Abstract
Polyreactive antibodies, a major component of the natural antibody repertoire, bind with low affinity to a variety of structurally unrelated antigens. Many of these antibodies are germline or near germline in sequence. Little is known, however, about the function of these antibodies. In the present mini-review we show: (1) that the broad antibacterial activity of the natural antibody repertoire is largely due to polyreactive antibodies, which in the presence of complement lyse bacteria and enhance phagocytosis; (2) that polyreactive antibodies bind to UV- or human immunodeficiency virus-induced apoptotic cells and with complement enhance the phagocytosis of these cells by macrophages; and (3) that dinitrophenol can be used as a surrogate for quantitating the level of polyreactive antibodies in serum. We conclude that polyreactive antibodies protect the host against both foreign invaders and its own damaged/apoptotic cells.
Keywords: polyreactive antibody, natural antibody, apoptotic cells, phagocytosis, bacteria
Immunization of the host with a purified antigen results in the development of high-affinity antibodies that react with the immunizing antigen. However, for >100 years it has been known that normal serum contains low-affinity antibodies that react with self-antigens and some foreign antigens to which the host has never been exposed. These natural antibodies have been found in newborns and germ-free animals, adding to the enigma as to what triggers their production in the absence of antigenic stimulation [1].
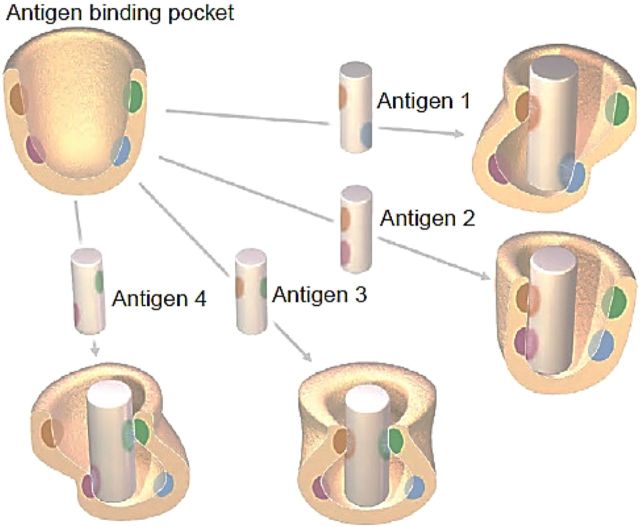
Studies beginning in the early 1980s from our laboratory at the National Institutes of Health and Stratis Avrameas's laboratory at the Pasteur Institute showed by making hybridomas that many of the resulting monoclonal antibodies bound with low affinity to a variety of structurally unrelated antigens [2–5]. In the past, antigen-antibody binding has been thought of in terms of a “lock and key” hypothesis with the antigen and antigen-binding pocket of the antibody having a rigid complementary structure. With the elucidation of the molecular structure of the antigen-binding pocket and the innumerable V-D-J combinations, it is now recognized that the antigen-binding pocket of polyreactive antibodies, many of which are germline or near germline in configuration, are more flexible than the antigen-binding pocket of classic monoreactive high-affinity antibodies. This has led to several explanations for polyreactivity [6–8]. The one that we favor the most [9] is illustrated in Figure 1. This shows that the antigen-binding pocket is flexible (ie, induced fit) and can accommodate antigens that bind to different residues within the binding pocket. Thus, alterations in conformation and clonal selection can be viewed as complementary processes that increase antibody diversity and are not mutually exclusive.
Figure 1.
Conformational/induced fit hypothesis to explain polyreactivity. In contrast to the rigid structure of the classic “lock and key” model of antigen-antibody binding, it is thought that the antigen-binding pocket of polyreactive antibodies are more flexible and can therefore accommodate different antigenic configurations. The graphic shows 4 antigens interacting with different amino acid residues with the antigen-binding pocket of a single broadly-reactive polyreactive antibody. Each interaction alters the folding or conformation of the antigen-binding pocket in a different way (from Ref. [9]).
BINDING OF POLYREACTIVE ANTIBODIES TO BACTERIA
During the last several years we have been attempting to elucidate the function of polyreactive antibodies which are a major component of the natural antibody repertoire. It has been known for some time that natural antibodies can react at low levels with a variety of bacteria and viruses to which the host has never been exposed [10, 11]. To see whether this reactivity was due to polyreactive antibodies within the natural antibody repertoire, we prepared a number of monoclonal polyreactive and monoclonal monoreactive antibodies by hybridoma technology and tested the binding capacity of these antibodies with a panel of gram-negative and gram-positive bacteria [12]. As seen in Table 1 the monoclonal monoreactive antibody (mAb2507) bound strongly to Escherichia coli 0157:H7 to which it was made, but it did not bind to the other 11 bacteria. In contrast, monoclonal polyreactive antibody (pAb2E4) bound strongly, moderately, or weakly to the same panel of bacteria to which mAb2075 did not bind.
Table 1.
Binding of Monoreactive (mAb2507) and Polyreactive (pAb2E4) Antibodies to Gram-Positive and Gram-Negative Bacteria as Determined by Fluorescence-Activated Cell Sorting Analysis
| Bacteria | Antibody Binding, % |
|
|---|---|---|
| Monoreactive | Polyreactive | |
| Streptococcus oralis J22 | 4.6 | 75.0 |
| S. oralis 10557 | 2.8 | 57.0 |
| Streptococcus mitis 15914 | 1.6 | 73.0 |
| Escherichia coli BL21 | 0.1 | 82.0 |
| S. oralis C104 | 1.5 | 37.0 |
| S. oralis 34 | 2.9 | 33.0 |
| Actinomyces naeslundii T14V | 1.0 | 31.0 |
| E. coli K12 | 1.0 | 36.0 |
| Streptococcus gordonii 38 | 3.0 | 11.0 |
| S. gordonii DL1 | 2.0 | 7.4 |
| A. naeslundii 12104 | 2.0 | 23.0 |
| E. coli 0157:H7 | 96.0 | 5.0 |
Further studies revealed that complement bound to the bacteria to which the monoclonal polyreactive antibody bound but not to the bacteria that had been incubated with monoclonal monoreactive antibody. Moreover, the gram-positive bacteria to which the polyreactive antibody and complement bound grew at a slower rate and were lysed and phaocytosed by macrophages (Figure 2). In contrast to gram-negative bacteria, gram-positive bacteria were not lysed by polyreactive antibodies and complement, but the binding of complement led to the release of C5a, a powerful chemotactic factor. These studies support the argument that the broad antibacterial activity of the natural antibody repertoire is in large part due to the presence of polyreactive antibodies.
Figure 2.
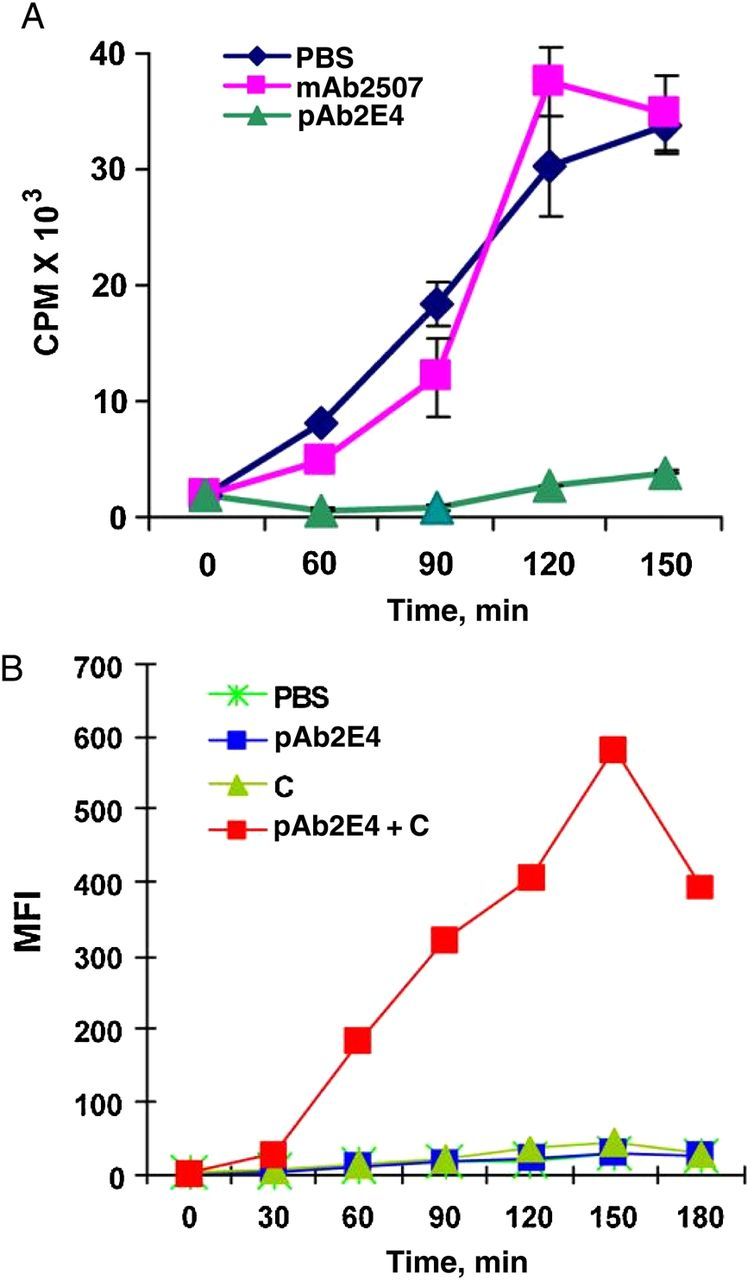
A, Inhibition in the growth of gram-positive Escherichia coli (BL21) by polyreactive antibody (pAb2E4), as measured by the incorporation of 3H-TdR in the presence of complement. Abbreviations: CPM, counts per minute; PBS, phosphate-buffered saline. B, Phagocytosis by macrophages of polyreactive antibody (pAb2E4)-treated E. coli (BL21) in the presence of complement, as determined by fluorescence-activated cell sorting analysis and measured by mean fluorescence intensity (MFI) (from Ref. [12]).
BINDING OF POLYREACTIVE ANTIBODIES TO APOPTOTIC CELLS
Very recently we found a second major function of polyreactive antibodies. In humans, it is thought that each day a billion or more cells undergo apoptosis. Numerous studies have shown that natural antibodies bind the apoptotic cells and enhance their phagocytosis by macrophages [13, 14]. To see whether polyreactive antibodies bind to apoptotic cells [15], human T cells were made apoptotic by short exposure to UV light. As seen in Figure 3, polyreactive antibodies bound to late apoptotic cells, but monoreactive antibodies did not. Moreover, that binding led to the fixation of complement, the generation of the chemotactic factor C5a, and the enhancement of phagocytosis by macrophages. Human T cells made apoptotic by infection with human immunodeficiency virus also led to the binding of polyreactive but not monoreactive antibody (Figure 4). This in turn resulted in the binding of complement and the enhancement of phagocytosis. We conclude from these studies that polyreactive antibodies contribute in a major way to the clearance of cells made apoptotic by a variety of natural and infectious processes.
Figure 3.
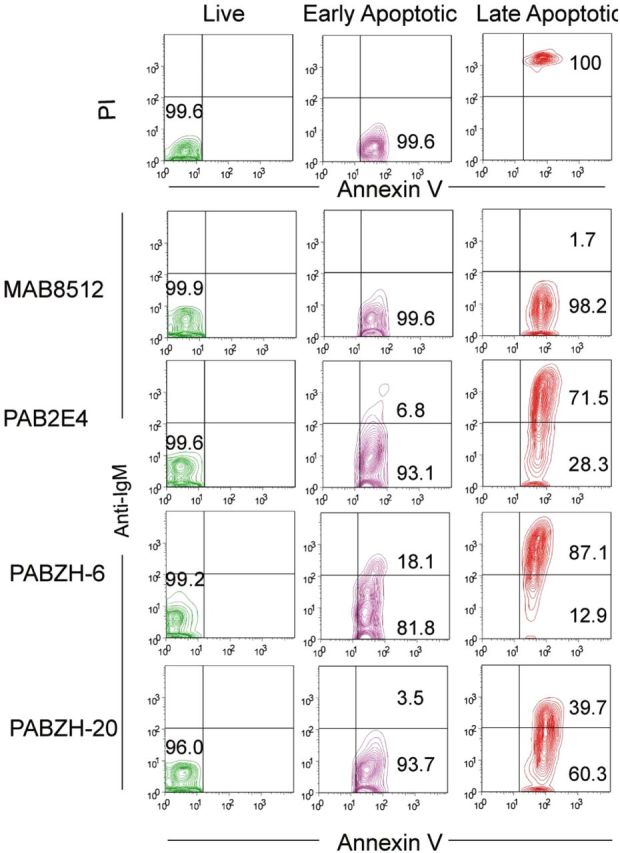
Human T cells exposed to UV light were sorted into live, early, and late apoptotic populations. The binding profile of monoreactive (mAb8512) and polyreactive (pAb2E4, pAbZH-6, pAbZH-20) antibodies shows that polyreactive antibodies, but not monoreactive ones, bind primarily to late apoptotic cells (from [15]). Abbreviations: IgM, immunoglobulin M; PI, propidium iodide.
Figure 4.
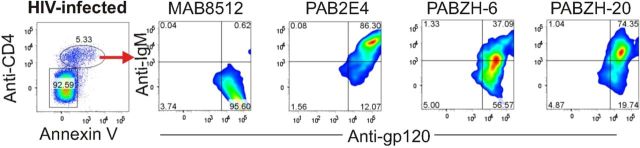
Polyreactive (pAb2E4, pAbZH-6, pAbZH-20) but not monoreactive (mAb8512), antibodies bind to human immunodeficiency virus (HIV)-induced apoptotic cells (from Ref. [15]). Abbreviation: IgM, immunoglobulin M.
QUANTIFICATION OF POLYREACTIVE ANTIBODIES IN SERUM
Most studies on monoclonal polyreactive antibodies have used antibodies from hybridomas. However, in serum, it is estimated that there are millions of different polyreactive antibodies, many of which show different binding patterns and affinities when evaluated with a large panel of antigens. Thus, measuring polyreactive antibodies in serum requires a different approach than used to measure an antigen-specific monoreactive antibody. Our studies showed that monoclonal polyreactive antibodies could bind to a variety of haptens and synthetic molecules. After extensively comparing a number of molecules, we decided to use dinitrophenol (DNP) as a surrogate for polyreactivity (unpublished data). DNP is a synthetic molecule not present in the environment, and individuals are not normally exposed to it. Therefore, if antibodies in the serum react with DNP, they would almost certainly have to be polyreactive antibodies, and their titer to DNP could serve as an index of polyreactivity.
To test this possibility, we used 2-fold serial dilutions of serum samples to determine the end-point titer of polyreactive anti-DNP, using enzyme-linked immunosorbent assay plates coated with DNP. Figure 5 shows a representative titration curve of anti-DNP polyreactive antibody using serum from 20 mice. Then we determined the titer of anti-DNP polyreactive antibodies in different strains of mice and in mice of different ages and sexes. The different strains of mice showed up to a 7-fold variation in polyreactive antibody titer, and 12-month-old male and female mice showed a 3–6-fold increase in polyreactive antibody titer compared with 2-month-old mice. The polyreactive antibody titer is now being used as an index to evaluate the range of polyreactive antibodies in various pathophysiologic conditions in mouse and human diseases.
Figure 5.
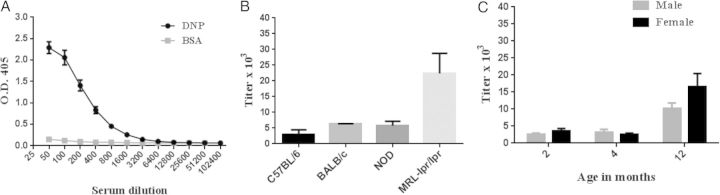
Quantification of polyreactive antibodies in serum samples using the titer of antibody to dinitrophenol (DNP) as a surrogate for polyreactivity. Enzyme-linked immunosorbent assay plates were coated with DNP, and the polyreactive antibody titer was determined by serial 2-fold dilutions. A, Representative titration curve from 20 mice. B, Polyreactive antibody titers in different strains of mice. C, Polyreactive antibody titers by age and sex of mice. Abbreviations: BSA, bovine serum albumin; OD405, optical density at 405 nm.
In conclusion, polyreactive antibodies have the capacity to protect the host against foreign invaders and their own apoptotic cells, and the titer of the antibody to DNP can be used as a index for measuring polyreactivity.
Notes
Disclaimer. The views expressed in this manuscript represent the opinions of the authors and do not necessarily represent the official views of the National Institutes of Health.
Financial support. This work was supported by the Intramural Research Program of the US National Institutes of Health.
Potential conflicts of interest. Both authors: No potential conflicts of interest.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Tauber AI, Podolsky SH. The generation of diversity: clonal selection theory and the rise of molecular immunology. Cambridge, MA: Harvard University Press, 1997. [Google Scholar]
- 2.Haspel MV, Onodera T, Prabhakar BS, et al. Multiple organ-reactive monoclonal autoantibodies. Nature 1983; 304:73–6. [DOI] [PubMed] [Google Scholar]
- 3.Satoh J, Prabhakar BS, Haspel MV, Ginsbergfellner F, Notkins AL. Human monoclonal autoantibodies that react with multiple endocrine organs. New Engl J Med 1983; 309:217–20. [DOI] [PubMed] [Google Scholar]
- 4.Prabhakar BS, Saegusa J, Onodera T, Notkins AL. Lymphocytes capable of making monoclonal autoantibodies that react with multiple organs are a common feature of the normal B-cell repertoire. J Immunol 1984; 133:2815–7. [PubMed] [Google Scholar]
- 5.Dighiero G, Lymberi P, Mazie JC, et al. Murine hybridomas secreting natural monoclonal-antibodies reacting with self antigens. J Immunol 1983; 131:2267–72. [PubMed] [Google Scholar]
- 6.Bosshard HR. Molecular recognition by induced fit: how fit is the concept? News Physiol Sci 2001; 16:171–3. [DOI] [PubMed] [Google Scholar]
- 7.James LC, Roversi P, Tawfik DS. Antibody multispecificity mediated by conformational diversity. Science 2003; 299:1362–7. [DOI] [PubMed] [Google Scholar]
- 8.Rini JM, Schulzegahmen U, Wilson IA. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science 1992; 255:959–65. [DOI] [PubMed] [Google Scholar]
- 9.Notkins AL. Polyreactivity of antibody molecules. Trends Immunol 2004; 25:174–9. [DOI] [PubMed] [Google Scholar]
- 10.Gordon J, Carter HS. The bactericidal power of normal serum. J Pathol Bacteriol 1932; 35:549–55. [Google Scholar]
- 11.Michael JG, Whitby JL, Landy M. Studies on natural antibodies to gram-negative bacteria. J Exp Med 1962; 115:131–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou ZH, Zhang YH, Hu YF, Wahl LM, Cisar JO, Notkins AL. The broad antibacterial activity of the natural antibody repertoire is due to polyreactive antibodies. Cell Host Microbe 2007; 1:51–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol 2009; 182:6031–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol 2012; 188:939–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou ZH, Wild T, Xiong Y, et al. Polyreactive antibodies plus complement enhance the phagocytosis of cells made apoptotic by UV-light or HIV. Sci Rep 2013; 3:2271. [DOI] [PMC free article] [PubMed] [Google Scholar]