Abstract
Context and Objectives:
Little is known regarding the influence of GH on brain development, especially in infants born very preterm (VP; <30 weeks' gestation). Preterm infants are thought to have higher levels of GH in the first days of life compared with full-term infants. VP infants experience cognitive difficulties in childhood and have a diffuse pattern of structural brain abnormalities. This study aimed to explore the relationship between postnatal GH concentrations following VP birth and its association with cognitive functioning and brain volumes at age 7 years.
Methods:
Eighty-three infants born VP had GH concentrations measured at eight time points postnatally, and 2- and 6-week area under the curve (AUC) summary measures were calculated. Followup at age 7 years included neuropsychological assessment and brain magnetic resonance imaging. Univariable and multivariable regression modeling were used where AUC for GH was the main predictor of neurodevelopmental outcome at age 7 years.
Results:
Univariable modeling revealed that higher GH levels (2-week AUC) were related to poorer performance on a verbal working memory (P = .04) and shifting attention task (P = .01). These relationships persisted on multivariable modeling and when the 6-week AUC was analyzed; working memory (P = .03), immediate spatial memory (P = .02), and delayed spatial memory (P = .03) deficits were found. Higher GH levels were also associated with larger amygdala volumes after adjustment for potential confounders (P = .002, 2-week AUC; P = .03, 6-week AUC).
Conclusions:
Higher postnatal GH levels may potentially contribute to the documented neurodevelopmental abnormalities seen in children born VP at school age.
GH is released from the anterior pituitary and is primarily involved in stimulating mitosis in cells, resulting in the growth, both in cell number and cell size, of all tissues in the body. GH is detectable in the human fetus by the ninth week of gestation (1). During gestational weeks 10–24, the pituitary develops to four times its weight and GH levels increase dramatically throughout this period. The weight of the pituitary increases another 10-fold between 24 weeks' gestational age and term-equivalent age, which is associated with a 5-fold increase in GH production (1). The regulation of GH by the central nervous system is not considered to be fully functional until the postnatal period.
Little is known regarding the influence of GH on brain development in humans. However, GH receptors have been found on neurons of the cerebral cortex in the fetal brain (2) and are prominent in cortical, hippocampal, and hypothalamic neurons in the brains of 10–12-day-old rats (3). In human adults, GH receptors are concentrated in the choroid plexus, pituitary, hippocampus, putamen, and hypothalamus (4).
The relationship between GH and cognition is also poorly understood. When GH therapy was given to children born small for gestational age (SGA), improvement to overall intellectual performances and behavioral symptomatology was found compared with untreated SGA children (5). Cognitive deficits, notably in the domains of attention, memory, and executive functioning, have also been noted in those with acromegaly who suffer from severely elevated levels of GH (6) and in children deficit in GH secretion (7). A meta-analysis examining cognitive functioning in adults with GH deficiency demonstrated that deficits were noted in the domains of attention, memory, and executive functioning when compared with matched controls (8).
Preterm (birth prior to 37 weeks' gestation) and very preterm (VP; <32 weeks' gestation) infants are thought to have altered GH levels in the neonatal period. GH reference intervals for the group of preterm infants investigated in this current study were recently published, and GH was shown to be highest during the first 7 days of life with levels up to 343 mU/L, which then declined over the following 4–6 weeks (9). Preterm infants have also been shown to have a differing GH profile when compared with term controls, possibly due to immature hypothalamic control and an underdeveloped negative feedback system (1, 10). Throughout infancy, plasma GH levels are known to decrease after birth and continue to decline over 2–3 months to reach the low basal levels commonly seen in children. Although few comparative studies have been conducted, higher GH levels have been documented in single serum samples taken from cord blood of preterm infants compared with those born at term (11). In addition, elevated circulating GH concentrations have been found in preterm infants in the first 6 hours (10) and 12 hours (12) of postnatal life compared with term infants. Despite these findings, the association between GH and neurodevelopmental impairments in preterm infants is unknown.
Preterm birth is associated with a diffuse pattern of cognitive deficits and structural brain abnormalities that are present throughout development and persist into adolescence. Overall, the brain volumes of those born preterm are smaller when compared with controls, including smaller volumes of both gray and white tissue, and a commensurate increase in cerebrospinal fluid (CSF) (13). Regional volumetric reductions have been noted in cerebellum, sensorimotor regions, midtemporal, parieto-occipital, and subgenual cortices, basal ganglia, amygdala, hippocampus, and corpus callosum (14). Regarding cognitive abilities, the general intellect of VP survivors is at least two thirds of a standard deviation below term-born peers (15). Specific cognitive domains such as attention and executive functioning (16), language (17), visuospatial processing and reasoning (18), learning and memory (19), and speed of information processing (20) have also been shown to be areas of concern in this population. These cognitive deficits have also been related to academic difficulties (21) and preterm children are at increased risk of developing mental health disorders and poor social functioning (22). Neonatal brain injury and volumetric reductions have been reported to be associated with neurodevelopmental impairments (16, 19). To the best of our knowledge no study has examined the influence of early postnatal GH levels on cognitive and brain development in VP children.
This study used extensive GH concentration data collected during the first 6 weeks of postnatal life following VP birth to examine its effects on neurodevelopment at age 7 years. This exploratory analysis aimed to assess the relationships between GH levels, cognitive functioning, and brain volumes at age 7 years in a group of children born VP.
Materials and Methods
Study design
Participants (n = 99) were a subgroup of children from the Victorian Infant Brain Studies (VIBeS) cohort, a prospective, longitudinal study examining the development of children born VP. Recruitment for the hormone subgroup occurred between June 2002 and December 2003 from the Royal Women's Hospital in Melbourne, Victoria, Australia. VP infants with a gestational age (GA) less than 30 weeks were considered eligible for recruitment. Infants with significant genetic or congenital abnormalities were excluded. At 7 years' corrected age, 83 participants from the original hormone subgroup were assessed (four withdrew from study; eight declined to participate in this study phase; two could not be contacted; two unavailable due to distance). Approval for the study was obtained from the Human Research and Ethics Committees of the Royal Women's Hospital and the Royal Children's Hospital. Written consent was obtained from parents.
Procedure and measures
During the neonatal period 0.5 mL of whole blood was collected from the cord at the time of delivery, and then from the infant on days 1, 4, 7, 14, 21, 28, and 42. Sample points were limited to eight occurrences throughout this 6-week period to adhere to ethical considerations around iatrogenic anemia. Specimen collection was timed with routine morning blood sampling, which occurred between 0700 and 0900 hours. Whole blood was centrifuged at 3000 rpm for 5 minutes. Serum was then collected into gel tubes and stored at −70°C until the hormone assays were performed in batches. GH was analyzed on the fully automated Immulite 2000 (Siemens Health Care Corporation) and measured using an immunometric assay, referenced to a pituitary-derived standard; international standard 80/505. The intra- and interassay coefficients of variation were acceptable (intra-assay = 3.4%; interassay range = 4.0–2.1%). Neonatal GH data were expressed as area under the curve (AUC) values, which represented a summary measure of hormone exposure over two designated time periods, birth to Day 14 and birth to Day 42. These two AUC measures were selected to account for the transient nature of these hormone changes as recovery to normal levels is expected within 2–3 weeks after birth. For those infants missing cord data (n = 49), birth (or cord levels) were extrapolated from Day 1 data. An analysis using an AUC starting from Day 1 as the main predictor of interest was conducted to ensure that this extrapolation procedure would not alter results (data not shown).
Further perinatal variables included: GA (in weeks), birth weight z score, and sex. GH levels are known to vary with illness state (23). To account for this, duration of intermittent positive pressure ventilation was included as a covariate and acted as a general marker of illness. At term-equivalent age, T1 and T2 images were acquired with a 1.5 Tesla General Electric magnetic resonance imaging (MRI) scanner at the Royal Children's Hospital (Signa LX Echospeed System; General Electric). A validated global cerebral abnormality score given by a neonatal neurologist who was blinded to GH status, with higher levels reflecting more severe pathology (24).
The 7-year followup included detailed neuropsychological evaluation and neuroimaging. Neuropsychological measures for the current study were selected to represent a broad range of cognitive domains (Table 1). Children who attempted a task but were too impaired to perform or had difficulty comprehending task instructions were assigned the lowest possible raw score for a subtest, or in the case of reaction time measures, the highest score recorded signifying a longer reaction time. The Social Risk Index parent questionnaire was also included, with higher scores representing greater social risk (25). This questionnaire is a composite measure of six aspects of social status known to contribute to development, including family structure, language spoken at home, maternal age at birth, education and occupation of the primary caregiver, and employment status of the primary income earner.
Table 1.
Neuropsychological Measures
| Test Battery | Description |
|---|---|
| Wechsler Abbreviated Scale of Intelligence (WASI) (35) | The 4-subtest version of the WASI was administered to estimate general intellectual functioning (FSIQ). Normative mean = 100; sd = 15. |
| Clinical Evaluation of Language Fundamentals—Fourth Edition—Australian Standardised Edition (CELF-IV) (36) | The Core Language Index from the CELF-IV was administered and used as a measure of general language ability. Normative mean = 100; sd = 15. |
| Working Memory Test Battery for Children (WMTBC) (37) | Three subtests from the WMTBC were administered to assess elements of immediate and working memory. 1) Digit Recall involves the child repeating a sequence of numbers, assessing immediate verbal memory. 2) Backward Digit Recall involves the child repeating a presented sequence of numbers in reverse order, assessing verbal working memory. 3) Block Recall requires the child to recall a sequence of blocks that the examiner taps on a standardized board, assessing spatial immediate memory. Raw scores for each task were used in the analyses. |
| California Verbal Learning Test— Children's Version (CVLT-C) (38) | The CVLT-C is a verbal learning and memory task in which the child is required to recall a list of 15 words, which is presented five times. Raw total learning score (total words recalled from trial 1 to 5) and long delay recall score were used as measures of verbal learning and verbal memory, respectively. |
| Children's Memory Scale (CMS) (39) | Dot Locations subtest from the CMS was administered to assess spatial learning and memory. The child is required to learn and remember the spatial location of an array of dots, which is presented three times. Raw total learning score (total score from trials 1 to 3) and long delay recall score were used as measures of spatial learning and spatial memory, respectively. |
| Test of Everyday Attention for Children (TEA-Ch) (40) | Raw scores for four selected subtests were used to assess different attention domains. 1) Sky Search (number of correct targets) assesses selective attention 2) Score! (number of correct trials) assesses sustained attention 3) Creature Counting (number of correct trials) assesses shifting attention, and 4) Sky Search Dual Task (proportion of correct counting games and identified targets) assesses divided attention. |
| Tower of London (TOL) (41) | The TOL is a measure of planning, organization, and problem solving. The total raw score was used which is a summary score for the 12 items where higher points are awarded for quicker completion times and points subtracted for planning errors. |
| CogState Research (42) | Two subtests from the CogState Research battery were selected to assess processing speed. 1) Detection assesses simple reaction time. 2) Identification assesses choice reaction time. The variable of interest for these two tasks was the mean of the log10 transformed reaction times for correct responses where lower scores indicated a better performance. |
Whole-brain images were acquired at 7 years' corrected age on a 3 Tesla Siemens Magnetom Trio (Tim system) scanner, at the Royal Children's Hospital, Melbourne, Australia (n = 65). Reasons for missing MRI data include: failing the MRI preparation session (n = 7), being too impaired to participate (n = 4), parents not consenting to MRI (n = 3), failing to attend appointment (n =1), refusal by the child (n = 1), cochlear implant (n = 1), and extreme movement in scanner (n = 1). Structural T1-weighted images were used for the purpose of the current study (flip angle = 9°; repetition time = 2.27 milliseconds; echo time = 1900 milliseconds; field of view = 210 × 210 mm; matrix = 256 × 256; 0.8 mm3 isotropic voxels). Data were processed by a single operator on Linux workstations using the automated FreeSurfer imaging processing suite (stable release version 4.4.0, http://surfer.nmr.mgh.harvard.edu). FreeSurfer output was inspected and manually edited as required. Those that were considered of unacceptably poor quality due to imaging artifact were excluded (n = 13), leaving 52 participants with brain volumes. Cortical and cerebellar gray and white matter, thalamus, caudate, putamen, pallidum, hippocampus, and amygdala volumes were estimated for each hemisphere, and volumes from both hemispheres combined. The total volumes for the caudate, putamen, and pallidum were combined to represent basal ganglia volume. Cerebellar volume was the sum of cerebellar white and gray matter. Total brain tissue was the combined volumes of all brain structures, excluding CSF.
Pituitary gland volumes were manually delineated for each participant by a single operator (Z.M.A.). Images were loaded on ITK-SNAP (version 2.2; http://www.itksnap.org/pmwiki/pmwiki.php) and viewed concurrently in three orthogonal planes with editing occurring primarily in the coronal plane, where it featured 9–12 slices. A hyperintense spot below the optic chiasm represented the emergence of the posterior pituitary and tracing progressed anteriorly until the pituitary gland was no longer visible. The pituitary gland boundaries were defined superiorly by the diaphragm sellae, inferiorly by the sphenoid sinus (sagittal plane), and bilaterally by the cavernous sinuses. Where the boundaries were unclear due to hyperintensity present in the surrounding CSF, movement, or artifact, the pituitary gland was edited in the sagittal plane. Intrarater reliability was calculated using intraclass correlation coefficients with absolute agreement, and was 0.93 (95% confidence interval [CI], 0.83–0.97; P < .0005) on 20 randomly chosen subjects (10 VP, 10 term controls).
Data analysis
Data were analyzed using Stata 13 (StataCorp, 2013). Attrition bias was assessed by comparing participants and nonparticipants using t tests or Mann-Whitney U test (continuous data) and χ2 (categorical data). The study's aim was assessed employing regression models using AUC for GH as the main predictor of outcome with models fitted in two ways, cord to Day 14 levels (14-d AUC), and cord to Day 42 (42-d AUC). Covariates included in all models were GA, birth weight z score, sex, duration of intermittent positive pressure ventilation, and global neonatal brain abnormality score and cognitive models additionally controlled for social risk. Results were reported for both unadjusted and adjusted analyses.
To account for the nonindependent effect of twins/triplets, robust standard errors using the Huber/White/sandwich method were used for all analyses. Standardized beta coefficients (β) (cut-points: 0.10, weak; 0.30, moderate; 0.50, strong) were used as measures of the magnitude of effect. Also, the proportion of variance explained by an independent predictor (R2 for unadjusted analyses; sr2 for adjusted analyses) was investigated and expressed as a percentage, where larger values were more desirable. Issues of multiple comparisons were closely considered given the exploratory nature of the current study and the large number of regression analyses conducted. As such, the pattern and strength of the relationships were interpreted in addition to statistical significance.
Results
Sample and GH characteristics
Table 2 outlines the characteristics of the sample as well as the MRI subgroup (ie, those with brain volumes). The mean age of the sample was 7.5 years (range, 6.8–8.3 y), 42% were male, and 59% were from single births. There were no characteristic differences between participants and nonparticipants (data not shown). The MRI subgroup had a significantly higher Full Scale Intelligence Quotient (FSIQ) than those in the sample without useable MRI data (b = 10.83; 95% CI, 3.02–18.65; P = .007).
Table 2.
Perinatal, Demographic, and Hormone Characteristics
| Characterisitic | 7-Year Sample | MRI Subgroup |
|---|---|---|
| n | 83 | 52 |
| Child characteristics | ||
| GA, wk, M (sd) | 27.3 (1.7) | 27.4 (1.6) |
| Birth weight, g, M (sd) | 975 (235) | 992 (216) |
| Males, n (%) | 35 (42.2) | 19 (36.5) |
| Singleton, n (%) | 49 (59.0) | 27 (51.9) |
| Perinatal medical factors | ||
| Small for gestational age, n (%) | 2 (2.4) | 2 (3.8) |
| Sepsis, n (%) | 34 (41.0) | 21 (40.4) |
| Patent ductus arteriosus, n (%) | 40 (48.2) | 23 (44.2) |
| Necrotising enterocolitis, n (%) | 9 (10.8) | 4 (7.8) |
| Bronchopulmonary dysplasia, n (%) | 26 (31.7) | 15 (28.8) |
| Antenatal corticosteroids, n (%) | 76 (91.6) | 50 (96.2) |
| Postnatal corticosteroids, n (%) | 10 (12.0) | 4 (7.7) |
| Intermittent positive pressure ventilation, h, median (25th, 75th percentile) | 98 (7, 431) | 88 (9, 376.5) |
| Cystic periventricular leukomalacia, n (%) | 1 (1.2) | 1 (1.9) |
| Grade III/IV intraventricular haemorrhage, n (%) | 3 (3.6) | 1 (1.9) |
| MRI brain injury score, median (25th, 75th percentile) | 5 (3, 8) | 5 (3, 8) |
| Neonatal GH characteristics | ||
| 14-d AUC, mU/L, M (sd) | 932.2 (358.1) | 965.0 (351.4) |
| 42-d AUC, mU/L, M (sd) | 2665.0 (814.1) | 2683.8 (792.1) |
| 7-y measures | ||
| Social risk score at age 7 y, median (25th, 75th percentile) | 2 (1, 3) | 2 (0, 3) |
| FSIQ, M (sd) | 95.5 (17.8) | 99.5 (12.7) |
Some sample sizes are less than the total sample due to missing data.
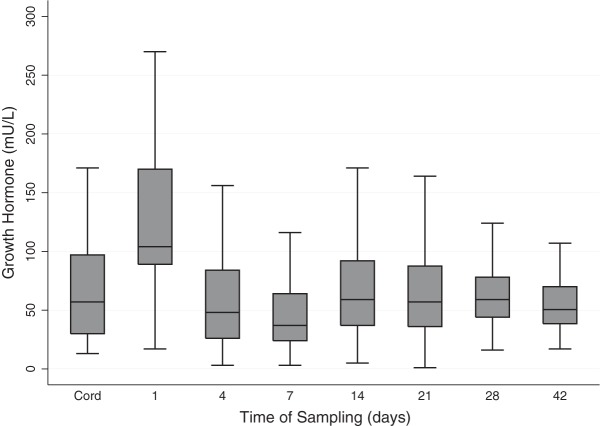
The 7-year sample did not differ in terms of GH levels over the 14-day (b = 2.45; 95% CI, −185.80–190.72; P = .98) or 42-day (b = 129.19; 95% CI, −308.99–567.37; P = .56) AUC to that of the entire hormone cohort. There were also no differences in GH levels between the MRI subgroup and those without useable MRI data over the 14-day AUC (b = 87.75; 95% CI, −73.77–249.28; P = .28) or 42-day AUC (b = 50.38; 95% CI, −319.28–420.04; P = .79). The GH profile of the sample included a prominent elevation on Day 1 followed by concentrations decreasing slightly below cord levels at Day 7, then a return to cord concentrations that remain stable, from Day 14 to Day 42 (Figure 1). GA, severity of illness, and sex did not significantly or independently affect GH concentrations over either the first 2 or 6 weeks of life (data not shown).
Figure 1.
Boxplot of the GH profile from cord to Day 42 for the assessed group. Values depicted as the median (solid line within box), 25th–75th percentiles (margins of the box), and range of the data. Outliers were removed.
Cognitive results
Regarding the 14-day AUC models, significant univariable relationships were observed with higher GH levels related to poorer performances on a verbal working memory and a shifting attention task (Table 3). In addition, these relationships persisted after controlling for confounders, with the 14-day AUC for GH independently accounting for 4.4% and 7.2% of the variance for these two outcomes, respectively, with moderate effect sizes observed (Table 3). On examination of the pattern of results, no positive associations were found between GH levels and cognitive outcomes. GH levels were either negatively associated with cognitive outcomes or not associated at all, with only the aforementioned relationships reaching significance.
Table 3.
Relationship between GH 14-Day AUC/42-Day AUC and 7-Year Cognitive Outcomes
| Cognitive Outcomes | 14-Day AUC (Unadjusted; n = 83) |
14-Day AUC (Adjusted; n = 79) |
42-Day AUC (Unadjusted; n = 83) |
42-Day AUC (Adjusted; n = 79) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 (%) | P | β | sr2 (%) | P | β | R2 (%) | P | β | sr2 (%) | P | |
| General intellectual ability | ||||||||||||
| FSIQ | −0.13 | 1.6 | .263 | −0.13 | 1.3 | .221 | −0.13 | 1.7 | .223 | −0.14 | 1.7 | .146 |
| Language | ||||||||||||
| General language ability | −0.11 | 1.2 | .288 | −0.09 | 0.6 | .354 | −0.11 | 1.3 | .325 | −0.16 | 2.2 | .119 |
| Verbal working memory, learning, and memory | ||||||||||||
| Immediate verbal memory | 0.02 | 0.02 | .870 | 0.03 | 0.08 | .780 | 0.04 | 0.2 | .687 | −0.03 | 0.1 | .765 |
| Working memory | −0.19 | 3.5 | .045 | −0.24 | 4.4 | .025 | −0.17 | 3.1 | .097 | −0.24 | 5.1 | .030 |
| Verbal learning | −0.03 | 0.09 | .787 | −0.07 | 0.3 | .604 | −0.03 | 0.09 | .764 | −0.12 | 1.2 | .280 |
| Verbal memory | −0.003 | <0.001 | .969 | −0.04 | 0.1 | .690 | −0.02 | 0.03 | .869 | −0.09 | 0.7 | .355 |
| Spatial working memory, learning, and memory | ||||||||||||
| Immediate spatial memory | 0.003 | <0.001 | .974 | −0.07 | 0.3 | .428 | −0.09 | 0.8 | .326 | −0.21 | 3.7 | .017 |
| Spatial learning | −0.10 | 1.0 | .404 | −0.16 | 2.0 | .181 | −0.06 | 0.3 | .567 | −0.18 | 2.7 | .062 |
| Spatial memory | −0.14 | 2.0 | .237 | −0.22 | 3.6 | .086 | −0.12 | 1.4 | .255 | −0.24 | 5.1 | .026 |
| Attention and executive functioning | ||||||||||||
| Selective attention | −0.009 | 0.01 | .931 | 0.006 | <0.001 | .955 | −0.07 | 0.5 | .527 | −0.11 | 1.1 | .333 |
| Sustained attention | −0.18 | 3.3 | .147 | −0.12 | 1.2 | .336 | −0.16 | 2.4 | .202 | −0.21 | 3.9 | .104 |
| Shifting attention | −0.26 | 6.8 | .010 | −0.31 | 7.2 | .017 | −0.19 | 3.6 | .061 | −0.16 | 2.2 | .175 |
| Divided attention | −0.19 | 3.5 | .075 | −0.21 | 3.3 | .122 | −0.14 | 2.0 | .182 | −0.17 | 2.6 | .154 |
| Planning | 0.003 | <0.001 | .972 | −0.05 | 0.2 | .648 | −0.08 | 0.6 | .477 | −0.16 | 2.1 | .139 |
| Speed of information processing and reaction time | ||||||||||||
| Simple reaction timea | −0.03 | 0.07 | .842 | −0.08 | 0.5 | .480 | 0.03 | 0.1 | .794 | 0.01 | 0.02 | .905 |
| Complex reaction timea | 0.11 | 1.2 | .238 | 0.05 | 0.2 | .618 | 0.08 | 0.6 | .490 | 0.02 | 0.03 | .878 |
Covariates for adjusted analyses included gestational age, birth weight z score, sex, social risk, marker of illness, and neonatal brain injury.
Some sample sizes are less than the total sample due to missing data.
Bold values denote statistical significance (P < .05).
Task is reverse scored where higher scores signify a slower reaction time.
No significant relationships were observed at a univariable level when the 42-day AUC was examined, although trends were again observed on the verbal working memory and shifting attention tasks. On multivariable analysis however, significantly poorer cognitive performance was related to higher GH levels on tasks of verbal working memory, immediate spatial memory, delayed spatial memory, and a trend for spatial learning (Table 3). Small effect sizes were observed and the 42-day AUC independently accounted for 3.7–5.1% of the variance for these outcomes. The above findings did not alter when SGA children (n = 3) were removed (data not shown).
Brain volume results
No unadjusted associations were found between postnatal GH levels over the first 2 or 6 weeks of life and 7-year brain volume (Table 4). Following adjustment for confounders, only one significant relationship was observed where higher GH levels were associated with larger amygdala volumes with small-to-moderate effect. Removing the SGA children did not alter the pattern of the findings (data not shown).
Table 4.
Relationship between GH 14-Day AUC/42-Day AUC and 7-Year Imaging Outcomes
| Imaging Outcomes | 14-Day AUC (Unadjusted; n = 52) |
14-Day AUC (Adjusted; n = 52) |
42-Day AUC (Unadjusted; n = 52) |
42-Day AUC (Adjusted; n = 52) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | R2 (%) | P | β | sr2 (%) | P | β | R2 (%) | P | β | sr2 (%) | P | |
| Total brain tissue | −0.02 | 0.04 | .881 | 0.04 | 0.09 | .743 | −0.19 | 3.7 | .261 | −0.07 | 0.4 | .491 |
| Cortical gray matter | 0.02 | 0.07 | .837 | 0.09 | 0.5 | .529 | −0.15 | 2.2 | .376 | −0.03 | 0.08 | .797 |
| Cortical white matter | −0.04 | 0.2 | .746 | 0.03 | 0.09 | .788 | −0.21 | 4.3 | .201 | −0.09 | 0.7 | .392 |
| Cerebellum | 0.13 | 1.7 | .507 | 0.17 | 2.0 | .191 | −0.05 | 0.2 | .803 | 0.08 | 0.5 | .505 |
| Pituitary | −0.04 | 0.2 | .639 | 0.06 | 0.3 | .509 | −0.07 | 0.5 | .452 | 0.04 | 0.1 | .665 |
| Thalamus | 0.13 | 1.7 | .438 | 0.06 | 0.3 | .613 | −0.03 | 0.09 | .851 | −0.04 | 0.1 | .729 |
| Basal ganglia | 0.12 | 1.4 | .425 | 0.21 | 3.1 | .109 | −0.02 | 0.02 | .910 | 0.08 | 0.6 | .400 |
| Hippocampus | 0.10 | 1.0 | .579 | 0.20 | 3.0 | .127 | −0.05 | 0.2 | .804 | 0.04 | 0.2 | .747 |
| Amygdala | 0.20 | 4.1 | .216 | 0.31 | 6.6 | .002 | 0.09 | 0.8 | .558 | 0.23 | 4.7 | .031 |
Covariates for adjusted analyses included gestational age, birth weight z score, sex, marker of illness, and neonatal brain injury.
Some sample sizes are less than the total sample due to missing data.
Bold values denote statistical significance (P < .05).
Discussion
In the current study, we observed that higher GH levels during the first 6 weeks of life were related to poorer working memory, spatial learning, and immediate and delayed spatial memory at age 7 years as well as larger amygdala volumes at age 7 years after controlling for potential confounders.
The GH profile for the assessed group included a rapid increase from cord to Day 1 followed by a nadir at Day 7 and then a return to cord levels the following week. This corresponded to a daily median (25th, 75th percentiles) level ranging from 37 (24, 64) to 104 (89, 170) mU/L. Reference intervals for GH levels in preterm infants are rare, but one study investigated GH levels from umbilical cord in infants born less than 37 weeks' gestation and the reported mean (SD) was 59.8 (28.6) mU/L (26). These GH cord blood levels were slightly lower than in the current study where we found a mean (SD) of 69.3 (57.0) mU/L, although the median was 57 mU/L. Ragetti et al (27) investigated serum samples on Day 4 (mean, 105.6; SD, 38.2 mU/L) and Day 30 (mean, 51.0; SD, 39.0 mU/L) in 18 infants born less than 36 weeks' gestation. Although sampling days differed, and older preterm infants were included by Ragetti et al (27), the current study is in agreement, showing that the GH levels of preterm infants seem to increase sharply in the first week of life followed by a decline to levels similar to cord blood. With regard to term-born populations, reference range data for GH levels are sparse; however, Soldin et al (28) reported the reference interval for 56 healthy children between the ages of 0 and 7 years to be <2.6–35.4 mU/L, which is much lower than the rates observed in the current study.
GH is known to cross the blood-brain barrier and it is this property that may allow GH to affect our observed neurodevelopmental impairments in learning, memory and attention. Our cognitive findings are consistent with published accounts of syndromes associated with GH deficiency, where poorer cognitive functioning, especially in the areas of attention and memory, have been reported (29). Although there is a dearth of information with regard to GH excess, individuals with acromegaly have also been found to have difficulties in the domains of attention, memory, and executive function (6). Similar findings have been reported in the animal literature where GH deficiency was related to poorer spatial learning memory performances (30). These findings, together with our results, suggest that attention, learning, and memory skills may be particularly vulnerable to a period of aberrant GH levels. A possible mechanism may exist as GH receptors are known to be expressed in the frontal cortex, which is crucial for attention and executive functions, and the hippocampus, which is vital to learning and memory functions. However, further investigation of this hypothesis is required as many of these studies are restricted to animal models (31).
In our study, high levels of GH were also related to larger amygdala volumes, after controlling for potential confounders. This finding is interesting and unexpected given that GH receptors have been found in high concentrations within the pituitary, hippocampus, putamen, hypothalamus, and cerebral cortex (2, 4); there is little mention of GH receptors located in the amygdala specifically. Despite this, the amygdala is a central region within the limbic system and intimately interconnected with the hippocampus. At a functional level, the amygdala is important for emotional learning and memory and can modulate both the encoding and the storage of hippocampal-dependent memories (32). This may therefore partly explain the lower performances observed on spatial learning and memory tasks in our study and the larger volumes may represent altered or dysmature development of the amygdala. Of note, this finding, although consistent across many statistical models was of small-to-moderate effect and as no other associations between postnatal GH levels and brain volume were found, this result should be interpreted with caution.
The abnormal postnatal hormone levels observed in preterm infants are thought to be transient with recovery to within expected levels noted 2–3 weeks following preterm birth. This return to expected range has been widely reported with respect to thyroid hormones and to a lesser extent the stress hormone, cortisol. This observation has led to a critical timing theory for thyroid hormone whereby exposure to low levels of thyroid hormone during a time of brain development dependent on these hormones is associated with poorer outcome (33). This model suggests that there are critical windows of thyroid hormone exposure during which aberrant levels may lead to later neurodevelopmental dysfunction. It is therefore possible that a window of vulnerability also exists for GH whereby deficient or excessive levels may contribute to poorer cognitive outcomes in childhood. In support of this van Dam et al (34) found that impaired hippocampal/mesial temporal function was greater in those with childhood-onset GH deficiency compared with adult-onset GH deficiency, suggesting a vulnerable period and a timing effect of GH levels. This potential timing effect may further explain the relationships found in our study.
The GH axis is known to be intimately connected with the IGF-I axis. Although IGF-I was also tested during the neonatal phase of this study, levels of IGF-I were below the sensitivity of the assay due to ethical limits of blood collection, making data from this hormone unusable. It is therefore possible that high GH levels led to changes in the IGF-I axis and it was this mechanism that led to our findings. Despite this limitation, this study was the first of its kind to comprehensively assess GH over the first weeks of postnatal life in a large sample of largely healthy VP infants. It is also the first to investigate the potential role of this hormone on later neurodevelopmental outcomes, namely cognition and brain volumes. Moreover, the use of the AUC measure allowed for an extrapolation of hormone functioning across time instead of relying on a single time point of hormone sampling.
In conclusion, this study was the first of its kind to investigate the relationship between these GH levels and cognitive functioning and brain development at 7 years of age. Past studies have largely focused on GH deficiency in SGA children with limited research available in contemporary cohorts of relatively healthy preterm infants. Findings from the current study suggest that high levels of GH during early infancy may partly explain the well-documented cognitive difficulties these preterm children experience during early school age. This finding has broader implications, especially with regard to the timing effect of GH.
Acknowledgments
This work was supported by the Australia's National Health and Medical Research Council (PhD Scholarship Grant 216757, Project Grants 237117, 491209, and Senior Research Fellowship to P.J.A., 628371), National Institutes of Health (R01, HD058056), and the Victorian Government's Operational Infrastructure Support Program. Funded by the National Institutes of Health (NIH).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AUC
- area under the curve
- CI
- confidence interval
- CSF
- cerebrospinal fluid
- FSIQ
- Full Scale Intelligence Quotient
- GA
- gestational age
- MRI
- magnetic resonance imaging
- SGA
- small for gestational age
- VIBeS
- Victorian Infant Brain Studies
- VP
- very preterm.
References
- 1. Kaplan SL, Grumbach MM, Shepard TH. The ontogenesis of human fetal hormones. I: Growth hormone and insulin. J Clin Invest. 1972;51:3080–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hill DJ, Riley SC, Bassett NS, Waters MJ. Localization of the growth hormone receptor, identified by immunocytochemistry, in second trimester human fetal tissues and in placenta throughout gestation. J Clin Endocrinol Metab. 1992;75:646–650. [DOI] [PubMed] [Google Scholar]
- 3. Lobie PE, García-Aragón J, Lincoln DT, Barnard R, Wilcox JN, Waters MJ. Localization and ontogeny of growth hormone gene expression in the central nervous system. Dev Brain Res. 1993;74:225–233. [DOI] [PubMed] [Google Scholar]
- 4. Harvey S, Hull K. Neural growth hormone: An update. J Mol Neurosci. 2003;20:1–14. [DOI] [PubMed] [Google Scholar]
- 5. Pareren YK, Duivenvoorden HJ, Slijper FSM, Koot HM, Hokken-Koelega ACS. Intelligence and psychosocial functioning during long-term growth hormone therapy in children born small for gestational age. J Clin Endocrinol Metab. 2004;89. [DOI] [PubMed] [Google Scholar]
- 6. Sievers C, Sämann PG, Pfister H, et al. Cognitive function in acromegaly: Description and brain volumetric correlates. Pituitary. 2012;15:350–357. [DOI] [PubMed] [Google Scholar]
- 7. Devesa J, Casteleiro N, Rodicio C, López N, Reimunde P. Growth hormone deficiency and cerebral palsy. Ther Clin Risk Manag. 2010;6:413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Falleti MG, Maruff P, Burman P, Harris A. The effects of growth hormone (GH) deficiency and GH replacement on cognitive performance in adults: A meta-analysis of the current literature. Psychoneuroendocrinology. 2006;31:681–691. [DOI] [PubMed] [Google Scholar]
- 9. Greaves RF, Zacharin MR, Donath SM, Inder TE, Doyle LW, Hunt RW. Establishment of hormone reference intervals for infants born <30 weeks' gestation. Clin Biochem. 2014;47:101–108. [DOI] [PubMed] [Google Scholar]
- 10. Wright NM, Northington FJ, Miller JD, Veldhuis JD, Rogol AD. Elevated growth hormone secretory rate in premature infants: Deconvolution analysis of pulsatile growth hormone secretion in the neonate. Pediatr Res. 1992;32:286–290. [DOI] [PubMed] [Google Scholar]
- 11. Cornblath M, Parker ML, Reisner SH, Forbes AE, Daughaday WH. Secretion and metabolism of growth hormone in premature and full-term infants. J Clin Endocrinol. 1965;25:209–218. [DOI] [PubMed] [Google Scholar]
- 12. Miller JD, Wright NM, Esparza A, et al. Spontaneous pulsatile growth hormone release in male and female premature infants. J Clin Endocrinol Metab. 1992;75:1508–1513. [DOI] [PubMed] [Google Scholar]
- 13. Inder TE, Wells SJ, Mogridge NB, Spencer C, Volpe JJ. Defining the nature of the cerebral abnormalities in the premature infant: A qualitative magnetic resonance imaging study. J Pediatr. 2003;143:171–179. [DOI] [PubMed] [Google Scholar]
- 14. Peterson BS, Vohr B, Staib LH, et al. Regional brain volume abnormailities and long-term cognitive outcome in preterm infants. JAMA. 2000;284. [DOI] [PubMed] [Google Scholar]
- 15. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: A meta-analysis. JAMA. 2002;288:728–737. [DOI] [PubMed] [Google Scholar]
- 16. Murray AL, Scratch SE, Thompson DK, et al. Neonatal brain pathology predicts adverse attention and processing speed outcomes in very preterm and/or very low birth weight children. Neuropsychology. 2014;28:552–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: A meta-analysis. J Pediatr. 2011;158:766–774.e1. [DOI] [PubMed] [Google Scholar]
- 18. Atkinson J, Braddick O. Visual and visuocognitive development in children born very prematurely. Prog Brain Res. 2007;164:123–149. [DOI] [PubMed] [Google Scholar]
- 19. Omizzolo C, Scratch SE, Stargatt R, et al. Neonatal brain abnormalities and memory and learning outcomes at 7 years in children born very preterm. Memory. 2014;22:605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mulder H, Pitchford NJ, Marlow N. Processing speed and working memory underlie academic attainment in very preterm children. Arch Dis Child Fetal Neonatal Ed. 2010;95:F267–F272. [DOI] [PubMed] [Google Scholar]
- 21. Johnson S, Wolke D, Hennessy E, Marlow N. Educational outcomes in extremely preterm children: Neuropsychological correlates and predictors of attainment. Dev Neuropsychol. 2011;36:74–95. [DOI] [PubMed] [Google Scholar]
- 22. Burnett AC, Anderson PJ, Cheong J, Doyle LW, Davey CG, Wood SJ. Prevalence of psychiatric diagnoses in preterm and full-term children, adolescents and young adults: A meta-analysis. Psychol Med. 2011;FirstView:1–12. [DOI] [PubMed] [Google Scholar]
- 23. Van den Berghe G. Dynamic neuroendocrine responses to critical illness. Front Neuroendocrinol. 2002;23:370–391. [DOI] [PubMed] [Google Scholar]
- 24. Kidokoro H, Neil JJ, Inder TE. New MR imaging assessment tool to define brain abnormalities in very preterm infants at term. Am J Neuroradiol. 2013;34:2208–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roberts G, Howard K, Spittle AJ, Brown NC, Anderson PJ, Doyle LW. Rates of early intervention services in very preterm children with developmental disabilities at age 2 years. J Paediatr Child Health. 2008;44:276–280. [DOI] [PubMed] [Google Scholar]
- 26. Samaan NA, Schultz PN, Johnston DA, Creasy RW, Gonik B. Growth hormone, somatomedin C, and nonsuppressible insulin-like activity levels compared in premature, small, average birth weight, and large infants. Am J Obstet Gynecol. 1987;157:1524–1528. [DOI] [PubMed] [Google Scholar]
- 27. Radetti G, Bozzola M, Paganini C, et al. Growth hormone bioactivity and levels of growth hormone, growth hormone-binding protein, insulinlike growth factor I, and insulinlike growth factor-binding proteins in premature and full-term newborns during the first month of life. Arch Pediatr Adolesc Med. 1997;151:170–175. [DOI] [PubMed] [Google Scholar]
- 28. Soldin OP, Hoffman EG, Waring MA, Soldin SJ. Pediatric reference intervals for FSH, LH, estradiol, T3, free T3, cortisol, and growth hormone on the DPC IMMULITE 1000. Clin Chim Acta. 2005;355:205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maruff P, Falleti M. Cognitive function in growth hormone deficiency and growth hormone replacement. Horm Res. 2006;64:100–108. [DOI] [PubMed] [Google Scholar]
- 30. Nieves-Martinez E, Sonntag WE, Wilson A, et al. Early-onset GH deficiency results in spatial memory impairment in mid-life and is prevented by GH supplementation. J Endocrinol. 2010;204:31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nyberg F, Hallberg M. Growth hormone and cognitive function. Nat Rev Endocrinol. 2013;9:357–365. [DOI] [PubMed] [Google Scholar]
- 32. Phelps EA. Human emotion and memory: Interactions of the amygdala and hippocampal complex. Curr Opin Neurobiol. 2004;14:198–202. [DOI] [PubMed] [Google Scholar]
- 33. Zoeller RT, Rovet J. Timing of thyroid hormone action in the developing brain: Clinical observations and experimental findings. J Endocrinol. 2004;16:809–818. [DOI] [PubMed] [Google Scholar]
- 34. van Dam PS, de Winter CF, de Vries R, et al. Childhood-onset growth hormone deficiency, cognitive function and brain N-acetylaspartate. Psychoneuroendocrinology. 2005;30:357–363. [DOI] [PubMed] [Google Scholar]
- 35. Wechsler D. Wechsler Abbreviated Scale of Intelligence. London, UK: Psychological Corporation; 1999. [Google Scholar]
- 36. Semel E, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals - Fourth Edition - Australian Standardised Edition. Marrackville, Australia: Harcourt Assessment; 2006. [Google Scholar]
- 37. Pickering SJ, Gathercole SE. Working Memory Test Battery for Children. London, UK: The Psychological Corporation; 2001. [Google Scholar]
- 38. Delis DC, Kramer JH, Kaplan E, Ober BA. The California Verbal Learning Test - Children's Version. San Antonio, TX: The Psychological Corporation; 1994. [Google Scholar]
- 39. Cohen MJ. Children's Memory Scale. Bloomington, MN: NCS Pearson, Inc; 1997. [Google Scholar]
- 40. Manly T, Robertson I, Anderson V, Nimmo-Smith I. Test of Everyday Attention for Children (TEA-Ch). Cambridge, UK: Thames Valley Test Company; 1998. [DOI] [PubMed] [Google Scholar]
- 41. Anderson PJ, Anderson V, Lajoie G. The Tower of London test: Validation and standarization for pediatric populations. Clin Neuropsychol. 1996;10:54–65. [Google Scholar]
- 42. CogState Ltd. Melbourne, Australia. [Google Scholar]