Abstract
Purpose
To decide the optimal treatment for breast cancer patients with locoregional recurrence (LRR), it is important to determine which group has the highest risk of subsequent distant metastasis (DM). We aimed to investigate the factors associated with DM in patients with LRR.
Methods
We reviewed the data of 208 patients with LRR as the first event after primary surgery for breast cancer at our institution between 1997 and 2010, to identify significant factors associated with DM. Subsequently, Kaplan-Meier curves and the Cox regression method were used to analyze the correlation between clinical factors and survival.
Results
DM occurred in 33.2% (68/208) of LRR patients. The median DM-free interval was 23 months. Some clinical factors were associated with DM in univariate analysis, including the type of primary surgery (p=0.026), tumor size (p=0.005), nodal status (p=0.011), and administration of initial adjuvant chemotherapy (p=0.001). In addition, regional rather than local recurrence and a disease-free interval (DFI; duration between primary surgery and LRR) ≤30 months were also significant (p<0.001 for both). However, only a shorter DFI reached significance in multiple logistic regression analysis. Cox regression analysis of DM-free survival showed that both a shorter DFI and regional recurrence were significant factors with hazard ratios of 2.1 (95% confidence interval [CI], 1.21-3.65) and 1.85 (95% CI, 1.04-3.28), respectively.
Conclusion
DFI was the most important factor associated with subsequent DM in patients with LRR as a first event of failure.
Keywords: Breast neoplasms, Local neoplasm recurrence, Neoplasm metastasis, Prognosis, Risk factors
INTRODUCTION
Breast cancer can recur locoregionally or systemically. Patients with locoregional recurrence (LRR) as a first event of failure have a higher risk of distant metastasis (DM) and poor survival compared to patients without LRR [1,2,3]. Despite treatment, some patients with LRR progress to systemic disease.
Little research has been carried out to determine if chemotherapy after surgery for local recurrence would benefit these patients. Recent results from the Chemotherapy as Adjuvant for Locally Recurrent Breast Cancer (CALOR) study suggest that chemotherapy after surgery for locally recurrent breast cancer improves both disease-free and overall survival (OS), especially in estrogen receptor-negative patients [4]. Therefore, it is important to determine which subgroup of patients will benefit from cytotoxic chemotherapy following LRR.
In general, to predict which patients will benefit from chemotherapy, their risk of DM should also be considered. However, few studies have investigated factors associated with a high risk of DM in patients with LRR.
In this study, we aimed to investigate the factors associated with DM and survival outcomes of patients with LRR. This analysis could help to select patients who require close monitoring or aggressive treatment following LRR.
METHODS
Patients and data collection
Of patients who underwent surgery for breast cancer at Seoul National University Hospital Breast Care Center between January 1995 and December 2010, 208 had LRR as a first event of failure after initial treatment. Ipsilateral breast or chest wall recurrence was regarded as local recurrence, and recurrence in the ipsilateral axilla, supraclavicular, or isolated internal mammary node as regional recurrence. Patients with contralateral breast recurrence, distant recurrence before LRR, or simultaneous DM and LRR were excluded. Electronic medical records were reviewed, and the patients' clinical and pathological data were collected including the patient's age, initial tumor stage, immunohistochemical results, initial treatment, type of LRR, and time to LRR. To assess tumor stage, staging guidelines from the America Joint Committee on Cancer, seventh edition, were used. Magnetic resonance imaging, bone scintigraphy, and computed tomography were used to detect DM. Biopsies were carried out on some of the metastatic lesions. To analyze the relationship between clinical factors and survival outcomes, dates of local recurrence, metastasis, and death were also collected. Hormone receptor status, human epidermal growth factor receptor 2 (HER2) overexpression, and Ki-67 levels were analyzed by immunohistochemistry. Tumor cells with ≥10% estrogen and progesterone receptor staining were regarded as positive. For HER2 overexpression analysis, cells with <10%, 10-30% or >30% staining were considered negative, indeterminate, or positive, respectively.
When HER2 immunohistochemistry results were reported as indeterminate, additional fluorescence in situ hybridization tests were performed. DM-free survival (DMFS) was defined as the duration between primary local recurrence and DM. OS was defined as the period from cancer diagnosis to death, including cancer-related death or death from other causes. Close resection margins implied that the tumor was ≤2 mm from the resection margin. This study was recognized by the Seoul National University Institutional Review Board (IRB number: 1409-100-601).
Statistical analysis
The chi-square test was used for univariate analysis of the association between different factors. Multiple logistic regression analysis was used for multivariate analysis. Kaplan-Meier survival curves with the log-rank test and the Cox regression method were used to analyze the relationship between risk factors and survival outcomes. Results with p<0.05 were regarded statistically significant. SPSS Statistics, version 21 (IBM Corp., Armonk, USA) was used for all statistical analyses.
RESULTS
The patients' median age at the time of primary breast cancer diagnosis was 45.8 years (range, 21-77 years). The median follow-up duration was 71 months (range, 6-229 months). Of 208 LRR patients, local recurrence occurred in 56.3% (117/208) and regional recurrence in 43.8% (91/208). Of these, 33% (69/208) progressed to systemic disease during the follow-up period. The median interval between initial surgery and LRR, defined as the disease-free interval (DFI), was 30 months (range, 1-204 months). The median interval between LRR and subsequent DM, defined as the DM-free interval, was 23 months (range, 0-167 months).
Table 1 shows the factors associated with DM in patients with LRR. Initial tumor size (≤2 cm vs. >2 cm), nodal status (negative vs. positive), and tumor stage (stage I vs. stage II vs. stage III) were associated with DM in univariate analysis (p=0.005, p=0.004, and p=0.011, respectively). The type of surgery (breast-conserving surgery vs. mastectomy), administration of adjuvant chemotherapy (no vs. yes), type of LRR (local vs. regional), and DFI (≤30 months vs. >30 months) were also associated with DM in univariate analysis (p=0.026, p=0.001, p<0.001, and p<0.001, respectively). However, hormone receptor status, HER2 status, and Ki-67 levels were revealed to be nonsignificant factors in this analysis. Multivariate analysis was performed on factors found to be significant in univariate analysis, and only DFI remained a significant risk factor associated with DM (odds ratio [OR], 4.78; 95% confidence interval [CI], 1.75-13.02).
Table 1. Analysis for characteristic of primary tumor and its treatment associated with subsequent distant metastasis in patients with locoregional recurrences.
| Characteristic | Distant metastasis | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|---|
| No No.(%) | Yes No.(%) | p-value | p-value | OR (95% CI, range) | |
| Age (yr) | 0.299 | 0.562 | 1.50 (0.38-5.84) | ||
| <35 | 19 (76.0) | 6 (24.0) | |||
| ≥ 35 | 120 (65.6) | 63 (34.4) | |||
| Operation type | 0.026 | 0.719 | 1.20 (0.44-3.33) | ||
| BCS | 71 (74.7) | 24 (25.3) | |||
| Mastectomy | 68 (60.2) | 45 (39.8) | |||
| Adjuvant chemotherapy | 0.001 | 0.123 | 3.24 (0.73-14.48) | ||
| Yes | 105 (62.5) | 63 (37.5) | |||
| No | 34 (89.5) | 4 (10.5) | |||
| Adjuvant radiotherapy | 0.623 | 0.512 | 1.38 (0.53-3.60) | ||
| Yes | 69 (68.3) | 32 (31.7) | |||
| No | 69 (65.1) | 37 (34.9) | |||
| Adjuvant hormone therapy | 0.284 | 0.092 | 3.17 (0.83-12.15) | ||
| Yes | 44 (62.0) | 27 (38.0) | |||
| No | 95 (69.3) | 42 (30.7) | |||
| Tumor size (cm) | 0.005 | 0.592 | 1.33 (0.14-3.70) | ||
| ≤2 | 60 (77.9) | 17 (22.1) | |||
| >2 | 74 (58.7) | 52 (41.3) | |||
| Lymph node | 0.004 | 0.761 | 1.16 (0.45-2.95) | ||
| Negative | 76 (76.8) | 23 (23.2) | |||
| Positive | 63 (57.8) | 46 (42.2) | |||
| Stage | 0.011 | ||||
| I | 46 (82.1) | 10 (17.9) | |||
| II | 64 (62.1) | 39 (37.9) | |||
| III | 26 (56.5) | 20 (43.5) | |||
| Histologic grade | 0.145 | 0.059 | 2.53 (0.97-6.64) | ||
| Low (grade 1, 2) | 40 (59.7) | 27 (40.3) | |||
| High (grade 3) | 89 (70.1) | 38 (29.9) | |||
| Close resection margin | 0.362 | 0.688 | 1.21 (0.48-3.00) | ||
| Yes | 34 (72.3) | 13 (27.7) | |||
| No | 105 (65.2) | 34 (34.8) | |||
| Estrogen receptor | 0.847 | 0.993 | 1.01 (0.30-3.38) | ||
| Negative | 52 (65.0) | 28 (35.0) | |||
| Positive | 71 (66.4) | 36 (33.6) | |||
| Progesterone receptor | 0.261 | 0.166 | 0.43 (0.13-1.43) | ||
| Negative | 45 (71.4) | 18 (28.6) | |||
| Positive | 79 (63.2) | 46 (36.8) | |||
| HER2 | 0.459 | 0.567 | 1.32 (0.51-3.45) | ||
| Negative | 80 (63.5) | 46 (36.5) | |||
| Positive | 32 (69.6) | 14 (30.4) | |||
| Ki-67 (%) | 0.609 | 0.416 | 1.45 (0.59-3.52) | ||
| ≥ 10 | 48 (65.8) | 25 (34.2) | |||
| <10 | 66 (69.5) | 29 (30.5) | |||
| Disease-free interval (mo) | < 0.001 | 0.002 | 4.78 (1.75-13.02) | ||
| > 30 | 79 (80.6) | 19 (19.4) | |||
| ≤ 30 | 60 (54.5) | 50 (45.5) | |||
| Type of recurrence | <0.001 | 0.183 | 1.87 (0.74-4.71) | ||
| Local | 90 (76.9) | 27 (23.1) | |||
| Regional | 49 (53.8) | 42 (46.2) | |||
OR=odds ratio; CI=confidence interval; BCS=breast-conserving surgery; HER2=human epidermal growth factor receptor 2.
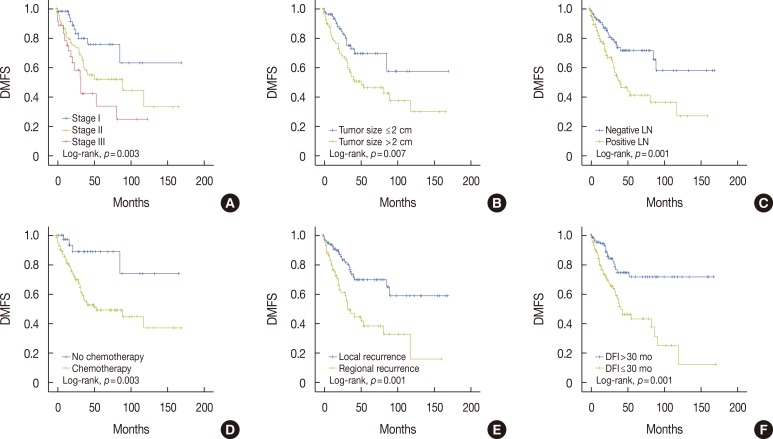
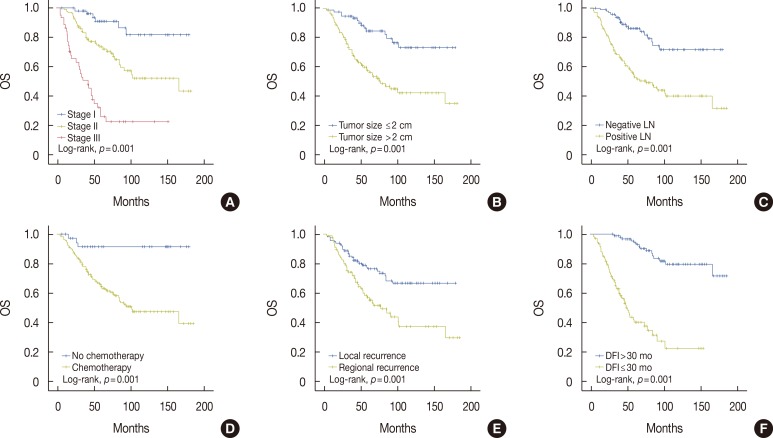
In survival analysis, there was a significant difference in DMFS and OS according to administration of adjuvant chemotherapy, tumor size, nodal status, overall tumor stage, type of LRR, and DFI (Figures 1, 2). Of these factors, a short DFI (≤30 months) and regional rather than local recurrence were significant risk factors associated with a reduced DMFS in Cox regression analysis (hazard ratio [HR], 2.10; 95% CI, 1.21-3.65 and HR, 1.85; 95% CI, 1.04-3.28, respectively). Patients with short or long DFIs had 5-year DMFS rates of 43.0%±6.4% or 71.4%±5.9%, respectively. The 5-year DMFS rates for patients with regional or local recurrence were 39.1%±6.9% or 70.3%±5.4%, respectively. In addition, a short DFI and positive lymph node metastasis were significantly associated with a reduced OS (HR, 6.96; 95% CI, 3.69-13.12 and HR, 2.20; 95% CI, 1.19-4.07, respectively). Patients with short or long DFIs had 5-year OS rates of 52.0%±5.2% or 96.8%±1.8%, respectively. The 5-year OS rate for patients with positive or negative lymph nodes were 61.2%±4.8% or 89.0%±3.3%, respectively (Table 2).
Figure 1. Kaplan-Meier analysis of distant metastasis-free survival (DMFS) in patients with locoregional recurrence (LRR). There was a significant difference in DMFS according to stage (A), tumor size (B), nodal status (C), administration of adjuvant chemotherapy (D), type of LRR (E), and disease-free interval (DFI) (F).
LN=lymph node.
Figure 2. Kaplan-Meier analysis of overall survival (OS) in patients with locoregional recurrence (LRR). There was a significant difference in OS according to stage (A), tumor size (B), nodal status (C), administration of adjuvant chemotherapy (D), type of LRR (E), and disease-free interval (DFI) (F).
LN=lymph node.
Table 2. Factors associated with survival outcomes in patients with locoregional recurrence.
| Variable | DMFS | OS | ||
|---|---|---|---|---|
| p-value | HR (95% CI) | p-value | HR (95% CI) | |
| Adjuvant chemotherapy (yes vs. no) | 0.060 | 0.48 (0.27-0.83) | 0.079 | 0.14 (0.08-0.27) |
| DFI (≤ 30 mo vs. > 30 mo) | 0.008 | 2.10 (1.21-3.65) | < 0.001 | 6.96 (3.69-13.12) |
| Type of LRR (regional vs. local) | 0.036 | 1.85 (1.04-3.28) | 0.808 | 1.07 (0.62-1.86) |
| Tumor size (> 2 cm vs. ≤ 2 cm) | 0.723 | 1.12 (0.60-2.10) | 0.240 | 1.49 (0.77-2.90) |
| Nodal status (positive vs. negative) | 0.314 | 1.37 (0.74-2.50) | 0.012 | 2.20 (1.19-4.07) |
DMFS=distant metastasis-free survival; OS=overall survival; HR=hazard ratio; CI=confidence interval; DFI=disease-free interval; LRR=locoregional recurrence.
DISCUSSION
This study mainly examined the factors associated with DM and survival outcomes in patients with LRR. Several clinical factors have been shown to be associated with disease progression and poor survival rates in breast cancer patients, such as patient's age, LRR, breast cancer subtype, initial tumor stage, histologic and nuclear grade, Ki-67 levels, HER2 and hormone receptor status [1,5,6,7,8,9,10,11]. In our study, some clinical factors were associated with DM in patients with LRR in univariate analysis.
Of the factors associated with initial tumor stage, initial tumor size and nodal status, surgery type, administration of adjuvant chemotherapy, and type of LRR were not associated with DM in multivariate analysis. Because patients with higher-stage disease are more likely to undergo mastectomy or adjuvant chemotherapy, we can think that these factors influence each other. In multivariate analysis, only DFI remained a significant factor. Patients with a short DFI (≤30 months) experienced more DM than patients with a long DFI (OR, 4.78; 95% CI, 1.75-13.02). This result shows that DFI is the most important factor associated with DM in patients with LRR.
Next, we analyzed the factors associated with survival outcome in patients with LRR. Previous studies have reported that initial local treatment (breast-conserving surgery vs. mastectomy), pathologic T-stage, LRR site (local vs. regional), and DFI were significant prognostic factors for DMFS and OS in patients with LRR [12,13,14]. In addition, studies examining the timing of local recurrence have shown that patients with early local recurrence have a worse outcome compared to those with late local recurrence [15,16,17,18]. In our study, the DFI and LRR site were prognostic factors associated with DMFS. A short DFI and positive initial lymph node status were associated with a worse OS. These results show that early LRR is a risk factor for DM and a short DFI is a prognostic factor for patients with LRR.
Early local or regional recurrence is thought to be due to rapid cell proliferation, and may explain the association between a short DFI and poor survival [19]. Courdi et al. [18,19] reported that early local recurrence was associated with high-grade tumors and a negative hormone receptor status. A high tumor grade and negative estrogen receptor status were significantly associated with a high percentage of S-phase cells as determined by the labeling index. In addition, late recurrences with a high mitotic count have the same poor prognosis as early recurrences [20]. However, in our study, initial tumor grade and hormone receptor status were not significantly associated with DM in LRR patients. In addition, Ki-67, a marker of cell proliferation, was not associated with DMFS.
There is some debate as to whether a late recurrence might be caused by a new primary lesion, thus, the prognosis is relatively better than if a true recurrence [21,22]. However, it was impossible to distinguish clearly between a new primary tumor and true recurrence in this study because the biology of the recurrent tumor was not determined in most of the patients. To identify the clonal relationship between LRR and the primary tumor, more research is required.
In our study, regional or local recurrence were not significant prognostic factors for OS, but were for DMFS. Isolated regional nodal recurrence without simultaneous local or distant recurrence is uncommon. The incidence of isolated nodal recurrence in patients who received breast-conserving treatment is approximately 1%-5.4% [23,24]. However, patients with regional recurrence are known to have a poorer prognosis than patients with isolated local recurrence [25,26]. Therefore, it is necessary to consider more aggressive salvage therapy for regional recurrence.
It has been shown in previous studies that breast-conserving surgery followed by radiotherapy results in a similar OS or disease-free survival compared with mastectomy for primary breast cancer [27,28]. However, several studies have reported that patients with LRR who underwent breast-conserving therapy for a primary tumor have more favorable outcomes than those who underwent mastectomy. Data on the survival outcome of patients with LRR according to type of primary local treatment are still controversial [12,21,29,30]. Multivariate analysis in our study showed that the type of surgery used for the primary tumor was not associated with DM, DMFS, or OS (p=0.716, p=0.562, or p=0.963, respectively).
Adjuvant chemotherapy reduces the risk of recurrence and death in patients with primary breast cancer. However, little research has been carried out to determine if chemotherapy after surgery for local recurrence would benefit patients. Although recent results from the CALOR study showed that chemotherapy after surgery for local recurrence significantly increased disease-free survival and OS, particularly in estrogen receptor-negative patients, the appropriate chemotherapy for breast cancer LRR patients is still a contentious issue. In general, to predict which patients will benefit from chemotherapy, their risk of DM should also be considered. The interval from primary surgery to LRR was a significant factor associated with the reduction in the risk of disease-free survival in CALOR study [4]. DFI was the most important factor associated with DM and survival outcomes in patients with LRR in our study. Therefore, we can hypothesize that chemotherapy will be more effective in patients with a short DFI. Patients in our study received various treatment modalities after LRR, such as surgery, radiotherapy, chemotherapy, and hormone therapy. However, unfortunately we were unable to analyze survival outcomes according to the treatment modality used because there was too much variation. Furthermore, as the clinical data were collected retrospectively, we were unable to determine why a particular modality was chosen for each patient. More studies are required to ascertain which subgroup of patients with LRR will benefit from chemotherapy in the future.
Our study involved a relatively large number of LRR patients with a long follow-up period. However, there were also some imitations. It was a retrospective study and there were missing data such as hormone receptor and HER2 status, and Ki-67 levels for some patients. A future prospective study could be designed to extend the results of our study.
In conclusion, DFI was the most significant factor associated with DM and survival outcome in patients with initial LRR after curative resection for breast cancer. Therefore, we should carefully monitor patients with LRR who have a short DFI for metastasis. Furthermore, it is necessary to consider more aggressive salvage treatment for LRR patients with a short DFI, regional recurrence, and an initial positive lymph node status.
Footnotes
This work was supported in part by a National Research Foundation of Korea (NRF) Grant funded by the Korea government (MSIP) (Grant 2012M3A9B2028834, 2012M3A9B2029131) and the Seoul National University Hospital (SNUH) Research Fund (Grant 03-2009-0270).
CONFLICT OF INTEREST: The authors declare that they have no competing interests.
References
- 1.Vicini FA, Kestin L, Huang R, Martinez A. Does local recurrence affect the rate of distant metastases and survival in patients with early-stage breast carcinoma treated with breast-conserving therapy? Cancer. 2003;97:910–919. doi: 10.1002/cncr.11143. [DOI] [PubMed] [Google Scholar]
- 2.Schmoor C, Sauerbrei W, Bastert G, Schumacher M. Role of isolated locoregional recurrence of breast cancer: results of four prospective studies. J Clin Oncol. 2000;18:1696–1708. doi: 10.1200/JCO.2000.18.8.1696. [DOI] [PubMed] [Google Scholar]
- 3.Fortin A, Larochelle M, Laverdière J, Lavertu S, Tremblay D. Local failure is responsible for the decrease in survival for patients with breast cancer treated with conservative surgery and postoperative radiotherapy. J Clin Oncol. 1999;17:101–109. doi: 10.1200/JCO.1999.17.1.101. [DOI] [PubMed] [Google Scholar]
- 4.Aebi S, Gelber S, Anderson SJ, Láng I, Robidoux A, Martín M, et al. Chemotherapy for isolated locoregional recurrence of breast cancer (CALOR): a randomised trial. Lancet Oncol. 2014;15:156–163. doi: 10.1016/S1470-2045(13)70589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stathopoulos GP, Malamos NA, Markopoulos C, Polychronis A, Armakolas A, Rigatos S, et al. The role of Ki-67 in the proliferation and prognosis of breast cancer molecular classification subtypes. Anticancer Drugs. 2014;25:950–957. doi: 10.1097/CAD.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zong Y, Zhu L, Wu J, Chen X, Huang O, Fei X, et al. Progesterone receptor status and Ki-67 index may predict early relapse in luminal B/HER2 negative breast cancer patients: a retrospective study. PLoS One. 2014;9:e95629. doi: 10.1371/journal.pone.0095629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contesso G, Mouriesse H, Friedman S, Genin J, Sarrazin D, Rouesse J. The importance of histologic grade in long-term prognosis of breast cancer: a study of 1,010 patients, uniformly treated at the Institut Gustave-Roussy. J Clin Oncol. 1987;5:1378–1386. doi: 10.1200/JCO.1987.5.9.1378. [DOI] [PubMed] [Google Scholar]
- 8.Vichapat V, Garmo H, Holmqvist M, Liljegren G, Wärnberg F, Lambe M, et al. Tumor stage affects risk and prognosis of contralateral breast cancer: results from a large Swedish-population-based study. J Clin Oncol. 2012;30:3478–3485. doi: 10.1200/JCO.2011.39.3645. [DOI] [PubMed] [Google Scholar]
- 9.Belkacémi Y, Penault-Llorca F, Gligorov J, Azria D. The use of breast cancer subtype classification to predict local and distant recurrence: a review. Cancer Radiother. 2008;12:577–583. doi: 10.1016/j.canrad.2008.08.272. [DOI] [PubMed] [Google Scholar]
- 10.Beadle BM, Woodward WA, Buchholz TA. The impact of age on outcome in early-stage breast cancer. Semin Radiat Oncol. 2011;21:26–34. doi: 10.1016/j.semradonc.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez RC, Acevedo CF, Petric GM, Galindo AH, Domínguez CF, León RA, et al. Survival of patients with metastatic breast cancer according to pathological types of tumors. Rev Med Chil. 2014;142:428–435. doi: 10.4067/S0034-98872014000400003. [DOI] [PubMed] [Google Scholar]
- 12.Shenouda MN, Sadek BT, Goldberg SI, Keruakous AR, Croft BJ, Abi Raad RF, et al. Clinical outcome of isolated locoregional recurrence in patients with breast cancer according to their primary local treatment. Clin Breast Cancer. 2014;14:198–204. doi: 10.1016/j.clbc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Komoike Y, Akiyama F, Iino Y, Ikeda T, Akashi-Tanaka S, Ohsumi S, et al. Ipsilateral breast tumor recurrence (IBTR) after breast-conserving treatment for early breast cancer: risk factors and impact on distant metastases. Cancer. 2006;106:35–41. doi: 10.1002/cncr.21551. [DOI] [PubMed] [Google Scholar]
- 14.Voogd AC, Cranenbroek S, de Boer R, Roumen RM, Rutten HJ, van der Sangen MJ. Long-term prognosis of patients with axillary recurrence after axillary dissection for invasive breast cancer. Eur J Surg Oncol. 2005;31:485–489. doi: 10.1016/j.ejso.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Neri A, Marrelli D, Rossi S, De Stefano A, Mariani F, De Marco G, et al. Breast cancer local recurrence: risk factors and prognostic relevance of early time to recurrence. World J Surg. 2007;31:36–45. doi: 10.1007/s00268-006-0097-2. [DOI] [PubMed] [Google Scholar]
- 16.Galper S, Blood E, Gelman R, Abner A, Recht A, Kohli A, et al. Prognosis after local recurrence after conservative surgery and radiation for early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2005;61:348–357. doi: 10.1016/j.ijrobp.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Veronesi U, Marubini E, Del Vecchio M, Manzari A, Andreola S, Greco M, et al. Local recurrences and distant metastases after conservative breast cancer treatments: partly independent events. J Natl Cancer Inst. 1995;87:19–27. doi: 10.1093/jnci/87.1.19. [DOI] [PubMed] [Google Scholar]
- 18.Courdi A, Largillier R, Ferrero JM, Lallement M, Raoust I, Ettore F, et al. Early versus late local recurrences after conservative treatment of breast carcinoma: differences in primary tumor characteristics and patient outcome. Oncology. 2006;71:361–368. doi: 10.1159/000107771. [DOI] [PubMed] [Google Scholar]
- 19.Courdi A, Héry M, Dahan E, Gioanni J, Abbes M, Monticelli J, et al. Factors affecting relapse in node-negative breast cancer: a multivariate analysis including the labeling index. Eur J Cancer Clin Oncol. 1989;25:351–356. doi: 10.1016/0277-5379(89)90029-1. [DOI] [PubMed] [Google Scholar]
- 20.Elkhuizen PH, Hermans J, Leer JW, van dE Vijver MJ. Isolated late local recurrences with high mitotic count and early local recurrences following breast-conserving therapy are associated with increased risk on distant metastasis. Int J Radiat Oncol Biol Phys. 2001;50:387–396. doi: 10.1016/s0360-3016(01)01469-9. [DOI] [PubMed] [Google Scholar]
- 21.Janni W, Shabani N, Dimpfl T, Starflinger I, Rjosk D, Peschers U, et al. Matched pair analysis of survival after chest-wall recurrence compared to mammary recurrence: a long-term follow up. J Cancer Res Clin Oncol. 2001;127:455–462. doi: 10.1007/s004320100238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang E, Buchholz TA, Meric F, Krishnamurthy S, Mirza NQ, Ames FC, et al. Classifying local disease recurrences after breast conservation therapy based on location and histology: new primary tumors have more favorable outcomes than true local disease recurrences. Cancer. 2002;95:2059–2067. doi: 10.1002/cncr.10952. [DOI] [PubMed] [Google Scholar]
- 23.Harris EE, Hwang WT, Seyednejad F, Solin LJ. Prognosis after regional lymph node recurrence in patients with stage I-II breast carcinoma treated with breast conservation therapy. Cancer. 2003;98:2144–2151. doi: 10.1002/cncr.11767. [DOI] [PubMed] [Google Scholar]
- 24.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 25.Lee JS, Kim SI, Park HS, Lee JS, Park S, Park BW. The impact of local and regional recurrence on distant metastasis and survival in patients treated with breast conservation therapy. J Breast Cancer. 2011;14:191–197. doi: 10.4048/jbc.2011.14.3.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukens JN, Vapiwala N, Hwang WT, Solin LJ. Regional nodal recurrence after breast conservation treatment with radiotherapy for women with early-stage breast carcinoma. Int J Radiat Oncol Biol Phys. 2009;73:1475–1481. doi: 10.1016/j.ijrobp.2008.06.1955. [DOI] [PubMed] [Google Scholar]
- 27.Veronesi U, Salvadori B, Luini A, Greco M, Saccozzi R, del Vecchio M, et al. Breast conservation is a safe method in patients with small cancer of the breast: long-term results of three randomised trials on 1,973 patients. Eur J Cancer. 1995;31A:1574–1579. doi: 10.1016/0959-8049(95)00271-j. [DOI] [PubMed] [Google Scholar]
- 28.Fisher B, Redmond C, Poisson R, Margolese R, Wolmark N, Wickerham L, et al. Eight-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1989;320:822–828. doi: 10.1056/NEJM198903303201302. [DOI] [PubMed] [Google Scholar]
- 29.Fodor J, Major T, Polgár C, Orosz Z, Sulyok Z, Kásler M. Prognosis of patients with local recurrence after mastectomy or conservative surgery for early-stage invasive breast cancer. Breast. 2008;17:302–308. doi: 10.1016/j.breast.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 30.van Tienhoven G, Voogd AC, Peterse JL, Nielsen M, Andersen KW, Mignolet F, et al. EORTC Breast Cancer Cooperative Group and the Danish Breast Cancer Cooperative Group. Prognosis after treatment for loco-regional recurrence after mastectomy or breast conserving therapy in two randomised trials (EORTC 10801 and DBCG-82TM) Eur J Cancer. 1999;35:32–38. doi: 10.1016/s0959-8049(98)00301-3. [DOI] [PubMed] [Google Scholar]