Abstract
The traditional Japanese phytomedicine rikkunshito is traditionally used for the treatment of gastrointestinal motility disorders, cachexia and nausea. These effects indicate 5-HT3 receptor antagonism, due to the involvement of these receptors in such pathophysiological processes. E.g., setrons, specific 5-HT3 receptor antagonists are the strongest antiemetics, developed so far. Therefore, the antagonistic effects of the eight rikkunshito constituents at heterologously expressed 5-HT3Areceptors were analyzed using the two-electrode voltage-clamp technique. The results indicate that tinctures from Aurantii, Ginseng, Zingiberis, Atractylodis and Glycyrrhiza inhibited the 5-HT3A receptor response, whereas the tinctures of Poria cocos, Jujubae and Pinellia exhibited no effect. Surprisingly, the strongest antagonism was found for Glycyrrhiza, whereas the Zingiberis tincture, which is considered to be primarily responsible for the effect of rikkunshito, exhibited the weakest antagonism of 5-HT3A receptors. Rikkunshito contains various vanilloids, ginsenosides and flavonoids, a portion of which show an antagonistic effect on 5-HT3 receptors. A screening of the established ingredients of the active rikkunshito constituents and related substances lead to the identification of new antagonists within the class of flavonoids. The flavonoids (-)-liquiritigenin, glabridin and licochalcone A from Glycyrrhiza species were found to be the most effective inhibitors of the 5-HT-induced currents in the screening. The flavonoids (-)-liquiritigenin and hesperetin from Aurantii inhibited the receptor response in a non-competitive manner, whereas glabridin and licochalcone A exhibited a potential competitive antagonism. Furthermore, licochalcone A acts as a partial antagonist of 5-HT3A receptors. Thus, this study reveals new 5-HT3A receptor antagonists with the aid of increasing the comprehension of the complex effects of rikkunshito.
Keywords: rikkunshito, Glycyrrhiza, flavonoids, hesperetin, (-)-liquiritigenin, glabridin, licochalcone A, 5-HT3A receptor
Introduction
The 5-HT3 receptor channels are the only ionotropic receptors within the 5-HT receptor family and belong to the cys-loop family of ligand-gated ion channels (Derkach et al., 1989; Hannon and Hoyer, 2008). These channels occur in the PNS and are highly expressed in the trigeminus (Manteniotis et al., 2013) and the enteric nervous system (Niesler et al., 2003). In the CNS, they are expressed in the striatum, substantia nigra, amygdala, hippocampus and nucleus accumbens (Boess and Martin, 1994). Other 5-HT3 receptor expressing structures are the nucleus tractus solitarius and the area postrema (Boess and Martin, 1994), which are parts of the vomiting center that trigger nausea and vomiting.
5-HT3 receptors are involved in many pathophysiological processes, such as nociception and gastrointestinal motility disorders, and the development of nausea and vomiting, therefore showing broad clinical relevance (Doak and Sawynok, 1997; Gershon, 2004; Jeggo et al., 2005; Costedio et al., 2007). Specific 5-HT3 receptor antagonists, such as ondansetron, are mainly used for the treatment of nausea in various conditions, such as chemotherapy-induced nausea and vomiting (CINV), and nausea during the postoperative phase (PONV) (Cubeddu et al., 1994; Gyermek, 1995).
Many 5-HT3 receptor antagonists have been identified to date. In addition to the competitively acting setrons (Hope et al., 1996), non-competitive antagonists have been identified within the family of cannabinoids, such as Δ9-THC, anandamide and cannabidiol (Barann et al., 2002). Moreover, steroids (Barann et al., 1999) and the anesthetics ketamine, propofol, methohexital and pentobarbital (Barann et al., 1993, 2000a,b, 2008) have been shown to antagonize 5-HT3 receptors. Many plant compounds also act as 5-HT3 receptor antagonists. For example, alkaloids, such as nicotine (Schreiner et al., 2014), hot substances and terpenes, e.g., bilobalide and ginkgolide B (Thompson et al., 2011), gingerols (Walstab et al., 2013) and many others, were reviewed by Walstab et al. (2010).
Kampo is a traditional Japanese phytomedicine that has its seeds in traditional Chinese medicine. Rikkunshito, a combination of eight constituents, is one of the most famous and prescribed kampo medicines (Tominaga and Arakawa, 2015), and it has a well-known physiological effect on the gastrointestinal system, shows orexigen and antiemetic effects, and takes part in the regulation of peristalsis (Takeda et al., 2008; Tominaga et al., 2011; Yanai et al., 2013; Fujitsuka and Uezono, 2014). These effects indicate an antagonism of 5-HT3 receptors. Therefore, the antagonistic effects of the eight constituents of rikkunshito (Aurantii pericarpium, Ginseng radix, Zingiberis rhizoma, Jujubae (Zizyphi) frucutus, Pinellia tuber, Atractylodis rhizoma, Glycyrrhiza radix and Poria cocos (Hoelen) were investigated as ethanol tinctures. Furthermore, we investigated the established ingredients of the active rikkunshito constituents to identify new 5-HT3A receptor antagonists. Although the antagonistic and hence the antiemetic effect of Ginseng and Zingiberis due to the action of ginsenosides, gingerols and shogaols is well-described (Ernst and Pittler, 2000; Kim et al., 2005; Lee et al., 2007; Haniadka et al., 2012; Ding et al., 2013), there is currently little knowledge of the effect of the residual rikkunshito constituents on 5-HT3 receptors.
The aim of this study was the evaluation of the relative contribution of the single constituents of rikkunshito to 5-HT3 receptor antagonism and the identification of new antagonists. Therefore, we tested the modulatory effect of tinctures and single substances on heterologously expressed human 5-HT3A receptors using the two-electrode voltage-clamp technique. Surprisingly, Glycyrrhiza was identified as the most effective antagonistic tincture among the rikkunshito constituents. Therefore, we concentrated on the investigation of Glycyrrhiza ingredients and identified several new flavonoids as 5-HT3A receptor antagonists. The drug Radix Glycyrrhiza is used in Kampo medicine for the treatment of pain, gastric ulcers and inflammations of the gastrointestinal and respiratory systems due to its antiphlogistic effect (Kim et al., 2008). A contribution of Radix Glycyrrhiza to the antiemetic effect of rikkunshito due to the action of flavonoids is conceivable.
Materials and methods
Expression system
The expression plasmid contains the cDNA coding for the 5-HT3A protein in pcDNA3 (Invitrogen) (Lobitz et al., 2001). cRNAs were prepared using the AmpliCap T7 high-yield message marker kit (Epicenter, Madison, WI, USA), following the manufacturer's protocol. Xenopus laevis oocytes were obtained as previously described (Sherkheli et al., 2010) and injected with a total amount of 7–20 ng of the receptor-coding cRNA using an injection-setup from WPI (Nanoliter 2000, Micro4). The injected oocytes were stored in ND 96 (96.0 mM NaCl, 2.0 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 5.0 mM HEPES, pH 7.2, 200 U/ml penicillin, and 200 μg/ml streptomycin) at 17°C. Measurements were performed one to 5 days after cRNA injection.
Electrophysiology
The electrophysiological recordings were performed using the two-electrode voltage-clamp technique as previously described (Saras et al., 2008). All of the measurements were performed in normal frog ringer (NFR) [115 mM NaCl, 2.5 mM KCl, 1.8 mM CaCl2, 10 mM HEPES; pH 7.2 (NaOH/HCl)] containing niflumic acid (NA) (100 μM) to block the Ca2+-induced currents mediated by the intrinsic chloride channel (TMEM16A) or under Ca2+-free conditions [115 mM NaCl, 2.5 mM KCl, 1.8 mM MgCl2, 10 mM HEPES; pH 7.2 (NaOH/HCl)]. All of the substances were applied after preincubation (30 s). The currents were recorded at a holding potential of typically −60 mV using the Cell Works 6.1.1. software (NPI).
Solvent controls
To exclude any unrequested effects of the solvents ethanol and DMSO, we tested their direct activation on non-injected and 5-HT3A receptor-expressing oocytes. At the maximal used concentration (1 Vol.-%), a negligible direct activation was observed. Moreover, the modulatory effect on the 5-HT3A receptor response was tested at concentrations of 1.0 Vol.-% for ethanol and DMSO. Ethanol exhibited an inhibition of 14.1 ± 2.6%, and DMSO exhibited an inhibition of 29.1 ± 4.7% (n = 6−11). Equivalent volumes of ethanol and DMSO were added to the reference 5-HT solutions. To resolve glycyrrhizin, the solution had to be acidified (pH 5.5). Therefore, we checked the modulatory effect of the pH values on 5-HT3A receptors. Low extracellular pH values inhibited the currents but high pH showed potentiating effects (Supplementary Figure 2).
Action of the tinctures on non-injected Xenopus oocytes
In the control experiments, at a concentration of 1 Vol.-%, the tinctures of Ginseng, Zingiberis and Atractylodis evoked currents in some non-injected oocytes with desensitizing responses (data not shown). In our experiments with 5-HT3A receptor-expressing cells, oocytes were rejected if the amplitude of this direct activation was greater than 10% of the 5-HT-induced current; thus, the direct action of the extracts could not prevent the identification of pronounced blocking effects. Moreover, in our blocking experiments, these currents were desensitized during the 30-s preincubation with the tincture. At a concentration of 0.1 Vol.-%, none of the tinctures evoked any current different from that obtained from the control application of Ringer‘s solution.
Evaluation of competitive and non-competitive antagonists
To determine the apparent mode of antagonism of the identified tinctures and substances with antagonistic effects, we tested the inhibition of currents induced by low (2.5 μM) and high (30 μM) 5-HT concentrations. In the case of a non-competitive mechanism, the inhibition should be independent of the 5-HT concentration, whereas the efficacy of competitive antagonists decreases with increasing 5-HT concentrations. Alternatively to a competitive mechanism, the dependence of the inhibition on the agonist concentration can also be caused by an allosteric modulation. As a control, we tested the competitive antagonists ondansetron (1 nM) and d-tubocurarine (20 μM) (Hope et al., 1996) and the non-competitive antagonist picrotoxin (50 μM) (Das and Dillon, 2005). As expected, the inhibition of picrotoxin was independent of the 5-HT concentration, and the inhibition obtained with d-tubocurarine and ondansetron was reduced at higher 5-HT concentrations (Table 1). Thus, the method used may indicate the mode of antagonism. Nevertheless, a definitive differentiation between competitive, allosteric, and concentration-dependent antagonists must be performed using ligand binding assays.
Table 1.
Competitive and non-competitive action of the identified antagonists and tinctures with antagonistic effect.
| Tincture/Substance | Concentration | Mean inhibition ± SEM [%] | Significance level/mode of antagonism | |
|---|---|---|---|---|
| 5-HT [2.5 μM] | 5-HT [30 μM] | |||
| CONTROLS | ||||
| Picrotoxin | 50 μM | 72.5 ± 3.7 | 73.0 ± 2.8 | ns/nc |
| D-tubocurarine | 20 μM | 82.0 ± 3.5 | 45.9 ± 4.8 | **/c |
| Ondansetron | 1 nM | 82.4 ± 3.4 | 57.9 ± 5.3 | */c |
| TINCTURES | ||||
| Zingiberis | 1 Vol.-% | 20.1 ± 4.9 | 22.2 ± 4.2 | ns/nc |
| Aurantii | 1 Vol.-% | 37.3 ± 5.4 | 28.8 ± 2.6 | ns/nc |
| Ginseng | 1 Vol.-% | 44.6 ± 8.7 | 36.6 ± 7.3 | ns/nc |
| Atractylodis | 1 Vol.-% | 47.1 ± 5.6 | 27.4 ± 9.1 | */c |
| Glycyrrhiza | 1 Vol.-% | 64.5 ± 9.7 | 69.1 ± 7.4 | ns/nc |
| Unsweetened licorice | 1 Vol.-% | 44.6 ± 5.7 | 13.7 ± 6.6 | ***/c |
| SUBSTANCES | ||||
| Atractylenolide III | 1 mM | 55.0 ± 6.2 | 57.8 ± 6.0 | ns/nc |
| Licochalcone A | 1 mM | 76.1 ± 6.1 | 58.7 ± 2.6 | */c |
| Glabridin | 100 μM | 64.7 ± 2.1 | 17.3 ± 2.3 | ***/c |
| Hesperetin | 1 mM | 77.3 ± 9.0 | 79.7 ±6.1 | ns/nc |
| (-)-liquiritigenin | 0.5 mM | 84.5 ± 2.4 | 93.5 ± 0.5 | */nc |
The inhibitions of the 5-HT3A receptor responses by the controls (picrotoxin, ondansetron and d-tubocurarine) and different tinctures and substances at 5-HT concentrations of 2.5 and 30 μM are shown. The level of significance between the inhibitions obtained with two different 5-HT concentrations is indicated by asterisks in the last column (ns, not significant). Furthermore, the apparent mode of antagonism is listed in the last column (c, apparently competitive; nc, non-competitive) (n = 5–7).
Tinctures and substances
Ethanol tinctures of the rikkunshito constituents were obtained from Dr. Peter Lepke (Kronen Apotheke Wuppertal, Germany). Thus, plant preparations at appropriate quality for Japanese kampo medicine were extracted [200 g crushed plant material in 1 l ethanol (45–90% v/v)] for 10 days at room temperature. The tinctures were obtained by filtrating the supernatant, therefore containing no large solid parts of the plants. The dry weight of the extracted substances was determined by removing the solvent under vacuum (Supplementary Table 2). We used the following tinctures in our study:
Aurantii Pericarpium [Citrus reticulata Blanco (Rutaceae)] (chinpi, Chen Pi), White Ginseng Radix [Panax ginseng C.A.Mey. (Araliaceae)] (ninjin, Ren Shen), Zingiberis viridis Rhizoma [Zingiber officinale Roscoe (Zingiberaceae)] (shôkyô, Sheng Jiang), Jujubae Fructus [Ziziphus jujuba Mill. (Rhamnaceae)] (taisô, Da Zao), Pinelliae Tuber (Pinellia ternata [Thunb.) Makino (Araceae)] (hange, Ban Xia), Atractylodis macrocephala Rhizoma [Atractylodes macrocephala Koidz. (Asteraceae)] (sôjutsu, Bai Zhu), Glycyrrhiza Radix [Glycyrrhiza uralensis Fisch. (Fabaceae)] (kanzô, Gan Cao) and Poria cocos [Wolfiporia extensa (Peck) Ginns (Polyporaceae)] (bukuryô, Fu Ling).
Unsweetened licorice (Liquirizia purissima from R. De Rosa, Italy) was inlayed in ethanol (70% v/v) under the same conditions. The chemicals were obtained from Sigma Aldrich (5-HT hydrochloride, niflumic acid (blocker for Ca2+ activated chloride channels), picrotoxin (non-competitive ion channel blocker), d-tubocurarine (competitive nACh and 5-HT3 receptor antagonist), ondansetron (specific, competitive 5-HT3 receptor antagonist), (-)-liquiritigenin, licochalcone A, hesperidin, hesperetin, glabridin and glycyrrhizin), Carl Roth (rutin) and PhytoLab (atractylenolide III). The substances were diluted in water, dimethyl sulfoxide (DMSO) or ethanol.
Data analysis
The test substances were applied in an alternating manner with 5-HT. Therefore, the currents of the test substances or the modulated currents were normalized to the mean of the 5-HT-induced currents before and after the test substance was applied. The concentration-response data were fitted with the Hill equation with variable slope using SigmaPlot 8.0 (SPSS). Thereby, the calculation of the EC50 and IC50-values was done. The deviations are represented by the standard error of the mean (SEM). The datasets were tested for statistically significant differences through Student's t-test using Excel 2010 (Microsoft) (*p < 0.05; **p < 0.005; ***p < 0.0005). For multiple comparisons, the significance levels were corrected via Bonferroni-correction.
Results
Effect of tinctures of rikkunshito constituents
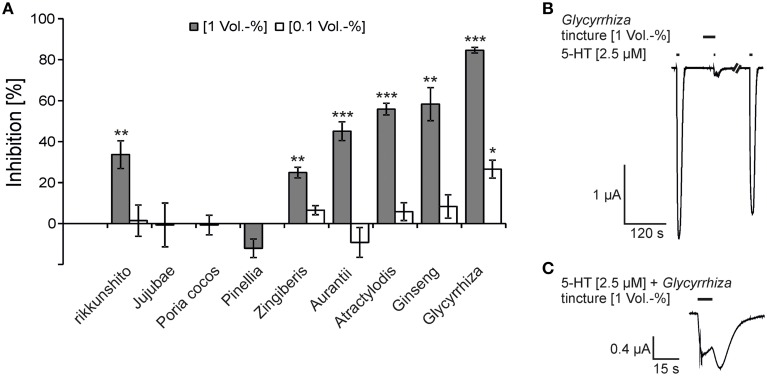
The gastroprokinetic and antiemetic effects of rikkunshito (Tominaga et al., 2011) could be explained by an antagonism of 5-HT3A receptors. To test this hypothesis, we tested the modulatory effects of the constituents of rikkunshito on the 5-HT3A responses. In the first experiments, we tested the effect of the respective tinctures to provide guidance for the identification of the most effective constituents. Under our experimental conditions an EC50 value for 5-HT of 2.39 ± 0.06 μM was determined for the 5-HT3A receptor (Supplementary Figure 1). This EC50 value is similar to previously reported EC50 values evaluated in Xenopus oocytes (about 2 μM 5-HT) (Lee et al., 2008; Schreiner et al., 2014). The tinctures from rikkunshito and its constituents Aurantii, Ginseng, Zingiberis, Atractylodis, and Glycyrrhiza, inhibited the 5-HT3A receptor responses (5-HT 2.5 μM, approximately EC50) at a concentration of 1 Vol.-%, whereas the tinctures of Poria cocos, Jujubae and Pinellia exhibited no effect (Figure 1A). Among the five tinctures that exerted an inhibitory effect, the tincture of Glycyrrhiza showed the strongest inhibition (84.6 ± 1.4%) (Figure 1B), and the weakest effect was obtained with the Zingiberis tincture (24.9 ± 2.6%). The inhibition obtained with the rikkunshito tincture (33.7 ± 1.5%) was close to that calculated by the addition of the effects of the tinctures of the eight constituents with regard to their mass distribution in the decoction (27.5%) (Supplementary Table 1). The 5-HT3A receptor responses showed a huge rebound when co-applied with Glycyrrhiza (Figure 1C). All of the six inhibitory tinctures were also tested at a lower concentration (0.1 Vol.-%). In these experiments, only the tincture of Glycyrrhiza showed a significant inhibition (26.6 ± 4.4%) (Figure 1A). All of the inhibitions were reversible after a 150-s washout.
Figure 1.
Inhibition of 5-HT3A receptors by tinctures of rikkunshito and its constituents (A) and original traces from the inhibition by the Glycyrrhiza tincture (B,C). (A) The dark bars represent the inhibition at 1 Vol.-%, and the bright bars represent that at a concentration of 0.1 Vol.-%. The inhibition from left to right are as follows: 33.7 ± 6.8%, 1.5 ± 7.6%, −0.7 ± 10.7%, −0.7 ± 4.8%, −12.0 ± 4.5%, 24.9 ± 2.6%, 6.5 ± 2.2%, 45.1 ± 4.6%, −9.2 ± 7.3%, 55.9 ± 2.8%, 5.8 ± 4.3%, 58.3 ± 8.1%; 8.4 ± 5.7%, 84.6 ± 1.4% and 26.6 ± 4.4% (n = 5 − 8) (*p < 0.05; **p < 0.005; ***p < 0.0005). (B,C) The time of application is denoted by the application bars above the trace. The inhibition was reversible after a 150-s washout (holding potential = −60 mV). (C) The 5-HT-induced currents showed a characteristic rebound when co-applied with the Glycyrrhiza tincture.
Modulatory effect of the rikkunshito ingredients and tincture of unsweetened licorice
The tinctures contain many different chemical substances. To identify the active compounds, we assessed the modulatory effects of some known ingredients of these plants on 5-HT3A receptors. We focused on the ingredients of Glycyrrhiza uralensis that showed the strongest antagonism within the rikkunshito constituents and other ingredients of further Glycyrrhiza species. Thus, we tested a tincture of unsweetened licorice because it is obtained from Glycyrrhiza glabra L. (Fabaceae). We used a concentration of 1 mM for all of the substances with the exception of glabridin (100 μM) and 1 Vol-% for the licorice tincture.
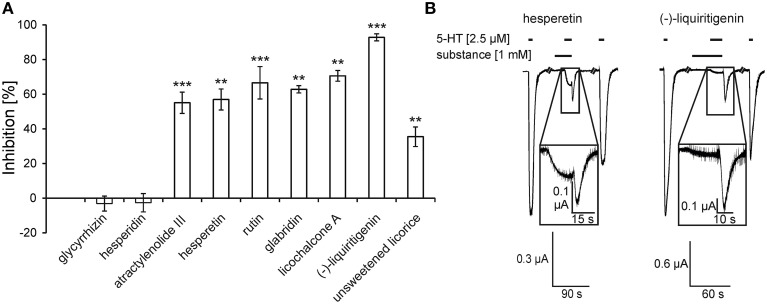
Four ingredients of Glycyrrhiza species were investigated. Glycyrrhizin and the flavonoid (-)-liquiritigenin are ubiquitous within the plants of the genus Glycyrrhiza, whereas the flavonoids glabridin and licochalcone A are restricted to G. glabra and G. inflata and G. eurycarpa (Xu et al., 1997; Rauchensteiner et al., 2005; Kondo et al., 2007). Glycyrrhizin exhibited no modulatory effect (−3.1 ± 4.3%), whereas glabridin revealed an inhibition of 62.8 ± 2.1%. Licochalcone A and (-)-liquiritigenin were the most effective antagonists, showing inhibitions of 70.6 ± 3.1% and 92.8 ± 2.2%, respectively (Figure 2A). Other flavonoids found in Aurantii, namely hesperetin and rutin, also exhibited antagonistic effects. Hesperidin, the most abundant ingredient in rikkunshito (Tominaga et al., 2011), revealed no effect. The flavonoids hesperetin and (-)-liquiritigenin caused 5-HT-induced responses with huge rebounds, similar to those obtained with the Radix Glycyrrhiza uralensis tincture (compare Figures 1C, 2B). The tincture of the unsweetened licorice caused an inhibition of 5-HT-induced currents of 35.5 ± 5.7%.
Figure 2.
5-HT3A receptor inhibition by the substances and the unsweetened licorice tincture (A) and original traces of the antagonistic effect of hesperetin and (-)-liquiritigenin (B). (A) Of the tested substances, the flavonoids exhibited the strongest inhibition with hesperetin and rutin from Aurantii and licochalcone A and (-)-liquiritigenin from Glycyrrhiza species. The inhibition from left to right are the following: −3.1 ± 4.3%, −2.6 ± 5.3%, 55.0 ± 6.2%, 57.0 ± 6.1%, 66.6 ± 9.3%, 62.8 ± 2.1%, 70.6 ± 3.1%, 92.8 ± 2.0%, and 35.5 ± 5.7% (n = 5 − 9) [tincture = 1 Vol.-%, substances = 1 mM except glabridin (100 μM)] (**p < 0.005; ***p < 0.0005). (B) The time of application is denoted by the application bars above the traces. The inhibition was reversible after a 150-s washout. The 5-HT-induced currents showed a characteristic rebound when co-applied with hesperetin or (-)-liquiritigenin (magnifications).
Competitive and non-competitive action of the identified antagonists
The tinctures of Atractylodis and unsweetened licorice, as well as glabridin and licochalcone A, exhibited an apparent competitive antagonism. The tincture of Zingiberis and Glycyrrhiza and (-)-liquiritigenin showed increased inhibition at the higher agonist concentration (30 μM), indicating a non-competitive blocking mechanism (Table 1).
Concentration-inhibition curves of the identified antagonists
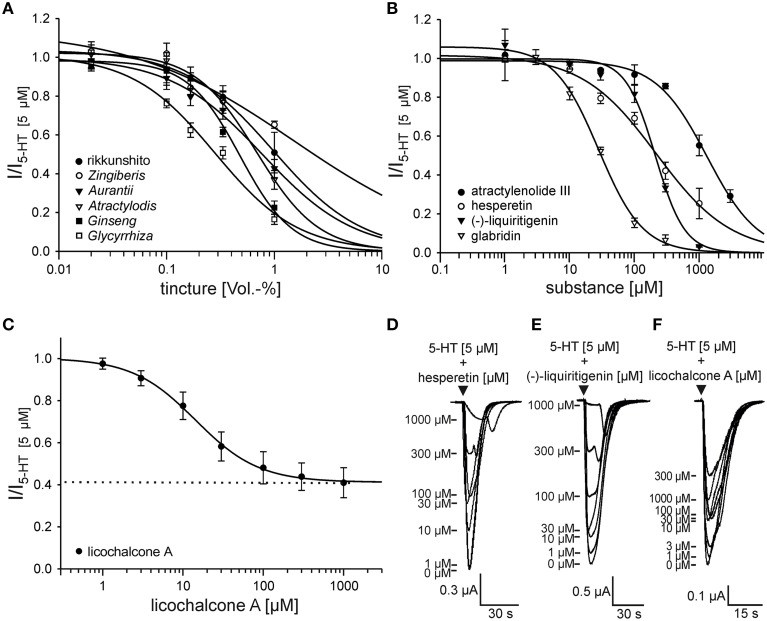
To characterize and quantify the action of the identified antagonists, we generated concentration-inhibition curves using 5 μM 5-HT. The tinctures inhibited the 5-HT3A receptor responses with regard to their IC50 values expressed as Vol.-% in the following order: Glycyrrhiza (0.281 ± 0.051) > Ginseng (0.481 ± 0.046) > Atractylodis (0.685 ± 0.028) > Aurantii (0.774 ± 0.096) > Zingiberis (1.517 ± 0.869) (Figure 3A). This ranking order was identical to that obtained previously (Figure 1A). The potency of the flavonoids hesperetin (IC50 = 218 ± 38 μM) and (-)-liquiritigenin (IC50 = 210 ± 25 μM) were similar (Figure 3B). The inhibition by licochalcone A stagnated at approximately 60%, indicating that it shows characteristics of a partial antagonist. In addition to glabridin (IC50 = 28.0 ± 2.8 μM), licochalcone A showed the highest potency (IC50 = 14.4 ± 1.3 μM) (Figures 3B,C).
Figure 3.
Concentration-inhibition curves of the antagonistic rikkunshito tinctures (A), substances (B,C) and original traces from the recordings of hesperetin (D), (-)-liquiritigenin (E) and licochalcone A (F). (A) The calculated IC50 values for the tinctures are the following: 1.025 ± 0.2 Vol.-% for rikkunshito (•), 1.517 ± 0.869 Vol.-% for Zingiberis (o), 0.774 ± 0.096 Vol.-% for Aurantii (▼), 0.685 ± 0.028 Vol.-% for Atractylodis (∇), 0.481 ± 0.046 Vol.-% for Ginseng (■) and 0.281 ± 0.051 Vol.-% for Glycyrrhiza (□) (n = 4 − 6). (B) The calculated IC50 values for the substances are the following: 1322 ± 145 μM for atractylenolide III (•), 218 ± 38 μM for hesperetin (o), 210 ± 25 μM for (-)-liquiritigenin (▼) and 28.0 ± 2.8 μM for glabridin (∇) (n = 5 − 7). (C) The inhibition by licochalcone A stagnated at 59.0 ± 7.1%, resulting in a residual current of approximately 40% remaining (dashed line). The IC50 value was 14.4 ± 1.3 μM (n = 5 − 8). (D–F) Application of 5-HT + inhibitor (▼) leads to currents, whose maximal amplitude (labeled with -) decreases with increasing concentrations of the inhibitor. The inhibition obtained with licochalcone A stagnated above a concentration of 10 μM, whereas the inhibition induced by hesperetin and (-)-liquiritigenin showing rebounds at concentrations greater than 300 μM and increased with increases in the concentrations until nearly the whole response was blocked.
Discussion
Rikkunshito is a Japanese herbal medicine that shows orexigen and antiemetic effects (Takeda et al., 2008; Fujitsuka and Uezono, 2014; Tominaga and Arakawa, 2015). Moreover, it is involved in the regulation of peristalsis and digestion (Tominaga et al., 2011) and therefore ameliorates symptoms of functional dyspepsia and irritable bowel syndrome (Oka et al., 2014). These effects and the antiemetic properties of rikkunshito may be explained by 5-HT3 receptor antagonism. Therefore, we assessed the modulatory effect of the eight rikkunshito constituents as ethanol tinctures to identify the most effective constituents and to find new specific antagonists for the 5-HT3A receptor.
We detected an antagonistic effect on 5-HT3A receptors exerted by the tinctures of rikkunshito, Aurantii, Ginseng, Zingiberis, Atractylodis, and Glycyrrhiza. Surprisingly, Zingiberis, which was initially thought to be mainly responsible for the effects of rikkunshito, was the weakest identified antagonist. However, we identified Radix Glycyrrhiza uralensis as the strongest 5-HT3A receptor antagonist among the tinctures (Figures 1A, 3A), presumably through the action of (-)-liquiritigenin.
The flavonoid glycoside hesperidin from Aurantii is one of the most abundant flavonoids in rikkunshito. It shows a gastroprokinetic effect similar to that of the specific 5-HT3 receptor antagonist ondansetron, suggesting that hesperidin is a 5-HT3 receptor antagonist (Tominaga et al., 2011). However, in our experiments, only hesperetin, the aglycone of hesperidin, exhibited 5-HT3A receptor antagonism. Tominaga et al. used an in vivo animal experimental paradigm in which hesperidin was applied orally. Therefore, it is possible that hesperidin is converted into the active substance by deglycosylation in vivo. The lack of 5-HT3A receptor antagonism for hesperidin may be explained by steric problems caused by glycosylation with the disaccharide rutinose. However, other glycosylated flavonoids are known, e.g., rutin from Aurantii, which shows an antagonistic effect on 5-HT3 receptors. However, also in this case, quercetin, the aglycone of rutin, is the more potent substance (Lee et al., 2008).
The tincture of Atractylodis, which is used in kampo medicine for the treatment of nausea and cachexia, showed a strong, apparently competitive antagonism. In our study, atractylenolide III, a weak antagonist (IC50 = 1322 ± 145 μM), was the only ingredient that was investigated. However, investigations of other ingredients, such as atractylol, atractylon or biatractylolid (Shao et al., 2014), could lead to the identification of competitive 5-HT3A receptor antagonists with higher potency.
Strong 5-HT3A receptor antagonism was also observed for the tincture of Ginseng, which is used due to its antiemetic effect. Kim et al. showed that Ginseng extracts reduce cisplatin-induced nausea in ferrets (Kim et al., 2005). Steroid glycosides, called ginsenosides, are accountable for the observed 5-HT3A receptor antagonism (Min et al., 2003; Lee et al., 2007), and their binding site in the pore region of 5-HT3A receptors has been identified (Lee et al., 2007).
The vanilloids gingerol and shogaol (Abdel-Aziz et al., 2005, 2006; Walstab et al., 2013) as well as the diterpene lactone galanolactone (Huang et al., 1991) are responsible for the antagonistic effect of the Zingiberis tincture. Abdel-Aziz et al. showed that gingerols and shogaols inhibit the contractions of isolated guinea pig and rat ilea induced by a specific 5-HT3 receptor agonist (Abdel-Aziz et al., 2005, 2006), indicating 5-HT3 receptor antagonism for the spasmolytic effects of Zingiberis and rikkunshito. Our study supports the proposal of these vanilloids as the active principles of Zingiberis due to the non-competitive antagonism of this tincture, which was previously reported for gingerol and shogaol (Walstab et al., 2013). In many clinical trials, Zingiberis was able to reduce nausea under various conditions, such as motion sickness, hyperemesis gravidarum, CINV and PONV (Ernst and Pittler, 2000; Haniadka et al., 2012; Ding et al., 2013).
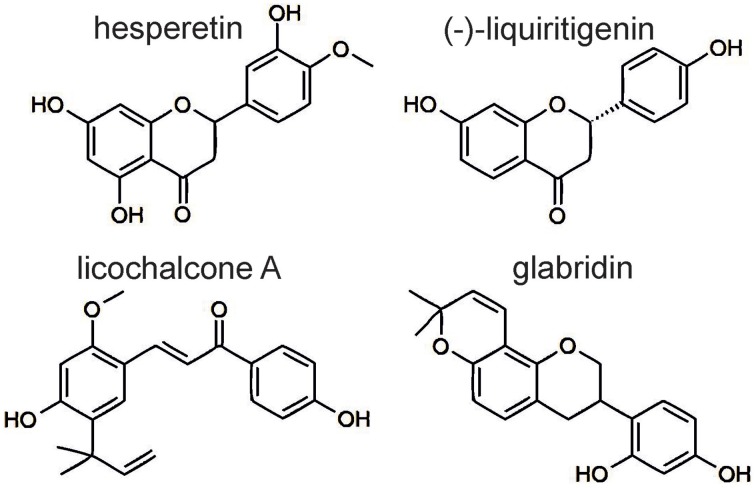
Radix Glycyrrhiza is used for the treatment of gastric ulcer and inflammations of the gastrointestinal and respiratory system (Kim et al., 2008). Among the rikkunshito constituents, Glycyrrhiza uralensis is the antagonistic tincture with the highest efficacy and potency. Moreover, we tested a tincture of unsweetened licorice, which is sourced from Glycyrrhiza glabra and usually consumed. This tincture was also able to inhibit the 5-HT3A receptor responses. We tested four Glycyrrhiza ingredients, namely the glycoside glycyrrhizin and the flavonoids glabridin, (-)-liquiritigenin and licochalcone A. Although (-)-liquiritigenin and glycyrrhizin are ubiquitous within the plants of the genus Glycyrrhiza, glabridin and licochalcone A are restricted to specific species. Glabridin is a specific ingredient of Glycyrrhiza glabra, and licochalcone A is detectable only in the species G. inflata and G. eurycarpa (Xu et al., 1997; Rauchensteiner et al., 2005; Kondo et al., 2007). Glycyrrhizin, which is used for the treatment of epilepsy, chronic gastritis and obstipation (Hänsel et al., 1993), showed no 5-HT3A receptor antagonism. The flavonoids glabridin, (-)-liquiritigenin and licochalcone A exhibited the strongest antagonism among our tested substances. (-)-Liquiritigenin appears to be the active component in Glycyrrhiza uralensis. Both showed non-competitive antagonism and lead to a huge rebound of the inhibited 5-HT3A receptor responses. In addition to (-)-liquiritigenin, hesperetin exhibited similar kinetics at high concentrations (Figures 3E,F). Because both flavonoids are chemically related (Figure 4), possess similar IC50 values and share a non-competitive antagonism, a common binding site at the receptor is hypothesized.
Figure 4.
Chemical structure of the investigated flavonoids. Hesperetin and (-)-liquiritigenin share similar molecular structures.
Kim et al. investigated the antiphlogistic effect of liquiritigenin and attributed this effect to the inhibition of NF-KB, an important transcription factor in the immune response (Kim et al., 2008). Therefore, liquiritigenin may contribute to the antiemetic and antiphlogistic effects of rikkunshito. In contrast, (-)-liquiritigenin cannot be accountable for the concentration-dependent 5-HT3A receptor antagonism of the licorice tincture. Instead, the apparently competitive blocker glabridin, which is a potentiator of the closely related GABAA receptor (Jin et al., 2013), may be the main antagonist of the unsweetened licorice, which is sourced from G. glabra. The blocker licochalcone A differs from hesperetin and (-)-liquiritigenin by its chemical structure and its action as a partial antagonist (Figures 3D–F, 4). The combination of concentration-dependency and partial blocker properties is unusual. Although these properties allude to the action of licochalcone A as a partial agonist, this consideration can be ruled out due to the absence of the direct activation of 5-HT3A receptors. The hypothesis of a concentration-dependent, allosteric antagonist whose maximal inhibition decreases with increasing agonist concentrations is more likely.
We showed that the Glycyrrhiza uralensis tincture exhibits the strongest inhibition of 5-HT3A receptor responses compared with the rest of the rikkunshito constituents, possibly due to the action of the flavonoid (-)-liquiritigenin. Other Glycyrrhiza species share this flavonoid-based 5-HT3A receptor antagonism, which is attributable to the antagonists glabridin and licochalcone A, a partial 5-HT3A receptor antagonist. These results contribute to a better understanding of the action of rikkunshito at a pharmacological level and allow the establishment of flavonoids as a new potent class of plant ingredients with regard to 5-HT3 receptor antagonism. Therefore, flavonoids appear to be at least equally active antagonists as gingerols and shogaols from Zingiberis and ginsenosides from Ginseng, which were thought to be responsible for the antiemetic properties of rikkunshito prior to this study. However, it should be mentioned, that the identified antagonists are inferior to already commonly used drugs like setrons due to their lower potency. Hence, a comparatively higher concentration of flavonoids must be reached to cause physiologically relevant effects. If those concentrations of substances with 5-HT3 receptor antagonism can be reached by rikkunshito under naturally-occuring conditions is hard to define. However, rikkunshito contains flavonoids, ginsenosides, and vanilloids, three well-investigated classes of plant ingredients that inhibit 5-HT3A receptors by binding to the receptor, presumably at independent binding sites. Hence, a synergistic drug interaction with additional or maybe mutual potentiating character is conceivable. In an exemplary experiment, we demonstrated that the combined action of distinct plant derived blockers can lead to an increased block of the 5-HT3A receptor (Supplementary Figure 3). In addition to that, a contribution of structurally related, unemployed substances, e.g., liquiritin and its apiosides and glycosides to the 5-HT3 receptor antagonism seems to be likely. In this study, we refuted the assumption that hesperidin, the main ingredient of rikkunshito, promotes gastric emptying via 5-HT3 receptor antagonism due to the lack of antagonism obtained in our screening. Instead, hesperetin, the aglycone of hesperidin, acts as a 5-HT3A receptor antagonist. Nevertheless, a 5-HT3 receptor mediated effect of hesperidin is assumed due to the occurrence of deglycosylation in vivo. This study contributes to a better understanding of the action of rikkunshito at a pharmacological level and emphasizes the importance of Glycyrrhiza and Aurantii for the antagonism of 5-HT3A receptors.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank U. Müller for the assistance provided. We especially thank Dr. P. Lepke for providing the kampo tinctures used in this study. This work was funded by the grants SFB 642 and SFB 874 from the German Research Foundation (Deutsche Forschungsgemeinschaft) to HH (TP A3).
Supplementary material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fphar.2015.00130
References
- Abdel-Aziz H., Nahrstedt A., Petereit F., Windeck T., Ploch M., Verspohl E. J. (2005). 5-HT3 receptor blocking activity of arylalkanes isolated from the rhizome of Zingiber officinale. Planta Med. 71, 609–616. 10.1055/s-2005-871265 [DOI] [PubMed] [Google Scholar]
- Abdel-Aziz H., Windeck T., Ploch M., Verspohl E. J. (2006). Mode of action of gingerols and shogaols on 5-HT3 receptors: binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur. J. Pharmacol. 530, 136–143. 10.1016/j.ejphar.2005.10.049 [DOI] [PubMed] [Google Scholar]
- Barann M., Dilger J. P., Bönisch H., Göthert M., Dybek A., Urban B. W. (2000a). Inhibition of 5-HT3 receptors by propofol: equilibrium and kinetic measurements. Neuropharmacology 39, 1064–1074. 10.1016/S0028-3908(99)00205-1 [DOI] [PubMed] [Google Scholar]
- Barann M., Göthert M., Brüss M., Bönisch H. (1999). Inhibition by steroids of [14C]-Guanidinium flux through the voltage- gated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 360, 234–241. 10.1007/s002109900089 [DOI] [PubMed] [Google Scholar]
- Barann M., Göthert M., Fink K., Bönisch H. (1993). Inhibition by anaesthetics of 14C-guanidinium flux through the voltage-gated sodium channel and the cation channel of the 5-HT3 receptor of N1E-115 neuroblastoma cells. Naunyn. Schmiedebergs. Arch. Pharmacol. 347, 125–132. 10.1007/BF00169256 [DOI] [PubMed] [Google Scholar]
- Barann M., Linden I., Witten S., Urban B. W. (2008). Molecular actions of propofol on human 5-HT3A receptors: enhancement as well as inhibition by closely related phenol derivatives. Anesth. Analg. 106, 846–857. 10.1213/ane.0b013e318162ca7c [DOI] [PubMed] [Google Scholar]
- Barann M., Meder W., Dorner Z., Bruss M., Bonisch H., Gothert M., et al. (2000b). Recombinant human 5-HT(3A) receptors in outside-out patches of HEK 293 cells: basic properties and barbiturate effects. Naunyn. Schmiedebergs. Arch. Pharmacol. 362, 255–265. 10.1007/s002100000288 [DOI] [PubMed] [Google Scholar]
- Barann M., Molderings G., Brüss M., Bönisch H., Urban B. W., Göthert M. (2002). Direct inhibition by cannabinoids of human 5-HT3A receptors: probable involvement of an allosteric modulatory site. Br. J. Pharmacol. 137, 589–596. 10.1038/sj.bjp.0704829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boess F. G., Martin I. L. (1994). Molecular biology of 5-HT receptors. Neuropharmacology 33, 275–317. 10.1016/0028-3908(94)90059-0 [DOI] [PubMed] [Google Scholar]
- Costedio M. M., Hyman N., Mawe G. M. (2007). Serotonin and Its Role in Colonic Function and in Gastrointestinal Disorders. Dis. Colon Rectum. 3, 376–388. 10.1007/s10350-006-0763-3 [DOI] [PubMed] [Google Scholar]
- Cubeddu L. X., Pendergrass K., Ryan T., York M., Burton G., Meshad M., et al. (1994). Efficacy of oral ondansetron, a selective antagonist of 5-HT3 receptors, in the treatment of nausea and vomiting associated with cyclophosphamide-based chemotherapies. Ondansetron study group. Am. J. Clin. Oncol. 17, 137–146. 10.1097/00000421-199404000-00010 [DOI] [PubMed] [Google Scholar]
- Das P., Dillon G. H. (2005). Molecular determinants of picrotoxin inhibition of 5-hydroxytryptamine type 3 receptors. J. Pharmacol. Exp. Ther. 314, 320–328. 10.1124/jpet.104.080325 [DOI] [PubMed] [Google Scholar]
- Derkach V., Surprenant A., North R. A. (1989). 5-HT3 receptors are membrane ion channels. Nature 339, 706–709. 10.1038/339706a0 [DOI] [PubMed] [Google Scholar]
- Ding M., Leach M. J., Bradley H. (2013). A systematic review of the evidence for topical use of ginger. Explore 9, 361–364. 10.1016/j.explore.2013.08.001 [DOI] [PubMed] [Google Scholar]
- Doak G. J., Sawynok J. (1997). Formalin-induced nociceptive behavior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience 80, 939–949. 10.1016/S0306-4522(97)00066-3 [DOI] [PubMed] [Google Scholar]
- Ernst E., Pittler M. H. (2000). Efficacy of ginger for nausea and vomiting: a systematic review of randomized clinical trials. Br. J. Anaesth. 84, 367–371. 10.1093/oxfordjournals.bja.a013442 [DOI] [PubMed] [Google Scholar]
- Fujitsuka N., Uezono Y. (2014). Rikkunshito, a ghrelin potentiator, ameliorates anorexia–cachexia syndrome. Front. Pharmacol. 5:271. 10.3389/fphar.2014.00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon M. D. (2004). Review article: serotonin receptors and transporters—roles in normal and abnormal gastrointestinal motility. Aliment. Pharmacol. Ther. 20(Suppl. 7), 3–14. 10.1111/j.1365-2036.2004.02180.x [DOI] [PubMed] [Google Scholar]
- Gyermek L. (1995). 5-HT3 receptors: pharmacologic and therapeutic aspects. J. Clin. Pharmacol. 35, 845–855. 10.1002/j.1552-4604.1995.tb04129.x [DOI] [PubMed] [Google Scholar]
- Haniadka R., Rajeev G., Palatty P. L., Arora R., Baliga M. S. (2012). (Ginger) as an anti-emetic in cancer chemotherapy: a review. J. Altern. Complement. Med. 5, 440–444. 10.1089/acm.2010.0737 [DOI] [PubMed] [Google Scholar]
- Hannon J., Hoyer D. (2008). Molecular biology of 5-HT receptors. Behav. Brain Res. 195, 198–213. 10.1016/j.bbr.2008.03.020 [DOI] [PubMed] [Google Scholar]
- Hänsel R., Keller K., Rimpler H., Schneider G. (eds.). (1993). Hagers Handbuch der Pharmazeutischen Praxis, Drogen E–O. 5. Berlin; Heidelberg: Springer-Verlag. [Google Scholar]
- Hope A. G., Peters J. A., Brown A. M., Lambert J. J., Blackburn T. P. (1996). Characterization of a human 5-hydroxytryptamine3 receptor type A (h5-HT3R-AS) subunit stably expressed in HEK 293 cells. Br. J. Pharmacol. 118, 1237–1245. 10.1111/j.1476-5381.1996.tb15529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q. R., Iwamoto M., Aoki S., Tanaka N., Tajima K., Yamahara J., et al. (1991). Anti-5-hydroxytryptamine3 effect of galanolactone, diterpenoid isolated from ginger. Chem. Pharm. Bull. 39, 397–399. 10.1248/cpb.39.397 [DOI] [PubMed] [Google Scholar]
- Jeggo R. D., Kellett D. O., Wang Y., Ramage A. G., Jordan D. (2005). The role of central 5-HT3 receptors in vagal reflex inputs to neurones in the nucleus tractus solitarius of anaesthetized rats. J. Physiol. 566, 939–953. 10.1113/jphysiol.2005.085845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z., Kim S., Cho S., Kim I. H., Han D., Jin Y. H. (2013). Potentiating effect of glabridin on GABAA receptor-mediated responses in dorsal raphe neurons. Planta Med. 79, 1408–1412. 10.1055/s-0033-1350698 [DOI] [PubMed] [Google Scholar]
- Kim J. H., Yoon I. S., Lee B. H., Choi S. H., Lee J. H., Lee J. H., et al. (2005). Effects of korean red ginseng extract on cisplatin-induced nausea and vomiting. Arch. Pharmacal Res. 28, 680–684. 10.1007/BF02969358 [DOI] [PubMed] [Google Scholar]
- Kim Y. W., Zhao R. J., Park S. J., Lee J. R., Cho I. J., Yang C. H., et al. (2008). Anti-inflammatory effects of liquiritigenin as a consequence of the inhibition of NF-kappaB-dependent iNOS and proinflammatory cytokines production. Br. J. Pharmacol. 154, 165–173. 10.1038/bjp.2008.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Shiba M., Nakamura R., Morota T., Shoyama Y. (2007). Constituent properties of licorices derived from glycyrrhiza uralensis, G. glabra, or G. inflata identified by genetic information. Biol. Pharm. Bull. 30, 1271–1277. 10.1248/bpb.30.1271 [DOI] [PubMed] [Google Scholar]
- Lee B. H., Lee J. H., Lee S. M., Jeong S. M., Yoon I. S., Lee J. H., et al. (2007). Identification of ginsenoside interaction sites in 5-HT3A receptors. Neuropharmacology 52, 1139–1150. 10.1016/j.neuropharm.2006.12.001 [DOI] [PubMed] [Google Scholar]
- Lee B. H., Pyo M. K., Lee J. H., Choi S. H., Shin T. J., Lee S. M., et al. (2008). Differential regulations of quercetin and its glycosides on ligand-gated ion channels. Biol. Pharm. Bull. 31, 611–617. 10.1248/bpb.31.611 [DOI] [PubMed] [Google Scholar]
- Lobitz N., Gisselmann G., Hatt H., Wetzel C. H. (2001). A single amino-acid in the TM1 domain is an important determinant of the desensitization kinetics of recombinant human and guinea pig alpha-homomeric 5-hydroxytryptamine type 3 receptors. Mol. Pharmacol. 59, 844–851. 10.1124/mol.59.4.844 [DOI] [PubMed] [Google Scholar]
- Manteniotis S., Lehmann R., Flegel C., Vogel F., Hofreuter A., Schreiner B. S. P., et al. (2013). Comprehensive RNA-seq expression analysis of sensory ganglia with a focus on ion channels and GPCRs in trigeminal ganglia. PLoS ONE 8:e79523. 10.1371/journal.pone.0079523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K. T., Koo B. N., Kang J. W., Bai S. J., Ko S. R., Cho Z. H. (2003). Effect of ginseng saponins on the recombinant serotonin type 3A receptor expressed in xenopus oocytes: implication of possible application as an antiemetic. J. Altern. Comp. Med. 9, 505–510. 10.1089/107555303322284794 [DOI] [PubMed] [Google Scholar]
- Niesler B., Frank B., Kapeller J., Rappold G. A. (2003). Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene 310, 101–111. 10.1016/S0378-1119(03)00503-1 [DOI] [PubMed] [Google Scholar]
- Oka T., Okumi H., Nishida S., Ito T., Morikiyo S., Kimura Y., et al. (2014). Effects of kampo on functional gastrointestinal disorders. Biopsychosoc. Med. 8:5. 10.1186/1751-0759-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauchensteiner F., Matsumura Y., Yamamoto Y., Yamaji S., Tani T. (2005). Analysis and comparison of Radix Glycyrrhizae (licorice) from Europe and China by capillary-zone electrophoresis (CZE). J. Pharm. Biomed. Anal. 38, 594–600. 10.1016/j.jpba.2005.01.038 [DOI] [PubMed] [Google Scholar]
- Saras A., Gisselmann G., Vogt-Eisele A. K., Erlkamp K. S., Kletke O., Pusch H., et al. (2008). Histamine action on vertebrate GABAA receptors: direct channel gating and potentiation of GABA responses. J. Biol. Chem. 283, 10470–10475. 10.1074/jbc.M709993200 [DOI] [PubMed] [Google Scholar]
- Schreiner B. S. P., Lehmann R., Thiel U., Ziemba P. M., Beltrán L. R., Sherkheli M. A., et al. (2014). Direct action and modulating effect of (+)- and (-)-nicotine on ion channels expressed in trigeminal sensory neurons. Eur. J. Pharmacol. 728, 48–58. 10.1016/j.ejphar.2014.01.060 [DOI] [PubMed] [Google Scholar]
- Shao Q. S., Zhang A. L., Ye W. W., Guo H. P., Hu R. H. (2014). Fast determination of two atractylenolides in rhizoma atractylodis macrocephalae by fourier transform near-infrared spectroscopy with partial least squares. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 24, 499–504. 10.1016/j.saa.2013.10.035 [DOI] [PubMed] [Google Scholar]
- Sherkheli M. A., Vogt-Eisele A. K., Bura D., Beltrán L. R., Gisselmann G., Hatt H. (2010). Characterization of selective TRPM8 ligands and their structure activity response (S.A.R) relationship. J. Pharm. Pharm. Sci. 13, 242–253. [DOI] [PubMed] [Google Scholar]
- Takeda H., Sadakane C., Hattori T., Katsurada T., Ohkawara T., Nagai K., et al. (2008). Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 134, 2004–2013. 10.1053/j.gastro.2008.02.078 [DOI] [PubMed] [Google Scholar]
- Thompson A. J., Duke R. K., Lummis S. C. R. (2011). Binding sites for bilobalide, diltiazem, ginkgolide, and picrotoxinin at the 5-HT3 receptor. Mol. Pharmacol. 80, 183–190. 10.1124/mol.111.071415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K., Arakawa T. (2015). Clinical application of kampo medicine (rikkunshito) for common and/or intractable symptoms of the gastrointestinal tract. Front. Pharmacol. 6:7. 10.3389/fphar.2015.00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga K., Kido T., Ochi M., Sadakane C., Mase A., Okazaki H., et al. (2011). The traditional japanese medicine rikkunshito promotes gastric emptying via the antagonistic action of the 5-HT 3 receptor pathway in rats. Evid. Based Complement. Alternat. Med. 2011:248481. 10.1093/ecam/nep173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walstab J., Krüger D., Stark T., Hofmann T., Demir I. E., Ceyhan G. O., et al. (2013). Ginger and its pungent constituents non-competitively inhibit activation of human recombinant and native 5-HT3 receptors of enteric neurons. Neurogastroenterol. Motil. 25, 439–447. 10.1111/nmo.12107 [DOI] [PubMed] [Google Scholar]
- Walstab J., Rappold G., Niesler B. (2010). 5-HT(3) receptors: role in disease and target of drugs. Pharmacol. Ther. 128, 146–169. 10.1016/j.pharmthera.2010.07.001 [DOI] [PubMed] [Google Scholar]
- Xu X., Hou C., Zhang Y., Liu Q., Liu Y., Yang J. (1997). Chemical constituents of the roots of Glycyrrhiza eurycarpa P.C.Li. J. Chinese Mater. Med. 22, 679–80, 703–704. [PubMed] [Google Scholar]
- Yanai M., Mochiki E., Ogawa A., Morita H., Toyomasu Y., Ogata K., et al. (2013). Intragastric administration of rikkunshito stimulates upper gastrointestinal motility and gastric emptying in conscious dogs. J. Gastroenterol. 48, 611–619. 10.1007/s00535-012-0687-8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.