Abstract
Context:
HIV infection is associated with a greater risk for fasting hyperinsulinemia, impaired glucose tolerance, and higher incidence rates for vascular disease, myocardial infarction, or stroke despite effective combination antiretroviral therapy (cART). The underlying mechanism(s) may involve chronic low-grade systemic inflammation and immune cell activation. Dipeptidyl peptidase-4 inhibitors (sitagliptin) improve glucose tolerance and may possess immunomodulatory effects because leukocyte CD26 cell surface receptors express dipeptidyl peptidase-4 activity.
Objective:
Sitagliptin will reduce inflammatory and immune cell activation markers known to be elevated in cART-treated HIV-infected (HIV+) adults with impaired glucose tolerance.
Design:
This was designed as a prospective, randomized, placebo-controlled, double-blind trial of sitagliptin in HIV+ adults.
Setting:
The setting was an academic medical center.
Patients:
Patients were cART-treated HIV+ men and women (n = 36) with stable HIV disease and impaired glucose tolerance.
Interventions:
Interventions included sitagliptin 100 mg/d or placebo for 8 weeks.
Main Outcome Measures:
At baseline and week 8, plasma high-sensitivity C-reactive protein and C-X-C motif chemokine 10 concentrations (ELISA), oral glucose tolerance, and abdominal sc adipose mRNA expression for M1 macrophage markers (monocyte chemotactic protein-1, EGF-like module-containing, mucin-like hormone receptor 1).
Results:
Sitagliptin reduced glucose area under the curve (P = .002) and improved oral glucose insulin sensitivity index (P = .04) more than placebo. Sitagliptin reduced plasma high-sensitivity C-reactive protein and C-X-C motif chemokine 10 levels more than placebo (P < .009). Adipose tissue monocyte chemotactic protein-1 mRNA abundance declined significantly more (P = .01), and adipose EGF-like module-containing, mucin-like hormone receptor 1 mRNA expression tended to decline more (P = .19) in sitagliptin than placebo.
Conclusion:
Sitagliptin had beneficial systemic and adipose anti-inflammatory effects in cART-treated HIV+ adults with impaired glucose tolerance. Large-scale, long-term studies should determine whether sitagliptin reduces cardiovascular risk and events in HIV+ adults.
People living with HIV infection experience a 2-fold greater risk of vascular disease, myocardial infarction, or stroke, as well as a 2- to 4-fold greater incidence of elevated fasting glucose or hyperinsulinemia than the general population (1–3). The underlying mechanisms remain unclear. These non-AIDS comorbidities persist despite combination antiretroviral therapy (cART) and HIV suppression. They have been associated with chronic low-grade systemic inflammation, residual immune cell activation, and monocyte-macrophage migration into adipose depots (4, 5). Therapies targeted at reducing chronic immune cell activation and inflammation have been tested in cART-treated HIV-infected (HIV+) adults with suppressed viremia (6). However, no safe and effective treatment for reducing inflammation and immune cell activation in HIV+ adults exists.
Sitagliptin is a dipeptidyl peptidase-4 (DPP4) inhibitor (DPP4i) that represents a growing class of antidiabetic drugs that inhibit DPP4 enzyme activity and an exopeptidase that cleaves two N-terminal amino acids from the incretin hormones glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic polypeptide (GIP), which restricts their actions (7, 8). Sitagliptin inhibits circulating soluble DPP4 (sDPP4) enzyme activity and prolongs the circulating half-life of GLP-1 and GIP, indirectly enhancing insulin secretion and action (9). GLP-1 and GIP activate ubiquitously expressed G protein-coupled receptors (9) that exert several metabolic actions (9–14).
Sitagliptin also acts on DPP4 enzyme activity that resides in the CD26 cell surface receptor present on monocytes and T-lymphocytes (9), where it plays a role in proinflammatory signaling, immune regulation, and signal transduction (15). The number of DPP4/CD26+ T cells and sDPP4 enzyme activity are elevated in type 2 diabetes mellitus (T2DM) (15). Sitagliptin appears to have anti-inflammatory properties in T2DM, reduced circulating markers of inflammation (eg, high-sensitivity C-reactive protein [hsCRP] and IL-6), reduced monocyte expression of mRNA transcripts associated with inflammation (16), and reduced adipose-resident M1 macrophage polarization in obese rodents (17). sDPP4 may function as an adipokine and contribute to insulin resistance in obesity (18). Sitagliptin appears to have hematopoietic and vascular effects. In T2DM, sitagliptin mobilized bone-derived endothelial progenitor cells that can repair damaged vascular endothelium and improve function (19, 20). The potential beneficial pleiotropic actions of sitagliptin on systemic and adipose-resident inflammatory markers have not been adequately tested in cART-treated HIV+ adults.
Previously, we found that sitagliptin (100 mg/d, 16–24 wk) did not adversely affect virological or immune status in cART-treated HIV+ adults with normal glucose tolerance (21). Whether sitagliptin reduces inflammation and immune cell activation in HIV+ adults with impaired glucose tolerance (IGT) is unknown. If effective, sitagliptin may target two common conditions in HIV+ adults that can synergistically worsen cardiovascular disease (CVD) risk: IGT and inflammation/immune cell activation. We conducted a randomized, placebo-controlled, double-blind 8-week trial of sitagliptin in 36 HIV+ men and women with IGT. The primary hypothesis was that sitagliptin will reduce circulating levels of inflammatory markers known to be elevated in cART-treated HIV+ adults (hsCRP, IL-6, D-dimer, soluble CD14 [sCD14], C-X-C motif chemokine 10 [CXCL10]). The secondary hypotheses were that sitagliptin will reduce adipose-resident M1 macrophage mRNA transcript expression, increase circulating endothelial progenitor cell number, and improve glucose homeostasis and β-cell function in cART-treated HIV+ adults with IGT. We also hypothesized that reductions in inflammatory markers would be independent of improvements in glucose tolerance.
Subjects and Methods
Participants
HIV+ adults (18–65 y old) were recruited from the AIDS Clinical Trials Unit, the Infectious Diseases Clinics, and the Research Participant Registry at Washington University School of Medicine.
HIV+ participants were stable on cART for the prior 6 months (see Table 2) with stable immune (CD4+ T-cell count ≥ 300 cells/μL) and virological (plasma HIV RNA < 100 copies/mL) status. None of the participants had an AIDS-defining diagnosis, chronic kidney or liver dysfunction, heart failure, a history of pancreatitis, active malignancy, or T2DM, and none were taking antidiabetes medications. Participants had impaired fasting glucose or abnormal oral glucose tolerance on screening, fasting plasma glucose 100–125 mg/dL or 2-hour glucose concentration 140–200 mg/dL during a 75-g oral glucose tolerance test (oGTT), or fasting homeostatic model assessment of insulin resistance (HOMA-IR) of 2.5–6.0. This study was approved by the Human Research Protection Office at Washington University School of Medicine. All participants provided informed consent before participating. The study was registered with ClinicalTrials.gov (NCT 01552694).
Table 2.
Current cART
| Placebo | Sitagliptin | P Value | |
|---|---|---|---|
| NRTIs | 18 (100) | 17 (94) | .324 |
| ABC | 2 (11) | 4 (22) | .386 |
| FTC | 9 (50) | 12 (67) | .324 |
| 3TC | 3 (17) | 4 (22) | .684 |
| TDF | 15 (83) | 13 (72) | .437 |
| ZDV | 2 (11) | 2 (11) | 1.0 |
| Non-NRTIs | 10 (56) | 9 (50) | .747 |
| EFV | 7 (39) | 5 (28) | .494 |
| NVP | 3 (17) | 1 (6) | .302 |
| RPV | 0 | 3 (17) | .074 |
| Protease inhibitors | 7 (39) | 8 (44) | .744 |
| ATV | 1 (6) | 4 (22) | .157 |
| DRV | 4 (22) | 4 (22) | 1.0 |
| LPV | 2 (11) | 1 (6) | .560 |
| RTV | 6 (33) | 6 (33) | 1.0 |
| Integrase inhibitor RAL | 4 (22) | 3 (17) | .684 |
Abbreviations: ABC, abacavir; FTC, emtricitabine; 3TC, lamivudine; TDF, tenofovir; ZDV, zidovudine; EFV, efavirenz; NVP, nevirapine; RPV, rilpivirine; ATV, atazanavir; DRV, darunavir; LPV, lopinavir; RTV, ritonavir; RAL, raltegravir. Data are expressed as number (percentage) of participants taking each drug.
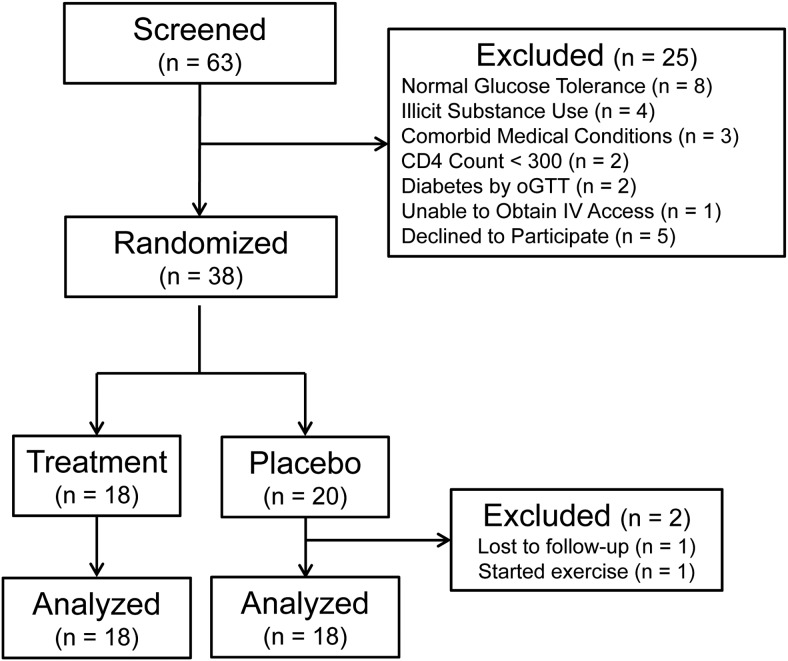
Figure 1 summarizes the screening and randomization for participants enrolled in this trial. Sixty-three potentially eligible candidates were screened, 25 were ineligible (Figure 1), and 38 were block randomized to placebo (n = 20) or sitagliptin 100 mg/d (n = 18) for 8 weeks. One placebo recipient was lost to follow-up, and one started an exercise program while on study. Both were excluded from data analysis, so the final analysis included 18 subjects per group (Table 1).
Figure 1.
The CONSORT flow diagram.
Table 1.
Baseline Characteristics
| Parameter | Placebo | Sitagliptin | P Value |
|---|---|---|---|
| n | 18 | 18 | |
| Gender (men/women), n | 13/5 | 13/5 | ns |
| Ethnicity (C/AA), n | 11 /7 | 2/16 | .002 |
| Age, y | 52 ± 6 | 49 ± 9 | .21 |
| Weight, kg | 102 ± 29 | 97 ± 21 | .59 |
| BMI, kg/m2 | 33.1 ± 8.2 | 32.7 ± 8.2 | .91 |
| Waist circumference, cm | 112 ± 23 | 106 ± 19 | .43 |
| Systolic blood pressure, mm Hg | 127 ± 14 | 124 ± 15 | .58 |
| Diastolic blood pressure, mm Hg | 78 ± 10 | 74 ± 9 | .26 |
| Heart rate, beats/min | 76 ± 15 | 72 ± 12 | .44 |
| HIV+ duration, y | 14.2 ± 7.5 | 14.9 ± 5.8 | .75 |
| CD4+ T-cell, cells/μL | 606 ± 218 | 731 ± 220 | .10 |
| CD8+ T-cell, cells/μL | 801 ± 294 | 1017 ± 542 | .16 |
| HIV RNA, copies/mL | <100 (100%) | <100 (100%) | ns |
Abbreviations: C, Caucasian; AA, African American; ns, not significant. Data are expressed as mean ± SD unless described otherwise.
Screening
A fasting, 2-hour oGTT with insulin, C-peptide, and glucagon monitoring was performed at screening and week 8. Glucose, insulin, C-peptide, and glucagon areas under the curve (AUCs) were quantified using the trapezoid rule. Validated algorithms were used to calculate glucoregulatory parameters: HOMA-IR = (fasting glucose [mg/dL] × fasting insulin [μU/mL])/405; homeostatic model assessment of β-cell function (HOMA-%β) = (360 × fasting insulin [μU/mL])/(fasting glucose [mg/dL] − 63); oral glucose insulin sensitivity index (OGIS120) (22); and insulinogenic index (IGI) = Δ insulin (0–30 min)/Δ glucose (0–30 min) (23). OGIS120 is an estimate of the glucose clearance rate (mL/min/m2 body surface area [BSA]) during a hyperinsulinemic glucose clamp. IGI is an estimate of first-phase insulin release during an oGTT.
At screening and week 8, fasting blood samples were collected for a complete metabolic panel, fasting lipid/lipoprotein panel, complete blood cell count, and CD4+ and CD8+ T-cell counts. These eligibility/safety analyses were conducted in the Washington University Core Laboratory for Clinical Studies (CLCS).
The research pharmacist distributed a 1-month supply of sitagliptin or matching placebo tablets to each participant at baseline and at the week 4 visit. Participants were instructed to take one tablet with their cART daily. Pill counts were used to determine adherence.
Circulating inflammatory markers
At baseline and week 8, fasting blood was collected, heparinized plasma and serum separated and stored (−80°C) and hsCRP and D-dimer were batch analyzed in the CLCS using particle-enhanced immuno-turbidimetric assay kits according to the manufacturer's instructions (Roche Diagnostics). D-dimer and hsCRP were complexed with latex particles coated with a monoclonal antibody directed against D-dimer or C-reactive protein epitopes, and the precipitate was assayed for turbidity on a Roche/Hitachi Cobas c System.
At baseline and week 8, fasting blood was collected into EDTA preservative, plasma was separated and stored (−80°C) until batch analyzed for sCD14 (diluted 1:1500; 100 μL/well), CXCL10 (at baseline, wk 4, and wk 8; undiluted; 75 μL/well) using ELISA, and IL-6 (undiluted; 100 μL/well) using an ultrasensitive ELISA (R&D Systems). Plates were read at 450 nm and corrected for 570 nm (or 690 nm for ultrasensitive ELISA) as described by the manufacturer. The intra-assay coefficient of variability for these analyses was < 12%.
Adipose-resident M1 macrophage gene transcripts
At baseline and week 8, participants reported to the Clinical Research Unit after an overnight fast and rested for 1 hour before an adipose tissue sample was obtained. Subcutaneous abdominal adipose tissue (1.5 ± 1.1 g) was aspirated through a small incision in the skin in the periumbilical region using a 4-mm liposuction cannula (Tulip Medical) after the region was anesthetized with lidocaine. The adipose sample was immediately rinsed with ice-cold saline, frozen in liquid nitrogen, and stored (−80°C) until batch analysis using quantitative RT-PCR for mRNA transcripts: CCL2 (monocyte chemotactic protein-1 [MCP-1]), and EMR1 (EGF-like module-containing, mucin-like hormone receptor 1), the human homolog of mouse F4/80 found on tissue-resident monocytes and macrophages (24).
Frozen tissue (100 mg) was suspended in 250 μL PureZOL (Bio-Rad) and homogenized using a RINO Pink Bead Lysis kit (Bullet Blender Blue 24; MidSci). Total RNA was extracted (Aurum Total RNA Fatty and Fibrous Tissue kit; Bio-Rad) according to the manufacturer's protocol and quantified using a NanoDrop 2000 UV-VIS spectrophotometer (Thermo Scientific).
Total RNA (200 ng) was reverse transcribed to cDNA in a 20-μL reaction volume (iScript Reverse Transcription Supermix containing RT buffer, MgCl2, dNTP mix, oligo [dT] random primers, RNase inhibitor, and iScript RNase H+ MMLV reverse transcriptase; Bio-Rad). Reverse transcription conditions followed the manufacturer's protocol using a Thermal Cycler 480 (Perkin-Elmer).
cDNA preamplification (SsoAdvance PreAmp Supermix; Bio-Rad) was required due to the small tissue sample size and relatively low target mRNA expression in adipose tissue. This reaction master mix is optimized for unbiased, target-specific cDNA preamplification (× 103) using PrimePCR PreAmp SYBR Green (Bio-Rad). Preamplification used 100 ng cDNA + 5 nmol primer template/target in 50-μL reaction volume, containing 25 μL SsoAdvanced Pre-Amp Supermix (antibody-mediated hot-start Sso7d fusion DNA polymerase, dNTPS, salts; Bio-Rad) and reaction conditions (hold 95°C for 3 min, 95°C for 15 s, 58°C for 4 min, hold 4°C until cycle end × 12 cycles) using an EP-Gradient S Mastercycler (Eppendorf). Before quantitative PCR, all preamplified cDNA templates were diluted 1:5 in water.
RT-PCR amplification and quantitation were performed in custom-designed 384-well Prime PCR Assay plates (Bio-Rad) using a C1000 Touch Thermal Cycler/CFX384 (Bio-Rad). Each well contained lyophilized primers for MCP-1, EMR1, HPRT-1 (reference gene; hypoxanthine phosphoribosyltransferase-1), mRNA degradation, and assay controls (RQ1/RQ2, gDNA, and negative PCR). Preamplified cDNA was added to each well with SsoAdvanced Universal SYBR Green supermix (Bio-Rad) to 10 μL total reaction volume. Quantitative PCR conditions were according to manufacturer's protocol and were followed by melt curve analysis.
Circulating endothelial progenitor cells
At baseline and week 8, 20 mL fasting blood was collected into EDTA tubes and diluted 1:1 with sterile saline, and peripheral blood mononuclear cells (PBMCs) were isolated using a modified density gradient separation (2 × 20 mL Lymphoprep tubes; Axis Shield). Mononuclear cells form a distinct band at the density gradient interface after centrifugation (800 × g, 15 min). PBMCs were harvested and concentrated by diluting with sterile saline and centrifugation (350 × g, 15 min). PBMC pellets were collected and suspended in red blood cell lysis buffer (5 mL, 5 min, FACS Lysing Solution; BD Biosciences), and the cleared PBMC pellet was harvested after addition of 5.0 mL PBS and centrifugation (350 × g). The pellet was resuspended in 1.0 mL FACS Blocking/Staining buffer (PBS, 1% BSA, 40% goat serum, 30 min), cell number was determined in a hemocytometer, and 1 × 106 cells/label reaction was used for fluorescence-assisted cell sorting.
To identify and count endothelial progenitor cells, cell aliquots were stained (30 min in the dark) with fluorescent antibodies (CD34-Fluorescein isothiocyanate; Santa Cruz Biotechnology; and VEGF-R2-KDR-Phycoerythrin; R&D Systems), along with respective IgG isotype controls. Stained cells were washed twice (PBS + 0.05% BSA), resuspended in 1% formalin buffered PBS, counted on a FACS Calibur-3 flow cytometer (Becton Dickinson), and data processed using FlowJo software.
Statistical analysis
Mean ± SD are reported. Continuous variables measured at baseline were compared using a two-sided t test, and categorical variables were compared using χ2 or Kruskal-Wallis analysis. After treatment, between-group comparisons (two-sided t test) were conducted by calculating each participant's delta score (week 8 − baseline) for each outcome, except adipose tissue mRNA expression. Adipose mRNA transcript data were expressed as fold change from baseline using the comparative CT (2−ΔΔCT) method; these were compared between groups using a two-sided t test. Spearman rank correlation examined relationships between changes in inflammatory/immune activation markers, fold change in adipose mRNA expression, and changes in glucose tolerance. A P value < .05 was accepted as statistically significant.
Results
Baseline characteristics
The groups were matched for baseline demographics, but more African Americans were randomized to sitagliptin than placebo (Table 1; P = .002). All 36 participants had undetectable (<100 HIV RNA copies/mL) plasma viremia and were stable on cART, and antiviral drug classes used were not different between groups (Table 2). Most participants (∼56%) were taking nucleoside reverse transcriptase inhibitor (NRTI) + non-NRTI-based regimens (most commonly emtricitabine + tenofovir + efavirenz [Atripla]), fewer (∼24%) were taking protease inhibitor-based regimens (ritonavir-boosted darunavir or lopinavir or atazanavir), and fewer still (∼20%) were taking raltegravir (integrase inhibitor). During the study, participants did not alter their cART, and plasma HIV RNA remained < 100 copies/mL in all participants. Despite randomization, baseline fasting triglycerides and insulin concentrations, HOMA-IR, and HOMA-%β were lower in the sitagliptin group (P < .04; Table 3). Baseline oGTT glucose, insulin, C-peptide, and glucagon AUCs, and total, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) cholesterol concentrations were not different between the groups (Table 3). Baseline plasma IL-6 and sCD14 concentrations were higher in placebo than sitagliptin (P < .02), but baseline CD4+/CD8+ helper/suppressor ratio, hsCRP, D-dimer, and CXCL10 concentrations were not different between the groups (Table 4).
Table 3.
Metabolic Parameters at Baseline and After 8 Weeks of Intervention
| Parameter | Group | Baseline | Wk 8 | Δ (Wk 8 − Baseline) |
P Value |
|
|---|---|---|---|---|---|---|
| Baseline | Δ Between Group | |||||
| Weight, kg | Placebo | 102 ± 29 | 101 ± 29 | −0.4 ± 2.4 | .59 | .79 |
| Sitagliptin | 97 ± 21 | 97 ± 20 | −0.6 ± 2.3 | |||
| BMI, kg/m2 | Placebo | 33.1 ± 8.2 | 32.7 ± 8.1 | −0.3 ± 1.2 | .91 | .57 |
| Sitagliptin | 32.7 ± 8.2 | 32.6 ± 8.4 | −0.1 ± 1.1 | |||
| Fasting glucose, mg/dL | Placebo | 97.7 ± 7.9 | 94.4 ± 7.9 | −3.3 ± 5.6 | .72 | .47 |
| Sitagliptin | 96.8 ± 7.5 | 92.2 ± 6.9 | −4.6 ± 5.3 | |||
| Fasting insulin, μU/mL | Placebo | 17.9 ± 10.7 | 15.5 ± 13.8 | −2.4 ± 8.0 | .03 | .24 |
| Sitagliptin | 11.4 ± 6.0 | 11.6 ± 6.4 | 0.2 ± 3.9 | |||
| HOMA-IR | Placebo | 4.3 ± 2.8 | 3.8 ± 3.8 | −0.5 ± 2.0 | .04 | .39 |
| Sitagliptin | 2.7 ± 1.5 | 2.6 ± 1.5 | −0.1 ± 1.0 | |||
| HOMA-%β | Placebo | 185 ± 111 | 171 ± 115 | −13 ± 152 | .04 | .36 |
| Sitagliptin | 120 ± 66 | 149 ± 93 | 29 ± 118 | |||
| OGIS120, mL/min/m2 BSA | Placebo | 362 ± 60 | 340 ± 64 | −22 ± 68 | .46 | .04 |
| Sitagliptin | 347 ± 62 | 366 ± 60 | 19 ± 40 | |||
| IGI | Placebo | 190 ± 89 | 178 ± 73 | −12 ± 58 | .88 | .15 |
| Sitagliptin | 195 ± 122 | 225 ± 142 | 30 ± 106 | |||
| oGTT glucose AUC | Placebo | 17 697 ± 1823 | 16 369 ± 2605 | −1327 ± 2158 | .52 | .002 |
| Sitagliptin | 18 092 ± 1852 | 14 720 ± 2291 | −3372 ± 1396 | |||
| oGTT insulin AUC | Placebo | 12 454 ± 6783 | 11 974 ± 8110 | −480 ± 6450 | .97 | .78 |
| Sitagliptin | 12 368 ± 7973 | 11 307 ± 8228 | −1061 ± 6106 | |||
| oGTT C-peptide AUC | Placebo | 1191 ± 321 | 1200 ± 468 | 9 ± 291 | .88 | .56 |
| Sitagliptin | 1140 ± 355 | 1099 ± 329 | −45 ± 250 | |||
| oGTT glucagon AUC | Placebo | 8016 ± 1629 | 7902 ± 1400 | −114 ± 769 | .25 | .42 |
| Sitagliptin | 8792 ± 2300 | 8405 ± 2788 | −387 ± 1197 | |||
| Fasting serum triglycerides, mg/dL | Placebo | 155 ± 106 | 140 ± 68 | −14 ± 78 | .03 | .21 |
| Sitagliptin | 94 ± 44 | 107 ± 61 | 13 ± 45 | |||
| Total cholesterol, mg/dL | Placebo | 178 ± 26 | 171 ± 25 | −7 ± 21 | .15 | |
| Sitagliptin | 163 ± 34 | 165 ± 40 | 2 ± 21 | |||
| LDL cholesterol, mg/dL | Placebo | 101 ± 33 | 97 ± 27 | −4 ± 18 | .48 | .32 |
| Sitagliptin | 94 ± 28 | 96 ± 30 | 2 ± 18 | |||
| HDL cholesterol, mg/dL | Placebo | 48 ± 17 | 46 ± 16 | −2 ± 6 | .67 | .92 |
| Sitagliptin | 51 ± 18 | 48 ± 18 | −3 ± 7 | |||
Data are expressed as mean ± SD.
Table 4.
Immune Activation and Inflammatory Parameters at Baseline and After 8 Weeks of Intervention
| Parameter | Group | Baseline | Wk 8 | Δ (Wk 8 − Baseline) |
P Value |
|
|---|---|---|---|---|---|---|
| Baseline | Δ Between Group | |||||
| CD4+/CD8+ ratio | Placebo | 0.85 ± 0.38 | 0.90 ± 0.35 | 0.05 ± 0.10 | .86 | .65 |
| Sitagliptin | 0.87 ± 0.48 | 0.87 ± 0.51 | 0.00 ± 0.15 | |||
| D-dimer, μg FEU/mL | Placebo | 0.28 ± 0.15 | 0.31 ± 0.20 | 0.03 ± 0.18 | .56 | .78 |
| Sitagliptin | 0.32 ± 0.20 | 0.33 ± 0.24 | 0.01 ± 0.18 | |||
| IL-6, pg/mL | Placebo | 2.61 ± 1.91 | 2.65 ± 1.83 | 0.18 ± 0.64 | 0.02 | 0.24 |
| Sitagliptin | 1.36 ± 0.74 | 1.30 ± 0.55 | −0.06 ± 0.58 | |||
| sCD14, ng/mL | Placebo | 3331 ± 1836 | 2917 ± 1523 | −414 ± 890 | .005 | .05 |
| Sitagliptin | 1899 ± 487 | 2024 ± 656 | 126 ± 668 | |||
| Adipose CCL2 (MCP-1) mRNA | Placebo (n = 7) | −0.4 ± 1.2 fold change | .01 | |||
| Sitagliptin (n = 11) | −2.5 ± 1.9 fold change | |||||
| Adipose EMR1 mRNA | Placebo (n = 6) | −0.7 ± 1.3 fold change | .19 | |||
| Sitagliptin (n = 11) | −1.9 ± 2.3 fold change | |||||
Abbreviations: CXCL10, C-X-C motif chemokine 10 (interferon-γ inducible protein; IP-10); CCL2, chemokine (C-C motif) ligand 2 (MCP-1). FEU, Fibrinogen equivalent units. Data are expressed as mean ± SD.
Weight and hormone concentrations
Adherence was 97 ± 2% (range, 93–100%) to placebo and 96 ± 5% (range, 83–100%; P = .28) to sitagliptin. Baseline body weight, body mass index (BMI), and waist circumference were not different between the groups, and small changes in weight and BMI were not significantly different between the groups during the intervention (Table 3). Both glucose AUC (P = .002) and the OGIS120 (P = .04) improved more in the sitagliptin group than the placebo group (Table 3). After 8 weeks, the small changes in insulin, C-peptide, and glucagon AUCs during oGTT and the small changes in serum triglycerides, total, LDL, and HDL cholesterol concentrations were not different between the groups (Table 3).
Circulating inflammatory and chemokine markers
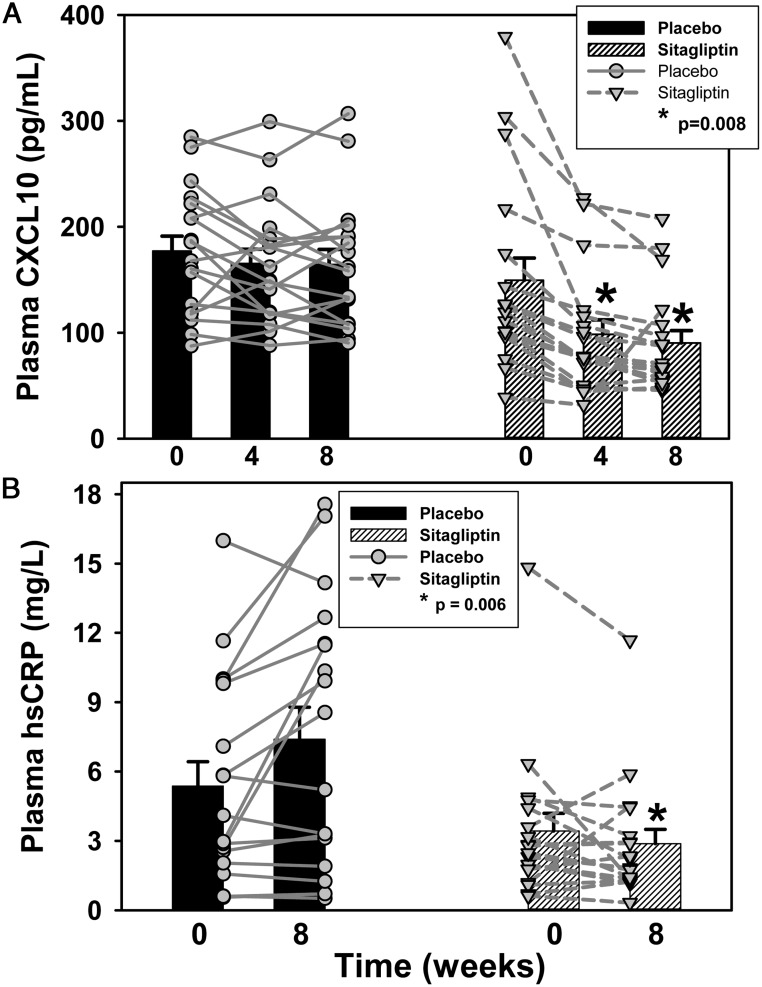
Plasma hsCRP at week 8 and CXCL10 concentrations at weeks 4 and 8 declined more in the sitagliptin group than the placebo group (P ≤ .008; Figure 2). Small changes in the CD4+/CD8+ helper/suppressor ratio and D-dimer and IL-6 concentrations were not significantly different between the groups (Table 4). Paradoxically, plasma sCD14 concentration declined more in the placebo group than the sitagliptin group.
Figure 2.
During sitagliptin administration to cART-treated HIV+ adults with IGT (n = 18), the reductions in plasma CXCL10 (P = .008) at weeks 4 and 8 (A) and hsCRP concentrations (P = .006) at week 8 (B) were greater than in the placebo group (n = 18). Bars represent mean ± SE. Symbols and lines represent results for individual participants.
Adipose tissue markers
Adipose tissue MCP-1 mRNA abundance declined significantly more in the sitagliptin group than the placebo group (P = .01; Table 4). Adipose tissue EMR1 mRNA abundance tended to decline more in the sitagliptin group (−1.9 ± 2.3 fold change from baseline) than in the placebo group (−0.7 ± 1.3 fold change), but this did not achieve statistical significance (P = .19).
Circulating endothelial progenitor cells
At baseline, circulating endothelial progenitor cells represented a small percentage of PBMCs in HIV+ adults (placebo, 0.9 ± 1.9% [n = 11], sitagliptin, 0.4 ± 0.8% [n = 12]). Sitagliptin increased endothelial progenitor cell number (0.7 ± 0.2%; Δ = +0.3 ± 0.6%) more than the decline after placebo (0.7 ± 0.1%; Δ = −0.2 ± 0.5%; P = .04).
Correlations
We conducted correlation analyses between the changes in hsCRP or CXCL10 or MCP-1 mRNA expression and the changes in OGIS120 or glucose AUC in the sitagliptin group. Reductions in CXCL10, hsCRP, and adipose MCP-1 mRNA transcript expression were not related to improvements in OGIS120 (r2 = 0.00012; 0.12; 0.67) or reductions in glucose AUC (r2 = 0.00011; 0.0084; 0.00002; all P > .05).
Discussion
The findings suggest that 8 weeks of sitagliptin had beneficial anti-inflammatory, immune regulatory, hematopoietic progenitor cell-mobilizing, and glucose-lowering effects in cART-treated virally suppressed HIV+ adults with IGT. In cART-treated HIV+ adults, plasma hsCRP is a marker for persistent immune cell activation and is associated with greater vascular endothelial activation markers, rates of thrombosis, and carotid intima media thickness—CVD risk factors (25–27). In this study, sitagliptin reduced plasma hsCRP concentration, whereas hsCRP increased in placebo-treated participants. Sitagliptin reduced the concentration of plasma CXCL10, a chemokine secreted from monocytes and endothelial cells in response to interferon-γ, HIV-related proteins (Tat, Nef, gp120), and lipopolysaccharide (LPS) binding to toll-like receptor-4 present on immune cells (28). Plasma CXCL10 binds a G protein-coupled receptor (CXCR3A/B) present on endothelial and vascular smooth muscle cells and functions as a chemoattractant to recruit monocytes/macrophages, T cells, and other immune cells to sites of inflammation (28), and CXCL10 promotes T-cell adhesion to endothelial cells during atheroma formation (29). These findings support our working hypothesis that sitagliptin-induced reductions in circulating inflammatory and chemokine markers, while increasing endothelial progenitor cell number, have the potential to reduce CVD risk and complications in cART-treated HIV+ adults with chronic, residual immune activation/inflammation that are predictors for CVD events.
Sitagliptin reduced MCP-1 mRNA abundance in sc abdominal adipose tissue. MCP-1 is a chemokine that recruits macrophages into adipose tissue (30) and contributes to adipose tissue inflammation. MCP-1 mRNA expression is often increased in obese or insulin-resistant adults (31) and can function as an adipokine that induces skeletal muscle and hepatic insulin resistance when released into the circulation. Adipose MCP-1 mRNA expression was elevated in cART-treated, lipodystrophic HIV+ adults and correlated with liver adipose content (5). Adipose MCP-1 expression was reduced after insulin-sensitizing interventions; eg, gastric bypass surgery (32) and pioglitazone in HIV-seronegative people with IGT (31). Because of its role in adipose tissue inflammation, dysfunction, and metabolic complications, MCP-1 could be a therapeutic target. This may be particularly relevant in cART-treated HIV+ adults with chronic immune activation, inflammation, metabolic complications, and increased risk of T2DM and CVD events. The observed reduction in adipose MCP-1 mRNA expression suggests that sitagliptin is a viable therapy for adipose inflammation and IGT in cART-treated HIV+ adults. Longer-term studies need to determine whether sitagliptin-induced reductions in immune cell activation, adipose and systemic inflammation markers, and improvements in glucose tolerance translate into fewer CVD events in HIV+ adults.
There were some limitations and unanticipated observations. In T2DM, sitagliptin typically reduces fasting insulin, C-peptide, and glucagon concentrations and AUCs during an oGTT. In the current study, there were trends for greater decrements in AUC for these glucoregulatory hormones during an oGTT, but between-group differences were not statistically significant. Perhaps sitagliptin would lower these hormone levels in HIV+ adults with T2DM. It is possible that a greater number of participants (insufficient statistical power; false negative finding), or a longer sitagliptin treatment period is required in HIV+ adults. The glucagon antibody used may lack specificity; several glucagon antibodies cross-react with other glucagon gene products (oxyntomodulin, glicentin, GLP-1, GLP-2); some of these are DPP4 substrates that could artifactually increase the measured glucagon concentration in sitagliptin-treated participants and explain our observation.
D-dimer and IL-6 concentrations are typically elevated in HIV+ individuals, especially cART-naive HIV+ adults, where these systemic inflammatory markers have been associated with undesirable comorbidities and mortality (25, 33). They were not reduced in either group after the intervention. D-dimer is a fibrin degradation product, and IL-6 is a proinflammatory cytokine produced by monocytes, lymphocytes, and vascular endothelial cells. Both are reduced upon cART initiation in HIV+ adults. When cART was interrupted (34), D-dimer and IL-6 concentrations increased and were correlated with increased plasma viremia. In the current study, plasma HIV RNA was undetectable in all participants at each time point, and this might explain the lack of change in D-dimer and IL-6 concentrations.
Despite randomization, baseline sCD14 concentrations were higher in the placebo than the sitagliptin group. CD14 is a monocyte cell surface receptor that is released into the circulation as sCD14 in response to pathogen-associated molecular patterns (eg, LPS, other microbial membrane lipids) (35). In HIV, LPS and other pathogen-associated molecular patterns are believed to translocate from an inflamed gastrointestinal tract, a phenomenon driven by latent HIV-infected CD4+ T cells present in the gut-associated lymphoid tissues, and potentially other latent HIV reservoirs (36). In HIV+ adults, plasma sCD14 concentration is typically elevated and is considered a marker for microbial translocation-associated monocyte activation that correlates with mortality and comorbid conditions (35, 37). In the sitagliptin group, the average baseline sCD14 concentration was unusually low (1899 ± 487 ng/mL), and it may not have been possible for sitagliptin to lower it further. In the placebo group, the average baseline sCD14 concentration was unusually high (3331 ± 1836 ng/mL) and declined modestly after 8 weeks (2917 ± 1523 ng/mL), but the 8-week value remained above normal. In the sitagliptin group, both baseline and 8-week sCD14 values were closer to normal (∼2000 ng/mL), significantly lower than that in the placebo group, and may explain the lack of a sCD14 response in the sitagliptin group. A higher baseline sCD14 concentration might be required for sitagliptin to reduce this monocyte activation marker.
In rodents, sitagliptin reduced adipose-resident macrophage number and F4/80 mRNA expression (17). In the current study, sitagliptin reduced adipose EMR1 mRNA expression, but not significantly more than in the placebo group. Adipose-resident macrophages are more abundant in rodent than human adipose tissue. We employed two amplification procedures in our human adipose samples to reliably quantify low-level EMR1 and MCP-1 transcript expression. Low-level expression may have limited our ability to detect a fold change difference in EMR1 between the groups. We obtained an adequate amount of adipose tissue for this analysis from a subset of participants (n = 11 sitagliptin; n = 6–7 placebo), so we may have been underpowered to detect a difference in EMR1 in the sitagliptin group. Many cART-treated HIV+ adults have sc lipoatrophy (5), and this can limit adipose tissue sample size.
Correlation analyses indicated that reductions in plasma hsCRP, CXCL10, and adipose tissue MCP-1 expression were not related to improvements in insulin sensitivity or glucose tolerance. This suggests that the anti-inflammatory effects of sitagliptin were independent of its beneficial actions on glucose homeostasis. This implies that IGT is not required to observe the anti-inflammatory actions of sitagliptin in HIV+ adults. Residual immune cell activation and inflammation are common among cART-treated HIV+ adults (4), and routine sitagliptin administration may be efficacious, regardless of their glycemic status.
In summary, sitagliptin had favorable anti-inflammatory, immune regulatory, progenitor cell-mobilizing, and glucoregulatory actions in cART-treated HIV+ adults with IGT. These short-term findings suggest that sitagliptin may reduce CVD risk and events in HIV+ adults, but large-scale, long-term treatment studies are needed. Taken together with prior findings obtained from HIV+ adults (21), sitagliptin does not adversely affect virological or immunological status, it improves postprandial hyperglycemia, and it effectively reduces inflammation and immune activation markers that are thought to contribute to non-AIDS comorbidities and mortality.
Acknowledgments
We thank all research participants for volunteering for clinical studies. Terri Pietka provided fluorescence-assisted flow cytometry expertise.
Merck Pharmaceuticals provided drug/placebo and financial support for the conduct of this study. The following National Institutes of Health (NIH) grants supported the personnel, cores, and resources utilized to conduct this study: Washington University Biomedical Mass Spectrometry Research Facility (P41 GM103422), AIDS Clinical Trials Unit (UM1 AI069495), Diabetes Research Center (P30 DK020579), Nutrition Obesity Research Center (P30 DK056341), Institute of Clinical and Translational Sciences (NCATS UL1 TR000448), Diabetes and Related Metabolic Diseases Postdoctoral Training grant (T32 DK007120), and Dean's Bridge Funds. The content is solely the responsibility of the authors and does not necessarily represent the official view of the NIH.
Trial Registration: ClinicalTrials.gov NCT 01552694.
Disclosure Summary: K.E.Y. received grant support and product from Merck Pharmaceuticals (October 2012 to December 2014). C.B., H.S., E.L., M.R., and D.N.R. have nothing to disclose.
Footnotes
- AUC
- area under the curve
- BMI
- body mass index
- BSA
- body surface area
- cART
- combination antiretroviral therapy
- CVD
- cardiovascular disease
- CXCL10
- C-X-C motif chemokine 10
- DPP4
- dipeptidyl peptidase-4
- DPP4i
- DPP4 inhibitor
- EMR1
- EGF-like module-containing, mucin-like hormone receptor-1
- GIP
- glucose-dependent insulinotropic polypeptide
- GLP
- glucagon-like peptide
- HDL
- high-density lipoprotein
- HIV+
- HIV-infected
- HOMA-%β
- homeostatic model assessment of β-cell function
- HOMA-IR
- homeostatic model assessment of insulin resistance
- hsCRP
- high-sensitivity C-reactive protein
- IGI
- insulinogenic index
- IGT
- impaired glucose tolerance
- LDL
- low-density lipoprotein
- LPS
- lipopolysaccharide
- MCP-1
- monocyte chemotactic protein-1
- NRTI
- nucleoside reverse transcriptase inhibitor
- oGTT
- oral glucose tolerance test
- PBMC
- peripheral blood mononuclear cell
- sCD14
- soluble CD14
- sDPP4
- soluble DPP4
- T2DM
- type 2 diabetes mellitus.
References
- 1. Brown TT, Li X, Cole SR, et al. Cumulative exposure to nucleoside analogue reverse transcriptase inhibitors is associated with insulin resistance markers in the Multicenter AIDS Cohort Study. AIDS. 2005;19:1375–1383. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan RC, Kingsley LA, Sharrett AR, et al. Ten-year predicted coronary heart disease risk in HIV-infected men and women. Clin Infect Dis. 2007;45:1074–1081. [DOI] [PubMed] [Google Scholar]
- 3. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS. 2015;29:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sevastianova K, Sutinen J, Kannisto K, Hamsten A, Ristola M, Yki-Järvinen H. Adipose tissue inflammation and liver fat in patients with highly active antiretroviral therapy-associated lipodystrophy. Am J Physiol Endocrinol Metab. 2008;295:E85–E91. [DOI] [PubMed] [Google Scholar]
- 6. Taiwo B, Barcena L, Tressler R. Understanding and controlling chronic immune activation in the HIV-infected patients suppressed on combination antiretroviral therapy. Curr HIV/AIDS Rep. 2013;10:21–32. [DOI] [PubMed] [Google Scholar]
- 7. Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62:3316–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mentlein R. Dipeptidyl-peptidase IV (CD26)–role in the inactivation of regulatory peptides. Regul Pept. 1999;85:9–24. [DOI] [PubMed] [Google Scholar]
- 9. Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr Rev. 2014;35:992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McIntosh CH, Widenmaier S, Kim SJ. Chapter 2: Pleiotropic actions of the incretin hormones. Vitam Horm. 2010;84:21–79. [DOI] [PubMed] [Google Scholar]
- 11. Ravassa S, Zudaire A, Díez J. GLP-1 and cardioprotection: from bench to bedside. Cardiovasc Res. 2012;94:316–323. [DOI] [PubMed] [Google Scholar]
- 12. Herzlinger S, Horton ES. Extraglycemic effects of glp-1-based therapeutics: addressing metabolic and cardiovascular risks associated with type 2 diabetes. Diabetes Res Clin Pract. 2013;100:1–10. [DOI] [PubMed] [Google Scholar]
- 13. Ishikawa S, Shimano M, Watarai M, et al. Impact of sitagliptin on carotid intima-media thickness in patients with coronary artery disease and impaired glucose tolerance or mild diabetes mellitus. Am J Cardiol. 2014;114:384–388. [DOI] [PubMed] [Google Scholar]
- 14. Ussher JR, Drucker DJ. Cardiovascular biology of the incretin system. Endocr Rev. 2012;33:187–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lee SA, Kim YR, Yang EJ, et al. CD26/DPP4 levels in peripheral blood and T cells in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab. 2013;98:2553–2561. [DOI] [PubMed] [Google Scholar]
- 16. Makdissi A, Ghanim H, Vora M, et al. Sitagliptin exerts an antinflammatory action. J Clin Endocrinol Metab. 2012;97:3333–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dobrian AD, Ma Q, Lindsay JW, et al. Dipeptidyl peptidase IV inhibitor sitagliptin reduces local inflammation in adipose tissue and in pancreatic islets of obese mice. Am J Physiol Endocrinol Metab. 2011;300:E410–E421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lamers D, Famulla S, Wronkowitz N, et al. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fadini GP, Avogaro A. Dipeptidyl peptidase-4 inhibition and vascular repair by mobilization of endogenous stem cells in diabetes and beyond. Atherosclerosis. 2013;229:23–29. [DOI] [PubMed] [Google Scholar]
- 20. Fadini GP, Boscaro E, Albiero M, et al. The oral dipeptidyl peptidase-4 inhibitor sitagliptin increases circulating endothelial progenitor cells in patients with type 2 diabetes: possible role of stromal-derived factor-1α. Diabetes Care. 2010;33:1607–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Goodwin SR, Reeds DN, Royal M, Struthers H, Laciny E, Yarasheski KE. Dipeptidyl peptidase IV inhibition does not adversely affect immune or virological status in HIV infected men and women: a pilot safety study. J Clin Endocrinol Metab. 2013;98:743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mari A, Pacini G, Murphy E, Ludvik B, Nolan JJ. A model-based method for assessing insulin sensitivity from the oral glucose tolerance test. Diabetes Care. 2001;24:539–548. [DOI] [PubMed] [Google Scholar]
- 23. Goedecke JH, Dave JA, Faulenbach MV, et al. Insulin response in relation to insulin sensitivity: an appropriate β-cell response in black South African women. Diabetes Care. 2009;32:860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schulz C, Gomez Perdiguero E, Chorro L, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90. [DOI] [PubMed] [Google Scholar]
- 25. Nixon DE, Landay AL. Biomarkers of immune dysfunction in HIV. Curr Opin HIV AIDS. 2010;5:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ross AC, O'Riordan MA, Storer N, Dogra V, McComsey GA. Heightened inflammation is linked to carotid intima-media thickness and endothelial activation in HIV-infected children. Atherosclerosis. 2010;211:492–498. [DOI] [PubMed] [Google Scholar]
- 27. Ross AC, Rizk N, O'Riordan MA, et al. Relationship between inflammatory markers, endothelial activation markers, and carotid intima-media thickness in HIV-infected patients receiving antiretroviral therapy. Clin Infect Dis. 2009;49:1119–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van den Borne P, Quax PH, Hoefer IE, Pasterkamp G. The multifaceted functions of CXCL10 in cardiovascular disease. Biomed Res Int. 2014;2014:893106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mach F, Sauty A, Iarossi AS, et al. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sell H, Eckel J. Monocyte chemotactic protein-1 and its role in insulin resistance. Curr Opin Lipidol. 2007;18:258–262. [DOI] [PubMed] [Google Scholar]
- 31. Di Gregorio GB, Yao-Borengasser A, Rasouli N, et al. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues: association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. [DOI] [PubMed] [Google Scholar]
- 32. Cancello R, Henegar C, Viguerie N, et al. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. [DOI] [PubMed] [Google Scholar]
- 33. Justice AC, Freiberg MS, Tracy R, et al. Does an index composed of clinical data reflect effects of inflammation, coagulation, and monocyte activation on mortality among those aging with HIV? Clin Infect Dis. 2012;54:984–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Abad-Fernández M, Vallejo A, Hernández-Novoa B, et al. Correlation between different methods to measure microbial translocation and its association with immune activation in long-term suppressed HIV-1-infected individuals. J Acquir Immune Defic Syndr. 2013;64:149–153. [DOI] [PubMed] [Google Scholar]
- 37. Lyons JL, Uno H, Ancuta P, et al. Plasma sCD14 is a biomarker associated with impaired neurocognitive test performance in attention and learning domains in HIV infection. J Acquir Immune Defic Syndr. 2011;57:371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]