Abstract
The gut microbiota has a significant role in human health and disease. Dysbiosis of the intestinal ecosystem contributes to the development of certain illnesses that can be reversed by favorable alterations by probiotics. The published literature was reviewed to identify scientific data showing a relationship between imbalance of gut bacteria and development of diseases that can be improved by biologic products. The medical conditions vary from infectious and antibiotic-associated diarrhea to obesity to chronic neurologic disorders. A number of controlled clinical trials have been performed to show important biologic effects in a number of these conditions through administration of prebiotics, probiotics, and synbiotics. Controlled clinical trials have identified a limited number of prebiotics, probiotic strains, and synbiotics that favorably prevent or improve the symptoms of various disorders including inflammatory bowel disease, irritable bowel syndrome, infectious and antibiotic-associated diarrhea, diabetes, nonalcoholic fatty liver disease, necrotizing enterocolitis in very low birth weight infants, and hepatic encephalopathy. Studies have shown that probiotics alter gut flora and lead to elaboration of flora metabolites that influence health through 1 of 3 general mechanisms: direct antimicrobial effects, enhancement of mucosal barrier integrity, and immune modulation. Restoring the balance of intestinal flora by introducing probiotics for disease prevention and treatment could be beneficial to human health. It is also clear that significant differences exist between different probiotic species. Metagenomics and metatranscriptomics together with bioinformatics have allowed us to study the cross-talk between the gut microbiota and the host, furthering insight into the next generation of biologic products.
Keywords: probiotics, prebiotics, synbiotics, lactobacilli, bifidobacteria
The human gastrointestinal tract is a complex ecosystem that, although sterile at birth, becomes rapidly colonized by microorganisms with a vast microbial population comprising tens of trillions of bacteria and hundreds of different species. The density and diversity increase exponentially moving from the stomach to the colon, where the microbial content is at its highest concentration. The fecal microbiota has been found to be relatively stable over time in individuals, but differs between subjects [1, 2]. The human gut microbiota is mostly dominated by the phyla Firmicutes and Bacteroidetes [2–4] and contains a core microbiome with shared functionality [5]. The microbiota facilitates digestion and aids in providing nutrition and in the shaping of our immune system [6].
Studies in germ-free animals show that commensal microorganisms are necessary for the development and maturation of the intestinal epithelium and immune system [7]. The intestinal microbiota contributes to the defense against pathogens by the mechanism of colonization resistance and fermentation of nondigestible carbohydrates, occurring mostly in the proximal colon. The main products produced by are short chain fatty acids (SCFAs), which include acetate, propionate, and butyrate. Butyrate is a major energy source for intestinal epithelial cells; affects cell proliferation, cell differentiation, mucus secretion, and barrier function; and has anti-inflammatory and antioxidative potential [8]. Hence, the gut microbiota performs a wide variety of metabolic activities that are essential for the host's metabolism.
In this review, we examine the value of probiotics, prebiotics, and synbiotics in alteration of the gut microenvironment leading to favorable effects in a number of disorders, topics of growing scientific interest [9]. We will begin with definitions, present the various agents that have been evaluated in clinical settings, discuss mechanism of action of these flora-enhancing agents and their clinical value as seen in scientific and controlled trials, and end with a perspective on future studies and applications.
DEFINITIONS
Bacteriotherapy includes 3 slightly different agents: probiotics, prebiotics, and synbiotics. Probiotics are defined in this review as living bacteria or fungi that confer a health benefit for the host. Prebiotics are nondigestible compounds that lead to favorable changes in the intestinal microbiota, and synbiotics are defined as products that contain both probiotics and prebiotics.
CLASSIFICATION OF FLORA-ALTERING BIOLOGIC AGENTS
Table 1 lists the studied agents that have been evaluated in patients with one of several medical conditions, including inflammatory bowel disease, irritable bowel syndrome, Crohn disease, hepatic encephalopathy, obesity, atopic dermatitis, diabetes, cancer, necrotizing enterocolitis, and hepatic encephalopathy.
Table 1.
List of Potential Products in Development That Have Biologic Effects Through Improvement in Diversity of Intestinal Flora With Secondary Effects on the Immune System
| Product Classification | Compounds in Development | Therapeutic Target | References |
|---|---|---|---|
| Prebiotics | Inulin | Lipid control, cardiovascular effects, cancer prevention | [10–12] |
| Xylooligosaccharide | Lipid control, cancer prevention | [13, 14] | |
| Oligofructose | Cancer prevention, treatment of recurrent CDI | [12, 15, 16] | |
| Fructooligosaccharide | Lipid control, cardiovascular effects, prevention of atopic dermatitis | [17, 18] | |
| Probiotics | Saccharomyces boulardii | Prevention of AAD, prevention and treatment of infectious diarrhea, prevention of CDI, improvement in symptoms of IBS | [19–24] |
| Lactobacillus rhamnosus GG | Prevention of AAD, CDI, and infectious diarrhea; treatment of IBS and prevention of atopic dermatitis | [25–31] | |
| Lactobacillus reuteri (strains SD2112 and RC14) | Treatment of functional bowel disease (eg, IBS) and treatment of vaginosis/vaginitis | [32, 33] | |
| Lactobacillus plantarum 299V DSM 9843 | Treatment of IBS | [34, 35] | |
| Lactobacillus acidophilus (strain NCDO1748 and other strains) | Prevention of necrotizing enterocolitis, radiation enteritis, and vaginitis | [36–38] | |
| Lactobacillus casei DN-114001 | Prevention of AAD, infectious diarrhea, and CDI | [39, 40] | |
| Lactobacillus rhamnosus GR-1 | Treatment of vaginosis/vaginitis | [33] | |
| Lactobacillus gasseri SBT2055 | Associated with weight loss | [41] | |
| Escherichia coli DSM 17252 | Treatment of IBS | [42] | |
| Streptococcus faecalis | Treatment of IBS | [43] | |
| Bifidobacterium infantis B5624 | Treatment of IBS | [44] | |
| Bifidobacterium bifidum strain NCDO1463 | Treatment of necrotizing enterocolitis | [45] | |
| Bifidobacterium lactis | Prevention of atopic dermatitis | [46] | |
| Lactobacillus brevis CD2 | Reduce incidence of radiation- and chemotherapy-induced mucositis | [47] | |
| Lactobacillus casei, Lactobacillus acidophilus (Bio-K+ CL1285) | Prevention of AAD and CDI | [48] | |
| Eight probiotic strains (VSL#3)a | Management of IBS, IBD, and pouchitis; prevention of radiation-induced diarrhea | [49–55] | |
| Synbiotics | Lactobacillus acidophilus, Bifidobacterium bifidum, and fructooligosaccharides | Increase HDL cholesterol and reduce fasting glycemia | [56] |
| Bifidobacterium and fructooligosaccharides | Treatment of hepatic encephalopathy | [57] |
Abbreviations: AAD, antibiotic-associated diarrhea; CDI, Clostridium difficile infection; HDL, high-density lipoprotein; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome.
a Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus paracasei, Lactobacillus bulgaricus, Streptococcus thermophilus.
MECHANISMS OF ACTION OF PROBIOTICS
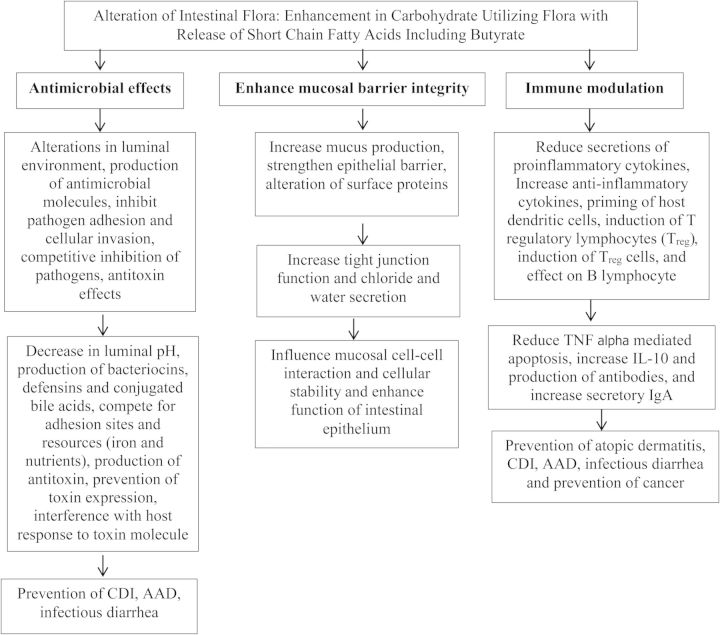
There are 3 general mechanisms by which probiotics appear to exert their beneficial effects, with important differences seen between probiotic species and strains: antimicrobial effects, enhancement of mucosal barrier integrity, and immune modulation (Figure 1). Probiotics exert antimicrobial effects by release in the intestinal environment of antimicrobial molecules and by taking up space, limiting growth of other microbes. The important benefits of probiotics come from their ability to metabolize complex carbohydrates and produce lactic acid and SCFAs such as butyrate [58, 59]. Butyrate reduces bacterial translocation, improves the organization of tight junctions [60], and stimulates the synthesis of mucin, a glycoprotein maintaining the integrity of the intestinal epithelium [61]. Understanding probiotic action along with increasing knowledge of probiotics on the host immune system is likely to offer useful and promising means to modulate host immunity for prevention and treatment of a broad range of human disorders. We will consider the 3 general functions of probiotics.
Figure 1.
Biologic effects and mechanisms of action of prebiotics, probiotics, and synbiotics. Abbreviations: AAD, antibiotic-associated diarrhea; CDI, Clostridium difficile infection; IgA, immunoglobulin A; IL-10, interleukin 10; TNF, tumor necrosis factor.
ANTIMICROBIAL EFFECTS OF MICROBIAL FLORA
Probiotic strains alter the luminal environment, decrease adhesion and cellular invasion, and can produce antibacterial products (eg, bacteriocins, hydrogen peroxide, and organic acids) that can inhibit the growth of pathogens. Several lactobacilli are responsible for producing bacteriocins [62]. The inhibitory action of these bacteriocins varies from inhibiting other lactobacilli to directly inhibiting a wider range of gram-positive, gram-negative bacteria, viruses, and certain fungi [63]. Another probiotic, Lactobacillus salivarius subspecies salivarius UCC118, produces a 2-peptide bacteriocin, ABP-118, which inhibits several pathogens including Enterococcus, Bacillus, Listeria, Staphylococcus, and Salmonella species [64].
Hydrolytic enzymes produced by some probiotics contribute to the increase of lactic acid, propionic acid, butyric acid, and other SCFAs in the intestinal lumen, reducing the luminal pH. Maintaining a lower pH creates a physiologically restrictive environment that can inhibit the growth and colonization by pathogenic bacteria. This was demonstrated in a study of mice infected with Shiga toxin–producing Escherichia coli O157:H7. Mice given the probiotic Bifidobacterium breve were found to have lower luminal pH via the production of a high concentration of acetic acid, consequently increasing animal survival [65]. This finding was confirmed in humans with ulcerative colitis given the probiotic preparation VSL#3, where a significant decrease in pH was seen [66].
ENHANCE MUCOSAL BARRIER INTEGRITY
Probiotics compete with pathogens and prevent their invasion through the epithelium by their ability to adhere to the intestinal epithelium and mucus. This mechanism inhibits the mucosal and epithelial adherence of pathogens in the intestinal system [67].
Probiotics also compete with other microorganisms for limiting resources. Iron is one such limited resource, as it is a necessary element for nearly all microorganisms. For example, the probiotic E. coli Nissle 1917 possesses multiple iron uptake mechanisms, enabling it to effectively take up this limited environmental iron, while simultaneously competitively inhibiting of the growth of other intestinal microbes and pathogens [68].
Intestinal barrier function is maintained by mucus production, chloride and water secretion, and tight junctions, which bind the apical portions of epithelial cells. Disruption of the epithelial barrier is seen in several conditions including infectious diarrhea [69], inflammatory bowel disease [70, 71], and autoimmune diseases including type 1 diabetes mellitus [72]. Enhancement of the mucosal barrier may be a crucial mechanism by which probiotic bacteria benefit the host in these diseases. In a study examining mice deficient in interleukin 10 (IL-10), the addition of Lactobacillus species was shown to improve barrier integrity in this fashion, preventing the development of colitis [73].
IMMUNE MODULATION
Probiotics can alter mucosal immunity considerably as they are able to affect many host cell types involved in the local and systemic immune responses, including epithelial cells, dendritic cells (DCs), T cells, regulatory T (Treg) cells, monocytes/macrophages, immunoglobulin A (IgA)–producing B cells, natural killer cells, and by induction of T-cell apoptosis [74].
Probiotic bacteria influence intestinal epithelial cells through pattern recognition molecules or Toll-like receptors (TLRs), such as TLR2 and TLR4. These interactions may stimulate the production of various protective cytokines, such as IL-10 and transforming growth factor β, that can inhibit epithelial cell apoptosis and enhance epithelial cell regeneration [75]. This effect is supported by a study in which the probiotic Lactobacillus rhamnosus GG prevented cytokine-induced apoptosis in intestinal epithelial cells [76].
Probiotic bacteria also have an effect on intestinal DCs, which extend processes through the epithelium into the gut lumen and are able to present antigens that are important in early bacterial recognition and in shaping T-cell responses. DCs have the ability to recognize and respond to different bacteria by linking the innate immune system to the adaptive immune response and to develop T- and B-cell responses [77–79]. In addition, Treg cells are also induced by some probiotics, and this may explain how probiotics can exert an anti-inflammatory effect and are beneficial in the treatment of a number of inflammatory diseases, including atopic dermatitis and Crohn disease [80]. Furthermore, probiotic bacteria may also modulate the immune response to protect against potentially harmful antigens via B lymphocytes and antibody production. Children with acute rotavirus diarrhea given L. rhamnosus GG were better able to potentiate a nonspecific humoral immune response, shown by increases in immunoglobulin G, IgA, and immunoglobulin M secretion from circulating lymphocytes, resulting in significantly shorter duration of diarrhea [81].
PREBIOTICS
Prebiotics are nondigestible oligosaccharides, such as fructooligosaccharides, galactooligosaccharides, lactulose, and inulin, which have the potential to stimulate growth of selective and beneficial gut bacteria, particularly lactobacilli and bifidobacteria [17, 82]. Because of their composition, prebiotics cannot be adsorbed until they reach the colon, where they can be fermented by a specific microbe into SCFAs and lactate [17]. Recent evidence shows that prebiotics are able to increase the production of SCFAs, which in turn modulates cytokine production within the gut mucosa by altering the gut flora composition. In human studies, administration of 10 g of transgalactooligosaccharides was shown to increase the number of bifidobacteria and modify the colonic fermentation metabolism of the gut flora [83].
Prebiotics can also be used as energy substrates by intestinal bacteria. When inulin-type fructan prebiotics were given to mice, the number of bifidobacteria increased significantly, with an inverse correlation with the levels of lipopolysaccharide, development of glucose tolerance, and fat mass [84, 85]. Additionally, in clinical trials using these inulin-type fructans [86], prebiotics have shown positive weight loss results in overweight and obese populations.
Prebiotics have also shown to be useful in hypercholesterolemia. A randomized, double-blind, crossover study in hamsters using inulin as a prebiotic resulted in a 29% decrease in total cholesterol and a 63% decrease in triglycerides compared to controls over a 5-week study [10]. In another study, 40 male Sprague-Dawley rats given xylooligosaccharide as a prebiotic showed a 27% reduction in triglycerides [14]. In a randomized crossover human study, inulin administration to 12 men with hypercholesterolemia led to a mean reduction in serum triglycerides by 40 mg/dL (P = .05) [11].
Prebiotics have also shown to reduce cancer incidence in animal models. Rats and mice fed with inulin and/or oligofructose had decreased numbers of chemically induced precancerous lesions [87, 88]. In another study testing inulin and oligofructose, breast cancer incidence in rats and mice [89] and large intestinal tumor incidence [90] was lowered by adding 5%–15% inulin or oligofructose to the diet. The result was even more striking when a combination of prebiotics and probiotics was given [91].
The recurrence of Clostridium difficile–associated diarrhea can also be decreased with prebiotics. In a randomized study of 142 patients with C. difficile–associated diarrhea receiving oligofructose or placebo for 30 days in addition to specific antibiotic treatment, the recurrence rate was lowered from 34.3% in controls to 8.3% in the oligofructose recipients (P < .001) [15].
Clinical data from the use of prebiotics in allergic conditions have been encouraging. A recent meta-analysis showed that using prebiotics resulted in a 32% reduction in the incidence of pediatric atopic dermatitis [92]. Another meta-analysis by Osborn and Sinn [93] exploring the effect of specific prebiotics in the prevention of allergy found that using a combination of galactooligosaccharide and fructooligosaccharide was associated with a significant reduction in eczema (relative risk, 0.68). The reduction of atopic eczema by prebiotics was also supported in another study of 200 infants who were administered fructooligosaccharide/galactooligosaccharide-enriched formula or placebo. At 6 months of age, the incidence of atopic eczema was reduced from 23.1% (95% confidence interval [CI], 16.0%–32.1%) in the placebo group to 9.8% (95% CI, 5.4%–17.1%) in the prebiotic-supplemented group [18].
PROBIOTICS
Probiotics have been included in a number of controlled clinical trials in patients with infectious diarrhea and for prevention of antibiotic-associated diarrhea, therapy and prevention of C. difficile infection, inflammatory bowel disease, irritable bowel syndrome, prevention of radiation- or chemotherapy-induced sequelae, necrotizing enterocolitis, hepatic encephalopathy, and atopic dermatitis (Table 2).
Table 2.
Clinical Evidence of Efficacy of Probiotics in Which Controlled Trials Have Been Conducted
| Target Condition | Probiotic Agent | Study Outcome | References |
|---|---|---|---|
| Treatment of infectious diarrhea in children | Lactobacillus rhamnosus strains | Randomized, double-blind, placebo-controlled trial. L. rhamnosus strains (573L/1, 573L/2, 573L/3) dose of 1.2 × 1010 CFU vs placebo, twice daily for 5 d. Mean duration of diarrhea in the treated group: 84 ± 56 h vs placebo: 96 ± 72 h (P = .36). In rotavirus infection: 76 ± 35 h vs 115 ± 67 h (P = .03), respectively. | [27, 94] |
| Prevention of infectious diarrhea | Bifidobacterium lactis and Lactobacillus reuteri | Randomized, double-blind, placebo-controlled trial. Infants in the control group were found to have a mean of 0.31 episodes of diarrhea (95% CI, .22–.44 episodes) vs 0.12 episodes (95% CI, .05–.21 episodes) and 0.02 episodes (95% CI, .01–.05 episodes) in the B. lactis and L. reuteri–supplemented study groups, respectively (P = .001). | [95] |
| Prevention of AAD | Lactobacillus casei, Lactobacillus acidophilus (Bio-K+ CL1285) | Randomized, double-blind, placebo-controlled dose-ranging study. Patients were randomized to 1 of 3 groups: high-dose (2 probiotics capsules/day) and low-dose probiotic (1 probiotic capsule, 1 placebo capsule/day) and placebo group (2 placebo capsules/day). High dose (15.5%) had a lower AAD incidence vs low dose (28.2%). Each probiotic group had a lower AAD incidence vs placebo (44.1%). In patients who acquired AAD, high dose (2.8 d) and low dose (4.1 d) had shorter symptom duration vs placebo (6.4 d). | [48] |
| Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus | Randomized, double-blind, placebo-controlled trial using 100 g (97 mL) probiotic mixture twice/day. The placebo group received a long-life sterile milkshake. Seven of 57 (12%) of the probiotic group developed diarrhea associated with antibiotic use vs 19/56 (34%) in the placebo group (P = .007). | [39] | |
| Lactobacillus acidophilus, Lactobacillus casei (Bio-K+ CL1285) | Randomized, double-blind, placebo-controlled trial, using 49 g (50 × 109 CFU of L. acidophilus CL1285 and L. casei (Bio-K+ CL1285) once daily for 2 d, followed by 98 g of Bio-K+ CL1285 once daily over duration of antibiotic treatment. AAD occurred in 7/44 patients (15.9%) in the Lactobacilli group and in 16/45 patients (35.6%) in the placebo group (OR, 0.34; 95% CI, .125–.944; P = .05). | [96] | |
| Saccharomyces boulardii | Double-blind, placebo-controlled, parallel group study. Lyophilized S. boulardii or placebo (1 g/day). Significantly fewer patients receiving S. boulardii (7/97 [7.2%]) developed AAD vs 14/96 (14.6%) on placebo (P = .02). The efficacy of S. boulardii for the prevention of AAD was 51%. | [19] | |
| Prevention of CDAD | Lactobacillus casei, Lactobacillus acidophilus (Bio-K+ CL1285) | Randomized, double-blind, placebo-controlled dose-ranging study. Patients were randomized to 1 of 3 groups: high-dose (2 probiotics capsules/day) and low-dose probiotic (1 probiotic capsule, 1 placebo capsule/day) and placebo group (2 placebo capsules/day). High-dose probiotic (1.2%) had a lower CDI incidence vs low-dose probiotic (9.4%). Each treatment group had a lower CDI incidence vs placebo (23.8%). | [48] |
| Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophilus | Randomized, double-blind, placebo-controlled trial of 100 g (97 mL) probiotic mixture twice/day. No one in the probiotic group and 9/53 (17%) in the placebo group had diarrhea caused by CDI (P = .001). The absolute risk reduction was 17% (95% CI, 7%–27%), and the NNT was 6 (NNT, 4–14). | [39] | |
| Saccharomyces boulardii | A meta-analysis of the 4 studies that used S. boulardii showed a trend toward lower CDI rates in the probiotic group, but this result was not significant (risk ratio, 0.70; 95% CI, .29–1.69) and there was more heterogeneity (I2 = 17.2%; P = .30). | [21] | |
| Prevention of recurrent CDAD | Saccharomyces boulardii | 500 mg S. boulardii given twice daily for 4 wk during and after antibiotic treatment for CDI yielded an overall CDI recurrence rate of 26.3% comparing to a 44.8% CDI recurrence rate in the placebo group (P = .05). | [22] |
| Saccharomyces boulardii | A significant decrease in recurrences was observed only in patients treated with high-dose vancomycin (2 g/day) and S. boulardii (1 g/day for 28 d) (16.7%), compared with those who received high-dose vancomycin and placebo (50%; P = .05). | [23] | |
| Lactobacillus rhamnosus GG | Five patients with multiple recurrences of CDI were treated successfully with LGG in an open-label study. In another open-label, uncontrolled study, 4 children with multiple recurrences of CDI had resolution of their infection after 2 wk of LGG administration. | [26, 97, 98] | |
| IBS | VSL#3 | Randomized, double-blind, parallel group study. Treatment with VSL#3 was associated with reduced flatulence over the entire treatment period (placebo: 39.5 ± 2.6 vs VSL#3: 29.7 ± 2.6; P = .011). Colonic transit was retarded with VSL#3 vs placebo (colon geometric center, 2.27 ± 0.20 vs 2.83 ± 0.19; P = .05). | [51] |
| Bifidobacterium bifidum MIMBb75 | Randomized, double-blind, placebo-controlled trial. B. bifidum MIMBb75(1 × 109 CFU) resulted in a greater reduction in global IBS symptoms, with more patients consuming the probiotic (47%) than the placebo (11%) reporting adequate relief of their symptoms. | [99] | |
| Lactobacillus plantarum (DSM 9843) | Randomized, double-blind, placebo-controlled trial. Study group received 400 mL per day of a rose-hip drink of 5 × 107 CFU/mL L. plantarum (DSM 9843) and 0.009 g/mL oat flour; placebo group received a plain rose-hip drink. Flatulence was rapidly and significantly reduced in the test group compared with the placebo group. Abdominal pain was reduced in both groups. At the 12-month follow-up, patients in the test group maintained better overall GI function than control patients. | [100] | |
| Lactobacillus plantarum 299V (LP299V) | Randomized, double-blind, placebo-controlled trial. All 10 patients in the LP299V group (5 × 107 CFU/mL) reported resolution of their abdominal pain compared with 11 patients from a placebo group (P = .0012). An improvement in IBS symptoms was noted in 95% of patients in the LP299V group vs 15% of patients in the placebo group (P < .0001). | [35] | |
| Lactobacillus plantarum 299v (DSM 9843) | Randomized, double-blind, placebo-controlled, parallel designed study, using 1 capsule (10 × 109 CFU per capsule) of L. plantarum 299v (DSM 9843) or placebo. After 4 wk, both pain severity (0.68 + 0.53 vs 0.92 + 0.57; P < .05) and daily frequency (1.01 + 0.77 vs 1.71 + 0.93; P < .05) were lower with L. plantarum 299v (DSM 9843) than with placebo. Similar results were obtained for bloating. At week 4, 78.1% of the patients scored the symptomatic effect of L. plantarum 299v (DSM 9843) as excellent or good vs only 8.1% for placebo (P < .01). | [34] | |
| Bifidobacterium infantis 35624 | B. infantis 35624 experienced a greater reduction in symptom scores. Another study showed that B. infantis 35624 at a dose of 1 × 108 CFU was significantly superior to placebo. The improvement in global symptom assessment exceeded placebo by >20% (P < .02). | [44, 101] | |
| Escherichia coli (DSM 17252) | Randomized, double-blind, placebo-controlled trial. The general symptom score to the drug was 27/148 (18.2%) vs placebo with 7/150 (4.67%) (P = .000397). The improvement in abdominal pain score was 28/148 (18.9%) vs 10/150 (6.67%) for placebo (P = .001649). | [42] | |
| Streptococcus faecium | Double-blind, placebo-controlled trial. After 4 wk, 81% of the Paraghurt-treated (freeze-dried culture of S. faecium) and 41% of the placebo-treated patients had improved according to physicians’ overall assessment (P = .002). | [43] | |
| Remission of ulcerative colitis | VSL#3 | Randomized, double-blind, placebo-controlled trial using 3.6 × 1012 CFU of VSL#3 vs placebo. There were no significant differences in obtaining clinical remission, but there was a significant clinical response in the VSL#3 group. | [50] |
| VSL#3 | Randomized, double-blind, placebo-controlled trial finding the VSL#3 (3.6 × 1012 CFU) group to have significantly higher remission rates (42.9% vs 15.9%) and endoscopic healing (32% vs 14.7%). | [49] | |
| E. coli Nissle 1917 | Randomized, double-blind, placebo-controlled trial. Doses of 40 mL, 20 mL, or 10 mL enemas containing ECN (1 × 108 CFU/mL) or placebo, concluding that remission rates significantly decreased according to dosing; 53%, 44%, and 27%, respectively. | [102] | |
| Maintenance of ulcerative colitis | E. coli Nissle 1917 | ECN at 200 mg/d was similar in efficacy to 1500 mg of mesalamine for maintaining UC in remission. In children with UC, VSL#3 also showed improved rates of maintenance of remission. Three of 14 (21.4%) patients treated with VSL#3 and IBD therapy and 11 of 15 (73.3%) patients treated with placebo and IBD therapy relapsed within 1 y of follow-up (P = .014; RR, 0.32; CI = .025–.773; NNT, 2). | [103, 104] |
| Crohn's disease | Studies have found Lactobacillus GG and other lactobacilli not to be superior to placebo for inducing or maintaining remission in CD or for the prevention of postoperative CD. In addition, there are also no solid data to support the use of ECN or S. boulardii in Crohn's disease. | [105–110] | |
| Prevention and remission of pouchitis | VSL#3 | Patients given 2 sachets twice daily (3.6 × 1012 CFU/day) for 4 wk; 16/23 patients (69%) were in remission after treatment. The median total Pouchitis Disease Activity Index scores before and after therapy were 10 (range, 9–12) and 4 (range, 2–11), respectively (P < .01). All 16 patients who went into remission maintained remission during maintenance treatment. | [52] |
| Maintenance of pouchitis | VSL#3 | Three randomized, placebo-controlled studies were performed. ITT analyses revealed significantly lower relapse rates after 9 or 12 mo intervention in UC patients with a pouch, either after inducing remission by antibiotics (n = 40 and n = 36) or starting 1 wk after ileostomy closure (n = 40). The 3 randomized placebo-controlled studies were included in the meta-analysis, revealing a pooled RR of 0.17 (95% CI, .09–.33). | [53, 54, 111] |
| Prevention of atopic dermatitis | Lactobacillus rhamnosus | Meta-analysis of double-blinded, randomized controlled trials of 25 clinical trials. Probiotics were effective in reducing total IgE (mean reduction: −7.59 U/mL; 95% CI, −14.96 to −.22; P = .044). Probiotics significantly reduced the risk of atopic sensitization when administered prenatally (RR: 0.88; 95% CI, .78–.99; P = .035) and postnatally (RR: 0.86; 95% CI, .75–.98; P = .027). | [28] |
| Treatment of atopic dermatitis | Probiotic mixture (Bifidobacterium bifidum, Lactobacillus acidophilus, Lactobacillus casei, and Lactobacillus salivarius strains) | Randomized, double-blind, placebo-controlled trial. Patients received 2 bags of 2 × 109 probiotic mixture. Probiotic group effectively reduced the SCORAD index and serum cytokines interleukin 5, interleukin 6, interferon γ, and total serum IgE levels vs the placebo group. | [112] |
| Prevention of radiation-induced diarrhea | VSL#3 | Randomized, double-blind, placebo-controlled trial. High-potency VSL#3 (1 sachet 3 times daily, each sachet of VSL#3 contained 4.5 × 1011/g) vs placebo starting from day 1 of radiation therapy. More placebo patients had radiation-induced diarrhea than VSL#3 patients (124/239 patients [51.8%] and 77/243 patients [31.6%]; P < .001), and more patients given placebo suffered grade 3 or 4 diarrhea vs VSL#3 recipients (55.4% and 1.4%; P < .001). Daily bowel movements were 14.7 ± 6 and 5.1 ± 3 among placebo and VSL#3 recipients, respectively (P < .05), and the mean time to the use of loperamide was 86 ± 6 h for placebo patients and 122 ± 8 h for VSL#3 patients (P < .001). | [55] |
| Reduce incidence of radiation- and chemotherapy-induced mucositis | Lactobacillus brevis CD2 | Randomized, double-blind, placebo-controlled trial. Six lozenges per day of 2 × 109 viable cells of L. brevis CD2 as the active ingredient were given. Grade III and IV mucositis developed in 52% of patients in the L. brevis CD2 group and 77% in the placebo group (P < .001). Anticancer treatment completion rates were 92% in the L. brevis CD2 group and 70% in the placebo group (P = .001). A larger proportion of patients remained free of mucositis when treated with L. brevis CD2 (28%) vs placebo (7%). | [47] |
| Prevention of necrotizing enterocolitis | Lactobacillus alone or in combination with Bifidobacterium | Meta-analysis. Enteral probiotic supplementation significantly reduced the incidence of severe NEC (stage II or more) (typical RR, 0.43; 95% CI, .33–.56; 20 studies, 5529 infants) and mortality (typical RR, 0.65; 95% CI, .52–.81; 17 studies, 5112 infants). The included trials reported no systemic infection with the supplemental probiotic organism(s). | [113] |
| Treatment of hepatic encephalopathy | Bifidobacterium combined with fructooligosaccharide | Bifidobacterium + FOS–treated patients compared with lactulose-treated patients showed a significant decrease of ammonia fasting HE1 (P < .001), and a significant increase of symbol digit modalities test (P < .001) and block design test (P < .001). | [57] |
Abbreviations: AAD, antibiotic-associated diarrhea; CD, Chrohn's disease; CDAD, Clostridium difficile–associated diarrhea; CDI, Clostridium difficile infection; CFU, colony-forming unit; CI, confidence interval; DSM, design structure matrix; ECN, Escherichia coli Nissle 1917; FOS, fructooligosaccharide; GI, gastrointestinal; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; IgE, immunoglobulin E; ITT, intent-to-treat; LGG, Lactobacillus rhamnosus GG; NEC, necrotizing enterocolitis; NNT, number needed to treat; OR, odds ratio; RR, relative risk; SCORAD, scoring atopic dermatitis; UC, ulcerative colitis.
Clinical trials have tested both single strains and mixtures of probiotics, with results depending upon the strain and the probiotic dose. The most common species that are used as single species and that have been studied are L. rhamnosus GG, Lactobacillus casei, Lactobacillus plantarum, Lactobacillus johnsonii, Bifidobacterium lactis, and Saccharomyces boulardii [114].
SYNBIOTICS
The concept of a synbiotic is to combine a probiotic and a prebiotic to facilitate the survival and activity of proven probiotics in vivo, as well as stimulating indigenous anaerobic bacteria. Probiotics and prebiotics work synergistically to provide a combined benefit. Some of the studies that have shown positive synergistic effects of synbiotics on obesity, diabetes, nonalcoholic fatty liver disease, necrotizing enterocolitis in very low birth weight infants, and treatment of hepatic encephalopathy are listed in Table 3.
Table 3.
Clinical Evidence of Efficacy of Synbiotics in Which Controlled Trials Have Been Conducted
| Target Condition | Synbiotic Agent | Study Outcome | Reference |
|---|---|---|---|
| Treatment of infectious diarrhea in children | Bifidobacterium lactis B94 plus inulin | Randomized, double-blind, placebo-controlled trial. N = 156. B. lactis B94 dose of 5 × 1010 CFU plus 900 mg inulin (Maflor sachet) was given once a day for 5 days. The duration of diarrhea was significantly reduced in the synbiotic group vs the placebo group (3.9 ± 1.2 d vs 5.2 ± 1.3 d, respectively; P < .001). The decrease was most pronounced in synbiotic-group cases of rotavirus diarrhea, (3.2 ± 1.3 d vs 5.2 ± 1.3 d, respectively; P = .001). | [115] |
| Constipation in adult women | Lactobacillus and Bifidobacterium strains plus FOS | Randomized, double-blind, placebo-controlled trial. N = 100. Each LACTOFOS sachet contained 6 g of FOS and 108–109 bacteria of Lactobacillus paracasei (Lpc-37), Lactobacillus rhamnosus (HN001), Lactobacillus acidophilus (NCFM), and Bifidobacterium lactis (HN019). Patients were given 2 daily doses of each for 30 days. Synbiotic group had increased frequency of evacuation, as well as stool consistency and shape nearer normal parameters than the placebo group, with significant benefits starting during the second and third weeks, respectively (interaction group/time, P < .0001). | [116] |
| Treatment of irritable bowel syndrome | Bacillus coagulans and FOS | Randomized, double-blind, placebo-controlled trial. N = 85. B. coagulans (15 × 107 CFU) and 100 g FOS (Lactol). Patients received synbiotic 3 × /d for 12 weeks. After treatment, more reduction in abdominal pain frequency was observed with synbiotic vs placebo (score reduction 4.2 ± 1.8 vs 1.9 ± 1.5; P < .001). Diarrhea frequency was decreased in the synbiotic group, but not in the placebo group (score reduction 1.9 ± 1.2 vs 0.0 ± 0.5; P < .001). | [117] |
| Crohn disease | Bifidobacterium longum and inulin/oligofructose (Synergy 1) | Randomized, double-blind, placebo-controlled trial. N = 35. B. longum, 2 × 1011 CFU plus 6 g of Synergy 1 were taken 2× daily for 6 months. Significant improvements in clinical outcomes occurred with synbiotic consumption, with reductions in both Crohn disease activity indices (P = .020) and histological scores (P = .018). Significant reductions occurred in TNF-α expression in synbiotic patients at 3 months (P = .041). Mucosal bifidobacteria proliferated in synbiotic patients. | [118] |
| Treatment of ulcerative colitis | Bifidobacterium longum plus psyllium | Randomized controlled trial. N = 120. B. longum 2 × 109 CFU and 8 g doses of psyllium. The primary endpoint was scores on the IBD Questionnaire, which assesses health-related quality of life in IBD at 4 weeks. Results showed a statistically significant improvement in scores (168 to 176; P = .03) for the synbiotic group at the end of the study. Individual scores for synbiotics group—systemic and social functions (P = .008 and P = .02). | [119] |
| Treatment of ulcerative colitis | Bifidobacterium breve strain Yakult and GOS | Randomized controlled study. B. breve strain Yakult (109 CFU/g) 3× a day, and 5.5 g of GOS per day for 1 year. There was significantly improvement of endoscopic grading (Matts classification) in the synbiotic group vs the standard therapy group (P < .05). | [120] |
| Necrotizing enterocolitis in very low birth weight infants | Bifidobacterium lactis plus inulin | Randomized, double-blind, placebo-controlled trial. N = 400. 30 mg of B. lactis (5 × 109 CFU) plus 900 mg of inulin. One sachet per day with breast milk or formula for 8 weeks before discharge or death. The rate of NEC was lower in probiotic (2.0%) and synbiotic (4.0%) groups vs prebiotic (12.0%) and placebo (18.0%) groups (P < .001). | [121] |
| Weight gain in children with failure to thrive | Bacillus coagulans plus FOS | Randomized, triple-blinded, placebo-controlled. N = 84. B. coagulans (1.5 × 108 CFU) and 100 mg FOS. Synbiotic mixture were administered for 6 months. The increase in weight was significantly higher in synbiotics group than in controls (P < .05). At the beginning, the mean weights were 10.25 ± 0.20 kg and 10.750 ± 0.160 kg in intervention and control groups, respectively. After 6 months, the mean weights became 12.280 ± 0.190 and 11.760 ± 0.17 kg in intervention and control groups, respectively. | [122] |
| Diabetes | Lactobacillus sporogenes plus inulin | Randomized double-blind, crossover controlled trial. N = 62. L. sporogenes (1 × 107 CFU) plus 0.04 g inulin, packed in 9-g packages taken 3× a day for 6 weeks. There was a significant decrease in serum insulin levels (changes from baseline: –1.75 ± 0.60 vs 0.95 ± 1.09 mIU /mL; P = .03), a significant decrease in hs-CRP levels (–1057.86 ± 283.74 vs 95.40 ± 385.38 ng/mL; P = 0.01), a significant increase in plasma total GSH (319.98 vs 19.73 mmol/L; P < 0.001) and serum uric acid levels (0.7 vs 0.1 mg/dL; P = .04). | [123] |
| Nonalcoholic fatty liver disease | Lactobacillus casei, Lactobacillus rhamnosus, Streptococcus thermophilus, Bifidobacterium breve, Lactobacillus acidophilus, Bifidobacterium longum, and Lactobacillus bulgaricus and FOS (Protexin) | Randomized, double-blind, placebo-controlled trial. N = 52. Each Protexin capsule contained 2 × 108 CFU of probiotic mixture and FOS. The synbiotic mixture was supplemented 2× daily for 28 wk. There was a significant reduction of ALT in the synbiotic group. ALT, −25.1 (−26.2, −24) vs −7.29 (−9.5, −5.1) IU/L, P < .001; AST, −31.33 (−32.1, −30.5) vs −7.94 (−11.1, −4.8) IU/L, P < .001; gamma-glutamyltransferase, −15.08 (−15.5, 214.7) vs −5.21 (−6.6, −3.9) IU/L, P < .001; hs-CRP, −2.3 (−3, −1.5) vs −1.04 (−1.5, −0.6) mmol/L, P < .05; TNF-α, −1.4 (−1.7, −1.1) vs −0.59 (−0.8, −0.3) mmol/L, P < .001; total nuclear factor kB p65, −0.016 (−0.022, −0.011) vs 0.001 (−0.004, −0.007) mmol/L, P < .001; and fibrosis score as determined by transient elastography, −2.98 (−3.6, −2.37) vs −0.77 (−1.32, −0.22) kPa, P < .001. | [124] |
| Lipid profile and glucose homeostasis in overweight or obese adults | Lactobacillus combined with inulin or Lactobacillus and Bifidobacterium combined with FOS | Meta-analysis study. Four synbiotic randomized controlled trials. A significant reduction in the triglycerides (SMD –0.43; 95% CI, –.70 to .15; P < .05) and fasting insulin (SMD –0.39; 95% CI –.75 to –.02; P = .04) after synbiotic supplementation. | [125] |
| Surgery for chronic pancreatitis | Streptococcus faecalis, Clostridium butyricum, Bacillus mesentericus, Lactobacillus sporogenes plus FOS | Randomized, single-blind, placebo-controlled trial. N = 75. S. faecalis T-110 (6 × 107), C. butyricum TOA (4 × 107 CFU), B. mesentericus TO-A (2 × 107 CFU), L. sporogenes (1 × 108 CFU) plus FOS. The synbiotic was given for 5 days prior and 10 days after the surgery. Primary study endpoint was the occurrence of postoperative infection during the first 30 days. The incidence of postoperative infectious complications (12.8% vs 39%; P < .05), duration of antibiotic therapy (P < .05), and length of hospital stay (P < .05) were significantly lower in the synbiotic group. | [126] |
| Radiation-induced acute proctitis | Lactobacillus reuteri plus inulin | Randomized, double-blind, placebo-controlled trial. N = 20. One sachet contained 5 g L. reuteri (108 CFU) and 4.3 g of inulin (Nestle). 1 sachet was given 1× a day for 1 wk before the beginning of radiation therapy, increasing the dose to 2 sachets/d for next 4 wk. The complete questionnaire score was higher in the second (23 [21–30] vs 26.5 [22–34], P < .05) and third (23 [21–32] vs 27.5 [24–33], P < .01) weeks in the placebo group. Proctitis symptoms were highest scored in the placebo group in both the second (19.5 [16–25]) and third (19 [17–24]) weeks than in the synbiotic group (week 2: 16.5 [15–20], P < .05; week 3: 17 [15–23], P < .01). | [127] |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CFU, colony-forming units; CI, confidence interval; FOS, fructooligosaccharides; GOS, galactooligosaccharide; GSH, glutathione; hs-CRP, high-sensitivity C-reactive protein; IBD, inflammatory bowel disease; LACTOFOS, symbiotic mixture includes Lactobacillus, Bifidobacterium strains and fructo-oligosaccharides; NEC, necrotizing enterocolitis; SMD, standardized mean difference; TNF, tumor necrosis factor; TOA, specific probiotic strain designation.
FUTURE STUDIES AND APPLICATION
Most of the currently commercialized probiotics used to treat and prevent medical conditions are limited to the Lactobacillus and Bifidobacterium strains previously discussed. The efficacy of the existing probiotics used for the treatment or prevention of medical conditions is limited. Information gained from previous studies are helping to set a rationale for selection of a next generation of probiotics such as Faecalibacterium prausnitzii [128], Clostridia clusters IV, XIVa, and XVIII [129], Akkermansia muciniphila [130], and Bacteroides uniformis [131]—the effects of some of which have been evaluated in preclinical trials, with promising results for inflammatory diseases and obesity.
New studies of probiotics in the treatment of various psychological states and autism are also beginning to be studied. The exact mechanism of how the microbiota influences gut-brain axis and behavior remains unknown, but there have been a few animal studies demonstrating the effects of probiotics to influence psychological states [132, 133]. In one such study, probiotics were tested as a delivery vehicle of neuroactive compounds due to their production of neurochemicals such as gamma-aminobutyric acid and other neurochemicals [134]. Similarly, emerging data have implied that a link between gut microbiome and autism may exist. Disruption of gut microbiota might promote the overproduction of neurotoxin-producing bacteria such as Clostridium tetani, which may contribute to autistic symptoms [135]. With better studies in humans, we await the results of appropriately designed placebo-controlled trials to further support the microbiome-gut-brain axis connection and identify a potential probiotic therapy for gastrointestinal and particular behavioral symptoms in human neurodevelopmental disorders.
CONCLUSIONS
The human gut microbiota plays an important role in human health, and the modulation of the gut microbiota may be used to treat and prevent an array of diseases. Prebiotics, probiotics, and synbiotics are appealing as preventive and therapeutic agents for human medical disorders. Their efficacy depends on the etiology of the disease and the probiotic strain. Future research will focus on well-designed human trials as well as mechanisms of action of probiotics, to provide more data on different probiotics strains and mixtures. With new advances in research using metagenomics and bioinformatic tools, the field of prebiotics, probiotics, and synbiotics will continue to grow as these agents are being evaluated in the modulation of intestinal health.
Notes
Financial support. This work was supported by discretionary funds from the University of Texas School of Public Health and from the Public Health Service (grant DK 56338), which funds the Texas Medical Center Digestive Diseases Center.
Supplement sponsorship. This article appeared as part of the supplement “Probiotics: Added Supplementary Value in Clostridium difficile Infection,” sponsored by Bio-K Plus International.
Potential conflicts of interest. H. L. D. has received consulting fees from Cubist, Merck, Salix, and Bio-K Plus and research grants through his university from GSK, Merck, Dr Falk Pharma, and Takeda. R. P. reports no potential conflicts.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Zoetendal EG, Akkermans AD, Akkermans-van Vliet WM, de Visser JAG, de Vos WM. The host genotype affects the bacterial community in the human gastrointestinal tract. Microb Ecol Health Dis 2001; 13:129–34. [Google Scholar]
- 2.Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science 2005; 308:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frank DN, Amand ALS, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A 2007; 104:13780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Takaishi H, Matsuki T, Nakazawa A, et al. Imbalance in intestinal microflora constitution could be involved in the pathogenesis of inflammatory bowel disease. Int J Med Microbiol 2008; 298:463–72. [DOI] [PubMed] [Google Scholar]
- 5.Qin J, Li R, Raes J, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010; 464:59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kau AL, Ahern PP, Griffin NW, Goodman AL, Gordon JI. Human nutrition, the gut microbiome and the immune system. Nature 2011; 474:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson-Chagoyán OC, Maldonado J, Gil A. Aetiology of inflammatory bowel disease (IBD): role of intestinal microbiota and gut-associated lymphoid tissue immune response. Clin Nutr 2005; 24:339–52. [DOI] [PubMed] [Google Scholar]
- 8.Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost F, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther 2008; 27:104–19. [DOI] [PubMed] [Google Scholar]
- 9.McFarland LV. Use of probiotics to correct dysbiosis of normal microbiota following disease or disruptive events: a systematic review. BMJ Open 2014; 4:e005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trautwein EA, Rieckhoff D, Erbersdobler HF. Dietary inulin lowers plasma cholesterol and triacylglycerol and alters biliary bile acid profile in hamsters. J Nutr 1998; 128:1937–43. [DOI] [PubMed] [Google Scholar]
- 11.Causey JL, Feirtag JM, Gallaher DD, Tungland BC, Slavin JL. Effects of dietary inulin on serum lipids, blood glucose and the gastrointestinal environment in hypercholesterolemic men. Nutr Res 2000; 20:191–201. [Google Scholar]
- 12.Klinder A, Forster A, Caderni G, Femia AP, Pool-Zobel BL. Fecal water genotoxicity is predictive of tumor-preventive activities by inulin-like oligofructoses, probiotics (Lactobacillus rhamnosus and Bifidobacterium lactis), and their synbiotic combination. Nutr Cancer 2004; 49:144–55. [DOI] [PubMed] [Google Scholar]
- 13.Langlands S, Hopkins M, Coleman N, Cummings J. Prebiotic carbohydrates modify the mucosa associated microflora of the human large bowel. Gut 2004; 53:1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hsu C-K, Liao J-W, Chung Y-C, Hsieh C-P, Chan Y-C. Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr 2004; 134:1523–8. [DOI] [PubMed] [Google Scholar]
- 15.Lewis S, Burmeister S, Brazier J. Effect of the prebiotic oligofructose on relapse of Clostridium difficile–associated diarrhea: a randomized, controlled study. Clin Gastroenterol Hepatol 2005; 3:442–8. [DOI] [PubMed] [Google Scholar]
- 16.Kolida S, Tuohy K, Gibson G. Prebiotic effects of inulin and oligofructose. Br J Nutr 2002; 87(suppl 2):S193–7. [DOI] [PubMed] [Google Scholar]
- 17.Bouhnik Y, Raskine L, Simoneau G, et al. The capacity of nondigestible carbohydrates to stimulate fecal bifidobacteria in healthy humans: a double-blind, randomized, placebo-controlled, parallel-group, dose-response relation study. Am J Clin Nutr 2004; 80:1658–64. [DOI] [PubMed] [Google Scholar]
- 18.Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child 2006; 91:814–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFarland LV, Surawicz CM, Greenberg RN, Elmer GW, et al. Prevention of beta-lactam-associated diarrhea by Saccharomyces boulardii compared with placebo. Am J Gastroenterol 1995; 90:439–48. [PubMed] [Google Scholar]
- 20.Szajewska H, Skorka A, Dylag M. Meta-analysis: Saccharomyces boulardii for treating acute diarrhoea in children. Aliment Pharmacol Ther 2007; 25:257–64. [DOI] [PubMed] [Google Scholar]
- 21.Johnson S, Maziade PJ, McFarland LV, et al. Is primary prevention of Clostridium difficile infection possible with specific probiotics? Int J Infect Dis 2012; 16:e786–92. [DOI] [PubMed] [Google Scholar]
- 22.McFarland LV, Surawicz CM, Greenberg RN, et al. A randomized placebo-controlled trial of Saccharomyces boulardii in combination with standard antibiotics for Clostridium difficile disease. JAMA 1994; 271:1913–8. [PubMed] [Google Scholar]
- 23.Surawicz CM, McFarland LV, Greenberg RN, et al. The search for a better treatment for recurrent Clostridium difficile disease: use of high-dose vancomycin combined with Saccharomyces boulardii. Clin Infect Dis 2000; 31:1012–7. [DOI] [PubMed] [Google Scholar]
- 24.Ciorba MA. A gastroenterologist's guide to probiotics. Clin Gastroenterol Hepatol 2012; 10:960–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guandalini S, Pensabene L, Zikri MA, et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: a multicenter European trial. J Pediatr Gastroenterol Nutr 2000; 30:54–60. [DOI] [PubMed] [Google Scholar]
- 26.Lawrence SJ, Korzenik JR, Mundy LM. Probiotics for recurrent Clostridium difficile disease. J Med Microbiol 2005; 54:905–6. [DOI] [PubMed] [Google Scholar]
- 27.Szymański H, Pejcz J, Jawień M, Chmielarczyk A, Strus M, Heczko P. Treatment of acute infectious diarrhoea in infants and children with a mixture of three Lactobacillus rhamnosus strains—a randomized, double-blind, placebo-controlled trial. Aliment Pharmacol Ther 2006; 23:247–53. [DOI] [PubMed] [Google Scholar]
- 28.Elazab N, Mendy A, Gasana J, Vieira ER, Quizon A, Forno E. Probiotic administration in early life, atopy, and asthma: a meta-analysis of clinical trials. Pediatrics 2013; 132:e666–76. [DOI] [PubMed] [Google Scholar]
- 29.Doron SI, Hibberd PL, Gorbach SL. Probiotics for prevention of antibiotic-associated diarrhea. J Clin Gastroenterol 2008; 42:S58–63. [DOI] [PubMed] [Google Scholar]
- 30.Katz JA. Probiotics for the prevention of antibiotic-associated diarrhea and Clostridium difficile diarrhea. J Clin Gastroenterol 2006; 40:249–55. [DOI] [PubMed] [Google Scholar]
- 31.Na X, Kelly C. Probiotics in Clostridium difficile infection. J Clin Gastroenterol 2011; 45:S154–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shornikova A-V, Casas IA, Isolauri E, Mykkänen H, Vesikari T. Lactobacillus reuteri as a therapeutic agent in acute diarrhea in young children. J Pediatr Gastroenterol Nutr 1997; 24:399–404. [DOI] [PubMed] [Google Scholar]
- 33.Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect 2006; 8:2772–6. [DOI] [PubMed] [Google Scholar]
- 34.Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol 2012; 18:4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niedzielin K, Kordecki H, ena Birkenfeld B. A controlled, double-blind, randomized study on the efficacy of Lactobacillus plantarum 299V in patients with irritable bowel syndrome. Eur J Gastroenterol Hepatol 2001; 13:1143–7. [DOI] [PubMed] [Google Scholar]
- 36.Ganguli K, Walker WA. Probiotics in the prevention of necrotizing enterocolitis. J Clin Gastroenterol 2011; 45:S133–8. [DOI] [PubMed] [Google Scholar]
- 37.Salminen E, Elomaa I, Minkkinen J, Vapaatalo H, Salminen S. Preservation of intestinal integrity during radiotherapy using live Lactobacillus acidophilus cultures. Clin Radiol 1988; 39:435–7. [DOI] [PubMed] [Google Scholar]
- 38.Hilton E, Isenberg HD, Alperstein P, France K, Borenstein MT. Ingestion of yogurt containing Lactobacillus acidophilus as prophylaxis for candidal vaginitis. Ann Intern Med 1992; 116:353–7. [DOI] [PubMed] [Google Scholar]
- 39.Hickson M, D'Souza AL, Muthu N, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007; 335:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pedone C, Arnaud C, Postaire E, Bouley C, Reinert P. Multicenter study on 928 children attending day care centers to evaluate the effect of milk fermented by Lactobacillus casei DN-114 001 on the incidence of diarrhoea. Int J Clin Pract 2000; 54:568–71. [PubMed] [Google Scholar]
- 41.Kadooka Y, Sato M, Imaizumi K, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 2010; 64:636–43. [DOI] [PubMed] [Google Scholar]
- 42.Enck P, Zimmermann K, Menke G, Klosterhalfen S. Randomized controlled treatment trial of irritable bowel syndrome with a probiotic E.-coli preparation (DSM17252) compared to placebo. Z Gastroenterol 2009; 47:209–14. [DOI] [PubMed] [Google Scholar]
- 43.Gade J, Thorn F. Paraghurt for patients with irritable bowel syndrome: a controlled clinical investigation from general practice. Scand J Prim Health Care 1989; 7:23–6. [DOI] [PubMed] [Google Scholar]
- 44.Whorwell PJ, Altringer L, Morel J, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol 2006; 101:1581–90. [DOI] [PubMed] [Google Scholar]
- 45.Lin H-C, Hsu C-H, Chen H-L, et al. Oral probiotics prevent necrotizing enterocolitis in very low birth weight preterm infants: a multicenter, randomized, controlled trial. Pediatrics 2008; 122:693–700. [DOI] [PubMed] [Google Scholar]
- 46.Panduru M, Panduru N, Sălăvăstru C, Tiplica GS. Probiotics and primary prevention of atopic dermatitis: a meta-analysis of randomized controlled studies. J Eur Acad Dermatol Venereol 2014. [DOI] [PubMed] [Google Scholar]
- 47.Sharma A, Rath G, Chaudhary S, Thakar A, Mohanti BK, Bahadur S. Lactobacillus brevis CD2 lozenges reduce radiation-and chemotherapy-induced mucositis in patients with head and neck cancer: a randomized double-blind placebo-controlled study. Eur J Cancer 2012; 48:875–81. [DOI] [PubMed] [Google Scholar]
- 48.Gao XW, Mubasher M, Fang CY, Reifer C, Miller LE. Dose–response efficacy of a proprietary probiotic formula of Lactobacillus acidophilus CL1285 and Lactobacillus casei LBC80R for antibiotic-associated diarrhea and Clostridium difficile-associated diarrhea prophylaxis in adult patients. Am J Gastroenterol 2010; 105:1636–41. [DOI] [PubMed] [Google Scholar]
- 49.Sood A, Midha V, Makharia GK, et al. The probiotic preparation, VSL# 3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 2009; 7:1202–9.e1. [DOI] [PubMed] [Google Scholar]
- 50.Tursi A, Brandimarte G, Papa A, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 2010; 105:2218–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim H, Vazquez Roque M, Camilleri M, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil 2005; 17:687–96. [DOI] [PubMed] [Google Scholar]
- 52.Gionchetti P, Rizzello F, Morselli C, et al. High-dose probiotics for the treatment of active pouchitis. Dis Colon Rectum 2007; 50:2075–84. [DOI] [PubMed] [Google Scholar]
- 53.Gionchetti P, Rizzello F, Helwig U, et al. Prophylaxis of pouchitis onset with probiotic therapy: a double-blind, placebo-controlled trial. Gastroenterology 2003; 124:1202–9. [DOI] [PubMed] [Google Scholar]
- 54.Mimura T, Rizzello F, Helwig U, et al. Once daily high dose probiotic therapy (VSL# 3) for maintaining remission in recurrent or refractory pouchitis. Gut 2004; 53:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delia P, Sansotta G, Donato V, et al. Use of probiotics for prevention of radiation-induced diarrhea. World J Gastroenterol 2007; 13:912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moroti C, Magri LFS, de Rezende Costa M, Cavallini DC, Sivieri K. Effect of the consumption of a new symbiotic shake on glycemia and cholesterol levels in elderly people with type 2 diabetes mellitus. Lipids Health Dis 2012; 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Malaguarnera M, Gargante MP, Malaguarnera G, et al. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol 2010; 22:199–206. [DOI] [PubMed] [Google Scholar]
- 58.Wong JM, Jenkins DJ. Carbohydrate digestibility and metabolic effects. J Nutr 2007; 137:2539S–46. [DOI] [PubMed] [Google Scholar]
- 59.Rolfe RD. The role of probiotic cultures in the control of gastrointestinal health. J Nutr 2000; 130:396S–402. [DOI] [PubMed] [Google Scholar]
- 60.Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis 2010; 16:1138–48. [DOI] [PubMed] [Google Scholar]
- 61.Burger-van Paassen N, Vincent A, Puiman P, et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem J 2009; 420:211–9. [DOI] [PubMed] [Google Scholar]
- 62.Klaenhammer TR. Bacteriocins of lactic acid bacteria. Biochimie 1988; 70:337–49. [DOI] [PubMed] [Google Scholar]
- 63.Nemcova R. Criteria for selection of lactobacilli for probiotic use [in Slovak]. Vet Med (Praha) 1997; 42:19–27. [PubMed] [Google Scholar]
- 64.Flynn S, van Sinderen D, Thornton GM, Holo H, Nes IF, Collins JK. Characterization of the genetic locus responsible for the production of ABP-118, a novel bacteriocin produced by the probiotic bacterium Lactobacillus salivarius subsp. salivarius UCC118. Microbiology 2002; 148:973–84. [DOI] [PubMed] [Google Scholar]
- 65.Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157: H7. Infect Immun 2004; 72:2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Venturi A, Gionchetti P, Rizzello F, et al. Impact on the composition of the faecal ora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther 1999; 13:1103–8. [DOI] [PubMed] [Google Scholar]
- 67.Servin AL, Coconnier M-H. Adhesion of probiotic strains to the intestinal mucosa and interaction with pathogens. Best Pract Res Clin Gastroenterol 2003; 17:741–54. [DOI] [PubMed] [Google Scholar]
- 68.Große C, Scherer J, Koch D, Otto M, Taudte N, Grass G. A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mole Microbiol 2006; 62:120–31. [DOI] [PubMed] [Google Scholar]
- 69.Sakaguchi T, Köhler H, Gu X, McCormick BA, Reinecker HC. Shigella flexneri regulates tight junction-associated proteins in human intestinal epithelial cells. Cell Microbiol 2002; 4:367–81. [DOI] [PubMed] [Google Scholar]
- 70.Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology 1999; 116:301–9. [DOI] [PubMed] [Google Scholar]
- 71.Wyatt J, Vogelsang H, Hübl W, Waldhoer T, Lochs H. Intestinal permeability and the prediction of relapse in Crohn's disease. Lancet 1993; 341:1437–9. [DOI] [PubMed] [Google Scholar]
- 72.Watts T, Berti I, Sapone A, et al. Role of the intestinal tight junction modulator zonulin in the pathogenesis of type I diabetes in BB diabetic-prone rats. Proc Natl Acad Sci U S A 2005; 102:2916–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. Lactobacillus species prevents colitis in interleukin 10 gene–deficient mice. Gastroenterology 1999; 116:1107–14. [DOI] [PubMed] [Google Scholar]
- 74.Zhang Z, Hinrichs DJ, Lu H, Chen H, Zhong W, Kolls JK. After interleukin-12p40, are interleukin-23 and interleukin-17 the next therapeutic targets for inflammatory bowel disease? Int Immunopharmacol 2007; 7:409–16. [DOI] [PubMed] [Google Scholar]
- 75.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 2004; 118:229–41. [DOI] [PubMed] [Google Scholar]
- 76.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 2002; 277:50959–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Akbari O, DeKruyff RH, Umetsu DT. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol 2001; 2:725–31. [DOI] [PubMed] [Google Scholar]
- 78.Iwasaki A, Kelsall BL. Freshly isolated Peyer's patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J Exp Med 1999; 190:229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Williamson E, Bilsborough JM, Viney JL. Regulation of mucosal dendritic cell function by receptor activator of NF-κB (RANK)/RANK ligand interactions: impact on tolerance induction. J Immunol 2002; 169:3606–12. [DOI] [PubMed] [Google Scholar]
- 80.Smits HH, Engering A, van der Kleij D, et al. Selective probiotic bacteria induce IL-10–producing regulatory T cells in vitro by modulating dendritic cell function through dendritic cell–specific intercellular adhesion molecule 3–grabbing nonintegrin. J Allergy Clin Immunol 2005; 115:1260–7. [DOI] [PubMed] [Google Scholar]
- 81.Kaila M, Isolauri E, Soppi E, Virtanen E, Laine S, Arvilommi H. Enhancement of the circulating antibody secreting cell response in human diarrhea by a human Lactobacillus strain. Pediatr Res 1992; 32:141–4. [DOI] [PubMed] [Google Scholar]
- 82.Vanhoutte T, De Preter V, De Brandt E, Verbeke K, Swings J, Huys G. Molecular monitoring of the fecal microbiota of healthy human subjects during administration of lactulose and Saccharomyces boulardii. Appl Environ Microbiol 2006; 72:5990–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bouhnik Y, Flourié B, D'Agay-Abensour L, et al. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J Nutr 1997; 127:444–8. [DOI] [PubMed] [Google Scholar]
- 84.Dewulf EM, Cani PD, Neyrinck AM, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARγ-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem 2011; 22:712–22. [DOI] [PubMed] [Google Scholar]
- 85.Cani PD, Neyrinck AM, Fava F, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007; 50:2374–83. [DOI] [PubMed] [Google Scholar]
- 86.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr 2009; 89:1751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bolognani F, Rumney CJ, Pool-Zobel BL, Rowland IR. Effect of lactobacilli, bifidobacteria and inulin on the formation of aberrant crypt foci in rats. Eur J Nutr 2001; 40:293–300. [DOI] [PubMed] [Google Scholar]
- 88.Pool-Zobel B, Van Loo J, Rowland I, Roberfroid M. Experimental evidences on the potential of prebiotic fructans to reduce the risk of colon cancer. Br J Nutr 2002; 87(suppl 2):S273–81. [DOI] [PubMed] [Google Scholar]
- 89.Taper H, Roberfroid M. Inulin/oligofructose and anticancer therapy. Br J Nutr 2002; 87(suppl 2):S283–6. [DOI] [PubMed] [Google Scholar]
- 90.Verghese M, Rao D, Chawan C, Williams L, Shackelford L. Dietary inulin suppresses azoxymethane-induced aberrant crypt foci and colon tumors at the promotion stage in young Fisher 344 rats. J Nutr 2002; 132:2809–13. [DOI] [PubMed] [Google Scholar]
- 91.Femia AP, Luceri C, Dolara P, et al. Antitumorigenic activity of the prebiotic inulin enriched with oligofructose in combination with the probiotics Lactobacillus rhamnosus and Bifidobacterium lactis on azoxymethane-induced colon carcinogenesis in rats. Carcinogenesis 2002; 23:1953–60. [DOI] [PubMed] [Google Scholar]
- 92.Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol 2014; 71:814–21. [DOI] [PubMed] [Google Scholar]
- 93.Osborn DA, Sinn J. Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev 2013; 3. [DOI] [PubMed] [Google Scholar]
- 94.Thomas DW, Greer FR. Probiotics and prebiotics in pediatrics. Pediatrics 2010; 126:1217–31. [DOI] [PubMed] [Google Scholar]
- 95.Weizman Z, Asli G, Alsheikh A. Effect of a probiotic infant formula on infections in child care centers: comparison of two probiotic agents. Pediatrics 2005; 115:5–9. [DOI] [PubMed] [Google Scholar]
- 96.Beausoleil M, Fortier N, Guénette S, et al. Effect of a fermented milk combining Lactobacillus acidophilus Cl1285 and Lactobacillus casei in the prevention of antibiotic-associated diarrhea: a randomized, double-blind, placebo-controlled trial. Can J Gastroenterol 2007; 21:732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gorbach S, Chang T-W, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet 1987; 330:1519. [DOI] [PubMed] [Google Scholar]
- 98.Biller J, Katz A, Flores A, Buie T, Gorbach S. Treatment of recurrent Clostridium difficile colitis with Lactobacillus GG. J Pediatr Gastroenterol Nutr 1995; 21:224–6. [DOI] [PubMed] [Google Scholar]
- 99.Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life–a double-blind, placebo-controlled study. Aliment Pharmacol Ther 2011; 33:1123–32. [DOI] [PubMed] [Google Scholar]
- 100.Nobaek S, Johansson M-L, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol 2000; 95:1231–8. [DOI] [PubMed] [Google Scholar]
- 101.O'Mahony L, McCarthy J, Kelly P, et al. Lactobacillus and Bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology 2005; 128:541–51. [DOI] [PubMed] [Google Scholar]
- 102.Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J. Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complement Altern Med 2010; 10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kruis W, Frič P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004; 53:1617–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A. Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 2009; 104:437–43. [DOI] [PubMed] [Google Scholar]
- 105.Schultz M, Timmer A, Herfarth HH, Sartor RB, Vanderhoof JA, Rath HC. Lactobacillus GG in inducing and maintaining remission of Crohn's disease. BMC Gastroenterol 2004; 4:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bousvaros A, Guandalini S, Baldassano RN, et al. A randomized, double-blind trial of Lactobacillus GG versus placebo in addition to standard maintenance therapy for children with Crohn's disease. Inflamm Bowel Dis 2005; 11:833–9. [DOI] [PubMed] [Google Scholar]
- 107.Rolfe VE, Fortun PJ, Hawkey CJ, Bath-Hextall F. Probiotics for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev 2006; 4. [DOI] [PubMed] [Google Scholar]
- 108.Rahimi R, Nikfar S, Rahimi F, et al. A meta-analysis on the efficacy of probiotics for maintenance of remission and prevention of clinical and endoscopic relapse in Crohn's disease. Dig Dis Sci 2008; 53:2524–31. [DOI] [PubMed] [Google Scholar]
- 109.Doherty G, Bennett G, Patil S, Cheifetz A, Moss AC. Interventions for prevention of post-operative recurrence of Crohn's disease. Cochrane Database Syst Rev 2009; 4. [DOI] [PubMed] [Google Scholar]
- 110.Meijer BJ, Dieleman LA. Probiotics in the treatment of human inflammatory bowel diseases: update 2011. J Clin Gastroenterol 2011; 45:S139–44. [DOI] [PubMed] [Google Scholar]
- 111.Gionchetti P, Rizzello F, Venturi A, et al. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 2000; 119:305–9. [DOI] [PubMed] [Google Scholar]
- 112.Yesilova Y, Calka O, Akdeniz N, Berktas M. Effect of probiotics on the treatment of children with atopic dermatitis. Ann Dermatol 2012; 24:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.AlFaleh KM, Bassler D. Cochrane review: probiotics for prevention of necrotizing enterocolitis in preterm infants. Evid Based Child Health 2010; 5:339–68. [DOI] [PubMed] [Google Scholar]
- 114.Saad N, Delattre C, Urdaci M, Schmitter J-M, Bressollier P. An overview of the last advances in probiotic and prebiotic field. Food Sci Technol 2013; 50:1–16. [Google Scholar]
- 115.Islek A, Sayar E, Yilmaz A, Baysan BO, Mutlu D, Artan R. The role of Bifidobacterium lactis B94 plus inulin in the treatment of acute infectious diarrhea in children. Turkish J Gastroenterol 2014; 25:628–33. [DOI] [PubMed] [Google Scholar]
- 116.Waitzberg DL, Logullo LC, Bittencourt AF, et al. Effect of synbiotic in constipated adult women—a randomized, double-blind, placebo-controlled study of clinical response. Clin Nutr 2013; 32:27–33. [DOI] [PubMed] [Google Scholar]
- 117.Rogha M, Esfahani MZ, Zargarzadeh AH. The efficacy of a synbiotic containing Bacillus coagulans in treatment of irritable bowel syndrome: a randomized placebo-controlled trial. Gastroenterol Hepatol Bed Bench 2014; 7:156–63. [PMC free article] [PubMed] [Google Scholar]
- 118.Steed H, Macfarlane GT, Blackett KL, et al. Clinical trial: the microbiological and immunological effects of synbiotic consumption—a randomized double-blind placebo-controlled study in active Crohn's disease. Aliment Pharmacol Ther 2010; 32:872–83. [DOI] [PubMed] [Google Scholar]
- 119.Fujimori S, Gudis K, Mitsui K, et al. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 2009; 25:520–5. [DOI] [PubMed] [Google Scholar]
- 120.Ishikawa H, Matsumoto S, Ohashi Y, et al. Beneficial effects of probiotic Bifidobacterium and galacto-oligosaccharide in patients with ulcerative colitis: a randomized controlled study. Digestion 2011; 84:128–33. [DOI] [PubMed] [Google Scholar]
- 121.Dilli D, Aydin B, Fettah ND, et al. The ProPre-Save Study: effects of probiotics and prebiotics alone or combined on necrotizing enterocolitis in very low birth weight infants. J Pediatr 2015; 166:p545–51.e1. [DOI] [PubMed] [Google Scholar]
- 122.Famouri F, Khoshdel A, Golshani A, Kheiri S, Saneian H, Kelishadi R. Effects of synbiotics on treatment of children with failure to thrive: a triple blind placebo-controlled trial. J Res Med Sci 2014; 19:1046–50. [PMC free article] [PubMed] [Google Scholar]
- 123.Asemi Z, Khorrami-Rad A, Alizadeh SA, Shakeri H, Esmaillzadeh A. Effects of synbiotic food consumption on metabolic status of diabetic patients: a double-blind randomized cross-over controlled clinical trial. Clin Nutr 2014; 33:198–203. [DOI] [PubMed] [Google Scholar]
- 124.Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled pilot study. Am J Clin Nutr 2014; 99:535–42. [DOI] [PubMed] [Google Scholar]
- 125.Beserra BT, Fernandes R, do Rosario VA, Mocellin MC, Kuntz MG, Trindade EB. A systematic review and meta-analysis of the prebiotics and synbiotics effects on glycaemia, insulin concentrations and lipid parameters in adult patients with overweight or obesity. Clin Nutr 2014; doi:10.1016/j.clnu.2014.10.004. [DOI] [PubMed]
- 126.Rammohan A, Sathyanesan J, Rajendran K, et al. Synbiotics in surgery for chronic pancreatitis: are they truly effective? A single blind prospective randomized control trial. Ann Surg 2015; doi:10.1097/SLA.0000000000001077. [DOI] [PubMed]
- 127.Nascimento M, Aguilar-Nascimento JE, Caporossi C, Castro-Barcellos HM, Motta RT. Efficacy of synbiotics to reduce acute radiation proctitis symptoms and improve quality of life: a randomized, double-blind, placebo-controlled pilot trial. Int J Radiat Oncol Biol Phys 2014; 90:289–95. [DOI] [PubMed] [Google Scholar]
- 128.Qiu X, Zhang M, Yang X, Hong N, Yu C. Faecalibacterium prausnitzii upregulates regulatory T cells and anti-inflammatory cytokines in treating TNBS-induced colitis. J Crohns Colitis 2013; 7:e558–68. [DOI] [PubMed] [Google Scholar]
- 129.Atarashi K, Tanoue T, Oshima K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 2013; 500:232–6. [DOI] [PubMed] [Google Scholar]
- 130.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci. 2013; 110:9066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cano PG, Santacruz A, Moya Á, Sanz Y. Bacteroides uniformis CECT 7771 ameliorates metabolic and immunological dysfunction in mice with high-fat-diet induced obesity. PLoS One 2012; 7:e41079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Desbonnet L, Garrett L, Clarke G, Bienenstock J, Dinan TG. The probiotic Bifidobacteria infantis: an assessment of potential antidepressant properties in the rat. J Psychiatr Res 2008; 43:164–74. [DOI] [PubMed] [Google Scholar]
- 133.Eutamene H, Lamine F, Chabo C, et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr 2007; 137:1901–7. [DOI] [PubMed] [Google Scholar]
- 134.Lyte M. Probiotics function mechanistically as delivery vehicles for neuroactive compounds: microbial endocrinology in the design and use of probiotics. Bioessays 2011; 33:574–81. [DOI] [PubMed] [Google Scholar]
- 135.Bolte E. Autism and Clostridium tetani. Med Hypotheses 1998; 51:133–44. [DOI] [PubMed] [Google Scholar]