Abstract
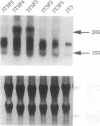
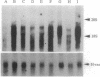
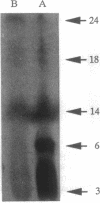
We have developed an efficient in vitro transformation system using N-methyl-N-nitrosourea that allows us to study the role of hormones and growth factors in mouse mammary tumorigenesis. Utilizing this system, we reported earlier that mammary tumors induced in vitro with N-methyl-N-nitrosourea in the presence of mammogenic hormones (progesterone and prolactin) contain predominately an activated c-Ki-ras protooncogene with a G35 --> A35 transitional mutation in the 12th codon. Mammary tumors induced in the presence of another mitogen, lithium (Li), do not have a mutation in the c-Ki-ras protooncogene. By using an expression cloning system, a plasmid clone containing a 1.75-kb cDNA insert has been isolated from this group of tumors. Nucleic acid sequence analysis of the insert reveals that it has a short open reading frame of 61 amino acids and that it does not have sequence homology with any known gene. The gene, designated MAT1, can neoplastically transform NIH 3T3 cells and also the mammary epithelial cell line TM3. Expression of this gene occurs in normal mouse tissues including mammary gland and is overexpressed in the original mammary tumors as indicated by Northern blot analysis. In vitro transcription and translation of the clone shows a protein product of 6000 Da, which agrees with the predicted open reading frame.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balmain A., Brown K. Oncogene activation in chemical carcinogenesis. Adv Cancer Res. 1988;51:147–182. doi: 10.1016/s0065-230x(08)60222-5. [DOI] [PubMed] [Google Scholar]
- Brown K., Buchmann A., Balmain A. Carcinogen-induced mutations in the mouse c-Ha-ras gene provide evidence of multiple pathways for tumor progression. Proc Natl Acad Sci U S A. 1990 Jan;87(2):538–542. doi: 10.1073/pnas.87.2.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christov K., Swanson S. M., Guzman R. C., Thordarson G., Jin E., Talamantes F., Nandi S. Kinetics of mammary epithelial cell proliferation in pituitary isografted BALB/c mice. Carcinogenesis. 1993 Oct;14(10):2019–2025. doi: 10.1093/carcin/14.10.2019. [DOI] [PubMed] [Google Scholar]
- Dandekar S., Sukumar S., Zarbl H., Young L. J., Cardiff R. D. Specific activation of the cellular Harvey-ras oncogene in dimethylbenzanthracene-induced mouse mammary tumors. Mol Cell Biol. 1986 Nov;6(11):4104–4108. doi: 10.1128/mcb.6.11.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Guerrero I., Calzada P., Mayer A., Pellicer A. A molecular approach to leukemogenesis: mouse lymphomas contain an activated c-ras oncogene. Proc Natl Acad Sci U S A. 1984 Jan;81(1):202–205. doi: 10.1073/pnas.81.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero I., Villasante A., Corces V., Pellicer A. Loss of the normal N-ras allele in a mouse thymic lymphoma induced by a chemical carcinogen. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7810–7814. doi: 10.1073/pnas.82.23.7810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman R. C., Osborn R. C., Bartley J. C., Imagawa W., Asch B. B., Nandi S. In vitro transformation of mouse mammary epithelial cells grown serum-free inside collagen gels. Cancer Res. 1987 Jan 1;47(1):275–280. [PubMed] [Google Scholar]
- Guzman R. C., Osborn R. C., Swanson S. M., Sakthivel R., Hwang S. I., Miyamoto S., Nandi S. Incidence of c-Ki-ras activation in N-methyl-N-nitrosourea-induced mammary carcinomas in pituitary-isografted mice. Cancer Res. 1992 Oct 15;52(20):5732–5737. [PubMed] [Google Scholar]
- Hori C., Oka T. Induction by lithium ion of multiplication of mouse mammary epithelium in culture. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2823–2827. doi: 10.1073/pnas.76.6.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imagawa W., Bandyopadhyay G. K., Nandi S. Regulation of mammary epithelial cell growth in mice and rats. Endocr Rev. 1990 Nov;11(4):494–523. doi: 10.1210/edrv-11-4-494. [DOI] [PubMed] [Google Scholar]
- Kumar R., Medina D., Sukumar S. Activation of H-ras oncogenes in preneoplastic mouse mammary tissues. Oncogene. 1990 Aug;5(8):1271–1277. [PubMed] [Google Scholar]
- Medina D. Hormones and mouse mammary tumorigenesis. Cancer Res. 1981 Sep;41(9 Pt 2):3819–3820. [PubMed] [Google Scholar]
- Medina D., Kittrell F. S., Liu Y. J., Schwartz M. Morphological and functional properties of TM preneoplastic mammary outgrowths. Cancer Res. 1993 Feb 1;53(3):663–667. [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. II. Dependence on hormone stimulation for tumorigenesis. J Natl Cancer Inst. 1974 Jul;53(1):223–226. doi: 10.1093/jnci/53.1.223. [DOI] [PubMed] [Google Scholar]
- Medina D. Mammary tumorigenesis in chemical carcinogen-treated mice. VI. Tumor-producing capabilities of mammary dysplasias in BALB/cCrgl mice. J Natl Cancer Inst. 1976 Nov;57(5):1185–1189. doi: 10.1093/jnci/57.5.1185. [DOI] [PubMed] [Google Scholar]
- Medina D. The preneoplastic state in mouse mammary tumorigenesis. Carcinogenesis. 1988 Jul;9(7):1113–1119. doi: 10.1093/carcin/9.7.1113. [DOI] [PubMed] [Google Scholar]
- Miki T., Fleming T. P., Crescenzi M., Molloy C. J., Blam S. B., Reynolds S. H., Aaronson S. A. Development of a highly efficient expression cDNA cloning system: application to oncogene isolation. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5167–5171. doi: 10.1073/pnas.88.12.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T., Matsui T., Heidaran M. A., Aaronson S. A. An efficient directional cloning system to construct cDNA libraries containing full-length inserts at high frequency. Gene. 1989 Nov 15;83(1):137–146. doi: 10.1016/0378-1119(89)90411-3. [DOI] [PubMed] [Google Scholar]
- Miyamoto S., Guzman R. C., Osborn R. C., Nandi S. Neoplastic transformation of mouse mammary epithelial cells by in vitro exposure to N-methyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1988 Jan;85(2):477–481. doi: 10.1073/pnas.85.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Sukumar S., Guzman R. C., Osborn R. C., Nandi S. Transforming c-Ki-ras mutation is a preneoplastic event in mouse mammary carcinogenesis induced in vitro by N-methyl-N-nitrosourea. Mol Cell Biol. 1990 Apr;10(4):1593–1599. doi: 10.1128/mcb.10.4.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintanilla M., Brown K., Ramsden M., Balmain A. Carcinogen-specific mutation and amplification of Ha-ras during mouse skin carcinogenesis. Nature. 1986 Jul 3;322(6074):78–80. doi: 10.1038/322078a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Tomooka Y., Imagawa W., Nandi S., Bern H. A. Growth effect of lithium on mouse mammary epithelial cells in serum-free collagen gel culture. J Cell Physiol. 1983 Dec;117(3):290–296. doi: 10.1002/jcp.1041170303. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]