Abstract
Objective
To determine the maximum tolerated dose (MTD), dose-limiting toxicity (DLT) and efficacy of sorafenib in combination with FOLFOX4 (oxaliplatin/leucovorin (LV)/5-fluorouracil) as first-line treatment for advanced gastric cancer, we performed a phase I dose-finding study in nine evaluable patients with unresectable locally advanced or metastatic gastric cancer or gastroesophageal junction adenocarcinoma.
Methods
According to modified Fibonacci method, the design of this study was to guide elevation of the sorafenib dosage to the next level (from 200 mg twice daily to 400 mg twice daily and then, if tolerated, 600 mg twice daily). If the patient achieved complete response (CR), partial response (PR) or stable disease (SD) after eight cycles of treatment, combination chemotherapy was scheduled to be discontinued and sorafenib monotherapy continued at the original dose until either disease progression or unacceptable toxicity.
Results
In sorafenib 200 mg twice daily group, DLT was observed in 1 of 6 patients, and in 400 mg twice daily group, it was observed in 2 of 3 patients. Seven of 9 (77.8%) evaluable patients achieved PR, with a median overall survival (OS) of 11.8 [95% confidence interval (CI): 8.9-14.7] months. Common adverse effects include hand-foot syndrome, leukopenia, neutropenia, anorexia, and nausea.
Conclusions
Twice-daily dosing of sorafenib 200 mg in combination with FOLFOX4 was proven effective and safe for the treatment of advanced gastric cancer, and could be an appropriate dosage for subsequent phase II clinical studies.
Keywords: Sorafenib, gastric cancer, FOLFOX4
Introduction
Gastric cancer is one of the most common malignant diseases worldwide. More than 50% of the world’s gastric cancer cases are in Eastern Asia and Chinese patients account for about 41% (1). Furthermore, gastric cancers are more frequently diagnosed at the advanced, unresectable stage. In general, the prognosis of advanced gastric cancer is poor and the efficacy of chemotherapy is unsatisfactory. Therefore, it is of great necessity to explore novel therapeutic regimens for this disease.
Sorafenib was an oral multikinase inhibitor, which targets Raf serine/threonine kinases (raf-1, b-raf), vascular endothelial growth factor receptor (VEGFR)-1/-2/-3, platelet-derived growth factor receptor (PDGFR)-β and Flt-3, c-kit and p38 tyrosine kinases (2). Sorafenib has been approved by US Food and Drug Administration (FDA) for the treatment of advanced renal cell carcinoma (RCC) in 2005 and unresectable hepatocellular carcinomas (HCC) in 2007 (3,4). Several clinical trials have shown that sorafenib could be used in combination with cytotoxic agents in different solid tumors, such as combined with interferon-α in RCC (5), with dacarbazine in melanoma (6-8), with doxorubicin in HCC (9), with gemcitabine in pancreatic and ovarian cancers (10-12), and with oxaliplatin or paclitaxel in advanced gastric cancer (13,14).
In vitro study, oxaliplatin was demonstrated to inhibit the growth of several gastric cancer cell lines (15). Oxaliplatin showed additive or synergistic activity when combined to 5-FU, even in 5-FU-resistant cell lines. It has been reported in several clinical trials that the response rates (RR) of oxaliplatin with 5-FU in first-line treatments for advanced gastric cancer are 43% to 44.9%, and about 26% in second-line treatments (16-20).
In this study, we investigated the maximum tolerated dose (MTD), dose-limiting toxicity (DLT) and efficacy of sorafenib in combination with FOLFOX4 to thereby determine the recommended dosage for subsequent phase II studies.
Materials and methods
Patients
Inclusion criteria in this study were: age >18 years; diagnosed gastric adenocarcinoma via histological examination; newly diagnosed or recurrent unresectable locally advanced or metastatic gastric cancer or gastroesophageal junction adenocarcinoma; no history of chemotherapy or radiation therapy; at least one lesion with a measurable diameter; an Eastern Cooperative Oncology Group (ECOG) performance status score of 0 or 1; an expected survival time of at least 3 months; hemoglobin >8.0 g/dL; absolute neutrophil count (ANC) >1.5×109/L; platelet count >100×109/L; total bilirubin <1.5× upper limit of normal (ULN); alanine aminotransferase (ALT) and aspartate aminotransferase (AST) <3× ULN (or for patients with liver metastases, <5× ULN); prothrombin time/international normalized ratio/partial thromboplastin time (PT/INR/PTT) <1.5× ULN; serum creatinine <1.5 mg/dL; and signed informed consent by patients prior to commencement of the study. Exclusion criteria were: a present or past medical history of other tumors, except for cured skin cancer (non-melanoma) or carcinoma in situ of the cervix; surgery, open biopsy, or obvious trauma within 28 d prior to enrollment; peritoneal seeding or intestinal obstruction; severe gastrointestinal bleeding; grade 1 or greater peripheral neuropathy [National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v.3.0]; a past medical history of serious neurological or psychiatric diseases; pregnancy or lactation; women of childbearing potential unwilling to use adequate contraception; a past medical history of heart disease such as greater than grade 2 New York Heart Association (NYHA) congestive heart failure, unstable coronary heart disease (patients having episodes of myocardial infarction earlier than 12 months prior to the study were allowed to enroll), arrhythmia requiring antiarrhythmic therapy (patients on β-blockers or digoxin were allowed to enroll), or uncontrolled hypertension; severe active infection (> grade 2 NCI CTCAE v.3.0); epileptic patients requiring medical treatment (such as corticosteroids or antiepileptic drugs); patients who had received immunotherapy within 4 weeks prior to or throughout the study or had received mitomycin C or nitrourea drugs within 4 weeks previously; allergies or possible allergies to the studied medications or other drugs administered during the study; any patient safety or compliance issues that might jeopardize their participation in the study; and an inability to swallow oral drugs.
This study was registered at ClinicalTrials.gov (No. 02209441), and was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. The study protocol and its amendments were reviewed and approved by the Ethics Committee of the Cancer Hospital, Chinese Academy of Medical Sciences (CAMS) & Peiking Union Medical College (PUMC). All eligible patients provided their written informed consent to participate in the study.
Study design
Dose escalation method
This study was designed as a single-center, open-label, dose-escalation investigation to determine the MTD and DLT of sorafenib in combination with FOLFOX4, to evaluate the safety profile and the preliminary efficacy of this combination regimen in patients with unresectable locally advanced or metastatic gastric cancer. According to modified Fibonacci method (21), sorafenib was scheduled to be administered at three dose levels (given twice daily from d 4 cycle 1) in combination with the standard FOLFOX4 regimen, oxaliplatin 85 mg/m2∙d intravenous (IV) infusion d 1; leucovorin (LV) 200 mg/m2∙d IV infusion d 1, d 2; and 5-FU 400 mg/m2∙d IV bolus d 1, d 2, and then 600 mg/m2 continuous intravenous (CIV) infusion for 22 h (22). The three dose levels to be investigated were:
Dose level 1: sorafenib 400 mg/d (in two divided doses) orally with FOLFOX4;
Dose level 2: sorafenib 800 mg/d (in two divided doses) orally with FOLFOX4;
Dose level 3: sorafenib 1,200 mg/d (in two divided doses) orally with FOLFOX4.
Each cycle was 14 d, and patients received the maximum of eight cycles. Those who achieved complete response (CR), partial response (PR) or stable disease (SD) after eight cycles were scheduled to be discontinued from combination chemotherapy and continued on sorafenib monotherapy at the original dose until either tumor progress or unacceptable toxicity.
Sorafenib dose escalation was performed according to modified Fibonacci method. Each dose group included at least three patients. If any of the three patients in each group reported DLT related to the study medication, another three patients were added to make a total of six patients. However, if none of the three original patients or less than 2 of the 6 patients in the expanded group reported DLT, all patients would then move to next dose level. The dosage used before the dose at which dose escalation was ceased was considered as the MTD.
DLT definition
DLT was defined as ANC <0.5×109/L lasting for more than 5 days; ANC <1.0×109/L, accompanied by fever with a body temperature ≥38.5 °C; grade 4 thrombocytopenia; any ≥ grade 3 non-hematologic toxicity, including fatigue, nausea, vomiting, diarrhea, and hand-foot syndrome; discontinuation of FOLFOX4 or sorafenib therapy due to adverse reactions; and delays in treatment of more than 14 days as a result of grade 2 or greater persistent toxicity.
Dosage adjustments
Patients with ANC ≥1.0×109/L and platelet counts ≥100×109/L could start the next cycle of treatment. However, if these parameters were not met, oxaliplatin, 5-FU and sorafenib therapy was discontinued or delayed until they had reverted to normal. For patients with ANC <0.5×109/L, the oxaliplatin dose was decreased by 25%, the 5-FU dose was decreased by 20%, and sorafenib treatment was interrupted in the next cycle until the adverse reaction recovered to grades 0 or 1. A lower dose level was then administered in the next cycle. Decreased doses of oxaliplatin (by 25%), 5-FU (by 20%) and a lower dose of sorafenib were administered to patients who experienced two episodes of severe hematologic toxicity. If severe hematologic toxicity occurred again after two dosage adjustments, oxaliplatin, 5-FU and sorafenib were completely discontinued. If persistent grade 2 or greater non-hematologic toxicity occurred, decreases in the doses of oxaliplatin (by 25%) and 5-FU by 20% were also conducted. Similarly, if grade 3 non-hematologic toxicity occurred with sorafenib, a lower dose level was administered. If two episodes of grade 3 non-hematologic toxicity or one episode of grade 4 hematologic toxicity occurred, the treatment was discontinued.
Evaluation criteria of efficacy and tolerability
The response was evaluated every three cycles according to Response Evaluation Criteria in Solid Tumors (RECIST) 1.1. The Kaplan-Meier method was used for survival analysis with SPSS software (SPSS Inc., Chicago, IL, USA). After the end of the study, patients were followed up every 3 months up to April 30 2013. Adverse reactions were evaluated in accordance with NCI CTCAE v.3.0.
Results
Patient characteristics
A total of ten patients who were diagnosed with advanced gastric cancer during March 2010 to November 2011 at the Cancer Hospital, CAMS & PUMC were enrolled in the study, and nine were evaluable including six males and three females with a median age of 59 (range, 42-72) years. Two patients had an ECOG performance status score of 0, and seven patients had a score of 1. The patient characteristics are shown in Table 1.
Table 1. Patients’ characteristics.
| Characteristics | No. of patients |
|---|---|
| Median age [range], years | 59 [42-72] |
| Gender | |
| Male | 6 |
| Female | 3 |
| ECOG performance status | |
| 0 | 2 |
| 1 | 7 |
| Primary tumor site | |
| Stomach | 5 |
| Gastroesophageal junction adenocarcinoma | 4 |
| Histological type | |
| Poorly differentiated adenocarcinoma | 4 |
| Signet-ring cell carcinoma | 2 |
| Well differentiated adenocarcinoma | 2 |
| Adenocarcinoma | 1 |
| Tumor stage | |
| Metastatic tumor | 9 |
| Metastasis sites | |
| Celiac lymph nodes | 4 |
| Liver | 3 |
| Pelvic cavity, mesentery | 4 |
| Others | 7 |
| Metastasis numbers | |
| 1-2 | 0 |
| ≥3 | 9 |
ECOG, Eastern Cooperative Oncology Group.
Dose escalations and treatment discontinuations
Six patients in the sorafenib 200 mg twice daily group received a mean of 8.2 (range, 4-49) treatment cycles, one of whom (16.67%) experienced DLT (hepatic impairment) in the second week of the 4th cycle at this dose level. Three patients in the sorafenib 400 mg twice daily group received a mean of 2.3 (range, 2-3) treatment cycles, two of whom (66.67%) discontinued chemotherapy due to DLT: one patient discontinued treatment due to grade 3 hand-foot syndrome and grade 4 neutropenia, the other discontinued after a 30 days treatment interruption due to grade 3 neutropenia complicated by fever in week 2 of the second cycle. The third patient discontinued treatment due to intestinal obstruction before commencement of the second cycle of treatment, which was confirmed as disease progression. The details are shown in Table 2.
Table 2. Patients’ treatment details.
| No. | Gender/age (years) | Sorafenib dose (mg twice daily) | Treatment cycles | Best response | Reasons for termination | PFS (months) | OS (months) | DLT/SAE |
|---|---|---|---|---|---|---|---|---|
| 1 | M/65 | 200 | 10 | PR | PD | 6.5 | 17.7 | None |
| 2 | F/42 | 200 | 6 | PR | Surgery | 3.0 | 11.8 | None |
| 3 | M/68 | 200 | 15 | PR | PD | 10.2 | 37.4+ | None |
| 4 | F/60 | 400 | 3 | PR | Patient refused to continue treatment due to grade 3 hand-foot syndrome | 1.8 | 18.6 | Grade 4 neutropenia; grade 3 hand-foot syndrome |
| 5 | M/52 | 400 | 2 | PD | Intestinal obstruction | 0.85 | 1.5 | Intestinal obstruction |
| 6 | F/57 | 400 | 2 | PR | Sorafenib withdrawal over 30 days due to grade 3 neutropenia and fever | 2.5 | 8.7 | Grade 3 neutropenia complicated fever |
| 7 | M/59 | 200 | 6 | PR | PD | 3.9 | 9.8 | None |
| 8 | M/59 | 200 | 4 | SD | Patient refused to continue treatment due to grade 3 hand-foot syndrome | 0.9 | 10.8 | Grade 3 hand-foot syndrome |
| 9 | M/71 | 200 | 8 | PR | Withdrawn due to hepatic dysfunction | 6.0 | 18.7 | Grade 4 hepatic dysfunction |
+, this patient still survived at the last follow up time; PFS, progression-free survival; OS, overall survival; DLT, dose-limiting toxicity; SAE, serious adverse event; M, male; F, female; PR, partial response; PD, progressive disease; SD, stable disease.
Efficacy
Response rate (RR)
Among the nine evaluable patients, seven cases (77.8%) achieved PR, one patient with SD and one patient with progression disease (PD). In sorafenib 200 mg twice daily group, five patients (83.3%) achieved PR, and one patient had SD. In sorafenib 400 mg twice daily group, two patients (66.67%) achieved PR, and one patient with PD.
Survival
With a median follow-up period of 15 (range, 5-35) months, the median progression-free survival (PFS) was 3.0 [95% confidence interval (CI): 1.5-4.5] months (Figure 1). The median overall survival (OS) was 11.8 (95% CI: 8.9-14.7) months (Figure 2).
Figure 1.
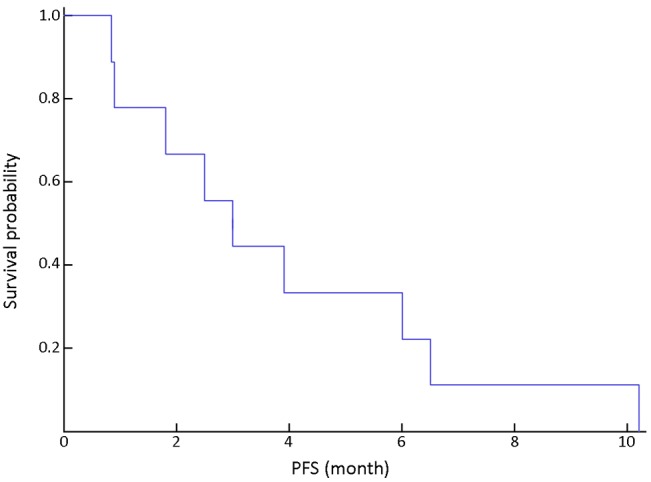
Progression-free survival (PFS) in all patients.
Figure 2.
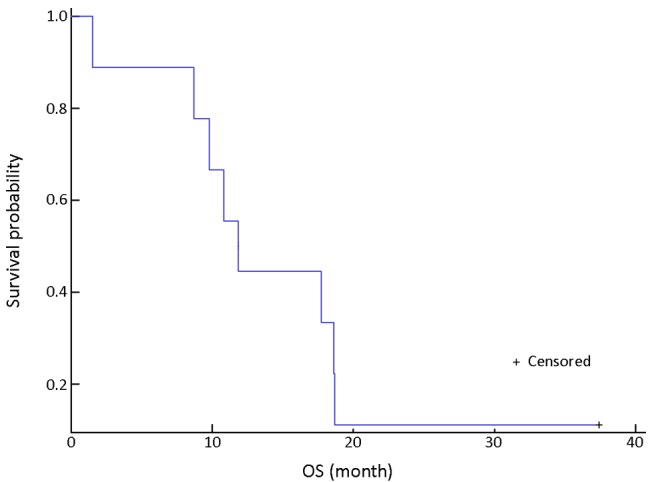
Overall survival (OS) in all patients.
Adverse events
In nine evaluable patients, common adverse events included hand-foot syndrome (100%), leukopenia (88.9%), neutropenia (88.9%), anorexia (88.9%) and nausea (66.7%). Grade 3 or 4 adverse events included neutropenia (77.8%), leukopenia (33.3%), hand-foot syndrome (22.2%) and hepatic dysfunction (11.1%) (Table 3).
Table 3. Occurrence of adverse events (n=9).
| Adverse events | N (%) |
|
|---|---|---|
| Grade 1 to 4 | Grade 3 to 4 | |
| Hematologic toxicity | ||
| Leukopenia | 8 (88.9) | 3 (33.3) |
| Neutropenia | 8 (88.9) | 7 (77.8) |
| Anemia | 3 (33.3) | |
| Thrombocytopenia | 1 (11.1) | |
| Non-hematologic toxicity | ||
| Cardiology | ||
| Hypertension | 3 (33.3) | |
| Dermatology | ||
| Hand-foot syndrome | 9 (100.0) | 2 (22.2) |
| Oral ulceration | 1 (11.1) | |
| Oral mucositis | 1 (11.1) | |
| Rash | 1 (11.1) | |
| Infectious disease | ||
| Fever | 2 (22.2) | |
| GI | ||
| Anorexia | 8 (88.9) | |
| Nausea | 6 (66.7) | |
| Vomiting | 3 (33.3) | |
| Constipation | 2 (22.2) | |
| Diarrhea | 2 (22.2) | |
| Liver dysfunction | ||
| ALT increase | 2 (22.2) | 1 (11.1) |
| AST increase | 1 (11.1) | 1 (11.1) |
| GGT increase | 1 (11.1) | 1 (11.1) |
| Billirubin increase | 1 (11.1) | 1 (11.1) |
| Others | ||
| Neurotoxicity | 1 (11.1) | |
| HTG | 1 (11.1) | |
| α-HBDH increase | 1 (11.1) | |
| LDH increase | 1 (11.1) | |
| Alopecia | 1 (11.1) | |
| Fatigue | 1 (11.1) | |
GI, gastrointestinal; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transferase; HTG, hypertriglyceridemia; α-HBDH, α-hydroxybutyrate dehydrogenase; LDH, lactate dehydrogenase.
Discussion
Chemotherapy is the major therapeutic method for advanced gastric cancer and gastroesophageal junction adenocarcinoma. But the efficacy of chemotherapy is unsatisfactory and the prognosis of advanced gastric cancer is poor. So there is still great unmeet medical need for this disease. Cytotoxic chemotherapy has already achieved a plateau and it is necessary to explore new therapeutic strategy to improve efficacy for this disease. FOLFOX4 was one of the recommended chemotherapy regimens in first-line treatment of advanced gastric cancer. The overall RR of FOLFOX4 was 36% to 50% and the median survival was 10 to 12 months which were all similar to other recommended chemotherapy regimens in first-line treatment of advanced gastric cancer (23-26). Sorafenib was an oral multikinase inhibitor, which targets Raf serine/threonine kinases (raf-1, b-raf), VEGFR-1/-2/-3, PDGFR-β and Flt-3, c-kit and p38 tyrosine kinases which can synergistically increase the efficacy of FOLFOX4 regimen (2). Promising efficacy and safety profile of sorafenib and oxaliplatin were shown in previous study (14). To better improve the efficacy of first-line treatment, we designed this phase I study to investigate the MTD, DLT and efficacy of sorafenib in combination with FOLFOX4 as first-line treatment in patients with advanced gastric cancer and gastroesophageal junction adenocarcinoma.
The overall RR of first-line chemotherapy for advanced gastric cancer was 25% to 50%, and the median survival was 8 to 13 months (16,17). In our study, the PR rate was 77.8%. The median OS was 11.8 months. This study demonstrated that FOLFOX4 regime plus sorafenib was associated with good efficacy in the first-line treatment of gastric cancer patients. In a phase II study of sorafenib combined with standard doses of docetaxel and cisplatin for patients with advanced gastric and gastroesophageal junction adenocarcinoma, the RR was 41% (26). Transtuzumab in combination with chemotherapy has been approved for the treatment of HER2-positive advanced gastric or gastroesophageal junction cancer patients. The Chinese subgroup analysis of the phase III, multi-center, randomized controlled trial (ToGA) comparing trastuzumab in combination with chemotherapy vs. chemotherapy alone for first-line treatment of HER2-positive advanced gastric or gastroesophageal junction cancer patients showed that the median survival of chemotherapy plus trastuzumab was 12.6 months. Additionally, 67% patients in our study are poorly differentiated adenocarcinoma and signet-ring cell carcinoma, and their histology types were more aggressive, which were associated with poorer survival. Even more aggressive histology types of gastric cancer patients are enrolled in our study, the FOLFOX4 plus sorafenib regimen still shows good efficacy which further confirmed the promising application prospect of this regimen.
The most common adverse events in this study were hand-foot syndrome, leukopenia, neutropenia, anorexia, and nausea. The incidences of grade 3 to 4 neutropenia and leucopenia were 77.8% and 33.3%, respectively. All patients developed hand-foot syndrome and 22.2% of the pateints were grade 3 to 4, especially in the FOLFOX4 plus sorafenib 400 mg twice daily group. Our study indicated that FOLFOX4 plus sorafenib was associated with more incidence of neutropenia and hand-food syndrome. So we reduced sorafenib dose to 200 mg twice daily even though the recommended dose of sorafenib was 400 mg twice daily.
Although the number of patients enrolled in our study was limited, this study is consistent with the phase I clinical trial standard to explore the MTD, DLT and efficacy of FOLFOX4 plus sorafenib regimen. For the reason that sorafenib has been widely used in various cancers (3,27-29), the pharmacokinetics and pharmacodynamics study of sorafenib was not investigated in our study. Based on the results of this study, we will carry out sample size enlarged phase II study to evaluate the efficacy of FOLFOX4 plus sorafenib in the near future. We also plan to conduct phase III clinical trials comparing FOLFOX4 plus sorafenib vs. standard first line FOLFOX4 for advanced gastric cancers. The exploration of molecular mechanism of patients who are sensitive to sorafenib and FOLFOX4 regimen is of great necessity but owing to the small sample size in this study, we did not do research in this aspect. The molecular mechanism is very important, and it could be further investigated in the phase II or phase III clinical trials.
Conclusions
Sorafenib (200 mg twice daily) in combination with FOLFOX4 shows good safety profile in patients with unresectable locally advanced or metastatic gastric cancer or gastroesophageal junction adenocarcinoma. Therefore, twice-daily dosing of sorafenib 200 mg in combination with FOLFOX4 could be an appropriate dosage for subsequent phase II clinical studies.
Acknowledgements
Funding: The study was supported by the Chinese National Major Project for New Drug Innovation (2008ZX09312, 2012ZX09303012), and we thank Bayer HealthCare Ltd. for partial financial support and Content Ed Net (Shanghai) Co., Ltd. for editorial support.
Disclosure: The authors declare no conflict of interest.
References
- 1.Sasako M, Inoue M, Lin JT, et al. Gastric Cancer Working Group report. Jpn J Clin Oncol 2010;40 Suppl 1:i28-37. [DOI] [PubMed] [Google Scholar]
- 2.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 2004;64:7099-109. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007;356:125-34. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [DOI] [PubMed] [Google Scholar]
- 5.Bracarda S, Porta C, Boni C, et al. Randomized prospective phase II trial of two schedules of sorafenib daily and interferon-α2a (IFN) in metastatic renal cell carcinoma (RAPSODY): GOIRC Study 0681. J Clin Oncol (Meeting Abstracts) 2007;25:5100.17991928 [Google Scholar]
- 6.Eisen T, Marais R, Affolter A, et al. An open-label phase II study of sorafenib and dacarbazine as first-line therapy in patients with advanced melanoma. J Clin Oncol (Meeting Abstracts) 2007;25:8529. [Google Scholar]
- 7.McDermott DF, Sosman JA, Hodi FS, et al. Randomized phase II study of dacarbazine with or without sorafenib in patients with advanced melanoma. J Clin Oncol (Meeting Abstracts) 2007;25:8511.18445842 [Google Scholar]
- 8.Soria J, Lazar V, Lassau N, et al. Sorafenib (S) and dacarbazine (D) combination in patients (pts) with advanced malignant solid tumors: Phase I study with tumor biopsy genomic analysis and dynamic contrast enhanced ultrasonography (DCE-US). J Clin Oncol (Meeting Abstracts) 2007;25:3556. [Google Scholar]
- 9.Richly H, Kupsch P, Passarge K, et al. Results of a phase I trial of BAY 43-9006 in combination with doxorubicin in patients with refractory solid tumors. J Clin Oncol (Meeting Abstracts) 2004;22:3049. [DOI] [PubMed] [Google Scholar]
- 10.Siu LL, Awada A, Takimoto CH, et al. Phase I trial of sorafenib and gemcitabine in advanced solid tumors with an expanded cohort in advanced pancreatic cancer. Clin Cancer Res 2006;12:144-51. [DOI] [PubMed] [Google Scholar]
- 11.Wallace JA, Locker G, Nattam S, et al. Sorafenib (S) plus gemcitabine (G) for advanced pancreatic cancer (PC): A phase II trial of the University of Chicago Phase II Consortium. J Clin Oncol (Meeting Abstracts) 2007;25:4608. [Google Scholar]
- 12.Welch S, Hirte H, Elit L, et al. CA-125 response as a marker of clinical benefit in patients with recurrent ovarian cancer treated with gemcitabine and sorafenib–A trial of the PMH Phase II Consortium. J Clin Oncol (Meeting Abstracts) 2007;25:5519.18048829 [Google Scholar]
- 13.Roth AD, Ajani J. Docetaxel-based chemotherapy in the treatment of gastric cancer. Ann Oncol 2003;14 Suppl 2:ii41-4. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Richard M, Gallego R, Pericay C, et al. Multicenter phase II study of oxaliplatin and sorafenib in advanced gastric adenocarcinoma after failure of cisplatin and fluoropyrimidine treatment. A GEMCAD study. Invest New Drugs 2013;31:1573-9. [DOI] [PubMed] [Google Scholar]
- 15.Eriguchi M, Nonaka Y, Yanagie H, et al. A molecular biological study of anti-tumor mechanisms of an anti-cancer agent Oxaliplatin against established human gastric cancer cell lines. Biomed Pharmacother 2003;57:412-5. [DOI] [PubMed] [Google Scholar]
- 16.Kim DY, Kim JH, Lee SH, et al. Phase II study of oxaliplatin, 5-fluorouracil and leucovorin in previously platinum-treated patients with advanced gastric cancer. Ann Oncol 2003;14:383-7. [DOI] [PubMed] [Google Scholar]
- 17.Chao Y, Yeh KH, Chang CJ, et al. Phase II study of weekly oxaliplatin and 24-h infusion of high-dose 5-fluorouracil and folinic acid in the treatment of advanced gastric cancer. Br J Cancer 2004;91:453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Louvet C, André T, Tigaud JM, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol 2002;20:4543-8. [DOI] [PubMed] [Google Scholar]
- 19.Al-Batran SE, Atmaca A, Hegewisch-Becker S, et al. Phase II trial of biweekly infusional fluorouracil, folinic acid, and oxaliplatin in patients with advanced gastric cancer. J Clin Oncol 2004;22:658-63. [DOI] [PubMed] [Google Scholar]
- 20.Suh SH, Kwon HC, Jo JH, et al. Oxaliplatin with biweekly low dose leucovorin and bolus and continuous infusion of 5-fluorouracil (modified FOLFOX 4) as a salvage therapy for patients with advanced gastric cancer. Cancer Res Treat 2005;37:279-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omura GA. Modified Fibonacci search. J Clin Oncol 2003;21:3177. [DOI] [PubMed] [Google Scholar]
- 22.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. [DOI] [PubMed] [Google Scholar]
- 23.Shi M, Ji J, Wu J, et al. Cetuximab combined with FOLFOX4 as the first-line treatment for advanced gastric cancer: report of 25 cases from a single institution. Hepatogastroenterology 2012;59:1054-8. [DOI] [PubMed] [Google Scholar]
- 24.Mohammad HA, Magdy FM, Mahmoud OM. FOLFOX (oxaliplatin and 5 fluorouracil/leucovorin) in patients with untreated metastatic gastric adenocarcinoma Phase II study. Indian J Cancer 2011;48:460-5. [DOI] [PubMed] [Google Scholar]
- 25.De Vita F, Orditura M, Matano E, et al. A phase II study of biweekly oxaliplatin plus infusional 5-fluorouracil and folinic acid (FOLFOX-4) as first-line treatment of advanced gastric cancer patients. Br J Cancer 2005;92:1644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun W, Powell M, O'Dwyer PJ, et al. Phase II study of sorafenib in combination with docetaxel and cisplatin in the treatment of metastatic or advanced gastric and gastroesophageal junction adenocarcinoma: ECOG 5203. J Clin Oncol 2010;28:2947-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boudou-Rouquette P, Thomas-Schoemann A, Bellesoeur A, et al. Sorafenib for patients with differentiated thyroid cancer. Lancet 2015;385:227-8. [DOI] [PubMed] [Google Scholar]
- 28.White PT, Cohen MS. The discovery and development of sorafenib for the treatment of thyroid cancer. Expert Opin Drug Discov 2015;10:427-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ni H, Yang M, Guo Z, et al. Sorafenib combined with cryoablation to treat unresectable hepatocellular carcinoma. Chin J Cancer Res 2011;23:188-93. [DOI] [PMC free article] [PubMed] [Google Scholar]


