Abstract
Objective
Human epididymis protein 4 (HE4) is a promising biomarker of epithelial ovarian cancer (EOC). But its role in assessing the primary optimal debulking (OD) of EOC remains unknown. The purpose of this study is to elucidate the ability of preoperative HE4 in predicting the primary cytoreductive outcomes in advanced EOC, tubal or peritoneal carcinoma.
Methods
We reviewed the records of 90 patients with advanced ovarian, tubal or peritoneal carcinoma who underwent primary cytoreduction at the Department of Obstetrics and Gynecology of Peking University People’s Hospital between November 2005 and October 2010. Preoperative serum HE4 and CA125 levels were detected with EIA kit. A receiver operating characteristic (ROC) curve was used to determine the most useful HE4 cut-off value. Logistic regression analysis was performed to identify significant preoperative clinical characteristics to predict optimal primary cytoreduction.
Results
OD was achieved in 47.7% (43/48) of patients. The median preoperative HE4 level for patients with OD vs. suboptimal debulking was 423 and 820 pmol/L, respectively (P<0.001). The areas under the ROC curve for HE4 and CA125 were 0.716 and 0.599, respectively (P=0.080). The most useful HE4 cut-off value was 473 pmol/L. Suboptimal cytoreduction was obtained in 66.7% (38/57) of cases with HE4 ≥473 pmol/L compared with only 27.3% (9/33) of cases with HE4 <473 pmol/L. At this threshold, the sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for diagnosing suboptimal debulking were 81%, 56%, 67%, and 73%, respectively. Logistic regression analysis showed that the patients with HE4 ≥473 pmol/L were less likely to achieve OD (odds ratio =5.044, P=0.002).
Conclusions
Preoperative serum HE4 may be helpful to predict whether optimal cytoreductive surgery could be obtained or whether extended cytoreduction would be needed by an interdisciplinary team.
Keywords: Human epididymis protein 4 (HE4), advanced epithelial ovarian cancer (EOC), optimal cytoreduction, CA125
Introduction
Ovarian carcinoma (OC) is a major cause of gynecologic cancer-related mortality worldwide. GLOBOCAN 2012 estimated that approximately 238,719 women would be newly diagnosed with ovarian carcinoma and nearly 151,917 would die from this disease annually (1). Among women with ovarian carcinoma, 70% are diagnosed in the advanced stages (stage III or IV) (2). Optimal primary surgical cytoreduction followed by platinum-based chemotherapy is the standard management for advanced epithelial ovarian cancer (EOC). Numerous studies have demonstrated that optimal debulking (OD) can extend progression free survival (PFS) and improve overall survival (OS) for advanced ovarian cancer (3,4). According to the Gynecologic Oncology Group (GOG), the current definition of “optimal cytoreduction” has been revised to less than 1 cm of residual disease at any anatomic site.
Over the past two decades, significant research has focused on developing applicable preoperative factors to predict the outcome of primary cytoreduction using serologic, radiological, and surgical tools. Preoperative serum CA125, one of the most studied strategies, has been used for years as a criterion standard biomarker for diagnosing ovarian cancer and monitoring the responses to therapy, and as a potential tool for predicting success rates of OD. Based on the assumption that higher preoperative serum CA125 values were directly correlated with larger tumor burdens, a useful cut-off level of CA125 was thought to help differentiate patients with ovarian tumors that were more likely to be optimally debulked. However, according to the last ten years of research, there is no consensus about whether preoperative CA125 levels can predict primary surgical cytoreduction (5-9). In 2000, Chi et al. (5) reported a study (n=100) on the role of CA125 in predicting optimal cytoreduction for advanced ovarian cancer, with sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 78%, 73%, 78% and 73%, respectively. In 2009, Chi et al. (6) published another retrospective study on 277 patients with advanced ovarian, tubal and peritoneal cancers using CA125 measurements before primary surgery. The study showed that preoperative CA125 did not predict OD of patients with advanced ovarian cancer following changes in surgical paradigm that extended upper abdominal procedures to attain OD. The current situation remains elusive, and more studies are needed to find other strategies to predict surgical resectability.
Identified by a recent research, human epididymis protein 4 (HE4) is considered to be one of the most promising new serum biomarkers for ovarian cancer. HE4 (gene name WFDC2) is a glycoprotein that was originally found in the epithelial cells of the human epididymis. Positive expression was found in 93% of serous, 100% of endometrioid and 50% of clear cell carcinomas (10). HE4 has equivalent sensitivity but higher specificity compared to CA125 for detecting ovarian malignancy, and a combination of the two markers could be complementary (11-13). In a prospective study (14) testing whether multiple serum tumor markers alone or in combination can improve the value of evaluating women with pelvic masses, HE4 was reported to display a sensitivity of 72.9% (specificity 95.0%) and serum CA125 only had a sensitivity of 43.3%. In combination, HE4 and CA125 achieved a sensitivity of 76.4% (specificity 95.0%), which was higher than either test alone. HE4 is less likely to be falsely elevated in benign neoplasms compared to serum CA125 and can be used to differentiate endometriomas and endometriosis from malignant ovarian tumors (15). However, the ability of preoperative HE4 levels in predicting tumor resectability is not well studied.
This study aims to explore the ability of preoperative HE4 levels to predict primary cytoreductive outcomes for advanced ovarian, tubal, and primary peritoneal carcinoma (PPC).
Materials and methods
Study population
We conducted a retrospective review in inpatients with EOC or PPC or primary fallopian tube carcinoma (PFTC) treated at the Department of Obstetrics and Gynecology of Peking University People’s Hospital. The study was approved by the institutional review board of the hospital, and the informed consent of all the patients was obtained. All patients with pathologically confirmed stage III or IV EOC, PPC or PFTC, who underwent primary cytoreductive surgery between November 2005 and October 2010, were identified from the tumor registry and pathology databases and screened for study inclusion. Patients with borderline cancers, recurrent ovarian tumors, ovarian metastases from other primaries, or those treated with neoadjuvant chemotherapy (NACT) prior to attempted cytoreduction were excluded. Two pathologists reviewed all histologic material of these patients with International Federation of Gynecology and Obstetrics (FIGO) stage III and IV EOC, PPC or PFTC, assigned the histological type according to the WHO criteria and graded the tumors to verify the diagnoses. The following demographic and clinical data were reviewed: age of diagnosis, extent of operation, sites and diameters of residual tumor nodule, ascites volume, tumor pathologic grade and FIGO stage, histologic subtype, lymph node status (positive or negative), preoperative serum CA125 level, and cytoreductive outcome (using the threshold of ≤1 cm residual disease to identify “optimal” or “suboptimal”). Residual tumor was defined as the maximal dimension of single largest cancer nodule at the end of cytoreductive surgery. We classified the residual disease as follows: no gross visible, gross residue 1-10, 11-20 and >20 mm. The surgery outcome (whether optimal or not) was confirmed by a chief gynecologic oncologist according to the operation note from the medical record database, The gynecologic oncologist was exempt from the surgeon and the assistant and didn’t know the result of CA125 and HE4.
Specimen collection and processing
The preoperative serum samples of these patients were selected from serum repository of Gynecology Oncology Center. Serum samples were collected from patients under fasting conditions before surgery using a vacuum blood collection tube without an anticoagulant. Collected samples were allowed to clot and centrifuged at 3,000 r/min for 10 min, and then were stored and frozen at −80 °C.
Measurement of HE4
Quantitative determination of HE4 and CA125 was performed using the EIA kits from CanAg Technical Consulting Service Ltd. (Beijing, China) according to manufacturer’s instructions. Frozen sera were thawed slowly at 4 °C, and the testing was performed at room temperature. The HE4 assay results were considered valid if the mean values of control duplicates were within the specified ranges. Three copies of high, moderate and low level samples and two copies of high and low level control materials were repeated for 10 times, then the coefficient of variation (CV) was calculated. The highest CV was 8.4%, which met the testing requirement. We repeated the experiment to confirm the reliability. The results were presented as median values.
Statistical analysis
The SPSS 17.0 software package (SPSS Inc., Chicago, IL, USA) was used in this study. Receiver operating characteristic (ROC) curves were used to calculate the most useful HE4 cut-off value. MedCalc (MedCalc Software bvba, Belgium) was used to compare the statistical difference between the ROC curves of HE4 and CA125. Quantitative variables were described by median and range (minimum-maximum) for abnormal distribution. The Chi-square test, Wilcoxon rank sum test, and the logistic regression analysis (Backward-Wald method, removal probability for stepwise was 0.10) were used for data analysis. P values were two-sided, and P<0.05 was considered statistically significant.
Results
Baseline characteristics
The study cohort included 90 patients. Primary disease sites were: ovary 73 (81%), tubal 6 (7%), and peritoneum 11 (12%). Stages were: IIIa 1 (1%), IIIb 4 (4%), IIIc 76 (84%); IV 9 (10%). Tumor grades were: grade 1, 3 (3.3%); grade 2, 13 (14.4%); grade 3, 74 (82.2%). All these patients underwent an attempt of maximal surgical cytoreduction unless there were unresectable diseases as determined by the attending surgeon. Primary cytoreduction surgery was performed for all cases by senior gynecologic oncologists.
The majority of patients underwent hysterectomy (89 cases, 99%); omentectomy was performed on all the patients; 13% (12/90) of patients underwent aggressive surgery in addition to standard surgery, including bowel resection (sigmoid resection with high anastomosis or colostomy, transverse colon resection, ileocecal resection, and/or small bowel resection), resection of mesentery disease and subtotal gastrectomy. Lymph node dissection (pelvic and/or para-aortic) was performed in 60 cases (67%).
The cytoreduction outcomes are as follows: no gross visible, 22/90 (24.4%); gross residue 1-10 mm, 21/90 (23.3%); gross residue 11-20 mm, 2/90 (2.3%); gross residue >20 mm, 45/90 (50.0%). The OD rate was 47.7%.
Table 1 shows patient characteristics and median values of serum HE4 based on FIGO stage, pathologic grade, histology type, lymph node status, surgery status, cytoreductive outcome, ascites volume and location of residual disease. The median age was 55 (range, 26-79) years old. The majority of cases had primary ovarian cancer (81%), stage III disease (90%), grade 3 tumor (81%) and serous histology (64%). Ascites were present in 90% of the patients with a median volume of 1,500 mL (range, 0-8,500 mL). Lymph node dissection (pelvic and/or para-aortic) was performed in 67% (60/90) of the patients, and 62% (37/60) of these patients had positive lymph nodes at final pathology.
Table 1. Median serum HE4 levels and clinical characteristics of patients (N=90).
| Characteristics | N | % | Serum HE4 [median (range)] (pmol/L) | P |
|---|---|---|---|---|
| FIGO stage | 0.995 | |||
| III | 81 | 90.0 | 633 (39-7,744) | |
| IV | 9 | 10.0 | 664 (46-2,131) | |
| Grade | 0.793 | |||
| 1 | 3 | 3.3 | 273 (87-896) | |
| 2 | 13 | 14.4 | 567 (78-7,744) | |
| 3 | 74 | 82.2 | 660 (39-6,671) | |
| Histopathology | 0.800 | |||
| Serous | 58 | 64.4 | 645 (46-2,986) | |
| Non-serous | 32 | 35.6 | 648 (39-7,744) | |
| Lymph node status | 0.314 | |||
| No lymphadenectomy | 30 | 33.3 | 751 (39-7,744) | |
| Negative | 23 | 25.6 | 406 (78-1,693) | |
| Positive | 37 | 41.1 | 618 (46-2,986) | |
| Residual disease (mm) | 0.330 | |||
| No gross visible | 22 | 24.4 | 326 (78-1,403) | |
| 1-10 | 21 | 23.3 | 567 (201-1,335) | |
| 11-20 | 2 | 2.3 | 925 (896-953) | |
| >20 | 45 | 50.0 | 820 (39-7,744) | |
| Optimal or suboptimal cytoreduction | <0.001 | |||
| Optimal | 43 | 47.8 | 423 (78-1,403) | |
| Suboptimal | 47 | 52.2 | 820 (39-7,744) | |
| Ascites [median (range)] (mL) | 0.015 | |||
| ≤1,000 [200 (0-1,000)] | 41 | 45.5 | 446 (39-2,986) | |
| >1,000 [3,000 (1,500-8,500)] | 49 | 54.4 | 745 (78-7,744) | |
| Location of residual tumor in suboptimal cytoreduction (n=47) | 0.287 | |||
| Pelvis | 27 | 57.4 | 782 (39-2,986) | |
| Omentum | 23 | 48.9 | 950 (191-7,744) | |
| Upper abdomen | 8 | 17.0 | 1,178 (477-2,825) | |
| Retroperitoneal node | 3 | 6.4 | 789 (618-1,033) | |
| Other | 2 | 4.3 | 919 (789-1,049) | |
HE4, human epididymis protein 4; FIGO, International Federation of Gynecology and Obstetrics.
The median preoperative serum HE4 level was645 pmol/L (range, 39-7,744 pmol/L). OD was achieved in 47.7% (43/90) of patients. The median value of preoperative HE4 for patients with OD vs. suboptimal debulking was 423 pmol/L (range, 78-1,403 pmol/L) and 820 pmol/L (range, 39-7,744 pmol/L), respectively (P<0.001). The OD rates for patients with ascites volume ≤1,000 mL and with ascites volume >1,000 mL were 61% and 37%, respectively (P=0.022). The median presurgical HE4 was 446 pmol/L (range, 39-2,986 pmol/L) in patients with ascites volume ≤1,000 mL and 745 pmol/L (range, 78-7,744 pmol/L) in patients with ascites volume >1,000 mL (P=0.015). Patients presenting higher circulatory levels of HE4 were more likely to have a suboptimal cytoreduction (P<0.001).
Correlation with cytoreductive outcomes
ROC curves are shown in Figure 1 (HE4) and Figure 2 (CA125). The areas under the curve (AUCs) of HE4 and CA125 in predicting suboptimal debulking vs. OD were 0.716 [95% confidence interval (95% CI): 0.611-0.822] and 0.599 (95% CI: 0.481-0.717), respectively. But there was no statistically significant difference by MedCalc analysis (P=0.080) (Figure 3). The most suitable clinical HE4 cut-off value was 473 pmol/L. At an HE4 cut-off value of 473 pmol/L, the analysis reached a sensitivity of 81% and a specificity of 56% for predicting suboptimal debulking with PPV of 67%, NPV of 73%, positive likelihood ratio of 1.84 and negative likelihood ratio of 0.34. Of the 57 patients with HE4 ≥473 pmol/L, OD was not achieved in 38 (67%) patients. Of the 33 patients with HE4 <473 pmol/L, OD was not achieved in 9 (27%) patients (P<0.001). At a CA125 cut-off value of 500 U/mL, the analysis reached a sensitivity of 80% and a specificity of 40% for predicting suboptimal debulking with PPV of 59%, NPV of 65%, positive likelihood ratio of 1.34 and negative likelihood ratio of 0.50. The relationship between clinical characteristics and cytoreductive outcomes are shown in Table 2.
Figure 1.
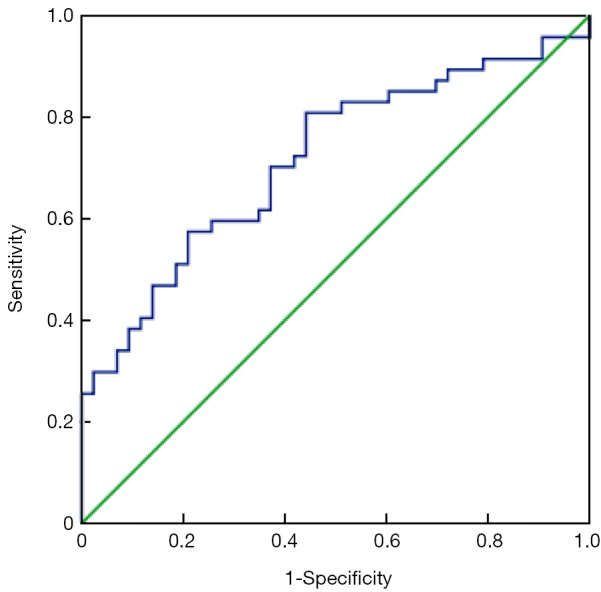
ROC curve shows the correlation between sensitivity and false-positive rate using serum HE4 level alone (AUC =0.716, 95% CI: 0.611-0.822). ROC, receiver operating characteristic; HE4, human epididymis protein 4; AUC, area under the curve; CI, confidence interval.
Figure 2.
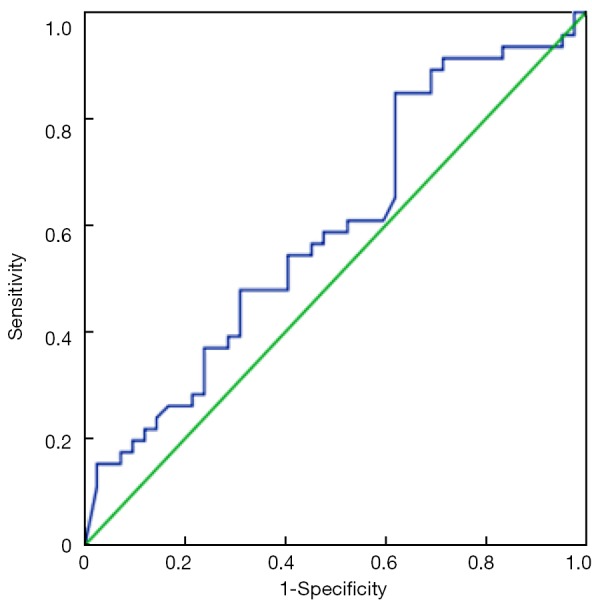
ROC curve shows the correlation between sensitivity and false-positive rate using serum CA125 level alone (AUC =0.599, 95% CI: 0.481-0.717). ROC, receiver operating characteristic; AUC, area under the curve; CI, confidence interval.
Figure 3.
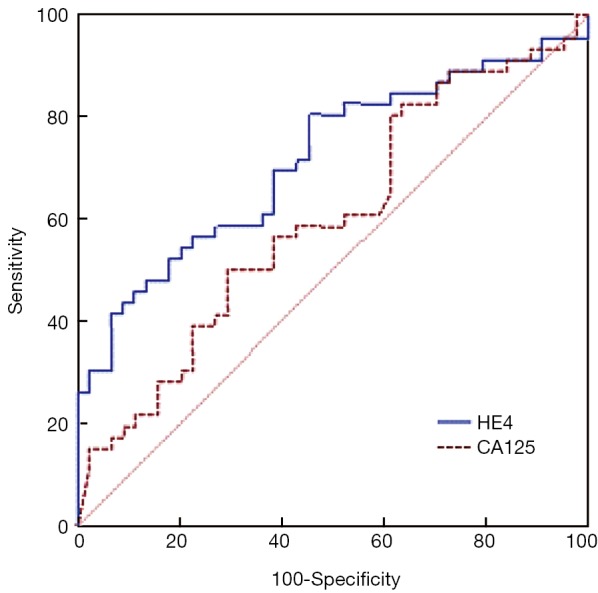
ROC curve analysis (using MedCalc) to compare HE4 and CA125 for prognosing the optimal outcome of primary surgery. No statistical difference in AUC is found between HE4 and CA125 (P=0.080). ROC, receiver operating characteristic; HE4, human epididymis protein 4; AUC, area under the curve.
Table 2. Relationship between clinical characteristics and cytoreductive outcomes.
| Characteristics | Optimal debulking* |
Suboptimal debulking** |
P | |||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| FIGO stage | 0.360 | |||||
| III | 40 | 49.4 | 41 | 50.6 | ||
| IV | 3 | 33.3 | 6 | 66.7 | ||
| Grade | 0.636 | |||||
| 1 | 2 | 66.7 | 1 | 33.3 | ||
| 2 | 5 | 38.5 | 8 | 61.5 | ||
| 3 | 36 | 48.6 | 38 | 51.4 | ||
| Histopathology | 0.059 | |||||
| Serous | 32 | 55.2 | 26 | 44.8 | ||
| Non-serous | 11 | 34.4 | 21 | 65.6 | ||
| Preoperative HE4 (pmol/L) | <0.001 | |||||
| <473 | 24 | 72.7 | 9 | 27.3 | ||
| ≥473 | 19 | 33.3 | 38 | 66.7 | ||
| Preoperative CA125 (U/mL) | 0.033 | |||||
| <500 | 17 | 65.4 | 9 | 34.6 | ||
| ≥500 | 26 | 40.6 | 38 | 59.4 | ||
| Lymph node status | <0.001 | |||||
| No lymphadenectomy | 2 | 6.7 | 28 | 93.3 | ||
| Negative | 17 | 73.9 | 6 | 26.1 | ||
| Positive | 24 | 64.9 | 13 | 35.1 | ||
| Ascites [median (range)] (mL) | 0.022 | |||||
| ≤1,000 [200 (0-1,000)] | 25 | 61 | 16 | 39 | ||
| >1,000 [3,000 (1,500-8,500)] | 18 | 36.7 | 31 | 63.3 | ||
*, “optimal” was identified using the threshold of ≤1 cm residual disease; **, “suboptimal” was with residual disease >1 cm. FIGO, International Federation of Gynecology and Obstetrics; HE4, human epididymis protein 4.
Correlation with prognostic factors
Binary logistic regression analysis was performed to analyze the preoperative clinical characteristics that influence the outcome of primary surgical cytoreduction, such as age of diagnosis, ascites volume, and preoperative HE4 and CA125 levels. The results showed that the significant predictor of the feasibility to achieve optimal cytoreduction was an HE4 level at a threshold of 473 pmol/L. Patients with HE4 ≥473 pmol/L were associated with a lower probability of achieving OD, with an odds ratio of 5.044 (P=0.002). Patients with CA125 ≥500 U/mL were associated with a lower probability of achieving OD (odds ratio =2.870, P=0.064) (Table 3). Based on these data, preoperative HE4 levels are more effective than CA125 levels for predicting primary surgical outcomes.
Table 3. Binary logistic regression analysis of factors influencing primary surgical cytoreduction outcomes.
| Variables | B | S.E. | Wald | df | Sig. | Exp(B) | 95% CI |
|---|---|---|---|---|---|---|---|
| Age | 0.045 | 0.027 | 2.779 | 1 | 0.096 | 1.046 | 0.092-1.104 |
| Preoperative HE4 | 1.618 | 0.517 | 9.812 | 1 | 0.002 | 5.044 | 1.832-13.883 |
| Preoperative CA125 | 1.054 | 0.569 | 3.427 | 1 | 0.064 | 2.870 | 0.940-8.760 |
| Constant | −2.945 | 1.567 | 3.533 | 1 | 0.060 | 0.053 | − |
B, coefficient of regression; S.E., standard error; df, degree of free; Sig., significance; Exp(B), odds ratio; CI, confidence interval; HE4, human epididymis protein 4.
Discussion
Maximal cytoreductive surgery that does not leave macroscopic disease or residual disease of less than 1 cm is beneficial to prolong PFS and improve OS in patients with advanced ovarian, tubal and peritoneal cancer.
There are numerous studies comparing the PFS/OS and peri-operative morbidities of patients treated with primary debulking surgeries (PDS) followed by adjuvant platinum-based chemotherapy or NACT, or followed by interval debulking surgery (IDS) in advanced ovarian cancer. Vergote et al. (16) concluded that in stage IIIc-IV ovarian cancer, NACT followed by debulking surgery produces similar OS and PFS outcomes compared to PDS. Due to the lower morbidity of IDS compared to PDS, NACT may be considered as a preferred treatment. Hou et al. (17) reported similar survival rates for these two groups, with OD rates of 95% and 71% for patients receiving NACT + IDS and PDS, respectively. Patients with extra-abdominal diseases, who had received carboplatin/paclitaxel as NACT had improved PFS and OS when compared to the PDS group with stage IV disease (15 vs. 9 months, P=0.015; 31 vs. 20 months, P=0.032, respectively). In the Vergote’s study, NACT was associated with a higher rate of optimal cytoreduction with reduced need for further aggressive surgery, lower peri-operative morbidity, less intra-operative blood loss, operating time, units of transfusion, shorter hospital stays and similar survival rates. Anyway, up to now there is no consensus on the NACT-IDS, and most gynecological oncologists prefer to choose maximal primary cytoreduction. Hence the need to address the ideal timing of cytoreduction has assumed greater clinical importance.
Optimal cytoreduction rates vary from 41.5% to 80.0% according to current literature as shown in Table 4 (5-9). Optimal cytoreduction rates, which vary from time to time, are based on experiences at different institutions, individual surgeons, population, and the adoption of extensive upper abdominal procedures. Higher optimal cytoreduction rates have been achieved with increasing operative morbidity, operative time and blood loss. Bristow (18) described the issue of “optimal cytoreduction” as a “moving target”. Therefore, the concept of the ability to predict an extremely variable outcome has proven to be impossible to achieve with sufficient adequacy with time. In this study, the optimal cytoreduction rate of 47.7% is comparable to rates achieved by many other contemporary institutions. Table 4 compares studies assessing the utility of preoperative CA125 and/or HE4 to predict OD.
Table 4. Comparison of studies assessing utility of preoperative CA125 and/or HE4 to predict OD.
| Study | No. of stage III and IV | Age (year)a | OD rate (%) | Item | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | AUC |
|---|---|---|---|---|---|---|---|---|---|---|
| Chi et al. (6) | 277b | 62 | 80 | CA125 | 500 U/mL | 45 | 64 | 83 | 23 | No area calculated |
| Vorgias et al. (7) | 426c | 62 | 41.5 | CA125 | 500 U/mL | 78.5 | 89.6 | 85 | 85 | No area calculated |
| Angioli et al. (8) | 57d | 59 | 63.2 | CA125 | 414 U/mL | 58 | 84 | 80 | 51 | 0.68 |
| HE4 | 262 pmol/L | 86 | 89 | 93 | 77 | 0.86 | ||||
| Barlow et al. (9) | 164e | 58 | 47 | CA125 | 500 U/mL | 66 | 59 | 64 | 36 | 0.67 |
| Tang et al. | 90 | 55 | 48 | CA125 | 500 U/mL | 80 | 40 | 59 | 65 | 0.60 |
| HE4 | 473 pmol/L | 81 | 56 | 61 | 73 | 0.72 |
a, median; b, ovarian cancer 232, tube cancer 9 and peritoneal carcinoma 36 (stage III 233, stage IV 44); c, ovarian cancer stage III and IV only; d, ovarian cancer only; e, ovarian cancer stage III and IV only; HE4, human epididymis protein 4; OD, optimal debulking; PPV, positive predictive value; NPV, negative predictive value; AUC, area under the curve.
In more utilization of extensive upper abdominal procedures and more collaboration by an interdisciplinary team, there was no threshold CA125 level for accurately predicting cytoreduction outcome of ovarian cancer. However, when preoperative CA125 is higher than 500 U/mL, extensive upper abdominal procedures were more necessary to achieve residual disease less than 1 cm (6).
In recent researches, HE4 is considered to be one of the most promising new serum biomarkers for ovarian cancer. Angioli et al. (8) published a prospective research, in which 59 patients affected by suspicious advanced ovarian cancer had serum CA125 and HE4 measured preoperatively. After a complete laparoscopy to assess the possibility of OD surgery, the patients were submitted to primary cytoreductive surgery or addressed to NACT. As a result, the HE4 level of 262 pmol/L is the best cut-off value to identify patient candidates to optimal cytoreduction with a sensitivity of 86.1% and a specificity of 89.5% better than CA125 with a sensitivity of 58.3% and a specificity of 84.0% at a cut-off value of 414 UI/mL (AUC is 0.860 and 0.680 respectively, P<0.001).
This study reported the utility of preoperative HE4 levels to predict the outcomes of primary cytoreduction surgery and compared with CA125 levels in the same patient cohort. The AUC to predict suboptimal vs. optimal surgery was greater for HE4 than for CA125 (0.716 vs. 0.599). We identified 38 of the 47 patients (81%) who underwent suboptimal cytoreduction using preoperative serum HE4 levels above 473 pmol/L. It is interesting that 7 of the 9 patients with preoperative HE4 levels below 473 pmol/L had preoperative CA125 levels above 500 U/mL. It is reasonable to hypothesize that preoperative HE4 levels combined with CA125 levels may improve the predictive values for suboptimal cytoreduction. Moreover, binary logistic regression supported HE4 levels as the most important factor of patient’s presurgical characteristics to influence the feasibility of optimal cytoreduction. The ROC curves between HE4 and CA125 showed no statistically significant difference, and it may be associated with limited sample size and retrospective analysis, so it is needed to expand sample size and design prospective studies in the future.
The cut-off value of the preoperative HE4 level above 473 pmol/L did not mean that all patients with preoperative serum HE4 values over 473 pmol/L were absolutely unable to achieve optimal cytoreduction. Instead, perhaps as a consequence of increased tumor burden, there is a higher likelihood that the tumor would diffuse to and implant in “unresectable” locations, such as the root of mesentery, colonic surface, porta hepatis, splenic hilum or other upper abdominal areas, where attempts to completely debulk become more difficult.
In our study, the proportion of optimal resection in patients with preoperative serum HE4 >473 pmol/L was 33% (19/57) and 0% in patients with preoperative HE4 >1,500 pmol/L, suggesting that patients with preoperative HE4 levels above 473 pmol/L still have a reasonable chance for OD compared with patients with HE4 levels above 1,500 pmol/L. According to our results, patients with HE4 levels greater than 1,500 pmol/L may be candidates for NACT.
The presence of clinically appreciable massive ascites was another deterrent to OD. Prognosis has been shown to be adversely affected by the presence of more than 1,000 mL of ascites (4). Although we did not find a significant association with optimal cytoreduction outcome by logistic regression analysis, we did discover that there was a statistically significant difference in the median of HE4 presurgical levels between those with ascites ≥1,000 mL and those with ascites <1,000 mL, suggesting that there was a greater degree of peritoneal disease with an increased volume of ascites. The volume of ascites may be helpful in predicting the scope of abdominal diseases.
Other investigations have analyzed the utility of computed tomography (CT) scans to predict cytoreduction outcomes. Researches which published long ago showed that it was difficult to devise universally applicable selection criteria or models of CT reports that reliably predict surgical outcomes across institutions and surgeons (19). Although the NPV of CT scans is often greater than that of CA125, Ferrandina et al. (20) investigated the role of CT scans and clinical evaluations to predict the OD outcome and concluded that CT scans still represent a valid tool to predict ovarian cancer optimal cytoreduction. The predictive ability would be improved by integrating clinical information. Suidan et al. (21) assessed the ability of preoperative CT scan of the abdomen/pelvis and serum CA125 to predict suboptimal primary cytoreduction. He developed a predictive model included age ≥60 years old (P=0.010), CA125 ≥500 U/mL (P<0.001), suprarenal retroperitoneal lymph nodes >1 cm (P<0.001), diffuse small bowel adhesions/thickening (P<0.001), lesions >1 cm in the small bowel mesentery (P=0.030), root of the superior mesenteric artery (P=0.003), perisplenic area (P<0.001), and lesser sac (P<0.001). The prognostic model combining these factors had a predictive accuracy of 0.758.
Most researched showed us that all clinical information such as age, complications, tumor markers and radiological findings by pelvic and abdominal CT or magnetic resonance imaging (MRI) should be integrated to make an optimal assessment for preoperative evaluation of primary cytoreduction. In general, preoperative serum HE4 may be helpful to predict whether optimal cytoreductive surgery will be obtained, or whether extended cytoreduction would be needed by an interdisciplinary team. Large prospective research is needed to verify the value of preoperative HE4 levels in predicting OD.
Acknowledgements
Funding: This study was supported by Natural Science Foundation of China (NSFC-81172454) and the Specialized Research Fund for Doctoral Program of Higher Education (SRFDR-20100001110079).
Disclosure: The authors declare no conflict of interest.
References
- 1.International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Accessed in 2015. Available online: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [DOI] [PubMed] [Google Scholar]
- 3.Bristow RE, Tomacruz RS, Armstrong DK, et al. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol 2002;20:1248-59. [DOI] [PubMed] [Google Scholar]
- 4.Peiretti M, Zanagnolo V, Aletti GD, et al. Role of maximal primary cytoreductive surgery in patients with advanced epithelial ovarian and tubal cancer: Surgical and oncological outcomes. Single institution experience. Gynecol Oncol 2010;119:259-64. [DOI] [PubMed] [Google Scholar]
- 5.Chi DS, Venkatraman ES, Masson V, et al. The ability of preoperative serum CA-125 to predict optimal primary tumor cytoreduction in stage III epithelial ovarian carcinoma. Gynecol Oncol 2000;77:227-31. [DOI] [PubMed] [Google Scholar]
- 6.Chi DS, Zivanovic O, Palayekar MJ, et al. A contemporary analysis of the ability of preoperative serum CA-125 to predict primary cytoreductive outcome in patients with advanced ovarian, tubal and peritoneal carcinoma. Gynecol Oncol 2009;112:6-10. [DOI] [PubMed] [Google Scholar]
- 7.Vorgias G, Iavazzo C, Savvopoulos P, et al. Can the preoperative Ca-125 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? A single institution cohort study. Gynecol Oncol 2009;112:11-5. [DOI] [PubMed] [Google Scholar]
- 8.Angioli R, Plotti F, Capriglione S, et al. Can the preoperative HE4 level predict optimal cytoreduction in patients with advanced ovarian carcinoma? Gynecol Oncol 2013;128:579-83. [DOI] [PubMed] [Google Scholar]
- 9.Barlow TS, Przybylski M, Schilder JM, et al. The utility of presurgical CA125 to predict optimal tumor cytoreduction of epithelial ovarian cancer. Int J Gynecol Cancer 2006;16:496-500. [DOI] [PubMed] [Google Scholar]
- 10.Drapkin R, von Horsten HH, Lin Y, et al. Human epididymis protein 4 (HE4) is a secreted glycoprotein that is overexpressed by serous and endometrioid ovarian carcinomas. Cancer Res 2005;65:2162-9. [DOI] [PubMed] [Google Scholar]
- 11.Bast RC, Jr, Badgwell D, Lu Z, et al. New tumor markers: CA125 and beyond. Int J Gynecol Cancer 2005;15 Suppl 3:274-81. [DOI] [PubMed] [Google Scholar]
- 12.Hellström I, Raycraft J, Hayden-Ledbetter M, et al. The HE4 (WFDC2) protein is a biomarker for ovarian carcinoma. Cancer Res 2003;63:3695-700. [PubMed] [Google Scholar]
- 13.Galgano MT, Hampton GM, Frierson HF, Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol 2006;19:847-53. [DOI] [PubMed] [Google Scholar]
- 14.Moore RG, Brown AK, Miller MC, et al. The use of multiple novel tumor biomarkers for the detection of ovarian carcinoma in patients with a pelvic mass. Gynecol Oncol 2008;108:402-8. [DOI] [PubMed] [Google Scholar]
- 15.Huhtinen K, Suvitie P, Hiissa J, et al. Serum HE4 concentration differentiates malignant ovarian tumours from ovarian endometriotic cysts. Br J Cancer 2009;100:1315-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vergote I, Tropé CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 2010;363:943-53. [DOI] [PubMed] [Google Scholar]
- 17.Hou JY, Kelly MG, Yu H, et al. Neoadjuvant chemotherapy lessens surgical morbidity in advanced ovarian cancer and leads to improved survival in stage IV disease. Gynecol Oncol 2007;105:211-7. [DOI] [PubMed] [Google Scholar]
- 18.Bristow RE. Predicting “unresectable” ovarian cancer: Taking aim at a moving target. Gynecol Oncol 2006;100:449-50. [DOI] [PubMed] [Google Scholar]
- 19.Salani R, Axtell A, Gerardi M, et al. Limited utility of conventional criteria for predicting unresectable disease in patients with advanced stage epithelial ovarian cancer. Gynecol Oncol 2008;108:271-5. [DOI] [PubMed] [Google Scholar]
- 20.Ferrandina G, Sallustio G, Fagotti A, et al. Role of CT scan-based and clinical evaluation in the preoperative prediction of optimal cytoreduction in advanced ovarian cancer: a prospective trial. Br J Cancer 2009;101:1066-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suidan RS, Ramirez PT, Sarasohn DM, et al. A multicenter prospective trial evaluating the ability of preoperative computed tomography scan and serum CA-125 to predict suboptimal cytoreduction at primary debulking surgery for advanced ovarian, fallopian tube, and peritoneal cancer. Gynecol Oncol 2014;134:455-61. [DOI] [PMC free article] [PubMed] [Google Scholar]


