Abstract
Purpose
According to previous studies, vitamin D exhibits protective effects against breast cancer via the vitamin D receptor (VDR). There is growing evidence that breast cancer incidence is associated with various polymorphisms of the VDR gene. This study investigates the association of VDR poly(A) microsatellite variants with 25-hydroxyvitamin D (25(OH)D) serum levels and breast cancer risk.
Methods
Polymorphism analysis was performed on a total of 261 blood samples, which were collected from 134 women with breast cancer and 127 controls. Single strand conformation polymorphism was assessed by polymerase chain reaction in combination with sequencing to detect poly(A) lengths for each sample. The vitamin D levels of samples were determined by electrochemiluminescence.
Results
The poly(A) variant L allele frequency was significantly higher in cancer patients than in controls (odds ratio [OR], 1.73; 95% confidence interval [CI], 1.16-2.57; p=0.006). Thus, carriers of the L allele (LS and LL genotypes) have a higher risk for breast cancer (OR, 1.86; 95% CI, 1.13-3.05; p=0.013). A larger increase in the risk for breast cancer was found in individuals with the L carrier genotype and lowered 25(OH)D levels.
Conclusion
The results primarily suggest that VDR gene polymorphism in the poly(A) microsatellite is associated with 25(OH)D levels and that it can affect the breast cancer risk in the female population from northern Iran.
Keywords: 25-Hydroxyvitamin D 2, Breast neoplasms, Calcitriol receptors, Genetic polymorphism, Microsatellite repeats
INTRODUCTION
Breast cancer is the most commonly diagnosed female-specific cancer, and shows an increasing trend in diagnosed cases [1]. Global statistics have shown that breast cancer is the second leading cause of cancer-related death in many women worldwide, second only to lung cancer [2]. Breast cancer constitutes 16% of all cancer diagnoses in Iran and is the fifth most common cause of death in Iran [3]. Many researchers have examined the cellular and molecular aspects of breast cancer. Despite of these investigations, many susceptibilities and risk factors for breast cancer are not well established [4]. There are many genes involved in growth, differentiation, and apoptosis, and variation in these genes may influence breast cancer susceptibility [5]. One group of these genes is involved in steroid hormone metabolism and transport [6].
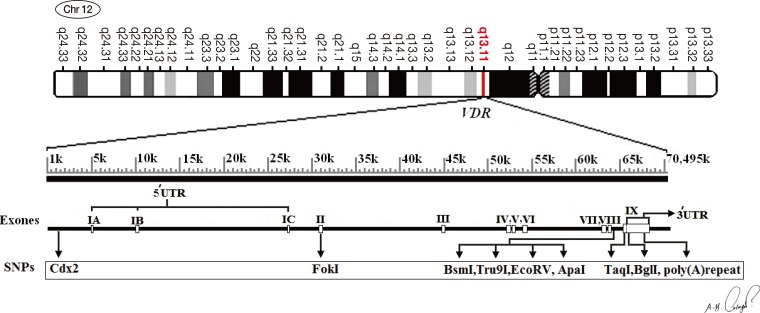
Previous preclinical evidence has demonstrated a significant inverse association between 25-hydroxyvitamin D (25(OH)D) serum levels and breast cancer risk [7]. In vitro and in vivo studies have shown apoptotic and antiproliferative effects of vitamin D against various types of cancers, including breast cancer [8]. The active form of vitamin D regulates growth, differentiation and apoptosis that is mediated by the vitamin D receptor (VDR) [9]. VDR is a member of the nuclear receptor family of steroid hormones that acts as transcriptional regulatory factor in most tissues [10]. This protein has an essential role in complex process of proliferation and differentiation of cells by controlling transcription of mediator or target genes [11]. VDR-vitamin D complex, along with the retinoid X receptor family, binds to vitamin D responsive elements in the target genes' promoters to induce or inhibit these genes [12]. A previous animal model study showed enhanced growth and proliferation of breast tissue in VDR knockout mice [13]. The human VDR gene is located on the long arm of chromosome 12 (12q12-14) and includes 11 exons (Figure 1) [14]. There are several single-nucleotide polymorphisms (SNP) on the VDR gene, and breast cancer prevalence is associated with various SNPs of this gene [15]. One of these polymorphisms, a variant length of the poly(A) microsatellite (rs17878969), is located in the 3'-untranslated region of the VDR gene (http://www.ncbi.nlm.nih.gov). According to previous studies, this locus contains a variable number of adenine (A) repeats that lead to different allelic lengths for the locus [16,17]. Variety in poly(A) length may affect the VDR gene expression by influencing posttranscriptional regulation [18]. In this study, we determined the length of the poly(A) microsatellite in 134 breast cancer patients and 127 matched controls from northern Iran and examined the relationship between the VDR poly(A) microsatellite length polymorphism and 25(OH)D levels, and how affect breast cancer risk.
Figure 1. Genomic structure of the vitamin D receptor (VDR) gene on chromosome 12q13, and locations of single nucleotide polymorphisms (SNPs)on VDR gene. The VDR chromosomal gene containing a total of 11 exon. VDR poly(A) variant is located in 3'-untranslated region.
METHODS
In a case-control study, all subjects were randomly selected from Shahid Rajaee Oncology Hospital (Babolsar, Iran), during 2009 to 2013. Patients gave informed consent, and a detailed medical and cancer history was obtained from all subjects. A case group comprising 134 patients with breast cancer as case group and a control group comprising 127 healthy women as control group were selected. Six milliliters of blood was collected from all participants in each group. Three milliliters were preserved in tubes containing ethylene diaminetetra-acetic acid-sodium salt (EDTANa2) at -20℃ for DNA extraction purposes. Sample volume was maintained across both groups for vitamin D estimation. All blood sample collection was conducted with the approval of the Medical Research Ethics Committee of the Babol University of Medical Sciences (IRB number: 8929931). Samples in the control group were matched to the patients in the case group for age.
DNA extraction
DNA extraction from blood was performed by salt precipitation [19]. To ensure the integrity of the extracted genome, 2 µL of each sample was electrophoresed and its concentration was determined at absorbance of 260 nm. Extracted DNA was used for poly(A) repeat genotyping in VDR gene.
Vitamin D estimation
We measured the serum 25(OH)D levels of 117 patients with breast cancer and 113 control subjects whose allele and genotype frequencies were in accordance with all samples (134 cases and 127 controls). Serum total levels of 25(OH)D were determined by electrochemiluminescent immunoassay on an Elecsys automated analyzer (Roche Diagnostics, Mannheim, Germany) and using Elecsys Total Vitamin D (25-OH) kit (Roche Diagnostics).
Polymerase chain reaction
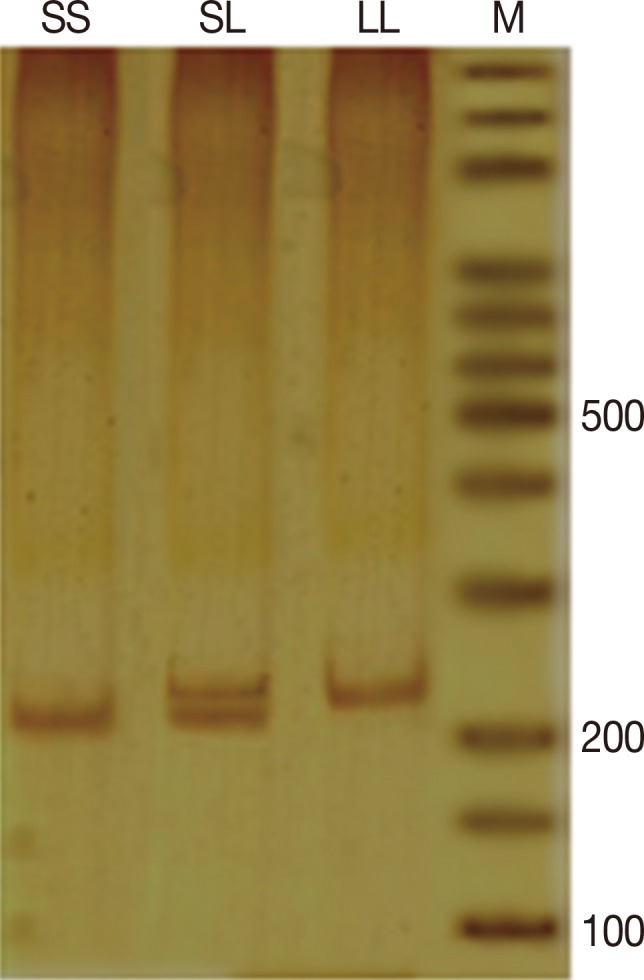
Poly(A) microsatellite genotyping was performed by single-strand conformation polymorphism (SSCP) polymerase chain reaction (PCR). For this purpose the entire genomic sequence of human VDR gene was deduced from the National Center for Biotechnology Information (NCBI) (AC number: NG_008731). Forward and reverse primers were designed by GeneRunner software (Hastings Software, New York, USA). Amplification of DNA fragments was performed by PCR in a final volume of 25 µL containing 1X PCR buffer, 0.8 mM MgCl2, 0.2 mM dNTPs, 0.2 mM of each primer (forward: 5'-CAGTTTGGGAGGTCGAGGTA, and reverse: 5'-TTGTTGTCCAGGTTGGAGAGTAACGG), 30 ng genomic DNA, and 1.25 units Taq DNA polymerase (all materials for PCR purchased from Fermentas, St. Leon-Rot, Germany). PCR was performed according to the following program: initial denaturation for 5 minutes at 94℃ followed by 30 repetitive cycles of denaturation at 94℃ for 30 seconds, annealing at 63.5℃ for 30 seconds and extension at 72℃ for 30 seconds. Final extension temperature was 72℃ for 5 minutes. The PCR products were used for SSCP purposes. Amplification success was detected by 8% polyacrylamide gel visualized by silver nitrate (AgNO3) staining (Figure 2).
Figure 2. Polymerase chain reaction product in nondenaturing polyacrylamide gel electrophoresis, visualized by silver nitrate staining: SS genotype with ~236-238 bp and LL genotype with ~241-243 bp.
Single-strand conformation polymorphism
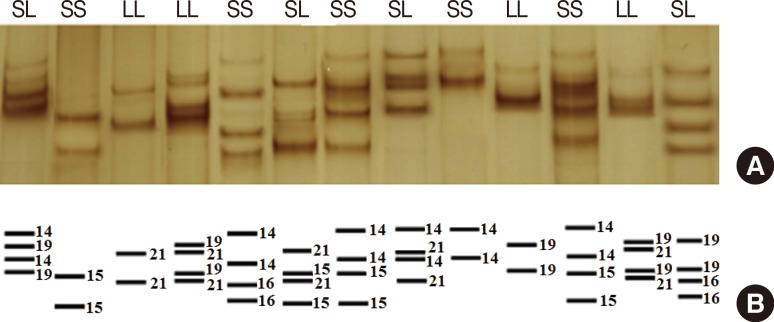
Detection of poly(A) length in PCR products was performed by SSCP. For this purpose, 3 µL PCR product was mixed with 12 µL SSCP dye (0.05% bromophenol blue, 0.05% xylene cyanol, 95% formamide, 20 mM EDTA). Before loading, the samples were denatured at 95℃ for 5 minutes and then placed on ice for 3 minutes. The samples were loaded on an 8% polyacrylamide gel and visualized by AgNO3 staining (Figure 3) [20].
Figure 3. Single-strand conformation polymorphism (SSCP) results of the polymerase chain reaction (PCR) product in denaturing polyacrylamide gel electrophoresis, visualized by silver nitrate staining: (A) PCR products of the SS genotype with ~236-238 bp and LL genotype with ~241-243 bp in SSCP gel; (B) Schematic of the adenine numeric repeat shown by number in single strand oligonucleotide bands or different alleles.
DNA sequencing
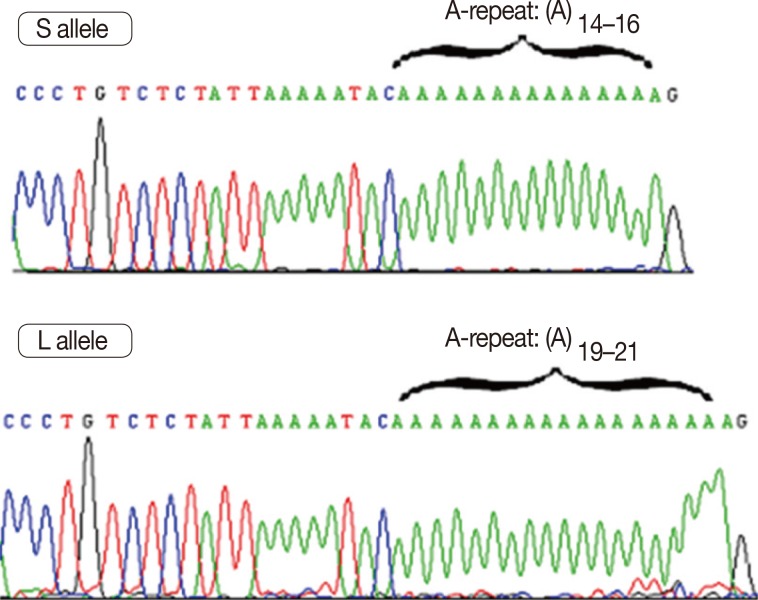
After categorizing samples by SSCP, some homozygote samples were randomly selected from each group and prepared for direct sequencing. For sequencing of heterozygote samples, each allele was extracted from polyacrylamide gel electrophoresis (PAGE) by crush and soak method [21] and then amplified by PCR. Direct DNA sequencing of the purified PCR product was performed on an ABI 3730XL DNA Analyzer (Bioneer, Daejeon, Korea) (Figure 4).
Figure 4. Deoxyribonucleic acid electropherogram analysis of poly(A) repeat polymorphism in the 3'-untranslated region of vitamin D receptor gene regions: S and L alleles with poly(A)-microsatellite A-repeats 14-16 and 19-21, respectively.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) for the case and control groups was analyzed, respectively. For various genotypes, odd ratios (ORs) with a 95% confidence interval (95% CI) were obtained using binary logistic regression. Differences in the frequency distributions of the alleles and genotypes between case and control groups were evaluated by a chi-square test. An independent t-test was used to compare numerical variables. Statistical analysis was carried out with the SPSS version 19 statistical software package (IBM Corp., Armonk, USA). All p-values less than 0.05 were considered to be statistically significant.
RESULTS
This analysis included a total of 261 subjects as case and control samples. There were no statistically significant differences in body mass index and age between the case and control groups. The mean ages were 48.72±9.60 and 47.04±12.07 years for the case and control groups, respectively. In this study, all of the breast cancer patients have been in stage II of cancer.
We determined the length of poly(A) microsatellite in 261 samples. Products were grouped based on the difference in numbers of Adenine-repeat or difference in single-strand conformation in an SSCP gel. Result of sequencing showed a series of alleles of different lengths for this locus that contained a variable number of A-repeats (from 14 to 21). The alleles' size distribution was bimodal and there was obvious segregation between alleles that separated them into two groups: short (S) and long (L) alleles. The length of poly(A) sequence in poly(A) S alleles was 14, 15, and 16 A-repeats, while in poly(A) L allele it was 19 or 21 A-repeats. Frequencies of alleles containing 14, 15, and 19 A-repeats were more common than others in this study. The poly(A) L and S alleles were distributed with frequencies of 26.3% (L) and 73.7% (S). We first analyzed the frequency of alleles and genotype relationship in susceptibility to breast cancer, regardless of 25(OH)D levels, and a significant association was seen. This result is presented in Table 1. HWE for both the case and control samples was examined, and no major deviation from expected HWE was found. Statistical analysis illustrated a highly significant difference between case and control sample for L allele carrier genotype (LL and LS) compared with SS genotype. The poly(A) variant L allele frequency was significantly higher among the patients compared with controls (p=0.007). When the SS genotype was used as a reference, the LL and LS genotypes were both significantly associated with breast cancer risk.
Table 1. Genotype and allele frequencies of the VDR poly(A) polymorphism among cases and controls and their association with risk of breast cancer.
| VDR poly(A) polymorphism | Case (n = 134) | Control (n = 127) | OR (95% CI)* | p-value† |
|---|---|---|---|---|
| Genotype | ||||
| SS | 64 | 80 | - | - |
| SL | 56 | 41 | 1.70 (1.01-2.87) | 0.043 |
| LL | 14 | 6 | 2.91 (1.06-8.01) | 0.032 |
| SL+LL | 70 | 47 | 1.86 (1.13-3.05) | 0.013 |
| Allele frequency | ||||
| S allele | 184 | 201 | - | - |
| L allele | 84 | 53 | 1.73 (1.16-2.57) | 0.006 |
VDR=vitamin D receptor; OR=odds ratio; CI=confidence interval.
*ORs were obtained from a binary logistic regression; †Two-sided chi-square test for distributions of genotype and allele frequencies between the cases and controls.
We performed a logistic regression analysis for serum 25(OH)D levels from patients with breast cancer and controls (Table 2). When the 25(OH)D levels were divided according to the median vitamin D level of the control, lower levels were associated with susceptibility to breast cancer. When the vitamin D level was divided into three groups (tertiles) according to vitamin D levels of the control, the lower tertile was associated with the development of breast cancer.
Table 2. Logistic regression analysis of 25-hydroxyvitamin D levels in patients with breast cancer and controls.
| Vitamin D level (ng/mL) | Case (n = 117) | Control (n = 113) | OR (95% CI)* | p-value† |
|---|---|---|---|---|
| By median | ||||
| < 14 | 87 | 56 | 2.95 (1.69-5.14) | < 0.001 |
| ≥ 14 | 30 | 57 | - | - |
| By tertile | ||||
| ≤9 | 76 | 37 | 3.90 (1.99-7.61) | < 0.001 |
| > 9.0, ≤ 16 | 21 | 38 | 1.05 (0.49-2.24) | 0.899 |
| > 16 | 20 | 38 | - | - |
OR=odds ratio; CI=confidence interval.
*ORs were obtained from a binary logistic regression; †Two-sided chi-square test.
When the genotype frequency of poly(A) polymorphism in combination with plasma vitamin D levels was studied in relation to breast cancer, a significant association was seen (Table 3). In this table, the poly(A) genotypes were divided into two categories, SS and genotypes containing the L alleles (LL+LS). Levels of 25(OH)D were divided according to Table 2. When the SS genotype with higher 25(OH)D median levels was used as the reference, patients with L alleles (LL+LS) who had lower 25(OH)D levels showed a significant and larger increase risk for breast cancer.
Table 3. Risk of breast cancer associated with VDR poly(A) genotypes by 25-hydroxyvitamin D levels.
| Vitamin D level (ng/mL) | SS genotype (case/control) | OR (95% CI)* | p-value† | SL+LL genotype (case/control) | OR (95% CI)* | p-value† |
|---|---|---|---|---|---|---|
| By median | ||||||
| < 14 | 39/36 | 2.23 (1.05-4.72) | 0.035 | 48/20 | 4.95 (2.24-10.93) | < 0.001 |
| ≥ 14 | 16/33 | - | - | 14/24 | 1.20 (0.49-2.92) | 0.683 |
| By tertile | ||||||
| ≤9 | 35/24 | 3.50 (1.41-8.62) | 0.006 | 41/13 | 7.56 (2.88-19.89) | < 0.001 |
| > 9.0, ≤ 16 | 10/21 | 1.14 (0.39-3.27) | 0.803 | 11/17 | 1.55 (0.53-4.47) | 0.414 |
| > 16 | 10/24 | - | - | 10/14 | 1.71 (0.57-5.13) | 0.335 |
VDR=vitamin D receptor; OR=odds ratio; CI=confidence interval.
*ORs were obtained from a binary logistic regression; †Two-sided chi-square test.
When the vitamin D level was divided into three groups (tertile) and SS genotype with higher 25(OH)D levels (upper tertile) was used as the reference, samples that had lower 25(OH)D levels (lower tertile) showed significant correlation with breast cancer risk, but larger increase in risk for breast cancer was observed in individuals with poly(A) L allele (LL+LS).
DISCUSSION
There has been much global investigation of the role of genetic susceptibility and environmental risk factors on the development of breast cancer [22]. There is growing evidence of protective effect of vitamin D against breast cancer, and vitamin D deficiency was identified as a risk factor for initiation and progression of breast cancer [10]. Despite compelling data in different studies, more investigations of VDR gene variants are required to provide sufficient data to obtain consistent and precise predictions for vitamin D supplementation for breast cancer prevention or treatment [23]. We demonstrated significant relation between serum levels of vitamin D and susceptibility to breast cancer. Our finding suggest that a deficiency in 25(OH)D may influence the development of breast cancer.
Because vitamin D is mediated by VDR as a ligand-dependent transcription factor, variants in VDR gene may influence receptor action and may be associated with increased risk of breast cancer [13]. Based our results, there is a significant association between polymorphism in VDR poly(A) microsatellites and susceptibility to breast cancer. We demonstrate a statistically significant increased risk of breast cancer associated with the VDR poly(A) L allele.
Previous results, some of which are controversial, for the association of VDR poly(A) length polymorphism with breast cancer risk are presented in Table 4 [16,17,24,25,26]. Our report is similar to results obtained from a United Kingdom Caucasian population in which the poly(A) L allele of this locus was associated with an increased risk of breast cancer [24]. It has also been reported that the LL genotype of poly(A) microsatellites was associated with a higher breast cancer risk [16]. In contrast, results obtained from a United States population indicate that the S allele of this locus is associated with an increased risk of breast cancer [17]. Furthermore a protective effect of the poly(A) L allele against breast cancer was reported among women from 21 states in the United States [25]. However, other studies did not record any significant association between poly(A) variants and breast cancer risk [26]. Zhang and Song [27] performed a meta-analysis to assess previous studies and did not find any significant association between the poly(A) variation and breast cancer risk. In contrast, we demonstrate a significant association between poly(A) variation and breast cancer risk. These diverse and conflicting reports demonstrate possible genetic differences of the VDR gene between ethnic groups, the influence of environmental factors, and gene-gene interactions.
Table 4. Reported result in previous study on relevance of VDR poly(A) variant and breast cancer susceptibility.
| Country (year) | No. of cases/controls | Cases sample | Poly-A repeat | Author(s) | |
|---|---|---|---|---|---|
| S allele | L allele | ||||
| India (2009) | 160/140 | Breast cancer | 8-11 | 16-18 | Chakraborty et al. [16] |
| USA (2007) | 1,631/1,435 | Breast cancer | - | - | Trabert et al. [26] |
| UK (2001) | 181/241 | Breast cancer | 13-17 | 18-24 | Bretherton-Watt et al. [24] |
| USA (2000) | 143/300 | Breast cancer | 14-17 | 18-22 | Ingles et al. [17] |
| USA (2007) | 500/500 | Breast cancer | 14-17 | 18-22 | McCullough et al. [25] |
VDR=vitamin D receptor.
Poly(A) microsatellites are located in the 3'-untranslated region (3'UTR) of the VDR gene, and it is established that this region does not affect the structure of the VDR protein. Since this region significantly determines the stability, localization, translation and degradation of messenger RNA [28] and considering the varying results for poly(A) L and S alleles in this study, we conclude that the poly(A) microsatellite sequence plays an important role in posttranscriptional regulation of the VDR gene either by altering mRNA stability or through the interaction of the mRNA with the translational apparatus. Therefore, the polymorphism within the 3'UTR of the VDR gene may influence the VDR gene expression level. Some previous studies have had controversial hypotheses for influence of this locus on mRNA stability. Whitfield et al. [18] suggested that the VDR poly(A) L allele may produce more stable mRNA. On the other hand, Durrin et al. [29] reported that the 3'UTR of the VDR gene does not affect the stability of mRNA. Additionally, previous studies have found that VDR has a critical role in formation of the biologically active form of vitamin D by binding to it [9]. Tiosano et al. [30] studied patients with a defective VDR. Their results exclude a role for VDR in 1α-hydroxylation. Result showed that 1α-hydroxylase activity increased by 3- to 6-fold in VDR-defective subjects compared with the controls. A similar increase in 1,25-(OH)2D (active form of vitamin D) levels was reported for VDR knockout mice. Hence, we conclude that VDR polymorphism may influence on vitamin D metabolism and 25(OH)D level.
Logistic regression for this analysis show higher risk for breast cancer in carriers of the poly(A) L allele (LL+LS genotype) who had lowered 25(OH)D levels. The findings in our study suggest that higher levels of vitamin D (especially in individuals with poly(A) L allele) can decrease susceptibility to breast cancer.
To summarize, we suggest that deficiency of the 25(OH)D may influence the development of breast cancer. Further, poly(A) polymorphisms of VDR gene may affect 25(OH)D levels and susceptibility to breast cancer, so that poly(A) L allele are more susceptible to breast cancer in the presence of low 25(OH)D statuses.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Rezaianzadeh A, Heydari ST, Hosseini H, Haghdoost AA, Barooti E, Lankarani KB. Prevalence of breast cancer in a defined population of iran. Iran Red Crescent Med J. 2011;13:647–650. doi: 10.5812/kowsar.20741804.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naghibi A, Shojaeezade D, Montazeri A, Yazdani J. Early detection of breast cancer among women in Mazandaran, Iran. IJHS. 2013;1:44–49. [Google Scholar]
- 3.Taghavi A, Fazeli Z, Vahedi M, Baghestani AR, Pourhoseingholi A, Barzegar F, et al. Increased trend of breast cancer mortality in Iran. Asian Pac J Cancer Prev. 2012;13:367–370. doi: 10.7314/apjcp.2012.13.1.367. [DOI] [PubMed] [Google Scholar]
- 4.Fang L, Barekati Z, Zhang B, Liu Z, Zhong X. Targeted therapy in breast cancer: what's new? Swiss Med Wkly. 2011;141:w13231. doi: 10.4414/smw.2011.13231. [DOI] [PubMed] [Google Scholar]
- 5.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 6.Beckmann L, Hüsing A, Setiawan VW, Amiano P, Clavel-Chapelon F, Chanock SJ, et al. Comprehensive analysis of hormone and genetic variation in 36 genes related to steroid hormone metabolism in pre- and postmenopausal women from the breast and prostate cancer cohort consortium (BPC3) J Clin Endocrinol Metab. 2011;96:E360–E367. doi: 10.1210/jc.2010-0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 8.Crew KD. Vitamin d: are we ready to supplement for breast cancer prevention and treatment? ISRN Oncol. 2013;2013:483687. doi: 10.1155/2013/483687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jurutka PW, Whitfield GK, Hsieh JC, Thompson PD, Haussler CA, Haussler MR. Molecular nature of the vitamin D receptor and its role in regulation of gene expression. Rev Endocr Metab Disord. 2001;2:203–216. doi: 10.1023/a:1010062929140. [DOI] [PubMed] [Google Scholar]
- 11.Thorne J, Campbell MJ. The vitamin D receptor in cancer. Proc Nutr Soc. 2008;67:115–127. doi: 10.1017/S0029665108006964. [DOI] [PubMed] [Google Scholar]
- 12.Barsony J, Prufer K. Vitamin D receptor and retinoid X receptor interactions in motion. Vitam Horm. 2002;65:345–376. doi: 10.1016/s0083-6729(02)65071-x. [DOI] [PubMed] [Google Scholar]
- 13.Welsh J, Wietzke JA, Zinser GM, Byrne B, Smith K, Narvaez CJ. Vitamin D-3 receptor as a target for breast cancer prevention. J Nutr. 2003;133(7 Suppl):2425S–2433S. doi: 10.1093/jn/133.7.2425S. [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto K, Kesterson RA, Yamamoto H, Taketani Y, Nishiwaki E, Tatsumi S, et al. Structural organization of the human vitamin D receptor chromosomal gene and its promoter. Mol Endocrinol. 1997;11:1165–1179. doi: 10.1210/mend.11.8.9951. [DOI] [PubMed] [Google Scholar]
- 15.Köstner K, Denzer N, Müller CS, Klein R, Tilgen W, Reichrath J. The relevance of vitamin D receptor (VDR) gene polymorphisms for cancer: a review of the literature. Anticancer Res. 2009;29:3511–3536. [PubMed] [Google Scholar]
- 16.Chakraborty A, Mishra AK, Soni A, Regina T, Mohil R, Bhatnagar D, et al. Vitamin D receptor gene polymorphism(s) and breast cancer risk in north Indians. Cancer Detect Prev. 2009;32:386–394. doi: 10.1016/j.canep.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Ingles SA, Garcia DG, Wang W, Nieters A, Henderson BE, Kolonel LN, et al. Vitamin D receptor genotype and breast cancer in Latinas (United States) Cancer Causes Control. 2000;11:25–30. doi: 10.1023/a:1008979417618. [DOI] [PubMed] [Google Scholar]
- 18.Whitfield GK, Remus LS, Jurutka PW, Zitzer H, Oza AK, Dang HT, et al. Functionally relevant polymorphisms in the human nuclear vitamin D receptor gene. Mol Cell Endocrinol. 2001;177:145–159. doi: 10.1016/s0303-7207(01)00406-3. [DOI] [PubMed] [Google Scholar]
- 19.Maurya R, Kumar B, Sundar S. Evaluation of salt-out method for the isolation of DNA from whole blood: a pathological approach of DNA based diagnosis. Int J Life Sci Biotechnol Pharma Res. 2013;2:53–57. [Google Scholar]
- 20.Green MR, Sambrook J. Molecular Cloning: a Laboratory Manual. 4th ed. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2012. pp. 104–110. [Google Scholar]
- 21.Sambrook J, Russell DW. The Condensed Protocols from Molecular Cloning: a Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2006. pp. 51–54. [Google Scholar]
- 22.Mavaddat N, Antoniou AC, Easton DF, Garcia-Closas M. Genetic susceptibility to breast cancer. Mol Oncol. 2010;4:174–191. doi: 10.1016/j.molonc.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta. 2006;371:1–12. doi: 10.1016/j.cca.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Bretherton-Watt D, Given-Wilson R, Mansi JL, Thomas V, Carter N, Colston KW. Vitamin D receptor gene polymorphisms are associated with breast cancer risk in a UK Caucasian population. Br J Cancer. 2001;85:171–175. doi: 10.1054/bjoc.2001.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCullough ML, Stevens VL, Diver WR, Feigelson HS, Rodriguez C, Bostick RM, et al. Vitamin D pathway gene polymorphisms, diet, and risk of postmenopausal breast cancer: a nested case-control study. Breast Cancer Res. 2007;9:R9. doi: 10.1186/bcr1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trabert B, Malone KE, Daling JR, Doody DR, Bernstein L, Ursin G, et al. Vitamin D receptor polymorphisms and breast cancer risk in a large population-based case-control study of Caucasian and African-American women. Breast Cancer Res. 2007;9:R84. doi: 10.1186/bcr1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang K, Song L. Association between vitamin D receptor gene polymorphisms and breast cancer risk: a meta-analysis of 39 studies. PLoS One. 2014;9:e96125. doi: 10.1371/journal.pone.0096125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hesketh J. 3'-Untranslated regions are important in mRNA localization and translation: lessons from selenium and metallothionein. Biochem Soc Trans. 2004;32(Pt 6):990–993. doi: 10.1042/BST0320990. [DOI] [PubMed] [Google Scholar]
- 29.Durrin LK, Haile RW, Ingles SA, Coetzee GA. Vitamin D receptor 3'-untranslated region polymorphisms: lack of effect on mRNA stability. Biochim Biophys Acta. 1999;1453:311–320. doi: 10.1016/s0925-4439(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 30.Tiosano D, Weisman Y, Hochberg Z. The role of the vitamin D receptor in regulating vitamin D metabolism: a study of vitamin D-dependent rickets, type II. J Clin Endocrinol Metab. 2001;86:1908–1912. doi: 10.1210/jcem.86.5.7448. [DOI] [PubMed] [Google Scholar]