Abstract
Context:
Recommendations for surgical, compared with lifestyle and pharmacologically based, approaches for type 2 diabetes (T2D) management remain controversial.
Objective:
The objective was to compare laparoscopic adjustable gastric band (LAGB) to an intensive medical diabetes and weight management (IMWM) program for T2D.
Design:
This was designed as a prospective, randomized clinical trial.
Setting:
The setting was two Harvard Medical School-affiliated academic institutions.
Interventions and Participants:
A 12-month randomized trial comparing LAGB (n = 23) vs IMWM (n = 22) in persons aged 21–65 years with body mass index of 30–45 kg/m2, T2D diagnosed more than 1 year earlier, and glycated hemoglobin (HbA1c) ≥ 6.5% on antihyperglycemic medication(s).
Main Outcome Measure:
The proportion meeting the prespecified primary glycemic endpoint, defined as HbA1c < 6.5% and fasting glucose < 7.0 mmol/L at 12 months, on or off medication.
Results:
After randomization, five participants did not undergo the surgical intervention. Of the 40 initiating intervention (22 males/18 females; age, 51 ± 10 y; body mass index, 36.5 ± 3.7 kg/m2; diabetes duration, 9 ± 5 y; HbA1c, 8.2 ± 1.2%; 40% on insulin), the proportion meeting the primary glycemic endpoint was achieved in 33% of the LAGB patients and 23% of the IMWM patients (P = .457). HbA1c reduction was similar between groups at both 3 and 12 months (−1.2 ± 0.3 vs −1.0 ± 0.3%; P = .496). Weight loss was similar at 3 months but greater 12 months after LAGB (−13.5 ± 1.7 vs −8.5 ± 1.6 kg; P = .027). Systolic blood pressure reduction was greater after IMWM than LAGB, whereas changes in diastolic blood pressure, lipids, fitness, and cardiovascular risk scores were similar between groups. Patient-reported health status, assessed using the Short Form-36, Impact of Weight on Quality of Life, and Problem Areas in Diabetes, all improved similarly between groups.
Conclusions:
LAGB and a multidisciplinary IMWM program have similar 1-year benefits on diabetes control, cardiometabolic risk, and patient satisfaction, which should be considered in the context of other factors, such as personal preference, when selecting treatment options with obese T2D patients. Longer duration studies are important to understand emergent differences.
The US Food and Drug Administration (FDA) approved the LAP-BAND in 2001 for weight loss in severely obese patients with a body mass index (BMI) of at least 40 kg/m2 or a BMI of at least 35 kg/m2 with an obesity-related comorbidity, such as diabetes or hypertension. Among surgeries for weight management, laparoscopic adjustable gastric band (LAGB) is associated with low mortality (under 0.1% in the first 30 d) and minimal nutritional deficiencies, and it is reversible. Patients require aftercare for maintenance, and reoperations and removals are common. In 2010, laparoscopic gastric banding use represented 46% of all bariatric operations (1). In 2011, the FDA expanded approval of LAGB to include obese individuals with BMI of 30–34 kg/m2 and other obesity-related comorbidities based on an open-label, nonrandomized study and clinical safety experience.
Meta-analyses of cohort studies demonstrate clinically meaningful improvements in weight, glucose tolerance, blood pressure, and lipids, including in those with type 2 diabetes (T2D) undergoing bariatric surgical procedures including LAGB, although weight loss and remission of obesity-related comorbidities is generally less after LAGB compared to Roux-en-Y gastric bypass (2, 3). Yet some feel surgical approaches for diabetes and weight management are draconian (4), especially given recent substantial improvements in diabetes pharmacotherapy and better understanding of the benefits of multidisciplinary-based approaches to promote lifestyle change (5, 6).
Currently, fewer than half of adults with T2D attain recommended goals for glycated hemoglobin (HbA1c), blood pressure, or cholesterol (7). These findings and the considerable individual and public health burden of diabetes-related micro- and macrovascular complications demonstrate the clinical need to understand the diverse therapeutic approaches to inform clinical decision-making to treat hyperglycemia and cardiovascular risk in patients with diabetes.
The Surgery or Lifestyle with Intensive Medical Management in Treatment of Type 2 Diabetes (SLIMM-T2D) trials were designed as two parallel randomized, controlled, pragmatic (in that all interventions were clinically available), single academic center studies to assess the feasibility of methods to conduct a larger multisite trial comparing the long-term impact of bariatric surgery to medical management to improve glycemic control and cardiometabolic risk in obese patients with T2D. We previously reported 1-year findings of Roux-en-Y gastric bypass compared to a multidisciplinary intensive medical diabetes and weight management (IMWM) program for diabetes called Why WAIT (Weight Achievement and Intensive Treatment), designed for application in real-world clinical practice (8). Why WAIT's cognitive behavioral support is based on the Diabetes Prevention Program (5) and the LookAHEAD study (9, 10), but it differs significantly in medication adjustment plan, amount of caloric reduction and dietary composition, exercise type and duration, and diabetes education sessions and is delivered in group sessions. We now report findings comparing the effectiveness of LAGB to the IMWM program.
Subjects and Methods
Trial design
The study was a randomized, parallel-group, pragmatic trial stratified for BMI above or below 35 kg/m2 with balanced randomization (1:1), conducted within one university.
Setting and participants
Participants were recruited from the health centers or by advertisements. Eligible participants were aged 21–65 years with at least 1 year of T2D, BMI of 30–45 kg/m2, a strong desire for substantial weight loss, and commitment to lifelong medical follow-up, and they were free from active cardiovascular or eye diseases prohibiting them from exercising safely or undergoing a bariatric surgical procedure. Additionally, potential participants had HbA1c above 7% regardless of ongoing treatment, or ≥ 6.5% on two oral antihyperglycemic medications or insulin, and with stable-dose treatment(s) for more than 8 weeks. Participants were excluded for detectable glutamic acid decarboxylase antibody, history of diabetic ketoacidosis, HbA1c > 12%, gastrointestinal diseases, malignancy within 5 years, significant cardiopulmonary or renal diseases, active eating disorders, drug and/or alcohol abuse, impaired mental status, weight loss > 3% within the previous 3 months, participation in an alternate weight-reduction program, or use of weight-reduction medications. Participants had to be nonsmoking for over 2 months (see Supplemental Data for additional information).
Randomization and interventions
The protocol was approved by the Partners Healthcare Institutional Review Board and the FDA. All participants provided written informed consent. An independent data monitoring committee reviewed patient safety. Potentially interested participants attended in-person orientations where study design, surgical options, and medical interventions were reviewed. People with a preference for a bariatric procedure other than LAGB were not enrolled. Those interested in the trial were screened for appropriateness for both surgical and medical interventions. Randomization was computer-generated in centrally allocated blocks of four.
LAGB was performed at Brigham and Women's Hospital. Patients were administered routine antibiotic and venous thromboembolism prophylaxis and standardized anesthesia per hospital protocols. Peritoneal attachments on the left crus of the diaphragm were dissected, and the band was wrapped around the upper portion of the stomach to create a “virtual pouch,” avoiding posterior dissection. To prevent slippage, the band was secured anteriorly with gastro-gastric sutures. The sc port was secured to the abdominal wall with stay sutures. The band was primed with fluid but not filled at the time of surgery.
The study participants randomized to surgery were managed in the same way as other patients undergoing bariatric surgery at our hospital. Initial postoperative care included a visit with the surgical team 2 weeks after surgery. The first fill was offered 6 weeks after surgery, when indicated. Follow-up appointments were recommended every 4–6 weeks thereafter. During these visits, diet and symptoms were reviewed to determine the need for band adjustment. A slow diet progression (3 d of liquid/soft food) was recommended after band adjustments with counseling to eat healthy solid foods, focusing on protein. Patients were encouraged to perform physical activity as tolerated. Program bariatric dietitians were available for appointments as needed, but the study did not provide any other formal support as an adjunct for ongoing postsurgical weight loss.
Medical arm-randomized participants enrolled in the Weight Achievement and Intensive Treatment (Why WAIT) program, designed for clinical diabetes practice and run quarterly at the Joslin Diabetes Center for groups of 10–15 patients (11). Why WAIT's multidisciplinary approach includes an endocrinologist, dietician, exercise physiologist, mental health provider, and diabetes nurse educator with 2-hour weekly group sessions over 12 weeks (initiation phase). Patients receive medication adjustments using a prespecified algorithm and participate in supervised group exercise and support/didactic sessions. Key aspects of Why WAIT include: weekly medication adjustments; up to 300 min/wk of graded, balanced, and individualized exercise, with emphasis on strength training (Supplemental Table 1); cognitive behavioral intervention and group education (Supplemental Table 2); structured modified dietary intervention with hypocaloric (1500–1800 kcal) diet with carbohydrates (40–45%), protein (1–1.5 g/kg or ∼30%), and reduced saturated fat below 7% (12). During the initial 6 weeks, breakfast and lunch were replaced by Boost Glucose Control (calories, 190; protein, 16 g; carbohydrate, 16 g; fiber, 3 g; fat, 7 g; Nestle Nutrition, Inc). Participants were instructed to eat two snacks of 100–200 kcal each and select a dinner from 14 structured menus. A maintenance phase of individual monthly counseling followed for the remainder of the year (see Supplemental Data for additional information).
Participants paid insurance copayments and deductibles for LAGB and Why WAIT interventions, although surgical costs were covered for participants with BMI < 35 kg/m2 by a grant from Covidien.
Follow-up and outcome assessments
Metabolic assessments were performed at baseline and repeated at 10% of initial body weight loss to obtain assessments at a comparable degree of weight loss. If 10% weight loss did not occur, metabolic assessments were performed at 3 months. Final assessments were repeated at 12 months, providing a time-based comparison. Assessments included: medications and dosing, weight (electronic scale, model 0501; Acme Scale Co), height (wall-mounted stadiometer, McKessen Medical-Surgical Inc), waist circumference (Gulak tape measure using NHLBI Clinical Guidelines), and seated blood pressure (blood pressure monitor, model BP742; Omron Healthcare). Body composition was estimated by bioelectrical impedance (body composition analyzer, model TFB-215; Tanita Corp). Exercise capacity was measured by a 6-minute walk test (13). Patient-reported outcomes were assessed using the following surveys: the Short Form 36 (SF-36) version 2 (14), Barriers to Being Active (15), Euro-QOL 5 Dimensions (EQ-5D) (16), Problem Areas in Diabetes (PAID) (17, 18), and Impact of Weight on Quality of Life (IWQOL)-Lite (19). The United Kingdom Prospective Diabetes Study (UKPDS) Risk Engine was used to calculate cardiovascular risk (20).
Laboratory
Quest Diagnostics (Cambridge, MA) performed clinical laboratory evaluations.
Statistical analysis
The primary outcome was attaining glycemic control (fasting plasma glucose levels below 7.0 mmol/L [126 mg/dL] and HbA1c below 6.5% [47.5 mmol/mol]) at 1 year of follow-up, whether or not patients were using pharmaceutical interventions.
Baseline characteristics are presented as mean ± SD, and outcome data are mean (95% confidence interval). Dichotomous and continuous variables were analyzed using logistic regression and a generalized linear mixed model, respectively, to test the null hypotheses of equal resolution of hyperglycemia and other major outcomes at 1 year. Continuous clinical endpoints were adjusted for baseline. The primary analysis was intention-to-treat, and it involved all randomly assigned patients who had at least one postrandomization assessment. For categorical data with missing values, we assumed no improvement at follow-up. We estimated sample size assuming that LAGB would result in resolution of hyperglycemia in 60% of patients (21) and medical management in 15% (11). Twenty-two subjects per group provided 80% power to detect a difference between groups with α = 0.05. Our intent was to enroll 30 per group to permit increased power, decreased effect size, or dropout. Interim analyses for superiority or futility were performed in October 2013, and enrollment was closed at 45 participants based on a modified futility analysis of the primary glycemic outcome. All subjects completed visits before data analysis.
Results
Participants
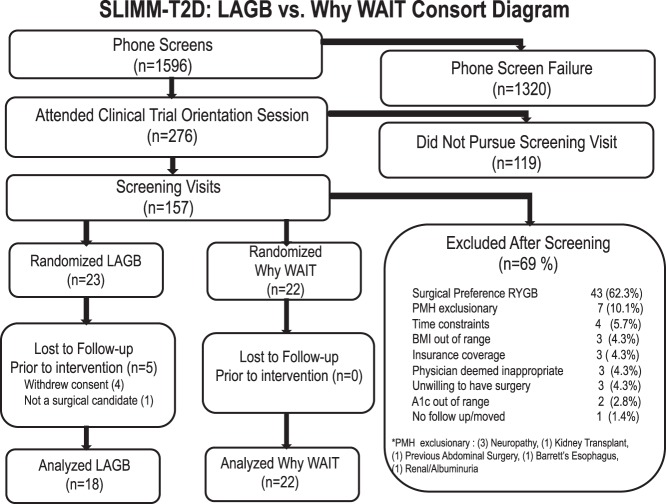
During recruitment (January, 2010, to November, 2013), 1596 potential subjects underwent phone screening, and 276 subsequently attended an orientation session (Figure 1). Of those, 157 underwent full medical screening. The most common reasons for screening failure were preference for an alternative surgical procedure or unwillingness to undergo surgery, exclusionary medical comorbidity, and time constraints. Forty-five participants were randomized to surgical (LAGB, n = 23) or IMWM (Why WAIT, n = 22) interventions. After randomization, five participants did not undergo surgery (four participants withdrew consent, one was found to have severe aortic dilatation) and are not included further in summary data because they were not evaluated beyond screening. Seven of the 18 LAGB and eight of the 22 IMWM participants had BMI below 35 kg/m2. Of those that initiated intervention, three missed 12-month assessments due to time constraints, and one moved out of state. Twelve-month retention rates were 94% in the LAGB group and 82% in the IMWM group.
Figure 1.
Enrollment, randomization, and retention of the study participants. A1c, glycated hemoglobin; n, number; PMH, past medical history.
Baseline demographics of participants undergoing intervention are provided in Table 1. Established microvascular complications were mild and infrequent.
Table 1.
Baseline Characteristics by Study Group
| Characteristic | Bariatric Surgery LAGB | IMWM (Why WAIT) |
|---|---|---|
| Age, y | 50.6 ± 12.6 | 51.4 ± 7.5 |
| Gender (male/female), n | 9/9 | 13/9 |
| Race and ethnicity | ||
| Caucasian | 11 (61) | 13 (59) |
| African American | 6 (33) | 8 (36) |
| Asian | 1 (6) | 0 (0) |
| Multiracial | 0 (0) | 1 (5) |
| Hispanica | 0 (0) | 4 (18) |
| BMI, kg/m2 | 36.4 ± 3.0 | 36.7 ± 4.2 |
| Subjects with BMI < 35 kg/m2 | 7 (39) | 8 (36) |
| Weight, kg | 106.8 ± 10.4 | 111.6 ± 17.9 |
| Systolic blood pressure, mm Hg | 129 ± 7 | 126 ± 13 |
| Diastolic blood pressure, mm Hg | 79 ± 5 | 81 ± 8 |
| Years since diagnosis | 10.4 ± 5.6 | 8.4 ± 4.2 |
| History of retinopathy | 1 (6) | 0 (0) |
| History of neuropathy | 1 (6) | 5 (23) |
| History of nephropathy | 1 (6) | 0 (0) |
| Insulin | 13 (72) | 4 (18) |
| Metformin | 13 (72) | 18 (82) |
| GLP-1 agonists | 5 (28) | 7 (32) |
| Symlin | 1 (6) | 0 (0) |
| Other glycemic medications | 2 (11) | 10 (45) |
| Statin | 14 (78) | 15 (68) |
| Other lipid-lowering medications | 4 (22) | 1 (5) |
| ACE inhibitor/ARB | 11 (61) | 14 (64) |
| Other antihypertensive medications | 9 (50) | 9 (41) |
| HbA1c, mmol/mol | 68.4 ± 12.1 | 64.8 ± 13.4 |
| HbA1c, % | 8.4 ± 1.1 | 8.1 ± 1.2 |
| Glucose, mmol/L | 9.27 ± 3.55 | 8.60 ± 2.66 |
| Glucose, mg/dL | 167 ± 64 | 155 ± 48 |
| Total cholesterol, mmol/L | 4.01 ± 0.88 | 4.17 ± 0.75 |
| Total cholesterol, mg/dL | 155 ± 34 | 161 ± 29 |
| Triglyceride, mmol/L | 1.99 ± 1.34 | 1.64 ± 1.18 |
| Triglyceride, mg/dL | 176 ± 119 | 145 ± 104 |
| HDL, mmol/L | 0.96 ± 0.23 | 1.09 ± 0.31 |
| HDL, mg/dL | 37 ± 9 | 42 ± 12 |
| LDL, mmol/Lb | 2.38 ± 0.70 | 2.36 ± 0.70 |
| LDL, mg/dL | 92 ± 27 | 91 ± 27 |
| UKPDS risk scores | ||
| Coronary heart disease risk | 10.5 ± 7.8 | 9.7 ± 8.1 |
| Fatal coronary heart disease risk | 6.5 ± 5.4 | 5.8 ± 5.3 |
| Stroke risk | 4.5 ± 3.4 | 3.7 ± 2.6 |
| Fatal stroke risk | 0.6 ± 0.5 | 0.5 ± 0.4 |
Data are expressed as mean ± SD or number (percentage). Laboratory assessments were made after an overnight fast. GLP-1, glucagon-like peptide-1; ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; UKPDS, United Kingdom Prospective Diabetes Study.
Hispanic may be of any race.
LDL measured directly.
Primary endpoint
Six of 18 subjects (33%) in the LAGB group reached the target HbA1c level below 6.5% and a fasting plasma glucose level below 7.0 mmol/L (126 mg/dL) at 12 months, compared to five of 22 (23%) in the IMWM group (P = .457) (Tables 2 and 3). One participant in the surgical group who achieved target glycemia was off all diabetes medications at 12 months, but none were off medications in the medical group.
Table 2.
Clinical and Biochemical Status by Treatment Group
| Dichotomous Clinical Endpoints | 12 Months, n (%) |
P Valuea | |
|---|---|---|---|
| LAGB | Why WAIT | ||
| n | 18 | 22 | |
| Primary Endpoint | |||
| HbA1c < 47.5 mmol/mol and FPG < 7.0 mmol/L (HbA1c < 6.5% and FPG < 126 mg/dL) | 6 (33) | 5 (23) | .457 |
| Meeting ADA treatment goals | |||
| HbA1c < 53.0 mmol/mol (<7.0%) | 10 (56) | 11 (50) | .726 |
| LDL-cholesterol < 2.59 mmol/L (<100 mg/dL) | 15 (83) | 10 (45) | .019 |
| Systolic blood pressure < 130 mm Hg | 9 (50) | 17 (77) | .078 |
| Meeting all three goals | 4 (22) | 3 (14) | .765 |
| Normoglycemia | |||
| HbA1c < 42.1 mmol/mol (<6.0%) | 5 (28) | 2 (9) | .260 |
| FPG < 5.55 mmol/L (<100 mg/dL) | 9 (50) | 9 (41) | .566 |
| Meeting both criteria | 4 (22) | 1 (5) | .231 |
Abbreviation: FPG, fasting plasma glucose; ADA, American Diabetes Association.
P values for differences between treatment groups from logistic regression (or exact logistic regression for cell counts less than 5).
Table 3.
Changes in Clinical and Biochemical Status from Baseline to 12 Months by Treatment Group
| Continuous Clinical Endpoints | Baseline, Mean ± SD |
10% Weight Loss or 3 Months, Baseline Adjusted Mean Change (95% Confidence Interval) |
12 Months, Baseline Adjusted Mean Change (95% Confidence Interval) |
P Valuea | |||
|---|---|---|---|---|---|---|---|
| LAGB | Why WAIT | LAGB | Why WAIT | LAGB | Why WAIT | ||
| Waist, cm | 115.7 ± 7.2 | 114.4 ± 9.3 | −7.4 (−9.5 to −5.3) | −7.8 (−9.7 to −5.9) | −9.2 (−13.5 to −4.8) | −7.8 (−12.2 to −3.4) | .816 |
| Lean mass, kg | 60.7 ± 10.8 | 65.3 ± 13.2 | −0.9 (−2.0 to 0.2) | −1.0 (−2.0 to −0.0) | −2.7 (−4.6 to −0.8) | −1.7 (−3.6 to 0.2) | .617 |
| Fat mass, kg | 43.9 ± 8.2 | 43.1 ± 11.0 | −8.0 (−9.6 to −6.4) | −6.6 (−8.0 to −5.1) | −10.4 (−13.0 to −7.8) | −6.7 (−9.3 to −4.2) | .053 |
| Systolic BP, mm Hg | 128.5 ± 7.4 | 126.2 ± 13.0 | 0.8 (−4.5 to 6.0) | −5.9 (−10.6 to −1.1) | 2.2 (−2.9 to 7.4) | −4.8 (−9.6 to 0.0) | .038 |
| Diastolic BP, mm Hg | 79.2 ± 5.3 | 80.9 ± 8.1 | −2.6 (−5.7 to 0.4) | −2.2 (−5.0 to 0.5) | −1.2 (−4.2 to 1.8) | −2.1 (−5.0 to 0.7) | .878 |
| Cholesterol, mmol/L | 4.01 ± 0.88 | 4.17 ± 0.75 | −0.28 (−0.58 to 0.01) | −0.47 (−0.75 to −0.20) | −0.05 (−0.39 to 0.28) | 0.12 (−0.21 to 0.44) | .955 |
| Triglycerides, mmol/L | 1.99 ± 1.34 | 1.64 ± 1.18 | −0.18 (−0.54 to 0.18) | −0.69 (−1.02 to −0.36) | −0.34 (−0.69 to 0.02) | −0.49 (−0.84 to −0.13) | .115 |
| HDL, mmol/L | 0.96 ± 0.23 | 1.09 ± 0.31 | 0.02 (−0.04 to 0.09) | 0.03 (−0.03 to 0.09) | 2.0 (0.11 to 0.30) | 0.14 (0.04 to 0.23) | .534 |
| LDL (directly measured), mmol/L | 2.38 ± 0.70 | 2.36 ± 0.70 | −0.23 (−0.48 to 0.01) | −0.23 (−0.46 to −0.00) | −0.16 (−0.44 to 0.12) | 0.14 (−0.14 to 0.42) | .301 |
| ALT, IU/L | 34.9 ± 17.7 | 28.5 ± 13.7 | −6.3 (−10.4 to −2.2) | −8.6 (−12.5 to −4.7) | −7.3 (−12.7 to −1.9) | −7.8 (−12.9 to −2.8) | .616 |
| AST, IU/L | 27.2 ± 11.4 | 23.9 ± 13.1 | −4.1 (−8.4 to 0.1) | −4.0 (−8.5 to 0.4) | −1.2 (−7.2 to 4.8) | −2.8 (−9.5 to 3.9) | .802 |
| Creatinine, μmol/L | 68.1 ± 13.3 | 69.8 ± 15.9 | −3.5 (−8.0 to 0.9) | 1.8 (−1.8 to 6.2) | −3.5 (−8.8 to 0.9) | −0.9 (−5.3 to 3.5) | .121 |
| Urinary albumin | 9.7 ± 10.1 | 17.2 ± 32.8 | ND | ND | −2.9 (−9.3 to 3.5) | −2.6 (−11.6 to 6.3) | .495 |
| Hematocrit, proportion of 1 | 0.380 ± 0.033 | 0.392 ± 0.034 | −0.016 (−0.028 to −0.004) | −0.09 (−0.020 to 0.002) | −0.006 (−0.019 to 0.007) | 0.002 (−0.010 to 0.014) | .293 |
| WBC, × 109/L | 6.7 ± 1.4 | 6.2 ± 1.7 | −0.9 (−1.3 to −0.5) | −0.6 (−1.0 to −0.2) | −0.7 (−1.2 to −0.3) | −0.4 (−0.8 to 0.0) | .185 |
| 6-min walk, m | 464 ± 59 | 518 ± 75 | −4.1 (−26.1 to 17.8) | 28.3 (8.3 to 48.3) | 29.3 (0.5 to 58.1) | 34.0 (4.6 to 63.4) | .215 |
| Heart rate recovery (1 min, BPM) | 95.2 ± 14.4 | 95.6 ± 13.9 | −7.0 (−11.7 to −2.4) | −3.4 (−7.7 to 0.9) | −6.0 (−12.7 to 0.6) | −5.4 (−12.2 to 1.3) | .527 |
Abbreviations, BP, blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BPM, beats per minute; WBC, white blood cells; ND, not determined. Urinary albumin was measured in micrograms of protein per milligram of creatinine in spot morning void.
P values for differences between treatment groups from linear mixed model corrected for baseline.
Weight and glycemia
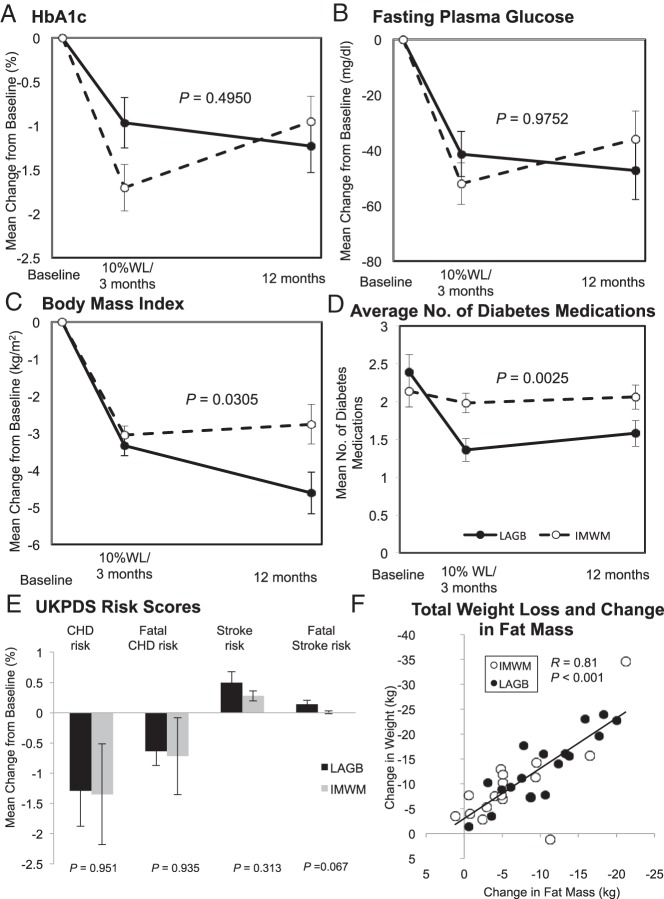
Both groups were successful in weight loss, with a similar rate and total weight loss at the early assessment. After the 2-week postoperative surgical assessment, LAGB participants attended a median of six postoperative-LAGB visits in the surgical clinic (range, 1–12), with a median of four band fills (range, 0–8). There was no correlation between the number of follow-up surgical visits or the number of band fills at these visits and change in weight or excess weight lost. Seven participants in each group achieved a 10% weight loss before 3 months from start of intervention. Additional weight loss occurred in the LAGB group over 12 months such that differences emerged over time (P = .027), with 12-month weight loss of −13.5 ± 1.7 kg (P < .001 vs baseline) in LAGB patients and −8.5 ± 1.6 kg (P < .001) in IMWM patients (Figure 2), and trend for 3.6 kg greater fat mass loss, estimated by bioelectrical impedance (P = .053) (Tables 2 and 3). Changes in fat free (lean) mass (P = .617) and reductions in waist circumference (P = .856) were similar.
Figure 2.
A–C, Glycemic measures of changes in HbA1c (A), fasting blood glucose (B), and BMI (C) are graphed by treatment group and time as baseline adjusted means with SE. P Values are for the difference between groups in linear mixed model adjusted for baseline. D, Mean number of diabetes medications is shown by treatment group and time. E, Changes from baseline for UKPDS risk scores are shown with SE for coronary heart disease (CHD), fatal CHD, stroke, and fatal stroke. Variance shown is SE. Black bars, LAGB; gray bars, IMWM. F, The relationship between total weight lost and change in fat by bioelectrical impedance is shown. ●, LAGB; ○, IMWM. WL, weight loss.
Overall, there was no difference in HbA1c reduction from baseline between groups over 12 months (P = .495). At the early assessment, HbA1c reductions trended greater in IMWM patients (P = .058) (Figure 2), but at 12 months, reductions were similar within LAGB (−1.23 ± 0.30%; P < .001 vs baseline) and IMWM (−0.95 ± 0.28%; P = .001). The pattern for reductions in fasting glucose was similar to HbA1c and between groups (P = .975) (Figure 2). Early reductions in fasting glucose were sustained and reduced from baseline by −47.3 ± 10.5 mg/dL (P < .001) within the LAGB group and −36.0 ± 10.2 mg/dL (P < .001) within the IMWM group. Other glycemic thresholds, including the proportion achieving HbA1c below 7% or below 6% and fasting glucose below 5.5 mmol/L (100 mg/dL) were likewise similar between groups (Tables 2 and 3).
Blood pressure and lipids
Systolic blood pressure was reduced more from baseline after IMWM than LAGB (P = .038), without a difference in change of diastolic blood pressure, total cholesterol, triglycerides, high-density lipoprotein (HDL) or low-density lipoprotein (LDL) cholesterol at 12 months (Tables 2 and 3). A greater proportion of persons achieved LDL-cholesterol below 2.59 mmol/L (100 mg/dL) after LAGB than IMWM (83 vs 45%; P = .019). Reductions in the use of antihypertensives were similar between groups, but there were greater reductions in lipid-lowering medications in LAGB (P = .029) (Supplemental Figure 1).
Cardiometabolic risk
The change in fitness, assessed by the 6-minute walk test distance and heart rate 1 minute after exercise, was similar in both groups. Changes in cardiometabolic risk scores for coronary heart disease, fatal coronary heart disease, stroke, and fatal stroke, estimated using the UKPDS risk engine, were all similar 12 months after LAGB and IMWM (Figure 2).
Patient-reported outcomes
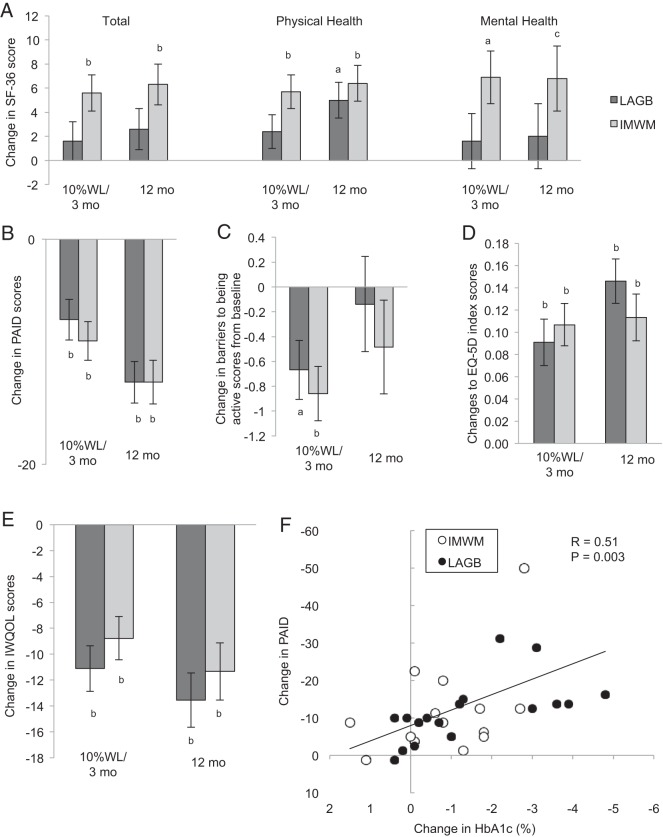
At baseline, subjects exhibited moderately low SF-36 total, physical health (PH), and mental health (MH), and moderately elevated IWQOL and PAID health status scores, consistent with moderate distress across all axes (Figure 3 and Supplemental Table 3).
Figure 3.
Patient-reported outcomes are shown for SF-36 including total score, PH, and MH subscores (A); PAID (B); Barriers to Being Active (C); EQ-5D (trademark of the EuroQol Group) index scale (D); and IWQOL-lite (E). Data are graphed by treatment group and time as baseline adjusted mean change from baseline and SE. Dark gray columns, LAGB; light gray columns, IMWM. Baseline mean and SD of all patient-reported outcomes are provided in Supplemental Table 3. Comparisons between groups were not statistically significant overall, or at early or 12-month assessment. F, The relationship between change in HbA1c and change in PAID scores is shown. ●, LAGB; ○, IMWM. a, P < .01; b, P < .001; and c, P < .05 (within group change from baseline, not between group differences).
There was no overall effect between treatment groups for change in SF-36 total, PH, and MH measures. IMWM, but not LAGB, had improvements in SF-36 total and MH scores at early and 12-month assessments. Physical subscale scores improved early only in IMWM, but both groups improved from baseline to 12 months without a difference between groups. Both groups realized similar magnitude improvements for IWQOL and PAID. At early assessment, IWQOL (−11 ± 2 vs −9 ± 2) and PAID (−7 ± 2 vs −9 ± 2) improved from baseline (all P < .05), with similar improvements in both groups. Changes from baseline in IWQOL (−14 ± 2 vs −11 ± 2) and PAID (−13 ± 2 vs −13 ± 2) further improved at 12 months in both groups, but again effects were similar in surgical and medical treatment arms. In the combined cohorts, improvement in patients' self-assessment of diabetes-specific emotional distress as assessed by PAID correlated with improvement in HbA1c (r = 0.51; P = .003), but not with a change in weight (P = .838). Both groups improved EQ-5D scores from baseline at early and 12-month assessments, with similar improvements between groups. Barriers to Being Active scores improved from baseline to a similar magnitude in both groups at early assessment, but change from baseline was not sustained at 12 months in either group, with no differences between groups.
Adverse events
No participant experienced severe hypoglycemia (requiring assistance). Surgical postintervention serious adverse events included: one patient who failed placement of LAGB due to unexpected large paraesophageal hernia; two patients who had prolonged hospital stays, one by 1 day for glycemic management, and one by 5 days for difficulty tolerating oral intake; and one patient who had surgical intervention for syringomyelia. One serious adverse event in the IMWM group was ischemic heart disease leading to coronary artery bypass surgery. All patients with adverse events were included in data analysis.
Discussion
Risks and benefits of bariatric surgery compared to nonsurgical medical management for obese T2D patients, particularly those with lesser-magnitude obesity, are of increasing interest. We and others confirm the ability to perform randomized clinical trials comparing these differing therapeutic approaches for T2D management and to inform clinical decision-making (8, 21–25). Although randomized trials are feasible, broad outreach is required to identify amenable patients. Despite efforts to assess the willingness to undergo surgical or nonsurgical interventions, more participants randomized to surgery withdrew before intervention. Because insurance coverage was secured for those with BMI above 35 kg/m2 and the cost was covered by the study for those below 35 kg/m2, concern about the surgery itself, rather than surgical cost may have impacted withdrawal. Given the resources required for recruitment, retention, and clinical care, conducting outcome trials may be cost prohibitive (26, 27), and the medical community may need to rely on findings from observational studies and multiple smaller randomized studies.
The proportion of patients achieving the primary outcome of both HbA1c < 6.5% and fasting glucose < 7 mmol/L and other relevant glycemic thresholds was comparable across both LAGB and IMWM therapeutic strategies. Weight loss was initially similar, but LAGB realized additional reductions over the year. The necessary weight loss for metabolic improvement is not clear, but resolution of obesity-related comorbidities is generally proportional to the extent of weight loss (2, 28). Improvement in systolic blood pressure was greater after IMWM, but the proportion achieving LDL-cholesterol below 2.59 mmol/L (100 mg/dL) was greater after LAGB, such that overall cardiometabolic risk improved similarly. In this group of patients willing to be randomized to medical or surgical management, satisfaction assessed by patient-reported outcomes was also similar, although similar-magnitude improvements in patient-reported diabetes burden were achieved in very different ways. Similarity of results with relatively advanced T2D may guide disease management and be reassuring for patient and provider when strong patient preferences are present.
Our trial is notable for the use of a clinically adapted IMWM program designed specifically for application in real-world clinical practice (11). The Why WAIT program, which provides more emphasis on dietary composition and the duration and type of exercise and adjusts diabetes medications to maximize weight loss, is more structured than previous models of lifestyle intervention used in the Diabetes Prevention Program and LookAHEAD studies (5, 6). Favorable glycemic and weight reductions occurred with medical and lifestyle intervention and were sustained over the first 12 months. This contrasts to the glycemic recidivism in the IMWM arm of our parallel study comparing gastric bypass to IMWM (using the same program) (8), which may be due to increased patient commitment to undergo two interventions that both require maintenance and active follow-up.
Advanced age, diabetes duration, insulin use, and HbA1c levels are inversely related to postoperative diabetes remission (29). Duration of diabetes and insulin or oral management as proxy for disease severity were not used for inclusion/exclusion criteria in our study. Our cohort was representative of a population with relatively advanced disease, yet relatively few diabetes-related complications. Notably, only one participant in the LAGB group achieved glycemic targets off medication. Of the two randomized trials with LAGB compared to nonsurgical management for T2D and follow-up of 1 year or more (21, 22), Dixon et al (21) demonstrated higher (73%) diabetes “remission” rates in the band cohort, which had substantially shorter diabetes duration (under 2 y), and LAGB subjects additionally received the comprehensive medical intervention. Courcoulas et al (22) report 27% partial and 23% complete diabetes remission 1 year after LAGB using modestly different glycemic criteria than ours, but our cohort is older, with longer baseline duration diabetes, higher use of insulin, and higher HbA1c. Thus, our study population informs a clinically relevant group where optimal management is highly uncertain. Furthermore, 37.5% of our cohort had initial BMI of 30–35 kg/m2, for whom comparative diabetes management data is particularly sparse (30). Although improved glycemia may not last indefinitely after surgery (31) or medical intervention, metabolic control sustained over a period of time may have delayed health benefits for T2D of both shorter and longer duration (32, 33) and may support aggressive interventions for patients who are appropriate for surgical risk and/or unsupervised exercise.
Observational studies suggest improved long-term survival for obese persons undergoing bariatric surgeries, including LAGB (31, 34–38). However, it is possible in nonrandomized studies that healthier persons were referred to or elected surgical approaches. Thus, long-term evaluation of randomized patients will be of continued value to inform clinical decision-making. LAGB intervention was not without risk, with one large esophageal hernia unrecognized preoperatively resulting in failed band placement, and two longer than average postoperative hospital stays including one for difficulty swallowing. These events did not adversely impact patient-reported outcomes compared with nonsurgical management. Serious adverse events were numerically more frequent in surgical patients, and possible debilitating surgical events (23) could substantially offset any favorable metabolic improvements. Individual and societal risk tolerance may differ. The potential impact for explants over time and lack of data on cardiovascular and mortality outcomes must additionally temper enthusiasm for endorsement of LAGB procedures for T2D management at this time.
This study had limitations. Because of the small sample size and 12-month follow-up of this feasibility trial, results related to efficacy outcomes should be considered preliminary and do not permit assessment of either cardiovascular or mortality outcomes or longer durability of metabolic responses. This is especially relevant if one were to make public health policy changes to recommend surgery as a therapeutic strategy for diabetes management (39). The American Recovery and Reinvestment Act (ARRA, Stimulus Act) feasibility funding for this randomized trial comparing bariatric and metabolic surgeries to medical approach did not permit extended follow-up. Although our study cohort spans a range of diabetes duration and severity, we cannot assess outcomes with earlier, milder disease or those with more advanced complications. Despite randomization, participants in the surgical arm had more insulin use at baseline and could be less likely to achieve the dichotomous endpoint. It is possible that participants willing to be randomized to surgery are not representative of motivated patients willing only to participate in an intensive medical-management program or vice versa—thus adversely impacting metabolic response in one group over the other. We did not study emerging surgical approaches, such as the gastric sleeve that is now used with increasing frequency (1, 40). Likewise, pharmacological agents and an additional device (the Maestro Rechargeable System) gained FDA approval since study initiation, and we do not address how these might augment either approach. This study was not masked to treatment assignment. We recognized that rates of response were lower than predicted in LAGB participants and higher than predicted in IMWM. In a modified futility analysis, if rates of meeting glycemic targets were met, approximately 180 participants would be needed to demonstrate superiority. Although increased numbers of participants were initially planned to permit increased power and detection of decreased effect size or to accommodate dropouts, enrollment was closed because obtaining evidence of superiority was substantially beyond funding limits.
In conclusion, after 12 months, weight loss was greater after LAGB; however, glycemic reduction was similar compared to an IMWM program. Improvement in blood pressure, triglycerides, and LDL-cholesterol did not differ, and the 5-year risk of coronary heart disease events, as determined by UKPDS risk engine scores, decreased comparably between groups. Patient self-reported outcomes, including the IWQOL and PAID, improved similarly with both treatment strategies. These findings suggest that LAGB and IMWM, as provided by the Why WAIT program, have generally similar benefits on diabetes control, cardiometabolic risk, and quality of life parameters. These results may be useful in guiding obese patients with T2D when they explore their options for glycemic and weight management.
Acknowledgments
We thank the study participants and Why WAIT team.
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (RC1-DK086918, R56-DK095451, and P30-DK03836), Patient-Centered Outcomes Research Institute (CE-1304-6756), the Marietta Blau Grant ICM-2010-02797 from the Österreichischer Austausdienst, and the Herbert Graetz Fund. Covidien provided funds for surgical costs of participants randomized to surgery with BMI < 35 kg/m2. Lifescan, a Division of Johnson & Johnson, provided home glucose monitoring supplies. Nestle Nutrition Inc provided Boost. Novo Nordisk provided FDA-approved medications to be administered according to label. We also acknowledge support from the Joslin Clinical Research Center and thank its philanthropic donors. No funding source had a role in trial design, conduct, data analysis, or manuscript preparation.
Trial Registration: Clinicaltrials.gov NCT01073020.
Author Contributions: A.B.G. and D.L. designed the study and secured the funding. D.C.S. monitored data collection, wrote the statistical analysis plan, analyzed the data, and reviewed the manuscript. All authors contributed to the development of data collection tools, recruitment efforts, participant visits, and participant safety. A.B.G. wrote the manuscript, and all authors reviewed, revised, and approved the final manuscript version.
Disclosure Summary: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: investigator initiated grant support from the National Institute of Health, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), Covidien, and the Herbert Graetz Fund for the submitted work, with supplies from Lifescan, a Division of Johnson and Johnson, Nestle Inc, and Novo Nordisk. A.B.G. has also received research grants and honoraria from the National Heart, Lung, and Blood Institute and the American Diabetes Association, has been on the Scientific Advisory Board for Novo Nordisk, consulted for the Colorado Prevention Center at University of Colorado Denver, and received supplies for investigator initiated work from Amneal Pharmaceuticals and Caraco Pharmaceuticals. D.L. reports personal fees from Covidien outside of the submitted work. M.W. received grant support from the Marietta Blau Grant from the Österreichischer Austausdienst. D.C.S. received funds from the Patient-Centered Outcomes Research Institute. O.H. reports grants from Metagenics Inc and Neurometrix and personal fees from Abbott Nutrition and Merck Pharmaceutical Inc, outside the submitted work. No other relationships or activities occurred that could appear to have influenced the submitted work.
For related article see page 2536
- BMI
- body mass index
- HbA1c
- glycated hemoglobin
- HDL
- high-density lipoprotein
- IMWM
- intensive medical and weight management
- LAGB
- laparoscopic adjustable gastric band
- LDL
- low-density lipoprotein
- MH
- mental health
- PH
- physical health
- T2D
- type 2 diabetes.
References
- 1. Hutter MM, Schirmer BD, Jones DB, et al. First report from the American College of Surgeons Bariatric Surgery Center Network: laparoscopic sleeve gastrectomy has morbidity and effectiveness positioned between the band and the bypass. Ann Surg. 2011;254:410–420; discussion 420–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248–256.e5. [DOI] [PubMed] [Google Scholar]
- 3. Puzziferri N, Roshek TB, 3rd, Mayo HG, Gallagher R, Belle SH, Livingston EH. Long-term follow-up after bariatric surgery: a systematic review. JAMA. 2014;312:934–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pinkney JH, Johnson AB, Gale EA. The big fat bariatric bandwagon. Diabetologia. 2010;53:1815–1822. [DOI] [PubMed] [Google Scholar]
- 5. Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Look AHEAD Research Group, Wing RR. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: four-year results of the Look AHEAD trial. Arch Intern Med. 2010;170:1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ali MK, Bullard KM, Saaddine JB, Cowie CC, Imperatore G, Gregg EW. Achievement of goals in U.S. diabetes care, 1999–2010. N Engl J Med. 2013;368:1613–1624. [DOI] [PubMed] [Google Scholar]
- 8. Halperin F, Ding SA, Simonson DC, et al. Roux-en-Y gastric bypass surgery or lifestyle with intensive medical management in patients with type 2 diabetes: feasibility and 1-year results of a randomized clinical trial. JAMA Surg. 2014;149:716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Look AHEAD Research Group, Wadden TA, West DS, Delahanty L, et al. The Look AHEAD study: a description of the lifestyle intervention and the evidence supporting it. Obesity (Silver Spring). 2006;14:737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Look AHEAD Research Group, Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamdy O, Carver C. The Why WAIT program: improving clinical outcomes through weight management in type 2 diabetes. Curr Diab Rep. 2008;8:413–420. [DOI] [PubMed] [Google Scholar]
- 12. Giusti J, Rizzotto JA. Interpreting the Joslin Diabetes Center and Joslin Clinic Clinical nutrition guideline for overweight and obese adults with type 2 diabetes. Curr Diab Rep. 2006;6:405–408. [DOI] [PubMed] [Google Scholar]
- 13. Beriault K, Carpentier AC, Gagnon C, et al. Reproducibility of the 6-minute walk test in obese adults. Int J Sports Med. 2009;30:725–727. [DOI] [PubMed] [Google Scholar]
- 14. McHorney CA, Ware JE, Jr, Lu JF, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. [DOI] [PubMed] [Google Scholar]
- 15. Barriers to Being Active Quiz. Centers for Disease Control and Prevention web site. http://www.cdc.gov/diabetes/ndep/pdfs/8-road-to-health-barriers-quiz-508.pdf Accessed February 7, 2015.
- 16. EuroQol Group Association web site. http://www.euroqol.org/about-eq-5d.html Accessed February 7, 2015.
- 17. Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabet Med. 2003;20:69–72. [DOI] [PubMed] [Google Scholar]
- 18. Polonsky WH, Anderson BJ, Lohrer PA, et al. Assessment of diabetes-related distress. Diabetes Care. 1995;18:754–760. [DOI] [PubMed] [Google Scholar]
- 19. Kolotkin RL, Crosby RD. Psychometric evaluation of the impact of weight on quality of life-lite questionnaire (IWQOL-lite) in a community sample. Qual Life Res. 2002;11:157–171. [DOI] [PubMed] [Google Scholar]
- 20. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. [DOI] [PubMed] [Google Scholar]
- 21. Dixon JB, O'Brien PE, Playfair J, et al. Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA. 2008;299:316–323. [DOI] [PubMed] [Google Scholar]
- 22. Courcoulas AP, Goodpaster BH, Eagleton JK, et al. Surgical vs medical treatments for type 2 diabetes mellitus: a randomized clinical trial. JAMA Surg. 2014;149:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ikramuddin S, Korner J, Lee WJ, et al. Roux-en-Y gastric bypass vs intensive medical management for the control of type 2 diabetes, hypertension, and hyperlipidemia: the Diabetes Surgery Study randomized clinical trial. JAMA. 2013;309:2240–2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schauer PR, Kashyap SR, Wolski K, et al. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mingrone G, Panunzi S, De Gaetano A, et al. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577–1585. [DOI] [PubMed] [Google Scholar]
- 26. Courcoulas AP, Yanovski SZ, Bonds D, et al. Long-term Outcomes of bariatric surgery: a National Institutes of Health Symposium. JAMA Surg. 2014;149:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lautz D, Halperin F, Goebel-Fabbri A, Goldfine AB. The great debate: medicine or surgery: what is best for the patient with type 2 diabetes? Diabetes Care. 2011;34:763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150:94–103. [DOI] [PubMed] [Google Scholar]
- 29. Wang GF, Yan YX, Xu N, et al. Predictive factors of type 2 diabetes mellitus remission following bariatric surgery: a meta-analysis. Obes Surg. 2015;25:199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Maggard-Gibbons M, Maglione M, Livhits M, et al. Bariatric surgery for weight loss and glycemic control in nonmorbidly obese adults with diabetes: a systematic review. JAMA. 2013;309:2250–2261. [DOI] [PubMed] [Google Scholar]
- 31. Arterburn DE, Bogart A, Sherwood NE, et al. A multisite study of long-term remission and relapse of type 2 diabetes mellitus following gastric bypass. Obes Surg. 2013;23:93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–1589. [DOI] [PubMed] [Google Scholar]
- 33. Gaede P, Lund-Andersen H, Parving HH, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med. 2008;358:580–591. [DOI] [PubMed] [Google Scholar]
- 34. Adams TD, Gress RE, Smith SC, et al. Long-term mortality after gastric bypass surgery. N Engl J Med. 2007;357:753–761. [DOI] [PubMed] [Google Scholar]
- 35. Sjöström L, Narbro K, Sjöström CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med. 2007;357:741–752. [DOI] [PubMed] [Google Scholar]
- 36. Sjöström L, Peltonen M, Jacobson P, et al. Bariatric surgery and long-term cardiovascular events. JAMA. 2012;307:56–65. [DOI] [PubMed] [Google Scholar]
- 37. Vest AR, Heneghan HM, Agarwal S, Schauer PR, Young JB. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–1777. [DOI] [PubMed] [Google Scholar]
- 38. Johnson BL, Blackhurst DW, Latham BB, et al. Bariatric surgery is associated with a reduction in major macrovascular and microvascular complications in moderately to severely obese patients with type 2 diabetes mellitus. J Am Coll Surg. 2013;216:545–556; discussion 556–548. [DOI] [PubMed] [Google Scholar]
- 39. Rubino F, Kaplan LM, Schauer PR, Cummings DE. The Diabetes Surgery Summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg. 2010;251:399–405. [DOI] [PubMed] [Google Scholar]
- 40. Buchwald H, Oien DM. Metabolic/bariatric surgery worldwide 2011. Obes Surg. 2013;23:427–436. [DOI] [PubMed] [Google Scholar]