Abstract
Primary inflammatory myofibroblastic tumors (IMTs) of the breast are uncommon and metastasis of IMTs is extremely rare. To date, the natural course of this disease is not fully understood. Although patients with IMTs should undergo regular follow-up after complete surgical resection of the tumor, the appropriate interval and method of follow-up are unclear. We report the case of a patient with an IMT of the breast that metastasized 2 years after complete surgical resection. This unusual case emphasizes the importance of preoperative examinations to determine whether the IMT has atypical features that should guide the interval and method of follow-up.
Keywords: Breast neoplasms, Granuloma, Metastasis, Plasma cells
INTRODUCTION
Inflammatory myofibroblastic tumors (IMTs) of the breast are rare tumors of unknown etiology that may be confused clinically and radiologically with malignancy [1]. The World Health Organization has classified IMTs as tumors of intermediate biological potential because they have a tendency to recur locally and are associated with a slight risk of distant metastasis. Recurrence of IMTs has been documented in up to 25% of cases and typically depends on the anatomical site of origin and the multifocality and completeness of the surgical resection [2,3]. Distant metastasis of IMTs is rare and occurs in less than 5% of cases, regardless of the site of origin. To date, there has only been one report of a patient with an IMT of the breast that developed distant metastases [4]. As a result, there are no clear consensus guidelines for the follow-up of patients with IMTs of the breast that are based on the early detection of metastases after complete surgical resection. We report a case of a 27-year-old woman with an IMT of the breast that metastasized 2 years after a complete surgical resection. Our aim was to identify imaging and histopathological findings that could predict metastasis or recurrence after complete surgical resection of an IMT of the breast.
CASE REPORT
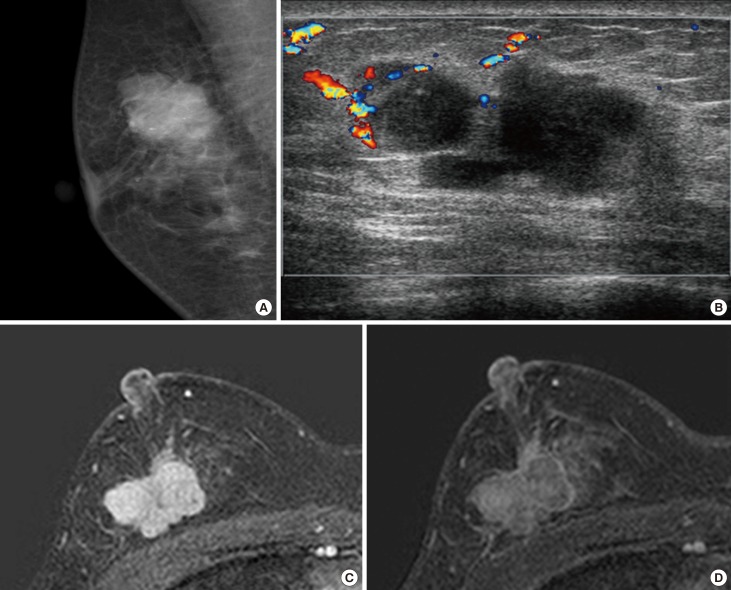
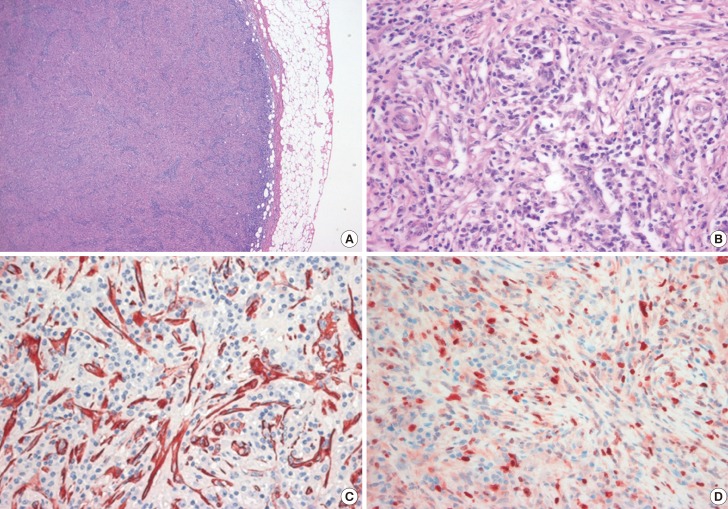
A 27-year-old woman was referred to our center with a painless palpable mass in the upper outer quadrant of the right breast. She had no family history of breast cancer and no personal history of breast injury. A physical examination revealed a solitary, hard, nontender, and nonmobile mass. There were no palpable lymph nodes in the axilla. Mammography showed an approximately 3×2 cm oval-shaped, microlobulated, hyperdense mass in the right upper outer quadrant of the breast. There were some lucent centered and coarse heterogeneous microcalcifications noted within the mass (Figure 1A). Ultrasonography (US) of the breast showed an irregularly shaped, microlobulated, hypoechoic mass with combined posterior features that was approximately 3 cm in diameter. A color Doppler study showed increased vascular flow to the peripheral portion of the lesion (Figure 1B). Magnetic resonance imaging (MRI) revealed an approximately 3×2 cm irregular mass with heterogeneous enhancement and dark internal septations on dynamic contrast-enhanced images. The MRI showed a rapid rate of enhancement in the initial period and washout in the delayed period of enhancement (Figure 1C, D). A kinetic curve of the lesion showed rapid initial enhancement and a washout pattern. The MRI showed no evidence of metastasis to the internal mammary, supraclavicular, and axillary lymph nodes, or to the mediastinum. We classified the mass as Breast Imaging Reporting and Data System category 4C. An US-guided core biopsy was performed and the pathologic results were consistent with an IMT with atypical features. Surgical excision of the tumor was then performed. Gross examination of the surgical specimen revealed an approximately 3×2 cm mass that was firm and yellow in color. Microscopy showed a well-circumscribed tumor with high cellularity. The tumor predominantly consisted of spindle cells with a swirling storiform-like pattern as well as inflammatory cells including plasma cells and lymphocytes (Figure 2A). Although the mass showed high mitotic activity (10 mitoses per 10 high-powered fields [HPFs]), the mitotic figures and the nuclear features were not significantly atypical (Figure 2B). Furthermore, the mass showed an absence of cells with epithelioid morphologies suggestive of carcinoma in situ or invasive carcinoma. Immunohistochemically, the spindle tumor cells were focally positive for smooth muscle actin (SMA) with a tram-track staining pattern (Figure 2C) and were focally positive for desmin, but were negative for cytokeratin (CK), low molecular weight CK, high molecular weight CK, p63, and anaplastic lymphoma kinase (ALK). Approximately 30% of the spindle-shaped tumor cells were positive for Ki-67 (Figure 2D). The resection margins were clear of tumor cells. Collectively, the histopathological and immunohistochemical findings supported the diagnosis of IMT, which resulted in the exclusion of the possibility of spindle cell metaplastic carcinoma. A diagnosis of IMT was made, and the patient was advised to have regular follow-up assessments given the rare cases of local recurrence that had occurred with a time to recurrence that varied between 3 months and 9 years [4,5]. Our patient had regular postoperative follow-up every 3-6 months for 1 year with US. However, the patient was lost to follow-up after 1 year.
Figure 1. Imaging findings of inflammatory myofibroblastic tumor at the time of initial diagnosis. (A) On mammography, an oval-shaped, microlobulated, hyperdense mass with some coarse heterogeneous microcalcifications was observed. (B) A color Doppler study showed an irregularly shaped, microlobulated, hypoechoic mass with combined posterior features and increased vascular flow in the peripheral portion of the lesion. Subtraction imaging on T1-weighted contrast-enhanced breast magnetic resonance imaging (MRI) showed an irregular mass with heterogeneous enhancement, with the periphery enhancing more than the center. In addition, MRI showed a fast rate of enhancement in the initial period (C) and a washout in the delayed period of enhancement (D).
Figure 2. Histologic, immunohistochemical analyses of inflammatory myofibroblastic tumor (IMT) at initial diagnosis. (A) A sample of the IMT obtained on excisional biopsy shows mainly composed of spindle cells forming swirling storiform-like patterns, as well as inflammatory cells including plasma cells and lymphocytes (H&E stain, ×100). (B) The mass showed high mitotic activity and mild cellular pleomorphism (H&E stain, ×400). (C) Immunohistochemical analysis shows positive staining for smooth muscle actin (SMA) in the spindle tumor cells (SMA, ×400) and (D) positive staining for Ki-67 in about 30% of the spindle tumor cells (Ki-67, ×400).
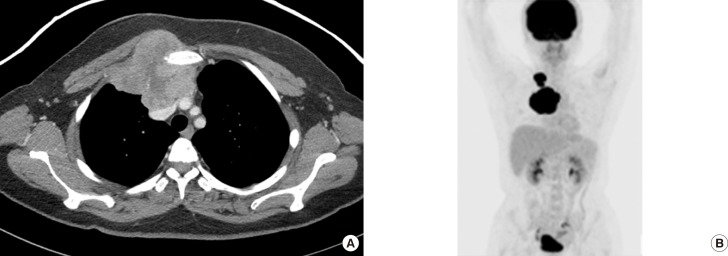
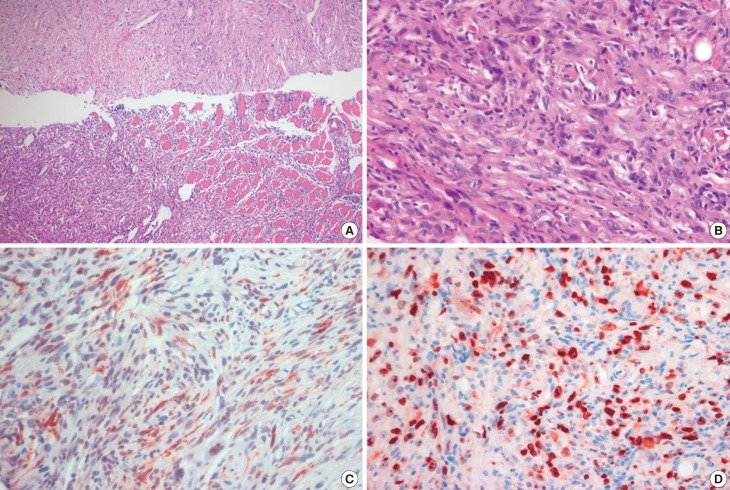
One year later, the patient was admitted to our breast center with newly developing masses in the upper inner quadrant of the right breast and right cervical area. A physical examination revealed a solitary hard, nontender, nonmobile mass that measured 5×4 cm in the upper inner quadrant of the right breast and a 3×1 cm mass in the right cervical region. Ultrasonography of the breast showed an approximately 7 cm, irregularly shaped, hypoechoic mass with a circumscribed margin in the right internal mammary chain and an enlarged, hypoechoic lymph node (approximately 3×1.7 cm) with eccentric cortical thickening in the right supraclavicular area. Chest computed tomography (CT) showed a 7.9×7.9 cm irregularly shaped, heterogeneously enhanced metastasis to the internal mammary chain and two supraclavicular lymph nodes measuring 3.3×1.6 cm and 1.5×1.5 cm on postcontrast enhanced images. The metastasis to the internal the mammary chain was noted to have anterior mediastinum, right pectoralis major muscle, and left innominate vein involvement that was confined to the superior vena cava. Furthermore, bone destruction in the sternum and right first and second ribs was noted on chest CT (Figure 3A). Combined 18F-fluorodeoxy-glucose positron emission tomography (PET) and CT showed metastasis to the internal mammary chain, supraclavicular lymph nodes, and mediastinal lymph nodes (Figure 3B). An US-guided core biopsy was performed, which showed that the tumor was mainly composed of proliferating spindle cells arranged in a loose fashion and surrounded by infiltrating inflammatory cells (Figure 4A). There was increased cellularity and spindle cell atypia compared to the findings from the first excisional biopsy. The tumor cells showed high mitotic activity (10 mitoses per 10 HPFs) (Figure 4B). In addition, partial adjacent muscular invasion was noted. Immunohistological studies revealed that the tumor exhibited positive staining for desmin, SMA (Figure 4C), and CK, but was negative for S-100, ALK, and p63. Positive Ki-67 staining was observed in 40% of the cells (Figure 4D). These clinical and pathological findings confirmed the diagnosis of metastatic IMT with malignant transformation.
Figure 3. Imaging findings of inflammatory myofibroblastic tumor at metastasis after complete surgical resection. (A) Two years after complete surgical resection, chest computed tomography (CT) showed an irregularly shaped, heterogeneous, enhancing metastasis to the internal mammary chain on post-contrast-enhanced images. (B) Positron emission tomography/CT showed increased 18F-fluorodeoxyglucose uptake in metastases to the internal mammary chain and two supraclavicular lymph nodes.
Figure 4. Histologic, immunohistochemical analyses of inflammatory myofibroblastic tumor (IMT) at metastasis diagnosis after complete surgical resection. (A) A sample of the IMT obtained on core needle biopsy shows that the tumor was mainly composed of a conspicuous proliferation of spindle cells arranged in a loose fashion and surrounded by infiltrating inflammatory cells (H&E stain, ×100). (B) Tumor showed increased cellularity and cellular atypia of spindle cells compared to first excisional biopsy. Tumor cells showed high mitotic activity (10 mitoses per 10 high-power field) (H&E stain, ×400). (C) Immunohistochemical analysis shows positive staining for smooth muscle actin (SMA) in the spindle tumor cells (SMA, ×400) and (D) positive staining for Ki-67 in about 40% of the spindle tumor cells (Ki-67, ×400).
DISCUSSION
IMTs of the breast are extremely rare; a literature search yielded only 20 cases in the English language [4,6,7,8]. The etiology of IMT remains unclear, though it has been proposed to result from an aberrant reactive or inflammatory response to local cytokines [3,7]. Recent studies have revealed cytogenetic clonal abnormalities and ALK expression in IMTs [2]. Currently IMTs are regarded as intermediate, locally recurring, and rarely metastasizing neoplasms. Previously published cases of metastatic IMTs have involved patients with a broad range of ages and primary sites, and metastases typically developed in the lungs, brain, liver, and bone. Prior to our case, there was only one reported case of metastatic IMT originating from the breast and involved metastasis to the left groin area 10 months after complete surgical resection [4]. Furthermore, metastasis to the lymph nodes or the mediastinum accounted for only two cases [9,10]. However, in these cases, the primary tumors originated in the mesentery and ileum rather than in the breast [9,10].
Pathologically, the mass in our case was initially diagnosed as an IMT with features of mild cellular pleomorphism, high mitotic activity, and positive Ki-67 nuclear staining in 30% of the cells. Although mitotic activity in IMTs has generally been reported to be low (0-2 mitoses per 10 HPFs), in our case the IMT exhibited high mitotic activity (10 mitoses per 10 HPFs) [2,3]. Both rapid tumor growth and a high Ki-67 labeling index have been associated with aggressive lesions. The tumor also exhibited substantial nuclear immunostaining of p53. Occasionally, IMT cases have involved disruption of an autoregulatory feedback loop involving p53, which may also be associated with varying degrees of biological aggressiveness [11]. A higher degree of nuclear pleomorphism, atypia, and atypical mitoses was observed for ALK-negative IMTs. Additionally, the absence of ALK expression was associated with subtle histological differences and biological aggressiveness including distant metastasis [2,12]. The potential for aggressive growth, recurrence, and malignant transformation is often correlated with a high degree of atypia, the presence of ganglion-like cells, increased mitotic figures, an elevated Ki-67 proliferative index, the absence of ALK reactivity, and oncogenic protein overexpression, such as overexpression of p53 [13]. In our case, the tumor exhibited high mitotic activity, pleomorphism, a high Ki-67 labeling index, p53 overexpression, absence of ALK reactivity, and the presence of ganglion-like cells. The patient suffered a relapse 2 years after complete surgical resection of the tumor, with aggressive metastatic tumor growth.
Radiographically, our case exhibited irregular margins on mammographic, sonographic, and MRI studies. The mammographic and sonographic features of IMTs of the breast are nonspecific [1,4,6,7,13]. Ultrasonography demonstrated a variable pattern of echogenicity, and the lesions were described as hypo- or hyperechoic with ill-defined or well-defined margins. In most previously reported cases, a well-defined border was observed on mammography [7]. There was one reported case in which MRI demonstrated strong contrast, medium early rim enhancement, and a central region of necrosis [13]. In our case, the MRI showed a lesion that was highly suspicious for malignancy in terms of kinetic curve and morphologic analysis. Although the mammographic and sonographic features of IMT are nonspecific, it should be emphasized that the MRI findings in both our case and in the one previously reported case of IMT of the breast with metastasis were suspicious for malignancy. In particular, a rapid initial enhancement and washout pattern on kinetic curve analysis was correlated with a low ratio of peripheral to central fibrosis and a high ratio of peripheral to central microvessel density. Dynamic contrast enhancement and kinetic curve analysis are functional derivatives of microvessel density and vascular endothelial growth factor-driven increases in perme-ability, both of which are increased in cases of malignancy [14]. In our case, color Doppler imaging also showed increased vascular flow in the peripheral portion of the lesion, further raising the suspicion of malignancy.
In conclusion, although metastasis of IMTs of the breast is extremely rare, the possibility of metastasis should be considered if the mass is initially diagnosed as IMT with atypical histopathologic features and if there are imaging findings that are suspicious for malignancy. The possibility of metastasis plays a critical role in the choice of a follow-up method and interval after complete surgical resection. This highlights the need for supplementary image modalities such as breast MRI or PET/CT in addition to mammography or US during the follow-up period for aggressive forms of IMT.
Footnotes
CONFLICT OF INTEREST: The authors declare that thy have no competing interests.
References
- 1.Haj M, Weiss M, Loberant N, Cohen I. Inflammatory pseudotumor of the breast: case report and literature review. Breast J. 2003;9:423–425. doi: 10.1046/j.1524-4741.2003.09516.x. [DOI] [PubMed] [Google Scholar]
- 2.Coffin CM, Hornick JL, Fletcher CD. Inflammatory myofibroblastic tumor: comparison of clinicopathologic, histologic, and immunohistochemical features including ALK expression in atypical and aggressive cases. Am J Surg Pathol. 2007;31:509–520. doi: 10.1097/01.pas.0000213393.57322.c7. [DOI] [PubMed] [Google Scholar]
- 3.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor): a clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859–872. doi: 10.1097/00000478-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Zhao HD, Wu T, Wang JQ, Zhang WD, He XL, Bao GQ, et al. Primary inflammatory myofibroblastic tumor of the breast with rapid recurrence and metastasis: a case report. Oncol Lett. 2013;5:97–100. doi: 10.3892/ol.2012.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vecchio GM, Amico P, Grasso G, Vasquez E, La Greca G, Magro G. Post-traumatic inflammatory pseudotumor of the breast with atypical morphological features: a potential diagnostic pitfall. Report of a case and a critical review of the literature. Pathol Res Pract. 2011;207:322–326. doi: 10.1016/j.prp.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 6.Kim SJ, Moon WK, Kim JH, Cho N, Chang CM. Inflammatory pseudotumor of the breast: a case report with imaging findings. Korean J Radiol. 2009;10:515–518. doi: 10.3348/kjr.2009.10.5.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilvan S, Celik V, Paksoy M, Cetinaslan I, Calay Z. Inflammatory myofibroblastic tumor (inflammatory pseudotumor) of the breast. APMIS. 2005;113:66–69. doi: 10.1111/j.1600-0463.2005.apm1130110.x. [DOI] [PubMed] [Google Scholar]
- 8.Sastre-Garau X, Couturier J, Derré J, Aurias A, Klijanienko J, Lagacé R. Inflammatory myofibroblastic tumour (inflammatory pseudotumour) of the breast: clinicopathological and genetic analysis of a case with evidence for clonality. J Pathol. 2002;196:97–102. doi: 10.1002/path.1004. [DOI] [PubMed] [Google Scholar]
- 9.Trojan A, Stallmach T, Kollias S, Pestalozzi BC. Inflammatory myofibroblastic tumor with CNS involvement. Onkologie. 2001;24:368–372. doi: 10.1159/000055109. [DOI] [PubMed] [Google Scholar]
- 10.Myint MA, Medeiros LJ, Sulaiman RA, Aswad BI, Glantz L. Inflammatory pseudotumor of the ileum: a report of a multifocal, transmural lesion with regional lymph node involvement. Arch Pathol Lab Med. 1994;118:1138–1142. [PubMed] [Google Scholar]
- 11.Hussong JW, Brown M, Perkins SL, Dehner LP, Coffin CM. Comparison of DNA ploidy, histologic, and immunohistochemical findings with clinical outcome in inflammatory myofibroblastic tumors. Mod Pathol. 1999;12:279–286. [PubMed] [Google Scholar]
- 12.Mergan F, Jaubert F, Sauvat F, Hartmann O, Lortat-Jacob S, Révillon Y, et al. Inflammatory myofibroblastic tumor in children: clinical review with anaplastic lymphoma kinase, Epstein-Barr virus, and human herpesvirus 8 detection analysis. J Pediatr Surg. 2005;40:1581–1586. doi: 10.1016/j.jpedsurg.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 13.Bosse K, Ott C, Biegner T, Fend F, Siegmann-Luz K, Wallwiener D, et al. 23-Year-old female with an inflammatory myofibroblastic tumour of the breast: a case report and a review of the literature. Geburtshilfe Frauenheilkd. 2014;74:167–170. doi: 10.1055/s-0033-1360185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tse GM, Chaiwun B, Wong KT, Yeung DK, Pang AL, Tang AP, et al. Magnetic resonance imaging of breast lesions: a pathologic correlation. Breast Cancer Res Treat. 2007;103:1–10. doi: 10.1007/s10549-006-9352-3. [DOI] [PubMed] [Google Scholar]