Abstract
Protein phosphorylation regulates virtually all biological processes. Although protein kinases are popular drug targets, targeting protein phosphatases remains a challenge. Here, we describe Sephin1 (selective inhibitor of a holophosphatase), a small molecule that safely and selectively inhibited a regulatory subunit of protein phosphatase 1 in vivo. Sephin1 selectively bound and inhibited the stress-induced PPP1R15A, but not the related and constitutive PPP1R15B, to prolong the benefit of an adaptive phospho-signaling pathway, protecting cells from otherwise lethal protein misfolding stress. In vivo, Sephin1 safely prevented the motor, morphological, and molecular defects of two otherwise unrelated protein-misfolding diseases in mice, Charcot-Marie-Tooth 1B, and amyotrophic lateral sclerosis. Thus, regulatory subunits of phosphatases are drug targets, a property exploited here to safely prevent two protein misfolding diseases.
A first line of defense against the accumulation of misfolded proteins in the endoplasmic reticulum (ER) consists of phosphorylating the α subunit of eukaryotic translation initiation factor 2 (eIF2α) on Ser51 to decrease protein synthesis, an adaptive stress response essential for survival (1-3). Guanabenz (GBZ) can prolong this adaptive response by selectively binding and inhibiting the regulatory subunit of the stress-induced eIF2α phosphatase composed of PPP1R15A and PP1c (4). GBZ spares the constitutive eIF2α phosphatase PPP1R15B-PP1c, avoiding persistent eIF2α phosphorylation, which would be lethal (5). As a result, GBZ increases the availability of chaperones to misfolded proteins and consequently rescues cells from proteostasis collapse (4). Correcting proteostasis defects could, in theory, benefit a broad range of diseases characterized by the accumulation of misfolded proteins (6). However, identifying a therapeutically valuable approach for progressive diseases represents a double challenge: achieving efficacy without adverse effects. In vivo, GBZ cannot be used to selectively inhibit PPP1R15A because it is a centrally active hypotensive drug with nanomolar affinity for the α2-adrenergic receptor (7).
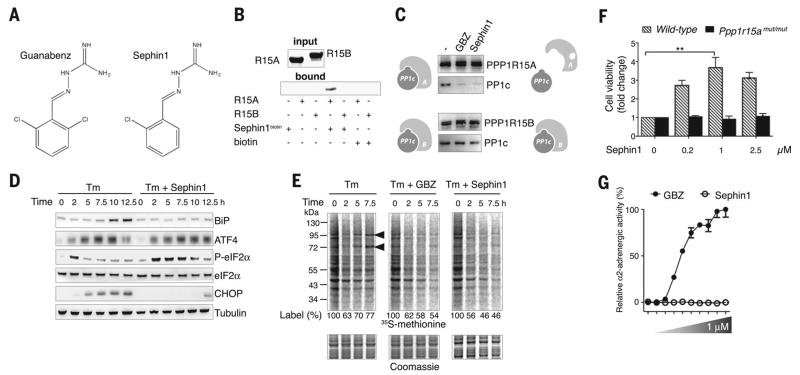
Searching for PPP1R15A inhibitors devoid of α2-adrenergic activity, we synthesized GBZ derivatives and identified Sephin1 (selective inhibitor of a holophosphatase) (Fig. 1A). Like GBZ (4), Sephin1 specifically bound a recombinant fragment of PPP1R15A (amino acids 325 to 636) but not the highly related PPP1R15B (amino acids 340 to 698) (Fig. 1B). In cells, Sephin1 selectively disrupted the PPP1R15A-PP1c complex but spared the related PPP1R15B-PP1c complex (Fig. 1C). As a result, Sephin1 prolonged eIF2α phosphorylation after stress (Fig. 1D), delaying translation recovery (Fig. 1E). Consequently, Sephin1 attenuated expression of stress genes such as CHOP, a pro-apoptotic protein (Fig. 1D and fig. S1), because this requires translation recovery (8, 9). Activating transcription factor 4 (ATF4) is selectively translated when eIF2α is phosphorylated (10), and this was prolonged in Sephin1- treated cells (Fig. 1D). In the absence of stress, Sephin1 did not affect eIF2α signaling (fig. S2), which was expected because PPP1R15A is only expressed upon stress (9). Confirming the selectivity of Sephin1 for PPP1R15A, Sephin1 did not inhibit the catalytic subunit PP1c (fig. S3). Selective inhibition of PPP1R15A by Sephin1 protected cells from cytotoxic ER stress (Fig. 1F and fig. S4), but this was abolished in cells lacking a functional allele of Ppp1r15a (Fig. 1F). Thus, all the cytoprotective activity of Sephin1 in ER - stressed cells resulted from selective inhibition of PPP1R15A. Sephin1 lacked any measurable α2-adrenergic agonist activity in a cell-based assay, in contrast to GBZ (Fig. 1G). Thus, Sephin1 is a selective PPP1R15A inhibitor.
Fig. 1. Sephin1 is a selective inhibitor of PPP1R15A.
(A) Structures of GBZ and Sephin1. (B) Coomassie-stained gel showing recombinant PPP1R15A325-636 and PPP1R15B340-698 (input). Biotinylated Sephin1 selectively captured PPP1R15A on neutravidin beads (bound). (C) Immunoprecipitations of PPP1R15 complexes from cells treated with vehicle, 50 μM GBZ, or Sephin1 for 6 hours analyzed with immunoblotting. (D) Immunoblots of the indicated proteins in lysates of HeLa cells treated with 2.5 μg/ml tunicamycin (Tm) in the presence or absence of 50 μM Sephin1 for the indicated time. (E) Newly synthesized proteins labeled with 35S-methionine in HeLa cells treated with Tm or with or without 50 μM GBZ or Sephin1 and revealed with autoradiography. (Bottom) Coomassie-stained gel. (F) Dose-dependent protection by Sephin1 of wild-type but not Ppp1r15a mutant (mut/mut) cells from Tm (2.5 μg/ml). Data are means ± SEM (n = 4 replicates). **P ≤ 0.001. (G) Adrenergic activity of GBZ and Sephin1 in cells expressing recombinant human adrenergic α2A receptor. Representative results of at least three independent experiments are shown in each panel.
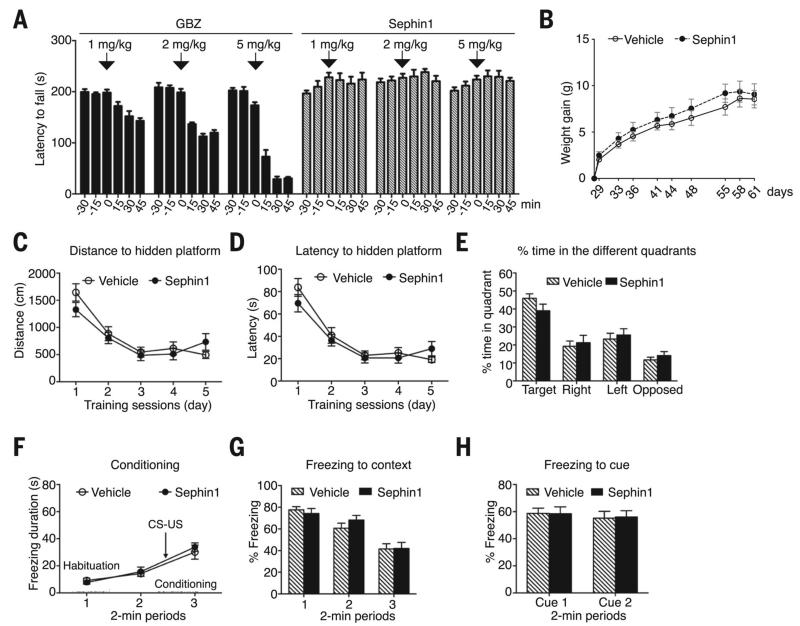
Pharmacokinetic analysis of Sephin1, administered orally at 1 or 10 mg/kg, revealed that the molecule rapidly disappeared from plasma (fig. S5) but concentrated in the nervous system, reaching concentrations 7 to 44 times higher in the brain and sciatic nerve (up to ~ 1 μM) than in the plasma (fig. S5, A and B), like GBZ does (11). In humans, the adrenergic agonist activity of GBZ has side effects, including drowsiness and coma, at high doses (12). In mice, GBZ (1 to 5 mg/kg) also exhibited side effects manifested by a rapid and dose-dependent decrease in rotarod performances (Fig. 2A). In contrast, Sephin1- treated mice (1 to 5 mg/kg) continued to run as before treatment (Fig. 2A). A chronic treatment (1 mg/kg for 1 month) with Sephin1 was also tolerable with no measurable adverse effects on body weight gain in mice (Fig. 2B). We next evaluated whether Sephin1 had adverse effects on memory because manipulations of PP1c (13) and eIF2α phosphorylation (14) affect memory. In the Morris water maze (15), Sephin1- treated mice showed normal spatial learning and improved their ability to locate a submerged platform as training progressed (Fig. 2, C and D) and remembered where the platform was, after it had been removed (Fig. 2E). In a fear conditioning paradigm, both Sephin1- and vehicle- treated mice showed similar basal fear responses during the conditioning phase (Fig. 2F) and after (Fig. 2, G and H). Thus, Sephin1 lacks the adverse effects of GBZ in vivo and has no measurable adverse effect on general health or memory in diverse experimental paradigms.
Fig. 2. Sephin1 is devoid of adverse effects on rotarod performances, total body weight gain, or memory.
(A) Motor performance of mice on an accelerating rotarod before or after the indicated treatments. Data are means ± SEM. (n = 5 mice). (B) Total body weight gain of mice treated orally with Sephin1 (1 mg/kg) or vehicle twice a day, from postnatal days 28 to 61. Data are means ± SEM (n = 6 mice). (C and D) Distance and latency to locate a hidden platform in the Morris water maze, in five trials a day for 5 consecutive days. (E) Quadrant occupancy after training and removal of the platform. (F) Freezing response during the conditioning session, where a light/tone [conditioned stimulus (CS)] and foot shock [aversive unconditioned stimulus (US)] were applied. (G and H) Freezing responses expressed as percentage of total time (2 min) mice spent immobile during context and auditory cue testing. In (C) to (H), data are means ± SEM (n = 12 mice). Mice were treated with 1 mg/kg Sephin1 or vehicle twice a day for 4 weeks. No statistical differences were found between Sephin1- or vehicle-treated mice [(B) to (H)].
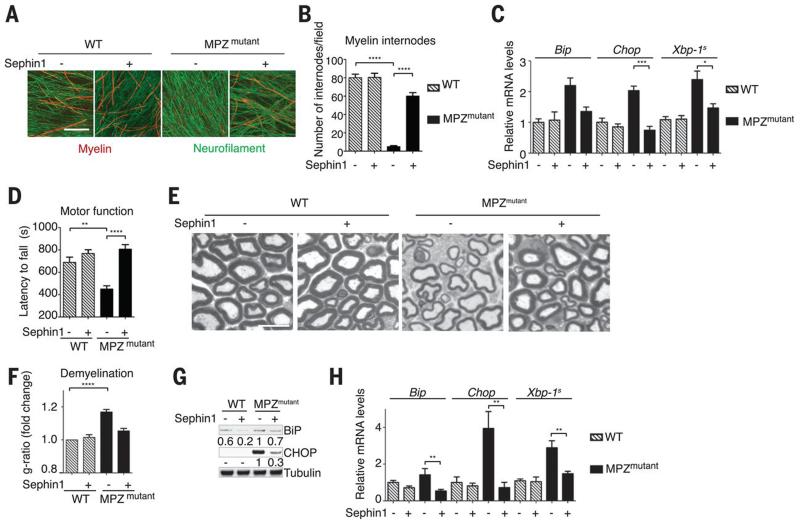
We next examined whether Sephin1 could correct a protein misfolding disease. Deletion of serine 63 of myelin protein zero (MPZmutant), a transmembrane protein produced by Schwann cells in the peripheral nervous system, causes the demyelinating neuropathy Charcot-Marie-Tooth 1B (CMT1B) in humans and a similar disorder in mice. MPZmutant causes CMT1B by a gain of toxic property associated with pathological signaling through CHOP and PPP1R15A (16, 17). As previously reported (16, 17), myelination defects were severe in dorsal root ganglia (DRG) cultures prepared from MPZmutant mouse embryos (Fig. 3, A and B). A 2-week treatment with 100 nM of Sephin1 rescued myelination (Fig. 3, A and B) and decreased the expression of ER-stress genes in mutant DRG cultures (Fig. 3C). Because of the potency of Sephin1 ex vivo (Fig. 3, A to C) and its pharmacokinetic properties (fig. S5), we next treated MPZmutant mice orally twice a day with 1 mg/kg of Sephin1. As reported (18), MPZmutant mice exhibited motor defects detectable with rotarod analysis at 4 months of age (Fig. 3D), but this was completely prevented by Sephin1 (Fig. 3D). Sephin1 also rescued myelin thickness around axons in sciatic nerves (Fig. 3, E and F) and reduced the levels of ER-stress markers in MPZmutant sciatic nerves (Fig. 3, G and H). Thus, without any obvious adverse effect, Sephin1 prevented the molecular, morphological, and motor defects of the MPZmutant mice.
Fig. 3. Sephin1 prevents the defects caused by misfolding-prone myelin MPZmutant protein ex vivo and in vivo.
(A and B) Representative images (A) and quantification (B) of myelin internodes revealed with immunostaining for myelin basic protein (MBP, red) from cultured DRG of the indicated genotype treated for 2 weeks with vehicle or Sephin1 (100 nM). In green, neurofilament (NF). Data are means ± SEM (n = 4 to 6 mice). (C) mRNA levels [by means of quantitative polymerase chain reaction (PCR)] of the indicated ER stress markers in cultures as (A). Data are means ± SEM (n = 5 to 7 mice). (D) Motor performance of 4-month-old wild-type or MPZmutant mice on a rotarod after a 3-month oral treatment with Sephin1 (1 mg/kg) or vehicle twice a day. Data are means ± SEM (n = 14 to 16 mice). (E) Myelin thickness revealed on toluidine blue–stained semithin sciatic nerve sections of 6-month-old mice of the indicated genotype after 5 months of oral Sephin1 treatment (1 mg/kg) or vehicle twice a day. Data are means ± SEM (n = 6 to 8 mice). (F) Demyelination expressed as increased g-ratio (ratio of axon diameter to fiber diameter) over wild-type mice. Data are means ± SEM (n = 5 or 6 mice). (G) Immunoblots on sciatic nerve lysates from mice. (H) mRNA levels (quantitative PCR) in sciatic nerves of 6-month-old mice as in (E). Data are means ± SEM (n = 5 to 7 mice). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. Scale bars, 100 μm.
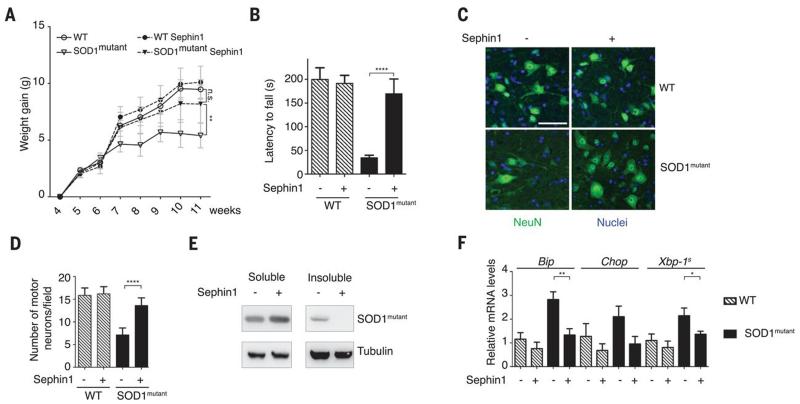
Selective inhibition of PPP1R15A could in principle ameliorate a broad range of protein misfolding diseases. Mutant and misfolding-prone superoxide dismutase 1 (SOD1) is associated with familial forms of amyotrophic lateral sclerosis (fALS), a motor neuron disease (19). SOD1 mutants bind to Derlin-1 on the cytosolic side of the ER membrane, blocking degradation of ER proteins and causing ER stress (20) with pathological PPP1R15A signaling (21). Transgenic mice expressing the human ALS-causing mutant SOD1G93A (SOD1mutant mice) develop a motor neuron disease that closely resembles ALS (22). The motor deficits in SOD1mutant mice were partially prevented by treatment with 1 mg/kg of Sephin1 twice a day (fig. S6). With 5 mg/kg of Sephin1 once a day, the progressive weight loss of SOD1mutant mice (Fig. 4A) as well as their motor deficits (Fig. 4B) were almost completely prevented, without adverse effects on weight gain or motor performance of wild-type mice (Fig. 4, A and 4B). The motor deficits of SOD1mutant mice were associated with motor neuron loss, which was prevented by Sephin1 (Fig. 4, C and 4D). Genetic ablation or pharmacological inhibition of PPP1R15A decreases translation rates and increases the availability of chaperones (4, 23). To assess whether Sephin1 could improve proteostasis in the cytosol, we monitored aggregation of SOD1mutant, a defining histopathological feature in ALS mouse models (22, 24). Sephin1 prevented the accumulation of insoluble SOD1mutant (Fig. 4E) and decreased ER stress markers in transgenic spinal cords (Fig. 4F). Thus, Sephin1 prevented the molecular and organismal defects of SOD1mutant mice.
Fig. 4. Sephin1 prevents motor deficits, motor neuron loss, and the molecular defects in SOD1mutant mice.
(A) Total body weight gain of wild-type or SOD1mutant mice treated orally with Sephin1 (5 mg/kg) or vehicle once a day from 4 to 11 weeks of age. Data are means ± SEM (n = 4 to 6 mice). (B) Rotarod analysis of 110-day-old wild-type or SOD1mutant mice treated as in (A). Data are means ± SEM (n = 4 to 6 mice). (C and D) Representative motor neuron staining (NeuN, green) and quantification (D) of sections of anterior horn of the lumbar region of spinal cord of 110-day-old mice treated as in (A). Nuclei, H33258 (blue). (D) Data are means ± SEM (n = 6 to 8 mice). (E) SOD1 immunoblots on soluble and insoluble fractions of spinal cord extracts from SOD1mutant mice treated as in (A). (F) mRNA levels (quantitative PCR) in lumbar spinal cord from 4-month-old mice of indicated genotype, after treatment with Sephin1 or vehicle as in (A). Data are means ± SEM (n = 4 to 6 mice). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001. Scale bar, 50 μm.
Here, we have shown that Sephin1 selectively inhibited PPP1R15A to prevent two otherwise unrelated protein misfolding diseases in mice. This was achieved while sparing PPP1R15B, a crucial property because the lack of both PPP1R15A and PPP1R15B is lethal in mice (25). Thus, PPP1R15A inhibitors could ameliorate a broad range of diseases caused by accumulation of misfolded proteins. Because many signaling pathways operate on the same dynamic—phosphorylation in the activation phase terminated by dephosphorylation— delaying the termination phase of signaling pathways through the selective inhibition of phosphatases may be of broad relevance to safely and selectively manipulate cellular functions for therapeutic benefit.
Supplementary Material
Acknowledgments
We thank P. Tsaytler for some initial experiments on Sephin1; H. Meziane for the studies on memory; E. Fisher for SOD1G93A mice; E. Pettinato and C. Ferri for technical assistance; R. Roberts for discussions on CMT; A. Segonds-Pichon for statistical analysis; and members of the Bertolotti laboratory, M. Goedert, M. Hastings and S. Munro for discussions. A.B. is an honorary fellow of the Clinical Neurosciences Department of Cambridge University. This work was supported by the Medical Research Council (UK) and the European Research Council (ERC) under the European Union’s Seventh Framework Programme (FP7/2007-2013) / ERC grant 309516. A. K. was supported by the European Molecular Biology Organization and Human Frontier Science Program, K. S. by the Swiss National Science Foundation, M. D. by the Italian Ministry of Health (GR-2011-02642791), and L. W. by NIH R01-NS55256. A.B. is a co-inventor on Great Britain patent WO 2014108520, covering benzylideneguanidine derivatives inhibitors of PPP1R15A. The data presented in this paper are tabulated in the main paper and the supplementary materials.
Footnotes
References and Notes
- 1.Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol. Cell. 2000;5:897–904. doi: 10.1016/s1097-2765(00)80330-5. [DOI] [PubMed] [Google Scholar]
- 2.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol. Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 3.Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/s1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 4.Tsaytler P, Harding HP, Ron D, Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332:91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 5.Scheuner D, Patel R, Wang F, Lee K, Kumar K, Wu J, Nilsson A, Karin M, Kaufman RJ. Double-stranded RNA-dependent protein kinase phosphorylation of the alpha-subunit of eukaryotic translation initiation factor 2 mediates apoptosis. J. Biol. Chem. 2006;281:21458–21468. doi: 10.1074/jbc.M603784200. [DOI] [PubMed] [Google Scholar]
- 6.Balch WE, Morimoto RI, Dillin A, Kelly JW. Adapting proteostasis for disease intervention. Science. 2008;319:916–919. doi: 10.1126/science.1141448. [DOI] [PubMed] [Google Scholar]
- 7.Holmes B, Brogden RN, Heel RC, Speight TM, Avery GS. Guanabenz. A review of its pharmacodynamic properties and therapeutic efficacy in hypertension. Drugs. 1983;26:212–229. doi: 10.2165/00003495-198326030-00003. [DOI] [PubMed] [Google Scholar]
- 8.Brostrom MA, Lin XJ, Cade C, Gmitter D, Brostrom CO. Loss of a calcium requirement for protein synthesis in pituitary cells following thermal or chemical stress. J. Biol. Chem. 1989;264:1638–1643. [PubMed] [Google Scholar]
- 9.Novoa I, Zhang Y, Zeng H, Jungreis R, Harding HP, Ron D. Stress-induced gene expression requires programmed recovery from translational repression. EMBO J. 2003;22:1180–1187. doi: 10.1093/emboj/cdg112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol. Cell. 2000;6:1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 11.Meacham RH, Ruelius HW, Kick CJ, Peters JR, Kocmund SM, Sisenwine SF, Wendt RL. Relationship of guanabenz concentrations in brain and plasma to antihypertensive effect in the spontaneously hypertensive rat. J. Pharmacol. Exp. Ther. 1980;214:594–598. [PubMed] [Google Scholar]
- 12.Hall AH, Smolinske SC, Kulig KW, Rumack BH. Guanabenz overdose. Ann. Intern. Med. 1985;102:787–788. doi: 10.7326/0003-4819-102-6-787. [DOI] [PubMed] [Google Scholar]
- 13.Genoux D, Haditsch U, Knobloch M, Michalon A, Storm D, Mansuy IM. Protein phosphatase 1 is a molecular constraint on learning and memory. Nature. 2002;418:970–975. doi: 10.1038/nature00928. [DOI] [PubMed] [Google Scholar]
- 14.Costa-Mattioli M, Gobert D, Stern E, Gamache K, Colina R, Cuello C, Sossin W, Kaufman R, Pelletier J, Rosenblum K, Krnjević K, Lacaille JC, Nader K, Sonenberg N. eIF2α phosphorylation bidirectionally regulates the switch from short- to long-term synaptic plasticity and memory. Cell. 2007;129:195–206. doi: 10.1016/j.cell.2007.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morris RGM, Garrud P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 16.Pennuto M, Tinelli E, Malaguti M, Del Carro U, D’Antonio M, Ron D, Quattrini A, Feltri ML, Wrabetz L. Ablation of the UPR-mediator CHOP restores motor function and reduces demyelination in Charcot-Marie-Tooth 1B mice. Neuron. 2008;57:393–405. doi: 10.1016/j.neuron.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Antonio M, Musner N, Scapin C, Ungaro D, Del Carro U, Ron D, Feltri ML, Wrabetz L. Resetting translational homeostasis restores myelination in Charcot-Marie-Tooth disease type 1B mice. J. Exp. Med. 2013;210:821–838. doi: 10.1084/jem.20122005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wrabetz L, D’Antonio M, Pennuto M, Dati G, Tinelli E, Fratta P, Previtali S, Imperiale D, Zielasek J, Toyka K, Avila RL, Kirschner DA, Messing A, Feltri ML, Quattrini A. Different intracellular pathomechanisms produce diverse Myelin Protein Zero neuropathies in transgenic mice. J. Neurosci. 2006;26:2358–2368. doi: 10.1523/JNEUROSCI.3819-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordlund A, Oliveberg M. SOD1-associated ALS: A promising system for elucidating the origin of protein-misfolding disease. HFSP J. 2008;2:354–364. doi: 10.2976/1.2995726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishitoh H, Kadowaki H, Nagai A, Maruyama T, Yokota T, Fukutomi H, Noguchi T, Matsuzawa A, Takeda K, Ichijo H. ALS-linked mutant SOD1 induces ER stress- and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 2008;22:1451–1464. doi: 10.1101/gad.1640108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Popko B, Roos RP. An enhanced integrated stress response ameliorates mutant SOD1-induced ALS. Hum. Mol. Genet. 2014;23:2629–2638. doi: 10.1093/hmg/ddt658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gurney ME, Pu H, Chiu A, Dal Canto M, Polchow C, Alexander D, Caliendo J, Hentati A, Kwon Y, Deng H. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. et. [DOI] [PubMed] [Google Scholar]
- 23.Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev. 2004;18:3066–3077. doi: 10.1101/gad.1250704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Farr GW, Zeiss CJ, Rodriguez-Gil DJ, Wilson JH, Furtak K, Rutkowski DT, Kaufman RJ, Ruse CI, Yates JR, 3rd, Perrin S, Feany MB, Horwich AL. Progressive aggregation despite chaperone associations of a mutant SOD1-YFP in transgenic mice that develop ALS. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1392–1397. doi: 10.1073/pnas.0813045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harding HP, Zhang Y, Scheuner D, Chen JJ, Kaufman RJ, Ron D. Ppp1r15 gene knockout reveals an essential role for translation initiation factor 2 α (eIF2α) dephosphorylation in mammalian development. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1832–1837. doi: 10.1073/pnas.0809632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang J, Farr GW, Hall DH, Li F, Furtak K, Dreier L, Horwich AL. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLOS Genet. 2009;5:e1000350. doi: 10.1371/journal.pgen.1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heiman-Patterson TD, Deitch JS, Blankenhorn EP, Erwin KL, Perreault MJ, Alexander BK, Byers N, Toman I, Alexander GM. Background and gender effects on survival in the TgN(SOD1-G93A)1Gur mouse model of ALS. J. Neurol. Sci. 2005;236:1–7. doi: 10.1016/j.jns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Schaeffer V, Lavenir I, Ozcelik S, Tolnay M, Winkler DT, Goedert M. Stimulation of autophagy reduces neurodegeneration in a mouse model of human tauopathy. Brain. 2012;135:2169–2177. doi: 10.1093/brain/aws143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. Fiji: An open-source platform for biological-image analysis. Nat. Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.