Abstract
Context:
Ecuadorian subjects with GH receptor deficiency (GHRD) have not developed diabetes, despite obesity.
Objective:
We sought to determine the metabolic associations for this phenomenon.
Design:
Four studies were carried out: 1) glucose, lipid, adipocytokine concentrations; 2) metabolomics evaluation; 3) metabolic responses to a high-calorie meal; and 4) oral glucose tolerance tests.
Setting:
Clinical Research Institute in Quito, Ecuador.
Subjects:
Adults homozygous for the E180 splice mutation of the GH receptor (GHRD) were matched for age, gender, and body mass index with unaffected control relatives (C) as follows: study 1, 27 GHRD and 35 C; study 2, 10 GHRD and 10 C; study 3, seven GHRD and 11 C; and study 4, seven GHRD and seven C.
Results:
Although GHRD subjects had greater mean percentage body fat than controls, their fasting insulin, 2-hour blood glucose, and triglyceride levels were lower. The indicator of insulin sensitivity, homeostasis model of assessment 2%S, was greater (P < .0001), and the indicator of insulin resistance, homeostasis model of assessment 2-IR, was lower (P = .0025). Metabolomic differences between GHRD and control subjects were consistent with their differing insulin sensitivity, including postprandial decreases of branched-chain amino acids that were more pronounced in controls. High molecular weight and total adiponectin concentrations were greater in GHRD (P = .0004 and P = .0128, respectively), and leptin levels were lower (P = .02). Although approximately 65% the weight of controls, GHRD subjects consumed an identical high-calorie meal; nonetheless, their mean glucose concentrations were lower, with mean insulin levels one-third those of controls. Results of the 2-hour oral glucose tolerance test were similar.
Main Outcome Measures:
Measures of insulin sensitivity, adipocytokines, and energy metabolites.
Conclusions:
Without GH counter-regulation, GHRD is associated with insulin efficiency and obesity. Lower leptin levels, despite higher percentage body fat, suggest that obesity-associated leptin resistance is GH dependent. Elevated adiponectin levels not correlated with percentage body fat indicate that GH signaling is necessary for their typical suppression with obesity.
In 1931, Bernardo Houssay commented, “An indirect action [of the pituitary] might be carried out by the regulation of the insulin secretion or the neutralization of its effects. We believe that in the future the study of the influence of the pituitary on carbohydrate metabolism will reveal further information on their interrelation” (1).
GH receptor deficiency (GHRD) resulting in attenuation of GH action and severe IGF-1 deficiency is a rare autosomal recessive condition affecting an estimated 300 subjects worldwide. One-third of the affected individuals originate from southern Ecuador, almost all of whom are homozygous for the E180 splice mutation in the GHR gene (2). This cohort, unmatched in size and homogeneity, offers a unique opportunity to compare characteristics of affected individuals to those of their unaffected relatives living under the same conditions. Despite a high frequency of obesity, no Ecuadorians with GHRD have developed diabetes, whereas the background population has an overt type 2 diabetes (T2D) prevalence of 6% (3).
In this report, we are presenting data relevant to insulin sensitivity in the GHRD population and relative control subjects. These observations provide information about the effects of the absence of GH action due to GHRD, documenting marked insulin sensitivity despite a high percentage of body fat, thereby supporting the hypothesis that GH signaling contributes to the development of insulin resistance associated with obesity.
Subjects and Methods
The investigation comprised four studies: 1) carbohydrate, lipid, and adipocytokine determinations in an adult population of 27 GHRD subjects and 35 age- (± 5 years) and body mass index (BMI) (± 3 kg/m2) matched control relatives; 2) metabolome analyses (metabolomics) of five males and five females with GHRD and age- and BMI-matched control relatives; 3) metabolic responses for 5 hours after a standard high-calorie meal in seven GHRD and 11 age- and BMI-matched control relatives; and 4) oral glucose tolerance tests (OGTTs) of seven GHRD and seven age- and BMI-matched control relatives.
BMI was calculated as weight in kilograms/height in meters squared. Body composition examinations were performed with Lunar dual-energy x-ray absorptiometry apparatus, and the results were analyzed for percentage body fat, ratio of lean to fat mass (L/F), and ratio of android to gynoid fat (A/G).
Insulin was measured by RIA; total cholesterol (TC), high-density lipoprotein-cholesterol (HDL-C), low-density lipoprotein-cholesterol (LDL-C), very LDL (VLDL)-cholesterol, apolipoprotein (Apo) A, Apo B, and triglycerides (TGs) were measured by standard methods (4, 5). Total and high molecular weight (HMW) adiponectin and leptin concentrations were determined by ELISA using kits from R&D Systems Inc and Millipore Corporation, respectively.
For the study of 27 GHRD and 35 control subjects, 1.5 g glucose/kg body weight was administered and samples were obtained at baseline and 2 hours. The Oxford online HOMA2 calculator (http://www.dtu.ox.ac.uk/homacalculator) was used to estimate HOMA β-cell function (HOMA2%B), insulin sensitivity (HOMA2%S), and insulin resistance (HOMA2-IR) (6, 7).
Metabolome analysis was performed as previously described (8) at the National Institutes of Health West Coast Metabolomics Center (UC Davis) at fasting in five males with GHRD and five sex-, age-, and BMI-matched control relatives and at fasting and 120 minutes after ingestion of 1.5 g glucose/kg body weight in five female GHRD subjects and five control relatives.
A meal providing 996 calories, consisting of 114 g of carbohydrate, 47 g of fat, and 40 g of protein, was administered after an overnight fast. Specimens for glucose, insulin, TG, and other components of the lipid profile were obtained at baseline and at 15-minute intervals for 1 hour, followed by 30-minute intervals for another 4 hours. The OGTT involved administration of 1.75 g glucose/kg body weight, and samples for glucose and insulin measurement were obtained at baseline, every 15 minutes for 1 hour, at 90 minutes, and at 2 hours. Areas under the curve (AUCs) were calculated for glucose, insulin, triglycerides; for the meal study, data were log-transformed because of the wide variability and small sample size. The 5-hour response to the high-calorie meal involved two waves, baseline to 150 minutes, and 180 to 300 minutes, necessitating separate AUC calculations for each of these periods.
Statistical comparisons of means were made using two-tailed t tests. Spearman's test was used to calculate correlations. Values are expressed as means ± SD in Tables 1 and 3 and as SEM in the figures. P values of <.05 were considered significant.
Table 1.
Anthropometric Data, Lipid Metabolism, Carbohydrate Metabolism, and Insulin Sensitivity Measures for 35 Controls and 27 GHRD Subjects
| Controls | GHRD | P | |
|---|---|---|---|
| Anthropometrics | |||
| Age, y | 39.8 (13) | 34.5 (11) | .09 |
| SDS ht | −1.7 (1.2) | −7.4 (1.2) | <.0001 |
| BMI, kg/m2 | 29.4 (4.4) | 27.6 (5.6) | .16 |
| A/G fat | 1.08 (0.18) | 1.07 (0.09) | .79 |
| % Fat | 41.1 (6.6) | 47.7 (8.9) | .0014 |
| L/F | 1.48 (0.47) | 1.18 (0.48) | .016 |
| Lipids | |||
| Total C, mg/dL | 199 (43.9) | 229 (47.3) | .0124 |
| HDL, mg/dL | 43.5 (13.7) | 50.9 (12.8) | .034 |
| HDL-C, mg/dL | 4.87 (1.33 | 4.65 (1.10) | .49 |
| LDL, mg/dL | 123.1 (37.5) | 157.6 (37.4) | <.0001 |
| Apo A, g/L | 1.24 (0.23) | 1.34 (0.23) | .0007 |
| Apo B, g/L | 0.95 (0.24) | 1.085 (0.23) | .029 |
| VLDL, mg/dL | 31.5 (18.7) | 20.2 (7.6) | .0044 |
| TG, mg/dL | 158.3 (95.3) | 100.7 (37.8) | .0001 |
| Carbohydrate metabolism, adipocytokines | |||
| Fasting glucose, mg/dL | 93.2 (22.4) | 88.6 (10.6) | .34 |
| Postprandial glucose, mg/dL | 94.1 (35.4) | 77.1 (13.4) | .027 |
| Fasting insulin, μU/mL | 13.8 (15.5) | 4.29 (0.74) | .0034 |
| HOMA2%B | 141 (103) | 90 (48) | .0206 |
| HOMA2%S | 108 (87) | 261 (133) | <.0001 |
| HOMA2-IR | 1.74 (1.84) | 0.59 (0.51) | .0025 |
| Leptin, ng/mL | 10.36 (5.24) | 7.32 (4.7) | .0212 |
| Adiponectin, mg/L | 6.92 (4.41) | 9.94 (4.84) | .0128 |
| HMW adiponectin, mg/L | 4.29 (2.89) | 7.59 (4.07) | .0004 |
Abbreviations: SDS ht, SD score for height; C, cholesterol. Data are shown as mean (SD). Conversion factors: glucose to mmol/L, multiply by 0.0555; insulin to pmol/L, multiply by 6.945; LDL and VLDL to mmol/L, multiply by 0.0259; TGs to mmol/ L, multiply by 0.0113.
Table 3.
Anthropometric Data and Results for Seven GHRD (One Male) and 11 Control Subjects (Two Males) Receiving a High-Calorie Meal and Tested for 5 Hours, and for Seven GHRD and Seven Control Subjects Undergoing 2-Hour OGTT
| High-Calorie Meal |
OGTT |
|||||
|---|---|---|---|---|---|---|
| Controls | GHRD | P | Controls | GHRD | P | |
| Age, y | 25.4 (3.7) | 29.9 (1.9) | .0093 | 25.1 (2.3) | 30.1 (6) | .0619 |
| BMI, kg/m2 | 26.2 (4.2) | 25.4 (3.9) | .6912 | 28.2 (3.9) | 28 (4.6) | .9315 |
| L/F | 1.83 (0.72) | 1.32 (0.61) | .1409 | 1.61 (0.43) | 1.26 (0.62) | .2432 |
| % Fat | 37.4 (8.4) | 45.8 (10.1) | .0736 | 39.5 (7.4) | 50.1 (6.8) | .0163 |
| Mean glucose, mg/dLa | 92.7 (6.1) | 83.9 (4.9) | .0062 | 106.7 (22.1) | 112.9 (29.5) | .6674 |
| AUC glucoseb | 4.30 (0.05) | 4.33 (0.05) | .3364 | |||
| AUC glucose 0–150 minb | 4.12 (0.06) | 4.08 (0.09) | .272 | |||
| AUC glucose 180–300 minb | 4.16 (0.05) | 4.1 (0.06) | .035 | |||
| Mean insulin, μU/mLa | 33.1 (15.5) | 11.4 (4.2) | <.0001 | 52.1 (26.7) | 18.5 (9.2) | .0085 |
| AUC insulinb | 4.0 (0.05) | 3.5 (0.26) | .0006 | |||
| AUC insulin 0–150 minb | 3.73 (0.20) | 3.19 (0.37) | .0009 | |||
| AUC insulin 180–300 minb | 3.5 (0.19) | 2.94 (0.38) | .0007 | |||
| AUC TG 0–150 minb | 4.41 (0.18) | 4.39 (0.11) | .7961 | |||
| AUC TG 180–300 minb | 4.52 (0.19) | 4.19 (0.37) | .0232 | |||
Data within parentheses are SD values. Conversion factors: glucose to mmol/L, multiply by 0.0555; insulin to pmol/L, multiply by 6.945.
Mean of the means for each time point (n = 13 for high-calorie meal, n = 7 for OGTT).
Log-transformed data.
The research protocol was approved by the ethics committees of the Instituto de Endocrinologia (IEMYR), Quito, Ecuador, and the University of Southern California.
Results
Anthropometrics, lipids, carbohydrate metabolism, and adipocytokines
Although the groups were matched for BMI, there was greater mean percentage body fat and lower mean L/F in the GHRD subjects despite no difference in A/G (Table 1). GHRD subjects had higher fasting TC, HDL-C, LDL-C, and Apo A and B concentrations; the mean ratio of TC to HDL-C did not differ between the two groups. Fasting insulin, 2-hour blood glucose, VLDL-cholesterol, and TG concentrations were lower in subjects with GHRD than in controls. HOMA2%B and HOMA2-IR were lower, and HOMA2%S was greater in those with GHRD (Table 1).
Total and HMW adiponectin concentrations were greater in the GHRD cohort than in controls. Mean leptin concentration was less in those with GHRD than in control subjects (Table 1). Correlations informative about insulin sensitivity, of percentage fat, serum insulin, leptin, total and HMW adiponectin concentrations, and HOMA2%S and HOMA2-IR are presented in Table 2. Although total and HMW adiponectin concentrations correlated with HOMA2%S in controls, there was no significant correlation for GHRD subjects. Leptin concentrations correlated with HOMA2%S and HOMA2-IR in GHRD subjects only. In neither group did insulin concentrations correlate with HMW adiponectin levels.
Table 2.
Correlation Coefficients Among Percentage Body Fat as Determined by Dual-Energy X-Ray Absorptiometry; Insulin, Leptin, Total and HMW Adiponectin Concentrations; and Indices for Insulin Sensitivity (HOMA2%S) and Resistance (HOMA2-IR)
| % Fat | Insulin | Leptin | Adiponectin |
HOMA2%S | HOMA2-IR | ||
|---|---|---|---|---|---|---|---|
| Total | HMW | ||||||
| % Fat | |||||||
| GHRD | 0.401 (.038) | 0.803 (<.0001) | 0.181 (.36) | 0.241 (.23) | −0.453 (.018) | 0.450 (.019) | |
| Controls | −0.166 (.34) | 0.600 (.0004) | −0.166 (.35) | −0.110 (.58) | −0.039 (.829) | 0.120 (.49) | |
| Insulin | |||||||
| GHRD | 0.401 (.038) | 0.499 (.008) | −0.247 (.21) | −0.221 (.27) | −0.825 (<.0001) | 0.998 (<.0001) | |
| Controls | −0.166 (.34) | −0.011 (.95) | −0.398 (.018) | −0.215 (.21) | −0.562 (.0009) | 0.997 (<.0001) | |
| Leptin | |||||||
| GHRD | 0.803 (<.0001) | 0.499 (.008) | 0.076 (.71) | 0.169 (.40) | −0.572 (.002) | 0.416 (.031) | |
| Controls | 0.600 (.0004) | −0.011 (.95) | −0.305 (.075) | −0.417 (.013) | −0.124 (.507) | −0.032 (.86) | |
| Total adiponectin | |||||||
| GHRD | 0.181 (.36) | −0.247 (.21) | 0.076 (.71) | 0.880 (<.0001) | −0.321 (.10) | −0.249 (.21) | |
| Controls | −0.166 (.075) | −0.398 (.018) | −0.305 (.075) | 0.877 (<.0001) | 0.646 (<.0001) | −0.408 (.015) | |
| HMW adiponectin | |||||||
| GHRD | 0.241 (.23) | −0.221 (.27) | 0.169 (.40) | 0.880 (<.0001) | 0.352 (.07) | −0.218 (.27) | |
| Controls | −0.110 (.58) | −0.215 (.21) | −0.417 (.013) | 0.877 (<.0001) | 0.463 (.005) | −0.226 (.19) | |
| HOMA2%S | |||||||
| GHRD | −0.453 (.02) | − 0.825 (<.0001) | −0.572 (.002) | −0.321 (.10) | 0.352 (.07) | 0.998 (<.0001) | |
| Controls | −0.039 (.829) | −0.562 (.0009) | −0.124 (.507) | 0.646 (<.0001) | 0.463 (.005) | 0.996 (<.0001) | |
| HOMA2-IR | |||||||
| GHRD | 0.450 (.019) | 0.998 (<.0001) | 0.416 (.031) | −0.249 (.21) | −0.218 (.27) | 0.998 (<.0001) | |
| Controls | 0.120 (.49) | 0.997 (<.0001) | −0.032 (.86) | −0.408 (.015) | −0.226 (.19) | 0.996 (<.0001) | |
Data are shown for 27 GHRD subjects and 35 control subjects for each parameter measured. P values are in parentheses.
Metabolomic analysis
The metabolome showed acute decreases of branched-chain amino acids (BCAAs; ie, valine and leucine) 2 hours after the glucose challenge that were significantly greater in the controls than in those with GHRD, consistent with their differing insulin sensitivity (Supplemental Figure 1 and Supplemental Table 1). Other amino acids, lipids, organic acids, and minor metabolites were increased or decreased in subjects with GHRD compared to controls (Supplemental Table 1).
Response to a high-calorie meal
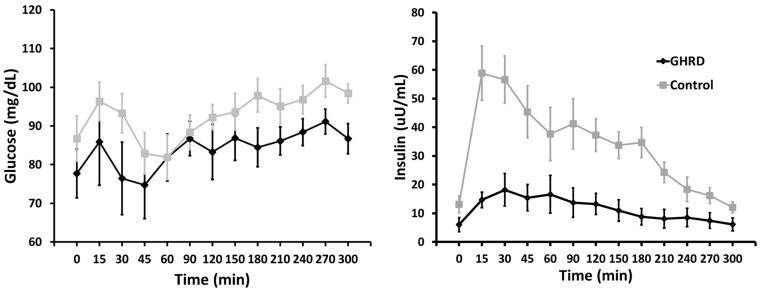
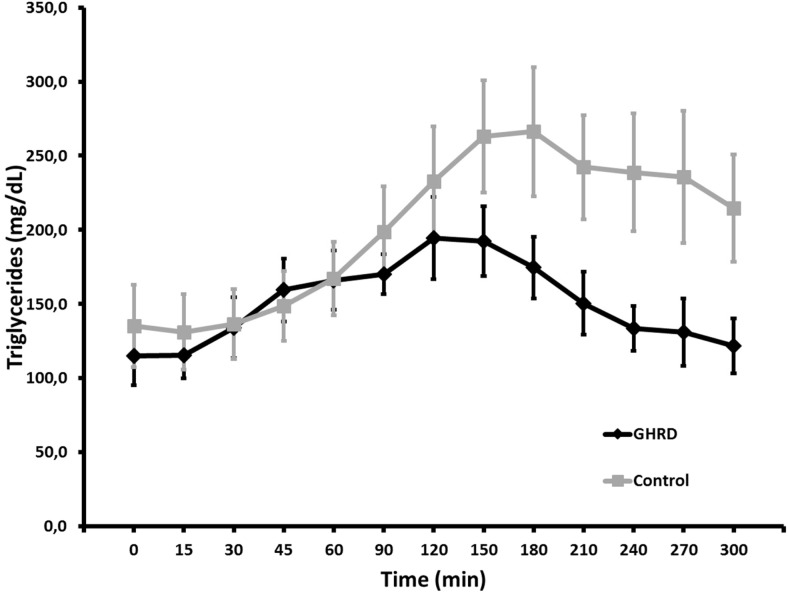
There were no differences in sex distribution, age, BMI, or L/F between the study groups, and percentage body fat was not quite significantly greater with GHRD in this subset (Table 3). Although glucose responses to the high-calorie meal were similar between GHRD and control subjects (Figure 1), the sum of the mean glucose levels at each time point was lower for GHRD subjects (Table 3). AUC analysis indicated that this was primarily due to the divergence during the second half of the 5-hour test period (Table 3). Insulin responses were markedly lower (Figure 2), and the sum of the insulin means and AUC reflected this significant difference (Table 3). Further analysis showed a significant inverse correlation between glucose and insulin levels in the GHRD group (r = −0.798; 95% confidence interval [CI], −0.950 to −0.339; P = .006), but not in the control group (r = −0.576; 95% CI, −0.885 to 0.084; P = .08). For TG, AUC did not differ between the groups during the initial 150 minutes after ingestion but diverged from 150 to 300 minutes, with GHRD subjects having an AUC significantly lower than that for controls (Figure 2 and Table 3). The other lipid concentrations were similar to those previously noted in larger cohorts (4) and in this study (Table 1) and did not diverge during the test (data not shown).
Figure 1.
Glucose and insulin responses to a high-calorie standard breakfast in seven subjects with GHRD and 11 control subjects (described in Table 3). Shown are means and SEM.
Figure 2.
TG responses to a high-calorie standard breakfast in seven subjects with GHRD and 11 control subjects (described in Table 3). Shown are means and SEM.
Results of glucose tolerance testing
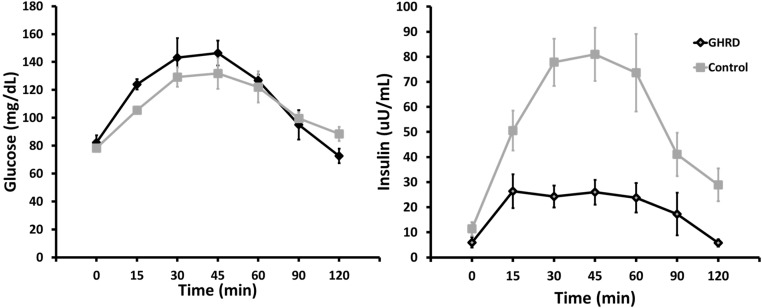
There were no significant differences from controls in age, BMI, or L/F in the GHRD group, but percentage fat was greater (Table 3). The mean glucose concentrations or AUC during the OGTT did not differ between the groups (Table 3). Insulin concentrations were greater at all timepoints after fasting in the control subjects (Figure 3), and the means and AUC for insulin reflected this marked difference (Table 3). Further analysis showed a significant correlation of glucose with insulin levels both in the GHRD group (r = 0.923; 95% CI, 0.444 to 0.992; P = .009) and in the control group (r = 0.996; 95% CI, 0.965 to 0.999; P < .0001).
Figure 3.
Glucose tolerance test results in seven subjects with GHRD and seven control subjects (described in Table 3). Oral glucose was ingested at time 0 in a dose of 1.5 g/kg body weight. Shown are means and SEM.
Discussion
We have found that the lifelong absence of GH action in individuals with GHRD is associated with markedly enhanced insulin sensitivity, despite a higher percentage body fat compared to their age- and BMI-matched unaffected relatives. In our earlier report (4) and in the current study, we have demonstrated elevated LDL-C, TC, and Apo B, consistent with dependence of the LDL receptor on GH action (9). These elevations might imply increased cardiovascular disease (CVD) risk; however, the elevated Apo A concentration and the higher HDL-C levels that result in a ratio of TC to HDL-C that is no different than in the control group suggest that the GHRD subjects do not have increased CVD risk. Indeed, we have not seen evidence of increased CVD in the Ecuadorian GHRD population (3). This is similar to observations in the GHRH receptor (GHRH-R)-deficient population with lifelong isolated GH deficiency (IGHD) (10).
T2D caused 5% of the deaths and had a prevalence of 6% among the relatives of subjects with GHRD (3); however, this disease has not been diagnosed in any individual from the Ecuadorian GHRD cohort of over 80 adult subjects. The studies we are reporting document the enhanced insulin sensitivity that explains the paradoxical coexistence of obesity and the absence of diabetes.
GHRD subjects and controls were given the same quantity of a high-calorie breakfast, although those with GHRD had a mean weight that was only 65% that of the controls. Nonetheless, the mean glucose responses of the GHRD subjects were significantly diminished, their insulin levels dramatically so, and TG responses were also lower (Table 3 and Figures 1 and 2). Our finding of low insulin levels after a high-calorie meal and during glucose tolerance testing in subjects with GHRD and the absence of diabetes contrasts with the observations of high insulin values, particularly in females, in Israeli subjects with GH insensitivity (11). Also noted in the Israeli cohort was the development of overt diabetes with severe cardiovascular complications, nephropathy, and retinopathy in two male patients of Iraqi and Yemenite Jewish origins, considered obese by skinfold measurements. This difference between Ecuadorian and Israeli GHRD subjects may be explained by variation in the timing of nutritional transitions that are known to influence the appearance of diabetes. Furthermore, the difference in diabetes risk may also be explained by a greater degree of obesity, especially in the Israeli females, ultimately exhausting the diminished β-cell reserve (12). This explanation would be consistent with our finding of significant correlations of percentage body fat with HOMA2%S and HOMA2-IR in the GHRD subjects only (Table 2). Thus, it is possible that diabetes will appear in the Ecuadorian GHRD population with the contemporary acceleration of westernization of the diet in southern Ecuador. Lower HOMA2%B in the subjects with GHRD likely reflects reduced β-cell mass, as has been demonstrated in mice with disruption of the GH receptor (13). Whereas controls had normal HOMA2%S, indicating normal insulin sensitivity, the GHRD subjects had over 2.5 times the sensitivity to insulin. Similarly, mice with disrupted GH receptor have increased insulin sensitivity and low circulating insulin and glucose concentrations, comparable to our GHRD cohort and in contrast to the observations in the Israeli population with this condition (13).
The population with severe lifelong IGHD due to mutation of the GHRH-R gene has also been described as having increased insulin sensitivity; however, their prevalence of diabetes was 16%, and the prevalence of impaired glucose tolerance was 38% (14). These abnormalities were attributed to decreased β-cell function as measured by HOMA2%B, which may not indicate diminished β-cell function or reserve, but may reflect a lesser requirement for insulin in the absence of GH counter-regulatory effects (6). The IGHD subjects also differ from our GHRD population in not having increased HDL-C or lower leptin concentrations than controls and also in not having lower TG levels, which would be expected if they truly had greater insulin sensitivity than controls (14).
Obese subjects with Prader-Willi syndrome have also been noted to have relative insulin sensitivity compared to overweight controls, which has been attributed to the fact that their fat excess is primarily sc (15, 16). However, subjects in the present study with GHRD had fat distribution comparable to BMI-matched controls, indicating that this variable was not a determinant of their enhanced insulin sensitivity.
Insulin sensitivity is reflected by characteristic metabolic fingerprints (17). Because amino acid profiles could aid in diabetes risk assessment (18), BCAAs are considered as a diagnostic test for T2D and are acutely decreased during OGTT (19). This is consistent with the observation that female control subjects in the present study had greater decreases in BCAAs after the glucose load than did those with GHRD (Supplemental Figure 1). Other amino acid levels were also different between GHRD subjects and controls; for example, proline and hydroxyproline, metabolites that may be involved in the redox state of cells (20), showed greater decline after glucose ingestion in the control females than in those with GHRD (Supplemental Table 1). An early marker for T2D is 2-hydroxybutyric acid (21), an organic acid that showed a greater decrease after the glucose load in GHRD subjects (Supplemental Table 1). These results, accompanying the enhanced insulin sensitivity, could clarify the role of specific metabolites in insulin action.
Elimination of the direct metabolic effects of GH can explain the dissociation of obesity and insulin resistance in the Ecuadorian cohort with GHRD. The increased fat mass in GHRD is presumably the result of the efficient action of insulin, unopposed by GH, that increases TG breakdown and the entry of glycerol and free fatty acids into fat stores and blocks their exit, with a net increase in the content of free fatty acids, glycerol, and TG in the adipocytes. The consequent paucity of glycerol in the circulation as a substrate for gluconeogenesis during fasting likely contributes to the commonly observed fasting hypoglycemia in children with GHRD and severe GH deficiency (11).
Elevated circulating concentrations of leptin are characteristic of obesity and are considered a contributory factor (22). GHRD subjects with higher total body fat, however, had lower leptin concentrations compared to the BMI-matched control population, and both groups had strong correlation of leptin levels with BMI (data not shown) and percentage body fat, the GHRD subjects at significantly lower leptin concentrations. Furthermore, leptin correlated with measures of insulin efficiency. The lower leptin levels of the GHRD subjects relative to controls in the present study contrast with elevations noted in adults with GHRD in Israel (23) and in children and adults with GH deficiency (24, 25). In those with IGHD due to GHRH-R deficiency, leptin levels were no different than in age-matched controls (10).
We observed higher levels of total and HMW adiponectin in subjects with GHRD compared to controls. Adiponectin, an anti-inflammatory cytokine produced by adipocytes, has been reported as correlating with insulin sensitivity and inflammation (26). Serum levels of adiponectin are reduced in obese subjects and subjects with T2D, CVD, hypertension, and metabolic syndrome. Our finding of elevated levels of adiponectin and HMW adiponectin is consistent with the observations in a small cohort of nine adults with GHRD (27) and with the observations in IGHD due to GHRH-R mutation (10). However, other GH-deficient adults and children do not differ in their adiponectin levels from age-, sex-, and BMI-matched controls (24, 25). Further inconsistent findings concerning the relationship of adiponectin concentrations with GH status include: the observation that untreated GH-deficient adolescents have lower levels than those receiving long-term recombinant GH therapy; that compared to age-, gender-, and BMI-matched controls (28), children with Prader-Willi syndrome have higher levels than controls, that increase further with GH therapy for 2 years (29); and that obese men with impaired fasting glucose treated for 18 months with GH had an increase in their levels (30). The absence of significant correlation of elevated adiponectin concentrations with measures of insulin sensitivity in the subjects with GHRD in this study and in Israeli adults (27) suggests that adiponectin in these individuals is not a major driver of insulin sensitivity (26).
In summary, subjects with GHRD do not develop diabetes because they lack the counter-regulatory effect of GH, thereby inducing a state of enhanced insulin sensitivity compared to control relatives without diabetes, and despite less insulin secretion. This critical role for GH action in the induction of insulin resistance is an important consideration in the frequent administration of pharmacological doses of recombinant human GH (rhGH) for growth promotion in children who do not have GH deficiency. A large postmarketing surveillance study noted a 6-fold increase in the development of T2D with rhGH therapy, which did not resolve when rhGH treatment was stopped (31). This is not unexpected, considering the long-recognized association of diabetes with acromegaly (1). We have also provided evidence that the obesity of GHRD can best be attributed to unopposed insulin action, unassociated with leptin resistance, and that the elevated adiponectin concentrations are an accompaniment rather than a cause of their enhanced insulin sensitivity.
Acknowledgments
This work was supported by Instituto de Endocrinologia, Metabolismo y Repróduccion (IEMYR) and Universidad San Francisco de Quito USFQ, Ecuador; the Intramural Research Program of the National Institutes of Health/National Institute on Aging; the University of Southern California Edna Jones Chair (to V.D.L.); The National Geographic Research Grant; and the Fundacion Alonso Martin Escudero (to I.A.).
This study was presented at The Endocrine Society meeting June 21–24, 2014, in Chicago, Illinois (Abstract LB-OR02-1).
Disclosure Summary: V.D.L. has equity interests in DSR Pharma Inc. V.D.L., J.G.-A., and P.B. have filed provisional patents related to the development of GHR blockers. All other authors have nothing to declare.
Footnotes
- A/G
- android to gynoid
- Apo
- apolipoprotein
- AUC
- area under the curve
- BCAA
- branched-chain amino acid
- BMI
- body mass index
- CI
- confidence interval
- CVD
- cardiovascular disease
- GHRD
- GH receptor deficiency
- GHRH-R
- GHRH receptor
- HDL-C
- high-density lipoprotein-cholesterol
- HMW
- high molecular weight
- HOMA
- homeostasis model of assessment
- HOMA2%B
- HOMA β-cell function
- HOMA2-IR
- HOMA insulin resistance
- HOMA2%S
- HOMA insulin sensitivity
- IGHD
- isolated GH deficiency
- LDL-C
- low-density lipoprotein-cholesterol
- L/F
- lean to fat mass
- OGTT
- oral glucose tolerance test
- rhGH
- recombinant human GH
- TC
- total cholesterol
- T2D
- type 2 diabetes
- TG
- triglyceride
- VLDL
- very LDL.
References
- 1. Houssay BA, Biasotti A. The hypophysis, carbohydrate metabolism and diabetes. Endocrinology. 1931;15:511–523. [Google Scholar]
- 2. Guevara-Aguirre J, Rosenbloom AL, Fielder PJ, Diamond FB, Rosenfeld RG. Growth hormone receptor deficiency in Ecuador: clinical and biochemical phenotype in two populations. J Clin Endocrinol Metab. 1993;76:417–423. [DOI] [PubMed] [Google Scholar]
- 3. Guevara-Aguirre J, Balasubramanian P, Guevara-Aguirre M, et al. Growth hormone receptor deficiency is associated with a major reduction in pro-aging signaling, cancer, and diabetes in humans. Sci Transl Med. 2011;3:70ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rosenbloom AL, Martinez V, Kranzier JH, Bachrach LK, Rosenfeld RG, Guevara-Aguirre J. Natural history of growth hormone receptor deficiency. Acta Paediatr Suppl. 1999;88:153–156, discussion 157. [DOI] [PubMed] [Google Scholar]
- 5. Hainline A, Jr, Karon J, Lippel K. Manual of Laboratory Operations: Lipid and Lipoprotein Analysis. 2nd ed Bethesda, MD: National Heart, Lung and Blood Institute, Lipid Research Clinics Program; 1982. NIH/HEW publication 75–628 (rev). [Google Scholar]
- 6. Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. [DOI] [PubMed] [Google Scholar]
- 7. Otten J, Ahrén B, Olsson T. Surrogate measures of insulin sensitivity vs the hyperinsulinaemic-euglycaemic clamp: a meta-analysis. Diabetologia. 2014;57:1781–1788. [DOI] [PubMed] [Google Scholar]
- 8. Fiehn O, Wohlgemuth G, Scholz M, et al. Quality control for plant metabolomics: reporting MSI-compliant studies. Plant J. 2008;53:691–704. [DOI] [PubMed] [Google Scholar]
- 9. Rudling M, Norstedt G, Olivecrona H, Reihnér E, Gustafsson JA, Angelin B. Importance of growth hormone for the induction of hepatic low density lipoprotein receptors. Proc Natl Acad Sci USA. 1992;89:6983–6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oliveira CR, Salvatori R, Meneguz-Moreno RA, et al. Adipokine profile and urinary albumin excretion in isolated growth hormone deficiency. J Clin Endocrinol Metab. 2010;95:693–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Laron Z. Insulin secretion and carbohydrate metabolism in patients with Laron syndrome: from hypoglycemia to diabetes mellitus. In: Laron Z, Kopchick JJ, eds. Laron Syndrome from Man to Mouse. Berlin, Heidelberg, Germany: Springer-Verlag; 2011: 259–272. [Google Scholar]
- 12. Laron Z, Ginsberg S, Lilos P, Arbiv M, Vaisman N. Body composition in untreated adult patients with Laron syndrome (primary GH insensitivity). Clin Endocrinol (Oxf). 2006;65:114–117. [DOI] [PubMed] [Google Scholar]
- 13. Liu JL, Coschigano KT, Robertson K, et al. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287:E405–E413. [DOI] [PubMed] [Google Scholar]
- 14. Vicente TA, Rocha IE, Salvatori R, et al. Lifetime congenital isolated GH deficiency does not protect from the development of diabetes. Endocr Connect. 2013;2:112–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haqq AM, Muehlbauer MJ, Newgard CB, Grambow S, Freemark M. The metabolic phenotype of Prader-Willi syndrome (PWS) in childhood: heightened insulin sensitivity relative to body mass index. J Clin Endocrinol Metab. 2011;96:E225–E232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldstone AP, Thomas EL, Brynes AE, et al. Visceral adipose tissue and metabolic complications of obesity are reduced in Prader-Willi syndrome female adults: evidence for novel influences on body fat distribution. J Clin Endocrinol Metab. 2001;86:4330–4338. [DOI] [PubMed] [Google Scholar]
- 17. Lucio M, Fekete A, Weigert C, et al. Insulin sensitivity is reflected by characteristic metabolic fingerprints–a Fourier transform mass spectrometric non-targeted metabolomics approach. PLoS One. 2010;5:e13317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang TJ, Larson MG, Vasan RS, et al. Metabolite profiles and the risk of developing diabetes. Nat Med. 2011;17:448–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Walford GA, Davis J, Warner AS, et al. Branched chain and aromatic amino acids change acutely following two medical therapies for type 2 diabetes mellitus. Metabolism. 2013;62:1772–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Phang JM, Liu W, Zabirnyk O. Proline metabolism and microenvironmental stress. Annu Rev Nutr. 2010;30:441–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ferrannini E, Natali A, Camastra S, et al. Early metabolic markers of the development of dysglycemia and type 2 diabetes and their physiological significance. Diabetes. 2013;62:1730–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jéquier E. Leptin signaling, adiposity, and energy balance. Ann NY Acad Sci. 2002;967:379–388. [DOI] [PubMed] [Google Scholar]
- 23. Laron Z, Silbergeld A, Lilos P, Blum FW. Serum leptin in obese patients with Laron syndrome before and during IGF-I treatment. J Pediatr Endocrinol Metab. 1998;11:653–656. [DOI] [PubMed] [Google Scholar]
- 24. Capalbo D, Mattace Raso G, Esposito A, et al. Cluster of cardiometabolic risk factors in children with GH deficiency: a prospective, case-control study. Clin Endocrinol (Oxf). 2014;80:856–862. [DOI] [PubMed] [Google Scholar]
- 25. Joaquin C, Aguilera E, Granada ML, et al. Effects of GH treatment in GH-deficient adults on adiponectin, leptin and pregnancy-associated plasma protein-A. Eur J Endocrinol. 2008;158:483–490. [DOI] [PubMed] [Google Scholar]
- 26. Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kanety H, Hemi R, Ginsberg S, et al. Total and high molecular weight adiponectin are elevated in patients with Laron syndrome despite marked obesity. Eur J Endocrinol. 2009;161:837–844. [DOI] [PubMed] [Google Scholar]
- 28. Lanes R, Soros A, Gunczler P, et al. Growth hormone deficiency, low levels of adiponectin, and unfavorable plasma lipid and lipoproteins. J Pediatr. 2006;149:324–329. [DOI] [PubMed] [Google Scholar]
- 29. Festen DA, van Toorenenbergen A, Duivenvoorden HJ, Hokken-Koelega A. Adiponectin levels in prepubertal children with Prader-Willi syndrome before and during growth hormone therapy. J Clin Endocrinol Metab. 2007;92:1549–1554. [DOI] [PubMed] [Google Scholar]
- 30. Herrmann BL, Saller B, Stratmann M, Berg C, Mann K, Janssen OE. Effects of a combination of rhGH and metformin on adiponectin levels in patients with metabolic syndrome. Horm Metab Res. 2005;37:49–52. [DOI] [PubMed] [Google Scholar]
- 31. Cutfield WS, Wilton P, Bennmarker H, et al. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet. 2000;355:610–613. [DOI] [PubMed] [Google Scholar]