(A) Schematic of a phenocopied embryonic lymphatic vessel undergoing remodeling in steps 1. Establishment of valve territory, 2. LEC reorientation and active migration at ridges of lymphatic vessels, 3. ECM organization in a fibrous ring and 4. Valve leaflet elongation. (B) Assumption of the same vessel in its mature form as a collecting lymphatic vessel showing fully developed intraluminal valves and LMCs.
Little is known about what determines the initiation of valve formation during the remodeling process, however, clustering of LECs in the future valve sites was recently shown to be flow-induced, and in vitro studies demonstrated upregulation of Prox1 and Foxc2 in primary LECs in response to oscillatory shear (Sabine et al., 2012). This in turn induced the expression of the Ca2+-sensitive transcription factor NFATc1 and the gap junction protein Connexin 37, while Connexin 43 was negatively regulated by this type of shear and in Prox1/Foxc2-high expressing LECs in vivo (Sabine et al., 2012). Foxc2, NFATc1 and Connexins 37 and 43 are all important for lymphatic valve development, and appear to have a conserved role in venous valve development (Kanady et al., 2011; Mellor et al., 2007; Munger et al., 2013; Norrmén et al., 2009; Petrova et al., 2004; Sabine et al., 2012). In addition, although Prox1, an important regulator of LEC identity (Hong et al., 2002), is strongly expressed in LECs establishing the territory of the future valves, its direct role in valve development is not yet known. Prox1 has been found to be important for the development of the lymphovenous valve, a specialized structure which empties the lymph from the thoracic duct at the interchange between the jugular and the subclavian veins (Srinivasan and Oliver, 2011). In contrast to intraluminal valves of collecting lymphatics, the lymphovenous valve contains Prox1- and Foxc2-expressing venous endothelium on the outflow side but only Prox1-expressing LEC on the inflow side (Srinivasan and Oliver, 2011). Its development relies on LEC interaction with activated platelets that initially create a ‘thrombus’ between the two vascular beds and this is important for the separation of the two systems throughout life (Abtahian et al., 2003; Bertozzi et al., 2010; Hess et al., 2014). There is a strong link between blood filled lymphatics with a dysfunctional lymphovenous valve or abnormal separation of the venous and lymphatic circulation (Abtahian et al., 2003; Bertozzi et al., 2010), but detailed molecular and architectural characteristics of this specialized structure are still missing. Recently, integrin-a5 was found to be important for the formation of lymphovenous valves (Turner et al., 2014), while previously it was reported to be expressed in lymphatic valves of collecting vessels at low levels (Bazigou et al., 2009). It would be interesting to examine the role of additional valve specific genes (see below) in the development and maintenance of the lymphovenous valve.
In mouse collecting lymphatic vessels, the establishment of the valve territory and the subsequent steps in leaflet formation were recently characterized in greater detail. Clustering of LECS expressing high levels of Prox1/Foxc2 is followed by collective migration of the clustered LECs (Tatin et al., 2013) and their arrangement in a perpendicular fashion across the vessel wall where they form transverse ridges (Bazigou et al., 2009; Tatin et al., 2013). Cells have been shown to align perpendicular to the direction of stretch (Richardson et al., 2011), and integrin-β1-induced LEC stretching has been reported in vivo in response to increased transmural pressure (Planas-Paz et al., 2012). The process of realignment in lymphatic vessels during valve development is orchestrated by members of the planar cell polarity pathway, where the atypical cadherin Celsr1-3/Flamingo recruits Strabismus/Van Ghogh-2 to the LEC membrane and together they regulate the distribution and stabilization of AJ (Bazigou et al., 2009; Tatin et al., 2013). Concomitant with this process, valve LEC express the cell matrix receptor integrin-α9 and start organizing the ECM around the vessel wall, containing the splice isoform of fibronectin containing the EIIIA domain (FN-EIIIA) and laminin-α5 (Bazigou et al., 2009; Sabine et al., 2012). These matrix proteins initially form a fibrous ring outside the LEC ridges (Bazigou et al., 2009). As the valve LECs elongate and migrate towards the lumen for the formation of leaflets (Tatin et al., 2013) FN-EIIIA and laminin-α5 fibers assemble and are deposited between the LECs to support the elongation of the valve leaflets (Bazigou et al., 2009). Optical microscopy imaging of rat collecting lymphatics has also identified elastin and axially oriented thick bands of collagen in the matrix core at the leaflet insertion points (Rahbar et al., 2012), suggesting variability in the composition depending on the vessel diameter or other physical parameters. The specific roles of elastin and collagen in lymphatic valve development or function still remain to be resolved.
The process of valve leaflet elongation is defective in mouse mutants for Itga9 and FNEIIIA, and loss of integrin-α9 manifests in accumulation of chylous fluid in the chest causing early postnatal lethality (Bazigou et al., 2009; Huang et al., 2000) and Itga9 missense mutations in human fetuses present the same phenotype (Ma et al., 2008). A similar manifestation is observed in a mouse model where the PDZ domain of Ephrin-B2, an otherwise arterial marker, is missing. Although lymphatic valves fail to form in these mutants, this might be due to a more general remodeling defect (Mäkinen et al., 2005). The importance of an orchestrated remodeling process in the development of lymphatic valves is highlighted in many additional mouse models, and implicates the signaling molecules Tie1 and Angiopoietin-2 (Dellinger et al., 2008; Shen et al., 2014) and the regulatory isoforms of the phosphoinositide 3-kinase (PI3K)/Akt pathway, p85α/p55α/p50α (Mouta-Bellum et al., 2009; Zhou et al., 2010). The specific roles of most of these pathways specifically in lymphatic valve development have not been delineated. However, in vitro studies have demonstrated a genetic hierarchical network linking Prox1, integrin-α9 and Ephrin-B2 under the control of the transcription factor Gata2, which is strongly expressed in lymphatic valves while patients with happloinsufficiency or loss of function mutations develop lymphedema (Kazenwadel et al., 2012). Importantly, both integrin-a9 together with its ligand FNEIIIA and Ephrin-B2 also actively regulate venous valve formation in mice (Bazigou et al., 2011) and mutations in Foxc2 cause venous valve reflux (Kanady et al., 2011; Mellor et al., 2007; Munger et al., 2013; Norrmén et al., 2009; Petrova et al., 2004; Sabine et al., 2012). These data suggest that valve LECs obtain a unique cellular identity that, in many cases, is shared between lymphatic and venous or cardiac valves, thus highlighting the importance of the valve function irrespective of the vascular bed in which these develop (Bazigou and Makinen, 2013). Maintenance of lymphatic valves in mature collecting lymphatic vessels requires a similar repertoire of molecular components such as NFTAc1 and Ephrin-B2, whereas integrin-α9 appears to be dispensable (Bazigou et al., 2011; Sabine et al., 2012). Tissue specific gene deletions that are temporally controlled will provide further insight into the role of the valve-specific genes in the development and maintenance of functional intraluminal valves. Importantly, the endothelial glycocalyx may be critical for the development and function of lymphatic valves, as suggested by their high expression of podocalyxin and Lycopersicon esculentum lectin on valve LEC (Bazigou et al., 2009; Sabine et al., 2012; Tammela et al., 2007), especially when considering reports that demonstrate flow responsive distribution of glycocalyx components (Henderson-Toth et al., 2012; Zeng and Tarbell, 2014).
FINAL REMARKS
The importance of a functional lymphatic network with competent one-way valve systems is highlighted in the lethal phenotypes observed in several animal models, as well as in the compromised quality of life in lymphedema patients. Despite these facts, the study of the lymphatic system has been hindered until recently due to lack of specific markers. At the same time, physical measurements of lymph formation and transport have been technically challenging, due to lack of sufficient spatial and temporal resolution in clinical imaging modalities. The flow velocities are also too small for detection by Doppler-based techniques. Fluorescence-based imaging allows for some visualization of superficial vessels (Tan et al., 2011) but does not result in reliable quantitative flow measurement.
Although there has been some progress in understanding the mechanisms behind the development and function of the two types of lymphatic valves, a number of important biological and physiological aspects of lymphatic behavior remain to be investigated. These include: the role of critical thresholds both in IF pressure, lymph volume and content (e.g. pH), and cell signaling for the development and maturation of primary valves in the different stromata and more specific links between the mechanical stimulation and molecular determinants of intraluminal valve development and maturation,. New emerging imaging technologies, in vitro 3D microfluidic systems as well as in vivo examination of lymphatic vessel development and remodeling will shed more light on these complex morphogenetic events. This will hopefully lead to a better understanding of normal physiology while suggesting more integrated treatment or rehabilitation methods for lymphedema patients.
Acknowledgments
We would like to acknowledge the British Heart Foundation for funding E. Bazigou. J.E. Moore wishes to acknowledge the support of The Royal Society, The Royal Academy of Engineering, The Sir Leon Bagrit Trust, and the USA National Institutes of Health (NHLBI and NCI, Grant HL-094269). We would also like to thank Dr. Michael J. Davis (University of Missouri) for providing the image of the cannulated lymphatic vessel.
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interest to state.
References
- Abtahian F, et al. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukland K, Nicolaysen G. Interstitial fluid volume: local regulatory mechanisms. Physiological reviews. 1981;61:556–643. doi: 10.1152/physrev.1981.61.3.556. [DOI] [PubMed] [Google Scholar]
- Aukland K, Reed R. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiological reviews. 1993;73:1–78. doi: 10.1152/physrev.1993.73.1.1. [DOI] [PubMed] [Google Scholar]
- Baluk P, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. The Journal of experimental medicine. 2007;204:2349–2362. doi: 10.1084/jem.20062596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank RA, et al. The increased swelling and instantaneous deformation of osteoarthritic cartilage is highly correlated with collagen degradation. Arthritis & Rheumatism. 2000;43:2202–2210. doi: 10.1002/1529-0131(200010)43:10<2202::AID-ANR7>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Bazigou E, et al. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. The Journal of clinical investigation. 2011;121:2984. doi: 10.1172/JCI58050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Makinen T. Flow control in our vessels: vascular valves make sure there is no way back. Cellular and Molecular Life Sciences. 2013;70:1055–1066. doi: 10.1007/s00018-012-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, et al. Integrin-α9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Developmental cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini C, Hennekam RC. Developmental Aspects of the Lymphatic Vascular System. Springer; 2014. Clinical Disorders of Primary Malfunctioning of the Lymphatic System; pp. 187–204. [DOI] [PubMed] [Google Scholar]
- Bertozzi CC, et al. Platelets regulate lymphatic vascular development through CLEC-2–SLP-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram C, et al. Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomechanics and modeling in mechanobiology. 2013a:1–16. doi: 10.1007/s10237-013-0505-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram C, et al. Incorporating measured valve properties into a numerical model of a lymphatic vessel. Computer methods in biomechanics and biomedical engineering. 2013b:1–16. doi: 10.1080/10255842.2012.753066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen HG, et al. Nitric oxide formation by lymphatic bulb and valves is a major regulatory component of lymphatic pumping. American Journal of Physiology-Heart and Circulatory Physiology. 2011;301:H1897. doi: 10.1152/ajpheart.00260.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen HG, et al. Phasic contractions of rat mesenteric lymphatics increase basal and phasic nitric oxide generation in vivo. American Journal of Physiology-Cell Physiology. 2009;66:H1319. doi: 10.1152/ajpheart.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvrée K, et al. Semaphorin3A, Neuropilin-1, and PlexinA1 are required for lymphatic valve formation. Circulation research. 2012;111:437–445. doi: 10.1161/CIRCRESAHA.112.269316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card CM, et al. Emerging roles of lymphatic endothelium in regulating adaptive immunity. The Journal of clinical investigation. 2014;124:943–952. doi: 10.1172/JCI73316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causey L, et al. Quantitative model for predicting lymph formation and muscle compressibility in skeletal muscle during contraction and stretch. Proceedings of the National Academy of Sciences. 2012;109:9185–9190. doi: 10.1073/pnas.1206398109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KL, et al. Crosslinking of collagen scaffolds promotes blood and lymphatic vascular stability. Journal of Biomedical Materials Research Part A. 2013 doi: 10.1002/jbm.a.34990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway DE, et al. Fluid shear stress on endothelial cells modulates mechanical tension across VE-cadherin and PECAM-1. Current Biology. 2013;23:1024–1030. doi: 10.1016/j.cub.2013.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danussi C, et al. Emilin1 deficiency causes structural and functional defects of lymphatic vasculature. Molecular and cellular biology. 2008;28:4026–4039. doi: 10.1128/MCB.02062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MJ, et al. Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol. 2011;301:H48–H60. doi: 10.1152/ajpheart.00133.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellinger M, et al. Defective remodeling and maturation of the lymphatic vasculature in Angiopoietin-2 deficient mice. Developmental biology. 2008;319:309–320. doi: 10.1016/j.ydbio.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon JB, et al. Lymph flow, shear stress, and lymphocyte velocity in rat mesenteric prenodal lymphatics. Microcirculation. 2006;13:597–610. doi: 10.1080/10739680600893909. [DOI] [PubMed] [Google Scholar]
- Galie P, Spilker RL. A two-dimensional computational model of lymph transport across primary lymphatic valves. Journal of biomechanical engineering. 2009;131:111004. doi: 10.1115/1.3212108. [DOI] [PubMed] [Google Scholar]
- Gashev A. The mechanism of the formation of a reverse fluid filling in the lymphangions. Fiziologicheskii zhurnal SSSR imeni IM Sechenova. 1991;77:63–69. [PubMed] [Google Scholar]
- Gashev A, et al. Regional variations of contractile activity in isolated rat lymphatics. Microcirculation. 2004;11:477–492. doi: 10.1080/10739680490476033. [DOI] [PubMed] [Google Scholar]
- Gnepp D, Green F. Scanning electron microscopic study of canine lymphatic vessels and their valves. Lymphology. 1980;13:91–99. [PubMed] [Google Scholar]
- Hedrick MS, et al. Lymphatic regulation in nonmammalian vertebrates. Journal of Applied Physiology. 2013;115:297–308. doi: 10.1152/japplphysiol.00201.2013. [DOI] [PubMed] [Google Scholar]
- Henderson-Toth CE, et al. The glycocalyx is present as soon as blood flow is initiated and is required for normal vascular development. Developmental biology. 2012;369:330–339. doi: 10.1016/j.ydbio.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Hess PR, et al. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. The Journal of clinical investigation. 2014;124:273. doi: 10.1172/JCI70422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holopainen T, et al. Effects of Angiopoietin-2-blocking antibody on endothelial cell–cell junctions and lung metastasis. Journal of the National Cancer Institute. 2012;104:461–475. doi: 10.1093/jnci/djs009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, et al. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Developmental dynamics. 2002;225:351–357. doi: 10.1002/dvdy.10163. [DOI] [PubMed] [Google Scholar]
- Huang X, et al. Fatal bilateral chylothorax in mice lacking the integrin α9β1. Molecular and cellular biology. 2000;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huveneers S, et al. Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. The Journal of cell biology. 2012;196:641–652. doi: 10.1083/jcb.201108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamalian S, et al. Parameter sensitivity analysis of a lumped-parameter model of a chain of lymphangions in series. American Journal of Physiology-Heart and Circulatory Physiology. 2013;305:H1709–H1717. doi: 10.1152/ajpheart.00403.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurisic G, et al. An Unexpected Role of Semaphorin3A–Neuropilin-1 Signaling in Lymphatic Vessel Maturation and Valve Formation. Circulation research. 2012;111:426–436. doi: 10.1161/CIRCRESAHA.112.269399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanady JD, et al. Connexin37 and Connexin43 deficiencies in mice disrupt lymphatic valve development and result in lymphatic disorders including lymphedema and chylothorax. Developmental biology. 2011;354:253–266. doi: 10.1016/j.ydbio.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazenwadel J, et al. Loss-of-function germline GATA2 mutations in patients with MDS/AML or MonoMAC syndrome and primary lymphedema reveal a key role for GATA2 in the lymphatic vasculature. Blood. 2012;119:1283–1291. doi: 10.1182/blood-2011-08-374363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedem Ot, Katchalsky A. Thermodynamic analysis of the permeability of biological membranes to non-electrolytes. Biochimica et biophysica acta. 1958;27:229–246. doi: 10.1016/0006-3002(58)90330-5. [DOI] [PubMed] [Google Scholar]
- Kim-Shapiro DB, et al. Unraveling the reactions of nitric oxide, nitrite, and hemoglobin in physiology and therapeutics. Arteriosclerosis, thrombosis, and vascular biology. 2006;26:697–705. doi: 10.1161/01.ATV.0000204350.44226.9a. [DOI] [PubMed] [Google Scholar]
- Kim H, et al. Inflammation-associated lymphangiogenesis: a double-edged sword? The Journal of clinical investigation. 2014;124:936–942. doi: 10.1172/JCI71607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruegel J, Miosge N. Basement membrane components are key players in specialized extracellular matrices. Cellular and Molecular Life Sciences. 2010;67:2879–2895. doi: 10.1007/s00018-010-0367-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak L, Burke J. Ultrastructural studies on the lymphatic anchoring filaments. The Journal of cell biology. 1968;36:129–149. [PMC free article] [PubMed] [Google Scholar]
- Lee Y-C, et al. Human recombinant soluble guanylyl cyclase: expression, purification, and regulation. Proceedings of the National Academy of Sciences. 2000;97:10763–10768. doi: 10.1073/pnas.190333697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutter S, Makinen T. Developmental Aspects of the Lymphatic Vascular System. Springer; 2014. Regulation of Lymphatic Vasculature by Extracellular Matrix; pp. 55–65. [DOI] [PubMed] [Google Scholar]
- Lutter S, et al. Smooth muscle–endothelial cell communication activates Reelin signaling and regulates lymphatic vessel formation. The Journal of cell biology. 2012;197:837–849. doi: 10.1083/jcb.201110132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkinen T, et al. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes & development. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale N, Meharg M. Co-ordination of pumping in isolated bovine lymphatic vessels. The Journal of physiology. 1992;450:503–512. doi: 10.1113/jphysiol.1992.sp019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHale N, Roddie I. The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. The Journal of Physiology. 1976;261:255–269. doi: 10.1113/jphysiol.1976.sp011557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor RH, et al. Mutations in FOXC2 are strongly associated with primary valve failure in veins of the lower limb. Circulation. 2007;115:1912–1920. doi: 10.1161/CIRCULATIONAHA.106.675348. [DOI] [PubMed] [Google Scholar]
- Mendoza E, Schmid-Schönbein GW. A model for mechanics of primary lymphatic valves. Journal of biomechanical engineering. 2003;125:407–414. doi: 10.1115/1.1568128. [DOI] [PubMed] [Google Scholar]
- Mirza M, et al. Essential role of the coxsackie- and adenovirus receptor (CAR) in development of the lymphatic system in mice. PloS one. 2012;7:e37523. doi: 10.1371/journal.pone.0037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer PS, Rockson SG. New developments in clinical aspects of lymphatic disease. The Journal of clinical investigation. 2014;124:915–921. doi: 10.1172/JCI71608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouta-Bellum C, et al. Organ-specific lymphangiectasia, arrested lymphatic sprouting, and maturation defects resulting from gene-targeting of the PI3K regulatory isoforms p85α, p55α, and p50α. Developmental Dynamics. 2009;238:2670–2679. doi: 10.1002/dvdy.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger SJ, et al. Absence of venous valves in mice lacking Connexin37. Developmental biology. 2013;373:338–348. doi: 10.1016/j.ydbio.2012.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmén C, et al. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. The Journal of cell biology. 2009;185:439–457. doi: 10.1083/jcb.200901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ny A, et al. A genetic Xenopus laevis tadpole model to study lymphangiogenesis. Nature medicine. 2005;11:998–1004. doi: 10.1038/nm1285. [DOI] [PubMed] [Google Scholar]
- Ohhashi T, et al. Current topics of physiology and pharmacology in the lymphatic system. Pharmacology & therapeutics. 2005;105:165–188. doi: 10.1016/j.pharmthera.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Pan WR, et al. The morphology of the human lymphatic vessels in the head and neck. Clinical Anatomy. 2010;23:654–661. doi: 10.1002/ca.21004. [DOI] [PubMed] [Google Scholar]
- Paupert J, et al. Lymphangiogenesis in post-natal tissue remodeling: lymphatic endothelial cell connection with its environment. Molecular aspects of medicine. 2011;32:146–158. doi: 10.1016/j.mam.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Petrova TV, et al. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nature medicine. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Pflicke H, Sixt M. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. The Journal of experimental medicine. 2009;206:2925–2935. doi: 10.1084/jem.20091739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas-Paz L, Lammert E. Developmental Aspects of the Lymphatic Vascular System. Springer; 2014. Mechanosensing in Developing Lymphatic Vessels; pp. 23–40. [DOI] [PubMed] [Google Scholar]
- Planas-Paz L, et al. Mechanoinduction of lymph vessel expansion. The EMBO journal. 2012;31:788–804. doi: 10.1038/emboj.2011.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar E, Moore JE., Jr A model of a radially expanding and contracting lymphangion. Journal of biomechanics. 2011;44:1001–1007. doi: 10.1016/j.jbiomech.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahbar E, et al. Passive pressure–diameter relationship and structural composition of rat mesenteric lymphangions. Lymphatic research and biology. 2012;10:152–163. doi: 10.1089/lrb.2011.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Miller NE. Lymphatic transport of high-density lipoproteins and chylomicrons. The Journal of clinical investigation. 2014;124:929–935. doi: 10.1172/JCI71610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson WJ, et al. A device to study the effects of stretch gradients on cell behavior. Journal of biomechanical engineering. 2011;133:101008. doi: 10.1115/1.4005251. [DOI] [PubMed] [Google Scholar]
- Roose T, Swartz MA. Multiscale modeling of lymphatic drainage from tissues using homogenization theory. Journal of biomechanics. 2012;45:107–115. doi: 10.1016/j.jbiomech.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Sabine A, et al. Mechanotransduction, PROX1, and FOXC2 cooperate to control connexin37 and calcineurin during lymphatic-valve formation. Developmental cell. 2012;22:430–445. doi: 10.1016/j.devcel.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Schmid-Schönbein GW. The second valve system in lymphatics. Lymphatic research and biology. 2003;1:25–31. doi: 10.1089/15396850360495664. [DOI] [PubMed] [Google Scholar]
- Shen B, et al. Genetic Dissection of Tie Pathway in Mouse Lymphatic Maturation and Valve Development. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1221–1230. doi: 10.1161/ATVBAHA.113.302923. [DOI] [PubMed] [Google Scholar]
- Solito R, et al. An Immunological Correlation Between the Anchoring Filaments of Initial Lymph Vessels and the Neighboring Elastic Fibers: A Unified Morphofunctional Concept. Lymphology. 1997;30:194–202. [PubMed] [Google Scholar]
- Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes & development. 2011;25:2187–2197. doi: 10.1101/gad.16974811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microscopy research and technique. 2001;55:92–99. doi: 10.1002/jemt.1160. [DOI] [PubMed] [Google Scholar]
- Tal O, et al. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. The Journal of experimental medicine. 2011;208:2141–2153. doi: 10.1084/jem.20102392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, et al. Therapeutic differentiation and maturation of lymphatic vessels after lymph node dissection and transplantation. Nature medicine. 2007;13:1458–1466. doi: 10.1038/nm1689. [DOI] [PubMed] [Google Scholar]
- Tan I, et al. Assessment of lymphatic contractile function after manual lymphatic drainage using near-infrared fluorescence imaging. Archives of physical medicine and rehabilitation. 2011;92:756–764.e1. doi: 10.1016/j.apmr.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatin F, et al. Planar cell polarity protein Celsr1 regulates endothelial adherens junctions and directed cell rearrangements during valve morphogenesis. Developmental cell. 2013;26:31–44. doi: 10.1016/j.devcel.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. The lymphatic edema safety factor: the role of edema dependent lymphatic factors (EDLF) Lymphology. 1990;23:111–123. [PubMed] [Google Scholar]
- Trzewik J, et al. Evidence for a second valve system in lymphatics: endothelial microvalves. The FASEB Journal. 2001;15:1711–1717. doi: 10.1096/fj.01-0067com. [DOI] [PubMed] [Google Scholar]
- Turner CJ, et al. Integrin-α5β1 Is not required for mural cell functions during development of Blood vessels but Is required for lymphatic-Blood vessel separation and lymphovenous valve formation. Developmental Biology. 2014 doi: 10.1016/j.ydbio.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- von der Weid P-Y. ATP-sensitive K+ channels in smooth muscle cells of guinea-pig mesenteric lymphatics: role in nitric oxide and β-adrenoceptor agonist-induced hyperpolarizations. British journal of pharmacology. 1998;125:17. doi: 10.1038/sj.bjp.0702026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von der Weid P-Y, Zawieja DC. Lymphatic smooth muscle: the motor unit of lymph drainage. The international journal of biochemistry & cell biology. 2004;36:1147–1153. doi: 10.1016/j.biocel.2003.12.008. [DOI] [PubMed] [Google Scholar]
- von der Weid P-Y, et al. Nitric oxide decreases pacemaker activity in lymphatic vessels of guinea pig mesentery. American Journal of Physiology-Heart and Circulatory Physiology. 2001;280:H2707–H2716. doi: 10.1152/ajpheart.2001.280.6.H2707. [DOI] [PubMed] [Google Scholar]
- Wiig H, et al. Interaction between the extracellular matrix and lymphatics: consequences for lymphangiogenesis and lymphatic function. Matrix Biology. 2010;29:645–656. doi: 10.1016/j.matbio.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiological Reviews. 2012;92:1005–1060. doi: 10.1152/physrev.00037.2011. [DOI] [PubMed] [Google Scholar]
- Wilson JT, et al. Confocal Image-Based Computational Modeling of Nitric Oxide Transport in a Rat Mesenteric Lymphatic Vessel. Journal of biomechanical engineering. 2013;135:051005. doi: 10.1115/1.4023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao L-C, et al. Plasticity of button-like junctions in the endothelium of airway lymphatics in development and inflammation. The American journal of pathology. 2012;180:2561–2575. doi: 10.1016/j.ajpath.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y, Tarbell JM. The Adaptive Remodeling of Endothelial Glycocalyx in Response to Fluid Shear Stress. PloS one. 2014;9:e86249. doi: 10.1371/journal.pone.0086249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, et al. Lymphangiogenic factors, mechanisms, and applications. The Journal of clinical investigation. 2014;124:878–887. doi: 10.1172/JCI71603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, et al. Akt/Protein kinase B is required for lymphatic network formation, remodeling, and valve development. The American journal of pathology. 2010;177:2124–2133. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]