Abstract
Purpose
Human epidermal growth factor receptor 2 (HER2)-positive luminal B type comprises estrogen receptor (ER)-positive and HER2-positive cancers, and HER2-negative luminal B type comprises ER-positive cancers showing a Ki-67 labeling index ≥14% or progesterone receptor (PR) expression of <20% according to the St. Gallen consensus 2013. The current study aimed to classify intrinsic subtypes according to the St. Gallen consensus 2013 and determine the differences in clinicopathological parameters and survival outcomes among the molecular types, especially among the luminal types.
Methods
Assessment of molecular types was performed for 267 invasive ductal carcinomas. The differences in clinicopathological parameters, disease-free survival (DFS), and overall survival (OS) among the molecular types were analyzed.
Results
The luminal B type was the most prevalent, at 44.9%, followed by the luminal A, triple-negative (including basal-like type), and HER2 type, at 21.7%, 18.7%, and 14.6%, respectively. There were statistically significant differences in size (p=0.003), nodal status (p=0.046), histologic grade (p<0.001), p53 (p<0.001) and cyclooxygenase 2 (COX-2) positivity (p=0.002), recurrence (p=0.001) and death rates (p=0.036), DFS (p=0.002), and OS (p=0.039) among the molecular types. Significant differences in size (p=0.009), nodal metastasis (p=0.019), histologic grade (p<0.001), p53 positivity (p=0.001), and PR expression (p<0.001) were noted between the luminal A and B types. Among the luminal B type cancers, the distributions of ER and PR scores showed significant differences (p=0.003, p=0.003). p53 positivity in the luminal B type cancers was related to shortened DFS (p=0.034). In luminal type cancers, COX-2 positivity was related to longer DFS (p=0.026).
Conclusion
Different management guidelines should be considered for the luminal A and luminal B breast cancer types. Positive p53 expression in luminal B type cancers and negative COX-2 expression in luminal type cancers seem to be related to poor clinical outcome.
Keywords: Breast neoplasms, Ki-67 antigen, Molecular type, Progesterone receptors
INTRODUCTION
The molecular classification of breast cancers into luminal A type, luminal B type, human epidermal growth factor receptor 2 (HER2)-positive or negative, and basal-like or triple-negative types is widely used. This conception of the molecular types of breast cancers was first introduced in 2000 by Perou et al. [1]. These molecular types correlated with prognosis and response to therapy, and thus had taken on clinical importance [2]. This classification of breast cancers based on gene expression patterns could be used as a prognostic marker for to overall survival (OS) and disease-free survival (DFS) in patients who had received uniform therapy [3].
In the St. Gallen consensus 2009 [4], the luminal A type was considered as the largest group, showing positive estrogen receptor (ER) and negative HER2 expression and the luminal B type comprised those cancers positive for both ER and HER2 expression [5,6,7]. ER and progesterone receptor (PR) statuses are important predictors of the response to hormonal therapy. The luminal A type is regarded as a low-risk group that shows a good response to endocrine therapy compared to the luminal B type, which is generally of higher grade and has a higher proliferative rate. Cancers that fail to express either ER or PR have a less than 10% likelihood of responding to hormonal therapy but are more likely to respond to chemotherapy [8,9].
Different treatment guidelines according to the Ki-67 index for ER-positive breast cancers were suggested in the St. Gallen consensus 2009 [4]. Cases with a high Ki-67 index (>30%) were managed with both chemotherapy and hormonal therapy, and cases with a low Ki-67 index (<10%), with hormonal therapy alone; management of cases with an intermediate index (16%-30%) was not certain. New surrogate definitions and different guidelines for the management for luminal A and B types were suggested in the St. Gallen consensus 2011 [10]. The cutoff point between "high" and "low" values for Ki-67 of 14% was the one best correlated with the gene expression definition of the luminal A type. A high Ki-67 labeling index, ≥14%, was used as the new surrogate definition for the HER2-negative luminal B type. In the St. Gallen consensus meeting 2013, another surrogate definition, decreased PR expression, was added for the HER2-negative luminal B type, with a PR expression cutoff point of ≥20% corresponding to the luminal A type (Table 1) [11].
Table 1. Surrogate definitions of the luminal type breast cancers.
| Subtype | Surrogate definitions | ||
|---|---|---|---|
| Before St. Gallen consensus 2011 | St. Gallen consensus 2011 | St. Gallen consensus 2013 | |
| Luminal A type | ER & PR (+) & HER2 (-) | ER & PR (+) & HER2 (-) & Ki-67 (< 14%) | ER & PR (+) & HER2 (-) & Ki-67 (< 14%) |
| HER2-positive luminal B type | ER & PR (+) & HER2 (+) | ER & PR (+) & HER2 (+) & Any Ki-67 | ER (+) & HER2 (+) & Any Ki-67 or Any PR |
| HER2-negative luminal B type | ER & PR (+) & HER2 (-) & Ki-67 (≥ 14%) | ER (+) & HER2 (-) & Ki-67 (≥ 14%) or PR (< 20%) | |
ER=estrogen receptor; PR=progesterone receptor; HER2=human epidermal growth factor receptor 2; Ki-67=Ki-67 protein.
In the St. Gallen consensus 2009, the importance of markers of proliferation was emphasized and it was suggested that these markers be applied to help guide the choice of adjuvant chemotherapy in the treatment of patients with hormone receptor-positive breast cancers [4]. After that, the importance of the Ki-67 labeling index among patients with hormone receptor-positive breast cancers [12,13] and the prognostic impact of the Ki-67 index according to its cutoff value [14,15] were reported. However, there were many limitations associated with the Ki-67 labeling index, related to differences in cutoff values and interpretation methods, interobserver variability, and heterogeneity of Ki-67 expression [16]. The varying results of immunohistochemical stains for Ki-67 may be due to a lack of consensus concerning methodology [17].
In the current study, we aimed to classify intrinsic molecular types of breast cancers according to the St. Gallen consensus 2013 [11] and determine the differences in clinicopathological parameters and survival outcomes among the molecular types. We applied the new criteria of the St. Gallen consensus 2013 to reassess the molecular types and then compared the differences in clinicopathological parameters such as age, tumor size, lymph node metastasis, histologic grade, p53 and cyclooxygenase 2 (COX-2) expression, recurrence and death rates, DFS, and OS among the new molecular types, especially between the luminal A and B types. In addition, we evaluated the differences in ER and PR expression between the luminal A and B types and the differences in recurrence and death rates, DFS, and OS according to p53 and COX-2 expression in the luminal type cancers.
METHODS
A total of 267 cases of invasive ductal carcinoma, regardless of histological type, were selected from the surgical pathology files of Busan Paik Hospital, from the period of January 2010 to December 2011. Clinical and pathological data including the expression of ER, PR, HER2, Ki-67, cytokeratin 5/6 (CK5/6), and epidermal growth factor receptor (EGFR) were recorded.
Clinicopathological data comprised age at diagnosis, tumor size (≤2.0 cm vs. >2.0 cm), lymph node metastasis (negative vs. positive), and histologic grade (low, intermediate, or high, according to modified Bloom-Richardson criteria).
Patients underwent surgical therapy with either breast conserving surgery or modified radical mastectomy, after which they received adjuvant therapy according to the National Comprehensive Cancer Network guidelines. We employed several chemotherapy regimens, including epirubicin and cyclophosphamide (100 and 600 mg/m2 every 21 days for four cycles); fluorouracil, epirubicin, and cyclophosphamide (500, 100, and 500 mg/m2 every 21 days for six cycles); and doxorubicin and cyclophosphamide (60 and 600 mg/m2 every 21 days for four cycles) followed by four cycles of paclitaxel (175 mg/m2) or docetaxel (75 mg/m2) according to molecular type and axillary nodal status. Trastuzumab was administered to patients with tumors larger than 1 cm or positive axillary nodes who had HER2 overexpression. Tamoxifen (20 mg/day for 5 years) or aromatase inhibitors were given to patients with positive hormone receptor status. Radiotherapy was given to patients undergoing breast conserving surgery or those with advanced disease stages.
The median follow-up period was 33 months (29 to 53 months). Thirty-one patients experienced the recurrence of breast cancer, regardless of local or systemic involvement. As of the last follow-up, a total of nine deaths were reported, including one breast cancer-associated death. Of the remaining eight deaths, one was not related to breast cancer and the causes of the others could not be identified.
Approval for the present study was obtained from the Institutional Review Board (IRB) of Busan Paik Hospital (IRB number: 13-172).
Assessment of molecular types
Immunohistochemical stains were performed using the VENTANA BenchMark ULTRA and ultraView Detection Kit (Roche Diagnostics Corp., Tucson, USA). Paraffin-embedded tissue sections were de-waxed in xylene and rehydrated in a graded alcohol series. Antigen retrieval was carried out in Tris-EDTA buffer (pH 8.0) except for the retrieval of EGFR, which was conducted in protease 1, for 36 to 52 minutes. The primary antibodies used were ER (1:90; Novocastra Laboratories Ltd., Newcastle upon Tyne, UK,), PR (1:170; Novocastra Laboratories Ltd.), HER2 (Ventana Medical Systems Inc., Tucson, USA), Ki-67 (1:280; Dako, Glostrup, Denmark), CK5/6 (1:100; Dako), EGFR (1:90; Invitrogen, Camarillo, USA), p53 (1:150; Dako), and COX-2 (1:160; NeoMarks, Fremont, USA). Visualization using DAB (Dako) and a counterstain with hematoxylin were conducted.
The expression of ER and PR was recorded as an immunohistochemical score of 0-8, similar to the Allred scoring system [18] based on the sum of intensity scores (1, weak; 2, moderate; 3, strong) and proportion scores (1, <1%; 2, 1%-10%; 3, 11%-33%; 4, 34%-66%; 5, ≥67%). Sixty-seven cases showing a PR score of 4-6 were re-examined to apply the cutoff point of 20% for decreased PR expression. HER2 expression was graded on a scale of 0 to 3+ according to the American Society of Clinical Oncology guidelines [19], and a score of 3+ was regarded as positive for HER2. A fluorescence in situ hybridization result for HER2 gene amplification was also included if performed. Expression of CK5/6 and EGFR was interpreted as positive if more than 10% of the tumor cells showed cytoplasmic or membranous expression.
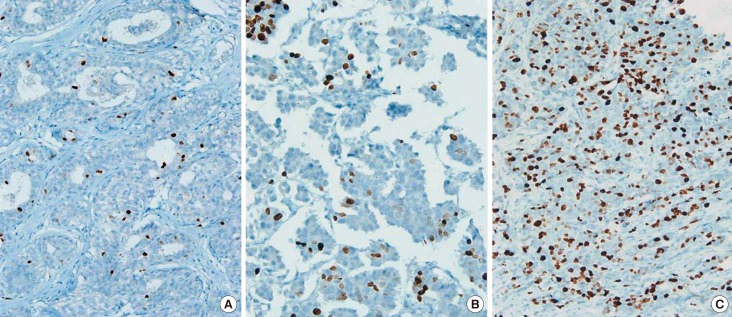
The Ki-67 labeling index was determined as follows: We selected any three foci in low-power fields, if the staining was homogenous, or we identified a hot spot and then selected three areas in a high-power field if the staining was heterogeneous. A three-step assessment was performed. The first step involved a visual assessment to label a low index in an area showing less than 10% expression and a high index in an area showing more than 30% expression. The second step was manual counting of "indeterminate cases" using only visual assessment. At least 500 cells were counted. The last step was to categorize each case as having a low or high labeling index using a cutoff value of 14% (Figure 1), based on the suggestion of the St. Gallen consensus meeting 2013 [11].
Figure 1. Immunohistochemical stains for Ki-67 in the breast cancers. Low labeling index (<10%) (A), intermediate (10%-20%) (B), and high labeling index (>20%) (C) based on Eye-10 method (×100).
Molecular types of breast cancers were classified based on ER and PR status; expression of HER2, CK5/6, and EGFR; and the Ki-67 labeling index [11]. The criteria to determine the luminal types are shown in Table 1. In this study, luminal B type cancers were described as B1, B2, and B3 according to the following criteria: luminal type B1 was HER2-positive, with ER-positive and any PR status or Ki-67 labeling index, and luminal types B2 and B3 were HER2-negative, with a high Ki-67 labeling index ≥14% and any PR status (B2) or any Ki-67 labeling index and PR expression of <20% (B3), respectively.
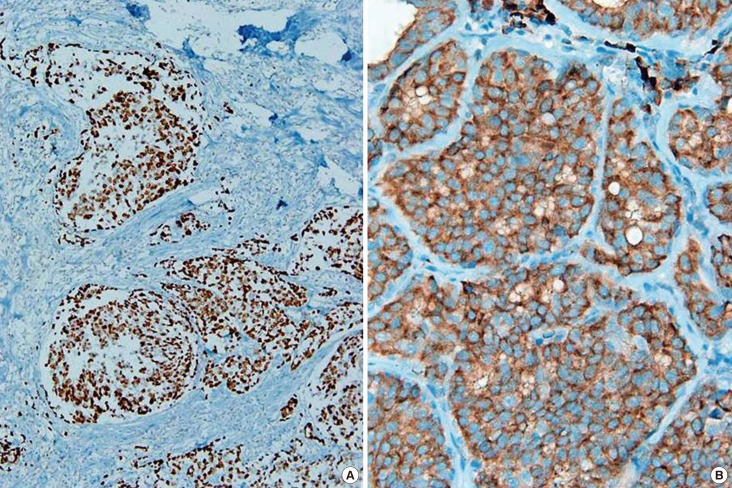
We also reviewed immunohistochemical stains for p53 and COX-2. Expression of p53 in more than two-thirds of tumor cells was regarded as p53 positivity (Figure 2A) [20], and diffuse (>50%) and strong expression for COX-2 was regarded as COX-2 positivity (Figure 2B) [21].
Figure 2. Immunohistochemical stains for p53 and cycloxygenase 2 (COX-2) in the breast cancers. More than two-thirds of the tumor cells is regarded as p53 positive (A, ×40), and diffuse (>50%) and/or strong reaction is regarded as COX-2 positive (B, ×100).
Statistical analysis
The differences in clinicopathological parameters and recurrence and death rates among the new molecular types and between luminal A and B types were analyzed using the chisquare test or Fisher exact test (SAS 9.3; SAS Institute Inc., Cary, USA). In addition, differences in ER and PR expression between the luminal A and B types were also analyzed using the above-mentioned tests. For age, the Wilcoxon rank sum test or Kruskal-Wallis test was used. The Kaplan-Meier method and log-rank test were used to analyze OS and DFS. A p-value less than 0.05 was considered to indicate statistical significance.
RESULTS
Distribution of molecular types
The proportions for each of the molecular types were 21.7% for luminal A, 44.9% for luminal B, 14.6% for HER2, and 18.7% for triple-negative, based on the criteria according to the St. Gallen consensus 2013 (Table 2).
Table 2. Changes in distribution of the molecular types of breast cancers.
| Surrogate definitions based on St. Gallen consensus | |||
|---|---|---|---|
| Before 2011 No. (%) | St. Gallen 2011 No. (%) | St. Gallen 2013 No. (%) | |
| Luminal A type | 149 (55.8) | 76 (28.5) | 58 (21.7) |
| Luminal B type | 29 (10.9) | 102 (38.2) | 120 (44.9) |
| HER2 type | 39 (14.6) | 39 (14.6) | 39 (14.6) |
| Triple-negative type* | 50 (18.7) | 50 (18.7) | 50 (18.7) |
HER2=human epidermal growth factor receptor 2.
*Including basal-like type.
The distributions of the luminal A and B types was changed according to the criteria for surrogate definitions for molecular types of breast cancers. When the criteria from before the 2011 St. Gallen consensus meeting were applied, the proportion of the luminal A type cancers was 55.8%, but that figure decreased to 21.7% based on the criteria according to the St. Gallen consensus 2013. In contrast, the proportion of luminal B type cancers showed an increase from 10.9% to 44.9% when the criteria from the St. Gallen consensus 2013 rather than the criteria from before 2011 were applied. There were 29 luminal B type cancers (24.2%) of the HER2-positive type (B1) and 91 luminal B type cancers of the HER2-negative type. The HER2-negative cancers included 73 cases (B2) showing ER-positive status, any PR expression, HER2-negative status, and a high Ki-67 labeling index (≥14%) and 18 cases (B3) showing ERpositive status, HER2-negative status, any Ki-67 labeling index, and low PR expression (<20%). The HER2 type comprised 39 cases (14.6%), and the triple-negative type included 50 cases (18.7%) (Table 2).
Differences in clinicopathological parameters and clinical outcomes according to molecular types
No significant differences in age were noted among the patients with the various molecular types. There was a statistically significant difference in tumor size (p=0.003), with tumors larger than 2.0 cm being more common in the triplenegative type than in the other types. Lymph node metastasis was commonly seen in the triple-negative type (48.0%), luminal B type (42.7%), HER2 type (33.3%), and luminal A type (24.1%), in decreasing order, and the difference among these was statistically significant (p=0.046). A high histologic grade was more frequently noted in triple-negative and HER2 types than in luminal types, with a statistically significant difference (p<0.001). Positive p53 and COX-2 expression was noted in 66 cases (27.3%) and 172 cases (64.4%), respectively, and this was significantly different (p<0.001 and p=0.002, respectively) among the molecular types.
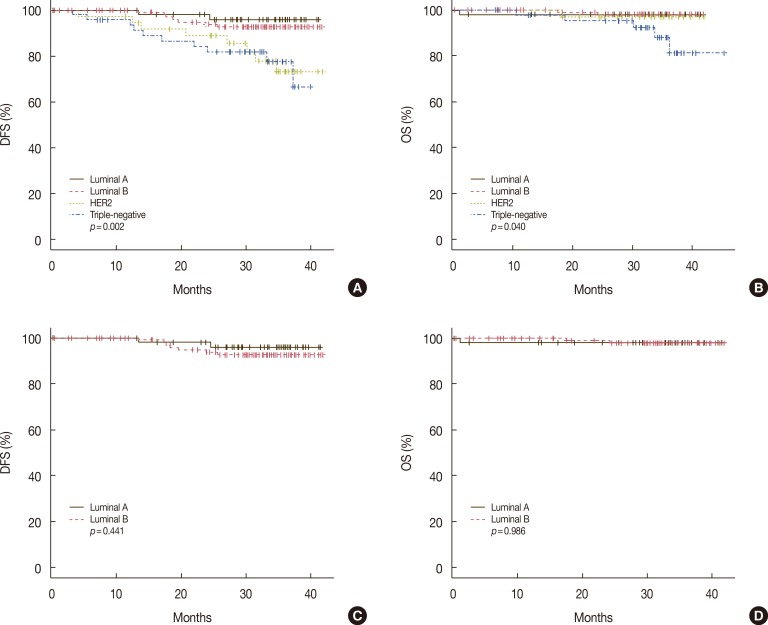
Recurrence was observed in 31 of 261 cases (11.8%), and death was noted in nine of 265 cases (3.4%). The recurrence rate was the highest in the HER2 type (25.6%), followed by the triple-negative, luminal B, and luminal A types at 20.8%, 6.8%, and 5.3%, respectively. In contrast, the death rate was the highest in the triple-negative type (10.2%), compared with the rates of the HER2 (2.6%) and luminal A and B types (1.7% and 1.7%, respectively). Among the molecular types, the recurrence rate (p=0.001), death rate (p=0.036), DFS (p=0.002), and OS (p=0.039) showed significant differences (Table 3, Figure 3A, B).
Table 3. Differences of clinicopathological parameters according to the molecular types based on St. Gallen Consensus 2013.
| Total (n = 267) No. (%) | Luminal A (n = 58) No. (%) | Luminal B (n = 120) No. (%) | p-value* | HER2 (n = 39) No. (%) | TN (n = 50) No. (%) | p-value† | |
|---|---|---|---|---|---|---|---|
| Age (yr)‡ | 56.30 ± 10.61 | 56.15 ± 10.75 | 55.39 ± 10.63 | 0.984 | 54.23 ± 10.13 | 57.94 ± 10.96 | 0.511 |
| Tumor size (cm) | 0.009 | 0.003 | |||||
| ≤ 2.0 | 167 (62.5) | 47 (81.0) | 74 (61.7) | 22 (56.4) | 24 (48.0) | ||
| > 2.0 | 100 (37.5) | 11 (19.0) | 46 (38.3) | 17 (43.6) | 26 (52.0) | ||
| LN | 0.019 | 0.046 | |||||
| Negative | 160 (61.5) | 41 (75.9) | 67 (57.3) | 26 (66.7) | 26 (52.0) | ||
| Positive | 100 (38.5) | 13 (24.1) | 50 (42.7) | 13 (33.3) | 24 (48.0) | ||
| Grade | < 0.001 | < 0.001 | |||||
| Low | 50 (18.7) | 32 (55.2) | 18 (15.0) | 0 | 0 | ||
| Intermediate | 116 (43.4) | 23 (39.6) | 73 (60.8) | 10 (25.6) | 10 (20.0) | ||
| High | 101 (37.8) | 3 (5.2) | 29 (24.2) | 29 (74.4) | 40 (80.0) | ||
| p53 | 0.001 | < 0.001 | |||||
| Negative | 194 (72.7) | 58 (100.0) | 101 (84.2) | 17 (43.6) | 25 (50.0) | ||
| Positive | 66 (27.3) | 0 | 19 (15.8) | 22 (56.4) | 25 (50.0) | ||
| COX-2 | 0.288 | 0.002 | |||||
| Negative | 95 (35.6) | 13 (22.4) | 36 (30.0) | 16 (41.0) | 30 (60.0) | ||
| Positive | 172 (64.4) | 45 (77.6) | 84 (70.0) | 23 (59.0) | 20 (40.0) | ||
| Recurrence | 0.689 | 0.001 | |||||
| No | 230 (88.2) | 54 (94.7) | 109 (93.2) | 29 (74.4) | 38 (79.2) | ||
| Yes | 31 (11.8) | 3 (5.3) | 8 (6.8) | 10 (25.6) | 10 (20.8) | ||
| Death | 0.983 | 0.036 | |||||
| Alive | 256 (96.6) | 57 (98.3) | 117 (98.3) | 38 (97.4) | 44 (89.8) | ||
| Dead | 9 (3.4) | 1 (1.7) | 2 (1.7) | 1 (2.6) | 5 (10.2) |
HER2=human epidermal growth factor receptor 2; TN=triple-negative type; LN=lymph node; COX-2=cycloxygenase 2.
*Between the luminal A type vs. luminal B type; †Among the molecular types; ‡Mean±SD.
Figure 3. Comparison of disease-free survival (DFS) and overall survival (OS) with respect to the molecular types of breast cancers. Statistically significant differences among the molecular types of DFS (A) and OS (B) are seen, but no significant differences of DFS (C) and OS (D) between the luminal A and B types.
Differences in clinicopathological parameters and clinical outcomes between luminal A and B types
A high histologic grade was more common in the luminal B type than in the luminal A type, and the difference was statistically significant (p<0.001). There were also statistically significant differences in tumor size (p=0.009), nodal metastasis (p=0.019), and p53 positivity (p=0.001), but no significant differences in age, COX-2 positivity, recurrence and death rates (Table 3), and DFS and OS between the luminal A and B types (Figure 3C, D).
No significant differences in ER expression were noted between the luminal A and B types (p=0153). However, there was a significant difference in PR expression (p<0.001); decreased PR expression, with a score ≤3, was noted in 27 out of 120 luminal B type cancers (22.5%). Within the luminal B type, the distributions of ER and PR scores were significantly different among the B1, B2, and B3 subtypes (p=0.003 and p=0.003, respectively). Cases showing a high ER score of ≥7 were less common among the HER2-positive luminal B type (B1) than among the HER2-negative luminal B type (B2 and B3) (Table 4).
Table 4. Distribution of estrogen receptor and progesterone receptor scores in the luminal type breast cancers based on St. Gallen Consensus 2013.
| Luminal A (n = 58) No. (%) | Luminal B (n = 120) No. (%) | p-value* | B1 (n = 29) No. (%) | B2 (n = 73) No. (%) | B3 (n = 18) No. (%) | p-value† | |
|---|---|---|---|---|---|---|---|
| ER score | 0.153 | 0.003 | |||||
| ≤3 | 0 | 7 (5.8) | 3 (10.3) | 3 (4.1) | 1 (5.5) | ||
| 4-5 | 2 (3.4) | 9 (7.5) | 1 (3.4) | 5 (6.8) | 3 (16.7) | ||
| 6 | 8 (13.8) | 19 (15.8) | 11 (37.9) | 8 (11.0) | 0 | ||
| 7-8 | 48 (82.8) | 85 (70.8) | 14 (48.3) | 57 (78.1) | 14 (77.8) | ||
| PR score | < 0.001 | 0.003 | |||||
| ≤3 | 0 | 27 (22.5) | 8 (27.6) | 14 (19.2) | 5 (27.8) | ||
| 4-5 | 3 (5.2) | 37 (30.8) | 12 (41.4) | 15 (20.5) | 10 (55.6) | ||
| 6 | 11 (19.0) | 16 (13.3) | 3 (10.3) | 10 (13.7) | 3 (16.7) | ||
| 7-8 | 44 (75.9) | 40 (30.0) | 6 (20.7) | 34 (46.6) | 0 |
B1=HER2-positive luminal B type; B2=HER2-negative luminal B type showing Ki-67 (≥14%) and any PR; B3= HER2-negative luminal B type showing any Ki-67 and PR <20%; ER=estrogen receptor; PR=progesterone receptor.
*Between the luminal A and B type; †Among subgroups of the luminal B type.
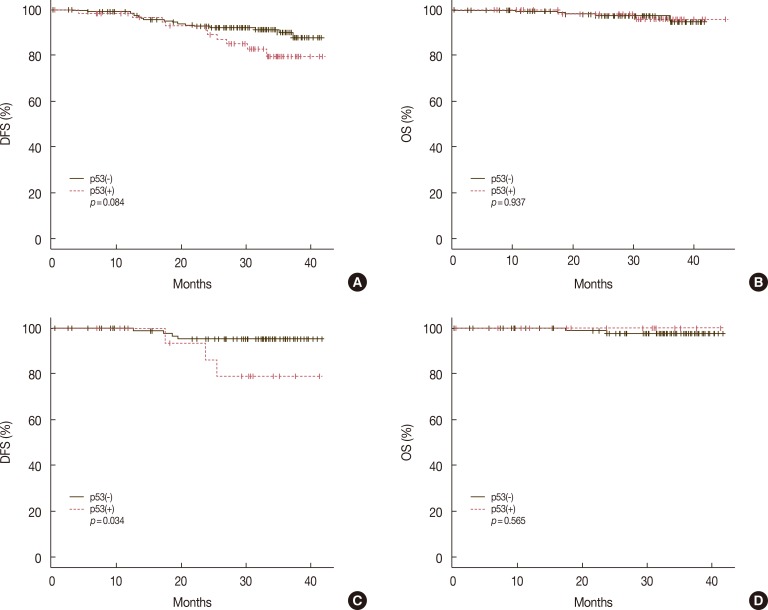
Positive p53 expression was noted in the luminal B type (10.7%), but not in the luminal A type. The recurrence rate in p53-positive luminal type cancers was 15.8%, compared with 5.2% in p53-negative luminal type cancers (p=0.072). The death rates also showed no significant differences according to p53 expression in the luminal type cancers (p=0.545) (Table 5). p53-positive luminal type cancers showed relatively shortened DFS, compared with p53-negative luminal type cancers, although the difference was not significant (p=0.084); likewise, no significant differences were found in OS according to p53 expression (Figure 4A, B). p53-positive luminal B type cancers showed significantly shortened DFS (p=0.034), but no significant differences in OS (p=0.565) (Figure 4C, D).
Table 5. Comparison of recurrence and death rates according to p53 and cycloxygenase 2 expressions in 178 cases of the luminal type breast cancers.
| No. | No. of recurrence (%) | p-value | No. of death (%) | p-value | |
|---|---|---|---|---|---|
| p53 | 0.072 | 0.545 | |||
| Negative | 155 | 8 (5.2) | 3 (1.9) | ||
| Positive | 19 | 3 (15.8) | 0 | ||
| COX-2 | 0.187 | 0.279 | |||
| Negative | 49 | 5 (10.2) | 0 | ||
| Positive | 125 | 6 (3.5) | 3 (1.7) |
COX-2=cycloxygenase 2.
Figure 4. Comparison of disease-free survivals (DFS) and overall survivals (OS) according to p53 expression in the luminal type cancers, and in the luminal B type cancers. Shortened DFS is noted in p53 positive luminal type cancers (p=0.084) (A), and in p53 positive luminal B type cancers (p=0.034) (C), but there were no significant differences of OS in the luminal type cancers (B) and the luminal type B cancers (D).
COX-2 positive luminal type cancers demonstrated a relatively lower recurrence rate of 3.5%, compared with 10.2% in the COX-2 negative luminal type cancers, but there were no significant differences in recurrence and death rates according to COX-2 expression (p=0.187 and p=0.279, respectively) (Table 5). COX-2 negative luminal type cancers displayed significantly shortened DFS compared with COX-2 positive luminal type cancers (p=0.026), but there was no difference in OS according to COX-2 expression (p=0.643) (Supplementary Figure 1A, B, available online). In luminal A and luminal B type cancers, there were no significant differences in DFS (p=0.370 and p=0.383) and OS (p=0.643 and p=0.367) according to COX-2 expression (Supplementary Figure 1C-1F, available online).
DISCUSSION
In our study, the proportions of each of the molecular types were 21.7% for the luminal A type, 44.9% for the luminal B type, 14.6% for the HER2 type, and 18.7% for the triple-negative type based on the criteria according to the St. Gallen consensus 2013. The luminal A type was the most common subtype according to the criteria used before the St. Gallen consensus 2011 (55.8%), but the luminal B type was the most common based on the application of the criteria of the St. Gallen consensus 2013 (44.9%). The proportion of the luminal A type decreased from 55.8% to 21.7%, while, in contrast, that of the luminal B type increased from 10.9% to 44.9% based on the changes in the St. Gallen consensus criteria from before 2011 to 2013.
The distribution of molecular types, especially the luminal type cancers, changed after the application of a cutoff value for a high Ki-67 labeling index of ≥14% and low PR expression of <20% for HER2-negative luminal type cancers. Braun et al. [5] used a combination of a cutoff value of 20% for the Ki-67 labeling index and 10% for PR expression for the luminal B type cancers, but the distribution of the molecular types was similar to that in this study; luminal B type was the most common at 50.6%, and the proportions of the others were 33.2% for the luminal A type, 8.8% for the HER2 type, and 7.4% for the triple-negative type.
The main reason for attempting a distinction between tumors of the luminal A type (more endocrine-sensitive, indolent, better prognosis) and the luminal B type (less endocrinesensitive, more aggressive, worse prognosis) was recognized to be the differing implications regarding the utility or futility of adjuvant cytotoxic therapy between these groups [11].
Recently, modified definitions for the HER2-negative luminal B type were reported [22], and luminal type cancers showing both a Ki-67 labeling index of 14%-19% and PR expression of <20% or showing a Ki-67 labeling index of ≥20% were regarded as being of the luminal B type. Braun et al. [5] reported slightly different cutoff values for the Ki-67 labeling index and PR expression for HER2-negative luminal breast cancers. They classified the luminal type cancers into low- and high-risk types (luminal A type/luminal B type), based on a combination of a cutoff value of 20% for the Ki-67 labeling index and a value of 10% for PR expression, due to the intratumoral variability in Ki-67 immunohistochemical stains.
The Ki-67 labeling index is important in the definition of luminal A and B types, and it is a useful marker to predict prognosis and relative responsiveness or resistance to chemotherapy or endocrine therapy. It is also used to estimate residual risk in patients on standard therapy before, during, and after neoadjuvant therapy [8]. A quantitative Ki-67 visual immunohistochemical score was first applied for the classification of luminal B type breast cancers in 2009 [23]. The optimal threshold of Ki-67 immunohistochemistry, 14%, was determined against an important distinction in the underlying biology of breast cancers. Researchers defined the luminal A subtype as ER- and/or PR-positive, HER2-negative, and a low Ki-67 labeling index (<14%), and the luminal B type as ERand/or PR-positive, HER2-negative, and a high Ki-67 labeling index (≥14%).
An international panel of investigators devised comprehensive recommendations regarding preanalytical and analytical assessment as well as interpretation and scoring of Ki-67, with the goal of achieving a harmonized methodology, through between-laboratory and between-study comparisons [16]. However, there were many limitations associated with the Ki-67 labeling index, related to the differences in cutoff values, heterogeneity of Ki-67 expression, interpretation methods, tumor region selection, counting method, subjective assessment of staining positivity, and interobserver variability [16,17]. The Eye-10 method using visual assessment of the percentage of Ki-67 positive cells in 10% intervals at a glance in micrographs taken at 100× and 200× fields including the hot spots was suggested by Hida et al. [24]. This method can exclude obvious high and low Ki-67 cases, leaving a "gray zone" around the cutoff point. In daily practice, combining the Eye-10 method and manual counting is a relatively simple and accurate way to assess the Ki-67 labeling index because it is not easy for a pathologist to count 1,000 cells to evaluate the Ki-67 labeling index in every single case.
In our study, we used a combination of the Eye-10 method and manual counting for the gray zone (10%-20%) to assess the Ki-67 labeling index, and a cutoff value of 14% for the Ki-67 labeling index and a cutoff value of 20% for decreased PR expression were applied for HER2-negative luminal B type cancers.
Quantitative PR gene and protein expression were found to be significantly higher in the luminal A type, and the presence of more than 20% PR-positive tumor cells had a statistically significant value for predicting survival differences in the luminal A type, independently of endocrine therapy administration [25]. In the St. Gallen consensus meeting 2013 [11], another surrogate definition, PR expression of <20%, was added for the HER2-negative luminal B type.
Patients with ER+/PR+ tumors experienced much more benefit from adjuvant tamoxifen therapy than those with ER+/PR- tumors [26]. Through a quantitative analysis of ER, PR, and HER2 in 1,595 tumors, Konecny et al. [27] reported that relatively low levels of HER2 were associated with a marked decrease of PR, but not of ER expression, thus causing tumors to be ER+/PR-, and amplification of HER2 genes ultimately led to the loss of ER expression and resulted in ER-/PR- breast cancers. Furthermore, three times as many ER+/PR- tumors as ER+/PR+ tumors expressed HER-1 and more than 50% showed HER2 overexpression, and loss of PR expression in ER-positive cancer might be a surrogate marker for increasing growth factor receptor tyrosine kinase activity, which results in lower PR expression and tamoxifen resistance [7]. Kim et al. [28] reported that the loss of PR expression was correlated with high HER2 expression and that loss of PTEN was associated with specific loss of PR expression but not with changes in ER levels; they suggested that ER+/PR- breast cancers should be treated with a combination of endocrine therapy and growth factor signaling inhibitors.
In this study, there was no significant difference in ER expression but there was a significant difference in PR expression between luminal A and B types. None of the luminal A type cancers showed decreased ER or PR expression (score ≤3), but the luminal B type cancers revealed decreased ER and PR expression (score ≤3) in 7 cases (4.4%) and 27 cases (20.9%), respectively. Among 120 luminal B type cancers, there were significant differences in ER and PR expression. These findings might be related to the complicated criteria for the luminal B type, consisting of HER2 status, Ki-67 labeling index, and decreased PR expression. Tumors showing high ER scores (≥7) related to good response to hormone therapy were less common among the HER2-positive luminal B type, compared with the HER2-negative luminal B type. Therefore different guidelines for management should be considered for the luminal A and B types, especially according to the subgroup of the luminal B type.
There were statistically significant differences in tumor size, nodal status, histologic grade, p53 and COX-2 expression, recurrence and death rates, DFS, and OS among the molecular types according to the St. Gallen consensus 2013. Braun et al. [5] also reported similar differences in tumor size and histologic grade among the molecular types.
Compared with the luminal A type, the luminal B type showed larger tumor size, more frequent nodal metastasis, high histologic grade, and a higher p53 positivity rate, which suggests that luminal B type cancers might be more aggressive biologically. However, no significant differences in recurrence and death rates, DFS, and OS were observed between the luminal A and B types, which is probably related to the relatively short follow-up period and the small number of cases enrolled in the current study.
In this study, p53 positivity was defined as nuclear expression in more than two-thirds of the tumor cells, similar to the report of Yemelyanova et al. [20]. They suggested that two immunohistochemical staining patterns, strong and diffuse expression, expression in more than 60% of the tumor cells, and a complete lack of expression were correlated with the presence of a TP53 mutation in ovarian carcinomas. Coates et al. [29] reported the interaction between p53 expression and ER status in node-negative breast cancer, and p53 expression was associated with a worse prognosis in the ER-positive luminal types. In the current study, positive p53 expression was noted in the luminal B type (15.8%) but not in the luminal A type, and p53-positive luminal type cancers showed a higher recurrence rate (p=0.072) and shortened DFS (p=0.084) compared with p53-negative luminal type cancers, with borderline statistical significance. p53-positive luminal B type cancers showed significantly shortened DFS (p=0.034). These results suggest that p53 expression would be a helpful marker to discriminate between the luminal A and B types and that p53- positive luminal B type cancers are related to worse clinical outcomes.
COX-2 is involved in the conversion of arachidonic acid to prostaglandins, which stimulate aromatase and increase the production of estrogen. High COX-2 expression, in more than 50% of the tumor cells, was common in hormone receptor- positive types, but was not found to be associated with breast cancer-specific survival or distant DFS [21]. In the current study, a cutoff value for positive COX-2 expression of >50% of tumor cells with strong intensity was applied. No significant differences in COX-2 positivity between the luminal A and B types, and no significant differences in recurrence and death rates or OS among the luminal type cancers were noted, similar to the results of Dhakal et al. [21]. However, COX-2-positive luminal type cancers showed significantly longer DFS than COX-2-negative luminal type cancers. Among the luminal A and luminal B type cancers, there were no significant differences in DFS and OS according to COX-2 expression. These findings suggest that COX-2 positivity in luminal type cancers might be an ancillary factor for predicting good clinical outcome.
In conclusion, our study found statistically significant differences in tumor size, nodal metastasis, histologic grade, p53 and COX-2 expression rates, recurrence and death rates, DFS, and OS among the molecular types of breast cancers reclassified according to the St. Gallen consensus 2013. The luminal B type might be more aggressive than the luminal A type, even though there were no significant differences in DFS or OS. Positive p53 expression in luminal B type cancers and negative COX-2 expression in luminal type cancers might be an adjunct tool for the estimation of poor clinical outcome as these findings were related to shorter DFS. However, there were some limitations in this study related to a relatively short follow-up period and a small study population. Therefore, further research regarding the treatment will be needed in a large series with sufficient follow-up data.
Footnotes
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
Supplementary Material
Comparison of disease-free survivals (DFS) and overall survivals (OS) according to cycloxygenase 2 (COX-2) expressions in the luminal type cancers, the luminal A type and the luminal B type cancers. Shortened DFS in COX-2 negative luminal type cancers (p=0.026) (A), but no significant differences of OS according to COX-2 expressions (B) are noted. There were no significant differences of DFS and OS according to COX-2 expression in the luminal A type cancers (C, D) and the luminal B type cancers (E, F).
References
- 1.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 2.Wang GS, Zhu H, Bi SJ. Pathological features and prognosis of different molecular subtypes of breast cancer. Mol Med Rep. 2012;6:779–782. doi: 10.3892/mmr.2012.981. [DOI] [PubMed] [Google Scholar]
- 3.Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuzick J, Dowsett M, Pineda S, Wale C, Salter J, Quinn E, et al. Prognostic value of a combined estrogen receptor, progesterone receptor, Ki-67, and human epidermal growth factor receptor 2 immunohistochemical score and comparison with the Genomic Health recurrence score in early breast cancer. J Clin Oncol. 2011;29:4273–4278. doi: 10.1200/JCO.2010.31.2835. [DOI] [PubMed] [Google Scholar]
- 5.Braun L, Mietzsch F, Seibold P, Schneeweiss A, Schirmacher P, Chang-Claude J, et al. Intrinsic breast cancer subtypes defined by estrogen receptor signalling-prognostic relevance of progesterone receptor loss. Mod Pathol. 2013;26:1161–1171. doi: 10.1038/modpathol.2013.60. [DOI] [PubMed] [Google Scholar]
- 6.Kim HS, Park I, Cho HJ, Gwak G, Yang K, Bae BN, et al. Analysis of the potent prognostic factors in luminal-type breast cancer. J Breast Cancer. 2012;15:401–406. doi: 10.4048/jbc.2012.15.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arpino G, Weiss H, Lee AV, Schiff R, De Placido S, Osborne CK, et al. Estrogen receptor-positive, progesterone receptor-negative breast cancer: association with growth factor receptor expression and tamoxifen resistance. J Natl Cancer Inst. 2005;97:1254–1261. doi: 10.1093/jnci/dji249. [DOI] [PubMed] [Google Scholar]
- 8.Dowsett M, Nielsen TO, A'Hern R, Bartlett J, Coombes RC, Cuzick J, et al. Assessment of Ki67 in breast cancer: recommendations from the International Ki67 in Breast Cancer working group. J Natl Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang SH, Cheung KY, Kim YS. Correlation between hormonal receptor status and clinicopathologic factors with prognostic assesment in breast cancer. J Korean Surg Soc. 2003;65:198–204. [Google Scholar]
- 10.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ, et al. Strategies for subtypes: dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colozza M, Azambuja E, Cardoso F, Sotiriou C, Larsimont D, Piccart MJ. Proliferative markers as prognostic and predictive tools in early breast cancer: where are we now? Ann Oncol. 2005;16:1723–1739. doi: 10.1093/annonc/mdi352. [DOI] [PubMed] [Google Scholar]
- 13.Luporsi E, André F, Spyratos F, Martin PM, Jacquemier J, Penault-Llorca F, et al. Ki-67: level of evidence and methodological considerations for its role in the clinical management of breast cancer: analytical and critical review. Breast Cancer Res Treat. 2012;132:895–915. doi: 10.1007/s10549-011-1837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono M, Tsuda H, Yunokawa M, Yonemori K, Shimizu C, Tamura K, et al. Prognostic impact of Ki-67 labeling indices with 3 different cutoff values, histological grade, and nuclear grade in hormone-receptor-positive, HER2-negative, node-negative invasive breast cancers. Breast Cancer. 2015;22:141–152. doi: 10.1007/s12282-013-0464-4. [DOI] [PubMed] [Google Scholar]
- 15.Ahlin C, Aaltonen K, Amini RM, Nevanlinna H, Fjällskog ML, Blomqvist C. Ki67 and cyclin A as prognostic factors in early breast cancer. What are the optimal cut-off values? Histopathology. 2007;51:491–498. doi: 10.1111/j.1365-2559.2007.02798.x. [DOI] [PubMed] [Google Scholar]
- 16.Polley MY, Leung SC, McShane LM, Gao D, Hugh JC, Mastropasqua MG, et al. An international Ki67 reproducibility study. J Natl Cancer Inst. 2013;105:1897–1906. doi: 10.1093/jnci/djt306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neri A, Marrelli D, Pedrazzani C, Caruso S, De Stefano A, Mariani F, et al. Prognostic relevance of proliferative activity evaluated by Mib-1 immunostaining in node negative breast cancer. Eur J Surg Oncol. 2008;34:1299–1303. doi: 10.1016/j.ejso.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 20.Yemelyanova A, Vang R, Kshirsagar M, Lu D, Marks MA, Shih IeM, et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Mod Pathol. 2011;24:1248–1253. doi: 10.1038/modpathol.2011.85. [DOI] [PubMed] [Google Scholar]
- 21.Dhakal HP, Naume B, Synnestvedt M, Borgen E, Kaaresen R, Schlichting E, et al. Expression of cyclooxygenase-2 in invasive breast carcinomas and its prognostic impact. Histol Histopathol. 2012;27:1315–1325. doi: 10.14670/HH-27.1315. [DOI] [PubMed] [Google Scholar]
- 22.Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, Dellapasqua S, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014;16:R65. doi: 10.1186/bcr3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101:736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hida AI, Oshiro Y, Inoue H, Kawaguchi H, Yamashita N, Moriya T. Visual assessment of Ki67 at a glance is an easy method to exclude many luminal-type breast cancers from counting 1000 cells. Breast Cancer. 2015;22:129–134. doi: 10.1007/s12282-013-0460-8. [DOI] [PubMed] [Google Scholar]
- 25.Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, Caballero R, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31:203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J Clin Oncol. 2003;21:1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 27.Konecny G, Pauletti G, Pegram M, Untch M, Dandekar S, Aguilar Z, et al. Quantitative association between HER-2/neu and steroid hormone receptors in hormone receptor-positive primary breast cancer. J Natl Cancer Inst. 2003;95:142–153. doi: 10.1093/jnci/95.2.142. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Cui X, Hilsenbeck SG, Lee AV. Progesterone receptor loss correlates with human epidermal growth factor receptor 2 overexpression in estrogen receptor-positive breast cancer. Clin Cancer Res. 2006;12:1013s–1018s. doi: 10.1158/1078-0432.CCR-05-2128. [DOI] [PubMed] [Google Scholar]
- 29.Coates AS, Millar EK, O'Toole SA, Molloy TJ, Viale G, Goldhirsch A, et al. Prognostic interaction between expression of p53 and estrogen receptor in patients with node-negative breast cancer: results from IBCSG Trials VIII and IX. Breast Cancer Res. 2012;14:R143. doi: 10.1186/bcr3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of disease-free survivals (DFS) and overall survivals (OS) according to cycloxygenase 2 (COX-2) expressions in the luminal type cancers, the luminal A type and the luminal B type cancers. Shortened DFS in COX-2 negative luminal type cancers (p=0.026) (A), but no significant differences of OS according to COX-2 expressions (B) are noted. There were no significant differences of DFS and OS according to COX-2 expression in the luminal A type cancers (C, D) and the luminal B type cancers (E, F).