Abstract
Context:
Severe obesity is the fastest growing subgroup of obesity in youth.
Objective:
We sought to explore the association between severe obesity and subclinical measures of cardiac and vascular structure and function in adolescents and young adults.
Design, Setting, and Participants:
This was a cross-sectional comparison of 265 adolescents and young adults with severe obesity (defined as body mass index [BMI] ≥120% of the 95th percentile) to 182 adolescents and young adults with obesity (defined as BMI ≥100–119th of the 95th percentile) at tertiary medical center.
Main Outcomes:
Noninvasive measures of cardiac and vascular structure and function were assessed.
Results:
Participants were a mean age of 17.9 years, 62% were non-Caucasian, and 68% were female. Systolic blood pressure, fasting insulin, C-reactive protein, IL-6, and frequency of type 2 diabetes were higher in participants with severe obesity (all P < .05). Arterial thickness and stiffness, cardiac structure, and diastolic function were also significantly worse in youth with severe obesity as measured by higher left ventricular mass index, worse diastolic function, higher carotid intima media thickness, and pulse wave velocity and lower brachial distensibility (all P < .05). Regression modeling showed that severe obesity (compared with obesity) was independently associated with each of the above outcomes after adjustment for age, race, sex, blood pressure, lipids, and inflammatory markers (P < .05).
Conclusions:
Adolescents and young adults with severe obesity have a more adverse cardiovascular risk profile and worse cardiac and vascular structure and function. More importantly, severe obesity is independently associated with these subclinical cardiac and vascular changes.
Severe obesity (defined as ≥120% of the 95th percentile) is the fastest growing subcategory of overweight and obesity in the United States and currently affects 4–6% of all youth (1, 2). Youth with severe obesity have a worse cardiometabolic risk profile including increased numbers of risk factors (3), and a more extreme risk profile including higher blood pressure (BP) (4), more dyslipidemia (5), and more inflammation (5).
Using noninvasive cardiovascular imaging techniques, our group has previously demonstrated that obesity is associated with adverse changes in cardiac and vascular structure and function (6–8). Similar changes have also been described in youth with severe obesity but comparisons have been made to normal weight controls (9–12). Therefore, the extent to which severe obesity (compared with less severe forms of obesity) is associated with subclinical cardiac and vascular changes has not been established.
In this study we sought to compare noninvasive cardiac and vascular structure and function in adolescents and young adults with severe obesity to an obese control group. In addition, we sought to evaluate the independent contributions of severe obesity to subclinical cardiac and vascular changes that are known to predict future myocardial infarction and stroke (13).
Materials and Methods
Participants
Participants included in this analysis were recruited as part of the Type 2 Cardiovascular Disease study, a cross-sectional study conducted in Cincinnati, Ohio, which was designed to compare cardiac and vascular structure and function in adolescents with type 2 diabetes to lean (<85th percentile) and obese (≥95th percentile) controls. Details of the larger study population have been previously published (6–8).
In the present article, we sought to evaluate the effects of severe obesity on cardiac and vascular structure and function. Thus, we divided the larger study group by age- and sex-specific body mass index (BMI) percentiles derived from Centers for Disease Control and Prevention growth charts. Here, only obese (BMI ≥ 100–119th of the 95th percentile or class I obesity) and severe obese (≥120% of the 95th percentile or class II and III obesity) (14) participants were compared. Classification of BMI as a percentage of the 95th percentile was chosen over waist circumference or waist-to-height ratio as a measure of adiposity because the latter two measures have limited use in persons with severe obesity due to difficulty in obtaining anatomic landmarks (15). We also chose this classification methodology over traditional BMI percentiles because a BMI percentile ≥ 99th performs poorly to define the severity of obesity (16). Lean and overweight controls are not included here given that we have previously published their cardiac and vascular data (6–8).
Written informed consent was obtained from subjects at least 18 years or from a parent/guardian with written assent for subjects less than 18 years of age, according to the guidelines established by the local institutional review board and in accordance with the Declaration of Helsinki.
Clinical parameters
Demographics, anthropometrics, BP, Tanner pubertal staging, fasting blood (> 8 h), and cardiac and vascular measurements were obtained as previously described (6–8). Briefly, height, weight, and waist circumference were obtained twice and averaged (6). BMI was calculated as weight in kilograms divided by the square of height in meters. Blood pressure was measured manually with a mercury sphygmomanometer (Baum Desktop model with V-Lok cuffs) three times and averaged according to the Fourth Report (17). Mean arterial pressure was calculated as two thirds times diastolic BP plus one third systolic BP (in mm Hg). Self report puberty data for breast (females) and pubic hair (males and females) was collected.
Laboratory
Fasting blood was drawn to measure lipids, glucose, insulin, glycosylated hemoglobin (A1c), C-reactive protein (CRP), and IL-6. Total cholesterol, high-density lipoprotein cholesterol (HDL-C) and triglycerides were measured in a National Heart Lung and Blood Institute–standardized laboratory with low-density lipoprotein cholesterol (LDL-C) calculated by using the Friedewald equation. If triglycerides were greater than 400 mg/dL, LDL-C was measured directly. Glucose was measured using a Hitachi model 704 glucose analyzer (Roche Hitachi) and insulin was measured by RIA with an anti-insulin serum raised in guinea pigs, 125I-labeled insulin (Linco) and a double antibody method to separate bound from free tracer. Hemoglobin A1c was measured in red blood cells by using HPLC. CRP and IL-6 were measured using high-sensitivity enzyme-linked immunoabsorbent assays. Diagnosis of type 2 diabetes was based on American Diabetes Association criteria, which included an elevated fasting plasma glucose levels of at least 126 mg/dL, or symptoms of hyperglycemia and random plasma glucose of at least 200 mg/dL, or 2-hour plasma glucose of at least 200 mg/dL during an oral glucose tolerance test (18). Negative islet cell antibody titers (glutamic acid decarboxylase, islet cell antigen 512, insulin autoantibodies) were also confirmed in individuals with type 2 diabetes from the time of diagnosis (Barbara Davis Center for Childhood Diabetes).
Physical activity
Physical activity was assessed using a multidirectional accelerometer (Actical, Phillips Respironics). Participants were instructed to wear the accelerometer on the right hip at waist level for 7 consecutive days, except when sleeping, bathing, or playing contact sports. Mean counts per minute were calculated per day. A valid day included 10–20 hours of wear time and at least 10 counts per minute.
Cardiac structure and function
The heart was noninvasively assessed with either a GE Vivid 5 or 7 or Philips Sonos 5500 ultrasound system with the participant in the left decubitus position to acquire parasternal long and short axis and apical four-chamber views. Left ventricular (LV) mass (a measure of hypertrophy) was calculated on each participant using the formula by Devereaux et al (19) and then indexed by dividing LV mass by height in meters raised to the power of 2.7 to give a measure of LV mass index, which standardizes the measure between different age, sex, and race groups (20).
Doppler measurements of the mitral inflow velocities were obtained to assess peak early filling (E wave: E) and late (A wave: A) left ventricular filling with the ratio (E/A) used to assess diastolic function. Tissue Doppler analyses of the myocardial wall velocities at both the septal and lateral annuli were also performed to obtain peak (Ea) and late (Aa) velocities, with the ratio (Ea/Aa) representing mitral annular flow. The ratio of mitral to myocardial early filling E/Ea lateral and septal were calculated as a measure of a diastolic function (21). Each of the above has been shown to correlate well with invasive measures of diastolic function and LV end-diastolic pressure (22). A total of three cardiac cycles were measured and averaged per subject. All measurements were read offline using Cardiology Analysis System (Digisonics).
Vascular structure and function
Vascular structure was measured as carotid intima media thickness (IMT) in bilateral carotid arteries using high-resolution B-mode ultrasonography (GE Vivid 7 ultrasound imaging system) with a 7.5 MHz linear array transducer. For each subject, the common, internal, and bulb far wall carotid segments were examined to identify the thickest area of IMT with the average value from the right and left artery used in the analysis. A trace technique using a Camtronic Medical System was employed to ascertain the maximum carotid thickness from leading edge of the lumen-intima to leading edge of the media-adventitia. Coefficients of variability for all carotid sites is ≤ 5.5% (23).
The SphygmoCor SCOR-PVx System (AtcorMedical) was used to obtain pulse wave velocity (PWV) and augmentation index. The PWV measurement is based on the principle that the pressure pulse generated by LV ejection travels at a speed determined by the size, shape, and properties of the artery (24). A tonometer is used to collect proximal (carotid) and distal (femoral) arterial waveforms gated by the R-wave on a simultaneously recorded electrocardiogram. PWV is then calculated as the distance from the carotid-to-femoral artery divided by the time delay measured between the feet of the two waveforms reported in m/sec (13). A higher PWV suggests higher peripheral vascular stiffness. Three measurements were obtained and averaged. Repeat measures show a coefficient of variation of < 7% (25).
Augmentation index, a mixed measure of central and peripheral vascular stiffness, was measured by placing the SphygmoCor tonometer over the right radial artery. The device analyzes pulse waves using a generalized transfer function validated in catheterization laboratory to calculate a central aortic pressure wave (13). Augmentation index is derived from the central pressure waveform by calculating the difference between the main outgoing wave and the reflected wave of the central arterial waveform, expressed as a percentage of the central pulse pressure. The magnitude of reflected wave represents the increased afterload that left ventricle copes with each cardiac cycle. A higher augmentation index indicates increased vessel stiffness. A negative number indicates reflections that happen late in cardiac cycle and are consistent with more pliable (less stiff) arteries. Augmentation index is influenced by heart rate; therefore, all values were adjusted to a standard heart rate of 75 beats per minute. Three measurements of augmentation were obtained per participant and averaged. Reproducibility studies demonstrated an intraclass correlation coefficient of 0.9 (25).
The DynaPulse pathway instrument (Pulse Metric) was used to measure brachial distensibility. Brachial distensibility assesses resting vascular function in a medium muscular artery (26). Brachial artery distensibility is derived from pressure curves generated from arterial pressure signals obtained from a standard BP cuff sphygmomanometer. A lower brachial destensibility (BrachD) indicates increased vascular stiffness. Three measures were averaged. Repeat measures show coefficients of variation of < 9% (25).
Statistics
All analyses were performed with SAS 9.3 (SAS Institute). Variance-stabilizing transformations were applied to continuous variables as appropriate. Group differences were evaluated using t tests. Regression modeling was performed to determine whether severe obesity was associated with independent risk beyond that of traditional cardiovascular risk factors. Therefore, the full model contained severe obesity group (vs obese group) and was adjusted for age, race, sex, mean arterial pressure, triglyceride/HDL-C ratio [representing small dense LDL particles (27)], CRP, IL-6, and diabetes status. Hemoglobin A1c, fasting glucose, and insulin were not included in the models as they were collinear with diabetes status. Strength of all models was assessed by R2. For all analysis, values of P < .05 were considered significant.
Results
The mean age of the study population was 17.9 years (age range, 10.2–23.9 y), 62% were non-Caucasian and 68% were female. Details of the demographic and metabolic variables by group are presented in Table 1. There were more non-Caucasians and females in the severe obesity group (P < .05). Thirty eight percent of participants in the obese group had type 2 diabetes compared with half of the participants in the severe obesity group (P < .05). Systolic BP, fasting insulin, CRP, and IL-6 were higher in youth with severe obesity (all P < .05). There were no significant differences in diastolic BP, lipids, fasting glucose, A1c, pubertal staging, or physical activity counts per minute between the two groups. All participants were pubertal (Tanner II or higher). Twenty four participants with severe obesity and eight participants with obesity, all whom had type 2 diabetes, reported taking an ACE inhibitor. Four participants also reported taking lipid-lowering medication.
Table 1.
Description of the Study Population
| Characteristic | Obese | Severe Obesity | P (t Test or χ2) |
|---|---|---|---|
| n | 182 | 265 | |
| Age, y | 18.4 ± 3.3 | 17.6 ± 3.1 | .022 |
| Race, n (%) non-Caucasian | 102 (56) | 176 (66) | .029 |
| Sex, n (%) female | 119 (65) | 184 (69) | .041 |
| Type 2 diabetes, n (%) | 69 (38) | 133 (50) | .012 |
| Height, cm | 168 ± 11 | 167 ± 10 | .780 |
| Weight, kg | 91.9 ± 15.0 | 120.0 ± 22.4 | <.001 |
| BMI, kg/m2 | 32.5 ± 2.9 | 42.7 ± 6.9 | <.001 |
| BMI, z score | 1.92 ± 0.16 | 2.46 ± 0.26 | <.001 |
| Systolic BP, mm Hg | 117 ± 11 | 121 ± 12 | <.001 |
| Diastolic BP, mm Hg | 63 ± 11 | 68 ± 13 | .084 |
| Total cholesterol, mg/dL | 173 ± 36 | 175 ± 35 | .794 |
| LDL-C, mg/dL | 106 ± 32 | 105 ± 29 | .613 |
| HDL-C, mg/dL | 46 ± 10 | 45 ± 11 | .246 |
| Triglycerides, mg/dL | 111 ± 54 | 122 ± 73 | .395 |
| Fasting glucose, mg/dL | 112 ± 56 | 119 ± 55 | .222 |
| Fasting insulin, mIU/mL | 20.9 ± 16.5 | 31.9 ± 32.3 | <.001 |
| Hemoglobin A1c, % | 6.3 ± 2.1 | 6.6 ± 2.0 | .184 |
| CRP, mg/L | 3.5 ± 3.7 | 5.8 ± 4.5 | <.001 |
| IL-6, pg/mL | 1.3 ± 1.2 | 2.3 ± 1.5 | <.001 |
Obese, BMI ≥100–119% of the 95th percentile; Severe obese, BMI ≥120% of the 95th percentile.
Data are mean and SD unless otherwise specified.
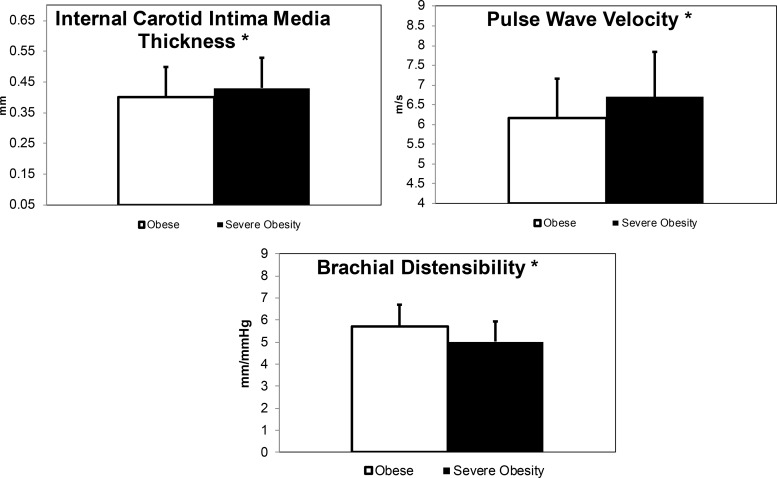
Table 2 lists the cardiac structure and diastolic function data by group. Vascular structure (thickness) and function (stiffness) measures are shown in the lower half of Table 2 and in Figure 1 for those that were significant. Cardiac structure, diastolic function, and vascular thickness and stiffness were worse in the severe obesity group compared with the obese group as measured by a higher LV mass index, E/Ea lateral, internal carotid, PWV femoral, and lower BrachD, respectively (all P < .05).
Table 2.
Cardiac and Vascular Measurements
| Characteristic | Obese | Severe Obesity | P by t Test |
|---|---|---|---|
| n | 182 | 265 | |
| LVM index, g/m2.7 | 35.9 ± 7.20 | 42.1 ± 9.6 | <.001 |
| E/A | 2.0 ± 0.06 | 1.9 ± .5 | .592 |
| Ea/Aa lateral | 11.5 ± 1.9 | 11.2 ± 1.9 | .150 |
| Ea/Aa_septal | 9.8 ± 1.4 | 10.0 ± 1.6 | .096 |
| E/Ea lateral | 6.0 ± 1.5 | 6.6 ± 1.5 | .002 |
| E/Ea septal | 8.0 ± 1.9 | 8.2 ± 1.7 | .383 |
| Common carotid IMT, mm | 0.50 ± 0.09 | 0.51 ± 0.09 | .254 |
| Bulb carotid IMT, mm | 0.51 ± 0.10 | 0.52 ± 0.12 | .615 |
| Internal carotid IMT, mm | 0.04 ± 0.10 | 0.43 ± 0.10 | .002 |
| Pulse wave velocity, m/s | 6.17 ± 1.00 | 6.7 ± 1.15 | <.001 |
| Augmentation Index, % | 2.54 ± 11.9 | 4.87 ± 11.6 | .045 |
| Brachial distensibility, mm/mm Hg | 5.72 ± 0.98 | 5.02 ± 0.91 | <.001 |
Obese, BMI ≥100–119% of the 95th percentile; Severe obese, BMI ≥120% of the 95th percentile.
Data are mean and SD.
Figure 1.
Comparisons of the vascular measurements in youth with obesity and severe obesity. Data are mean and SD. White indicates obese group, black indicates the severe obesity group.
Regression modeling was performed to determine whether severe obesity was independently associated with cardiac and vascular outcomes after adjustment for traditional cardiovascular risk factors including age, race, sex, mean arterial pressure, triglyceride/HDL-C ratio, CRP, IL-6 and diabetes status. Pubertal staging and physical activity were omitted from the models given that they were not different between groups. Only outcomes that were significantly different between the two groups were modeled. After adjustment for the above, severe obesity was a significant independent risk factor for LV mass index and E/Ea lateral, internal carotid IMT, PWV femoral, and BrachD (Table 3).
Table 3.
Independent Determinants of Vascular and Cardiac Outcomes
| Variable | LVM Index ht2.7, g/m | E/EA Lateral | Internal Carotid, mm | PWV Femoral, m/sec | BrachD, mm/mm Hg |
|---|---|---|---|---|---|
| Intercept | 3.375 ± 0.068 | 1.538 ± 0.133 | −1.240 ± 0.060 | 0.930 ± 0.074 | 1.845 ± 0.971 |
| Age, y | 0.015 ± 0.003 | 0.021 ± 0.003 | 0.024 ± 0.002 | 0.006 ± 0.003 | |
| Sex, female | −0.043 ± 0.023 | −0.101 ± 0.021 | 0.031 ± 0.014 | 0.077 ± 0.019 | |
| Race, non-Caucasian | 0.064 ± 0.014 | ||||
| Mean arterial pressure, mm Hg | 0.003 ± 0.001 | 0.004 ± 0.001 | −0.003 ± 0.001 | ||
| TG/HDL-C ratio | 0.006 ± 0.003 | ||||
| IL-6, pg/mL | 0.012 ± 0.005 | ||||
| Diabetes status (yes vs no) | 0.043 ± 0.022 | 0.074 ± 0.026 | 0.043 ± 0.020 | 0.039 ± 0.013 | −0.036 ± 0.018 |
| Severe obesity group (vs obese group) | 0.153 ± 0.023 | 0.080 ± 0.026 | 0.079 ± 0.020 | 0.078 ± 0.013 | −0.116 ± 0.018 |
| Model R2 | 0.20 | 0.09 | 0.18 | 0.46 | 0.21 |
Abbreviation: TG, triglyceride.
Data are parameter estimate and SE.
All of the variables were included in each of the models but only significant parameters (P < .05) are listed. Blanks indicate that parameter was P > .05.
Each model was repeated excluding participants with type 2 diabetes and the results were unchanged. Severe obesity was still independently associated with a higher LV mass index, E/Ea lateral, internal carotid IMT, PWV femoral, and lower BrachD.
Discussion
This study demonstrates two novel findings: Adolescents and young adults with severe obesity have a worse cardiac and vascular structure and function abnormalities compared with those with obesity and after adjustment for demographic and cardiovascular risk factors severe obesity is independently associated with adverse changes in the heart and vasculature. These data suggest that adolescents and young adults with severe obesity may be at higher risk for early cardiovascular complications.
Prior work has shown that severely obese youth have higher BP (3–5, 9) and insulin levels (4, 5) and more dyslipidemia most commonly with elevated triglycerides and low HDL-C (3, 4, 9) but comparisons have been made with normal-weight youth. Norris et al (5) was the first group to use an obese control group and found that youth with severe obesity also have higher inflammatory markers (IL-6 and CRP) and oxidized LDL levels. The present study is concordant with the latter and shows higher IL-6 and CRP levels as well as higher insulin levels and systolic BP in severely obese youth.
Prior work evaluating cardiac and peripheral vascular structure and function in the severely obese youth has used normal-weight controls. Specifically, Obert et al (10) has shown that severely obese youth mean age 14 years have higher indexed LV mass, diastolic filling pressure, and cardiac strain compared with their lean counterparts. Tounian et al (12) also documented lower common carotid artery distensibility and compliance as well as decreased brachial artery reactivity, whereas Kapiotis (9) found obese youth with severe obesity have higher mean carotid thickness and more endothelial dysfunction (measured by flow mediated dilation). By using normal-weight controls, the extent to which severe obesity (compared with less severe forms of obesity) is associated with early cardiac and vascular changes has not been established. Thus, the present study adds to the growing literature in this area but is able to document novel findings by using an obese control group. We show that the degree (or extent) of obesity is significant and specifically severe obesity is associated with additional risk for more advanced preclinical cardiac and peripheral vascular changes.
It is also important to note that for some measures, including the internal and bulb IMT, augmentation index, and several measures of diastolic function there were no statistical differences between groups. We know atherosclerosis develops in a nonuniform fashion (28, 29) so it is possible that these are later sites of development. In addition, each of the vascular outcomes, whereas all reliable, represent a different aspect of the arterial tree or the heart. PWV, a measure of central arterial stiffness, is considered the gold standard measure of subclinical arterial stiffness in both adults and children and has been shown to predict future cardiovascular events and mortality (30, 31). Augmentation index is a mixed measure of arterial stiffness that is influenced by central stiffness (PWV) and peripheral wave reflections (13) and has also been shown to predict all-cause mortality in adults with end-stage renal disease (32) and hypertension (33). BrachD is a nonultrasound measure of stiffness (arterial compliance) in a medium muscular artery (34) and is highly correlated with cardiovascular risk factors (34). Indexed LVM is a measure of cardiac hypertrophy and has been shown to predict future cardiovascular mortality (35). For the diastolic measures there is not one single noninvasive measurement that is proven reliable to predict future diastolic dysfunction, although E/Ea lateral best correlates with the reference standard LV end diastolic pressure measured invasively (22). Thus, given the differences between the various outcome measurements, it is not unexpected that each may be differentially affected by the extent of obesity and different cardiovascular risk factors.
Another subtle point of this study is that despite establishing severe obesity is an independent risk factor for cardiac and peripheral vascular changes, we were at best only able to explain 10–20% of the variance in the preclinical cardiac measures and 20–50% of the variance in the preclinical peripheral vascular measures. This suggests there are there are likely other risk factors in youth with obesity that contribute to subclinical cardiac and vascular disease not identified. Although we are not able to examine these risks in this study, adipokines and insulin resistance are important areas for further work.
This paper has limitations. First, the cross-sectional design of the present study prevents the assessment of temporal sequence of events. However, given that adiposity tracks from childhood to adulthood (36) and childhood adiposity increases future cardiovascular disease risk (37–40), it is reasonable to hypothesize that severe obesity contributes to our findings. Second, we have self-report pubertal staging and in males only pubic hair (not testicular staging). For this reason, age was included in the models to serve as a surrogate. Third, because of small numbers and an unclear the indication for the use of ACE inhibitors (BP lowering or microalbuminuria), we are unable to assess the effects of medications on our outcomes. Finally, although we accounted for the presence of type 2 diabetes in our regression models, these analyses were performed in an established group recruited to study the effects of type 2 diabetes, not severe obesity, so it is possible that the results may differ depending on the prevalence of diabetes in the cohort. However, when we excluded participants with type 2 diabetes, the results were unchanged.
In summary, this study establishes that adolescents and young adults with severe obesity have a worse cardiovascular risk profile and a greater degree of preclinical cardiac and peripheral vascular changes than obese youth. In addition, severe obesity is an independent risk factor for preclinical cardiac and peripheral vascular disease. Thus, youth with severe obesity seem to be at a greater risk for subclinical cardiovascular complications. These findings support the need for more aggressive management and intervention in this group.
Acknowledgments
We thank the participants who participated in the Cardiovascular Disease in Adolescents with Type 2 Diabetes (T2CVD) study in Cincinnati, Ohio.
This work was supported by National Institutes of Health (NIH) Grant R01 HL076269 (Cardiovascular Disease in Adolescents with Type 2 Diabetes). It was also supported in part by Grants UL1 RR026314 and K23HL118132.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- body mass index
- BP
- blood pressure
- BrachD
- brachial destensibility
- HDL-C
- high-density lipoprotein cholesterol
- hemoglobin A1c
- glycosylated hemoglobin
- IMT
- intima media thickness
- LDL-C
- low-density lipoprotein cholesterol
- LV
- left ventricular
- PWV
- pulse wave velocity.
References
- 1. Claire Wang Y, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among US children and adolescents, 1976–2006. Int J Pediatr Obes. 2011;6:12–20. [DOI] [PubMed] [Google Scholar]
- 2. Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA. 2012;307:483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ice CL, Murphy E, Cottrell L, Neal WA. Morbidly obese diagnosis as an indicator of cardiovascular disease risk in children: Results from the CARDIAC Project. Int J Pediatr Obes. 2011;6:113–119. [DOI] [PubMed] [Google Scholar]
- 4. Gidding SS, Nehgme R, Heise C, Muscar C, Linton A, Hassink S. Severe obesity associated with cardiovascular deconditioning, high prevalence of cardiovascular risk factors, diabetes mellitus/hyperinsulinemia, and respiratory compromise. J Pediatr. 2004;144:766–769. [DOI] [PubMed] [Google Scholar]
- 5. Norris AL, Steinberger J, Steffen LM, Metzig AM, Schwarzenberg SJ, Kelly AS. Circulating oxidized LDL and inflammation in extreme pediatric obesity. Obesity (Silver Spring). 2011;19:1415–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shah AS, Khoury PR, Dolan LM, et al. The effects of obesity and type 2 diabetes mellitus on cardiac structure and function in adolescents and young adults. Diabetologia. 2011;54:722–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Urbina EM, Kimball TR, Khoury PR, Daniels SR, Dolan LM. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Urbina EM, Kimball TR, McCoy CE, Khoury PR, Daniels SR, Dolan LM. Youth with obesity and obesity-related type 2 diabetes mellitus demonstrate abnormalities in carotid structure and function. Circulation. 2009;119:2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapiotis S, Holzer G, Schaller G, et al. A proinflammatory state is detectable in obese children and is accompanied by functional and morphological vascular changes. Arterioscler Thromb Vasc Biol 2006;26:2541–2546. [DOI] [PubMed] [Google Scholar]
- 10. Obert P, Gueugnon C, Nottin S, et al. Two-dimensional strain and twist by vector velocity imaging in adolescents with severe obesity. Obesity (Silver Spring) 2012;20:2397–2405. [DOI] [PubMed] [Google Scholar]
- 11. Schlager O, Willfort-Ehringer A, Hammer A, et al. Microvascular function is impaired in children with morbid obesity. Vasc Med. 2011;16:97–102. [DOI] [PubMed] [Google Scholar]
- 12. Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: A prospective study. Lancet. 2001;358:1400–1404. [DOI] [PubMed] [Google Scholar]
- 13. Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: Methodological issues and clinical applications. Eur Heart J. 2006;27:2588–2605. [DOI] [PubMed] [Google Scholar]
- 14. Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. Am J Clin Nutr. 2009;90:1314–1320. [DOI] [PubMed] [Google Scholar]
- 15. Kelly AS, Barlow SE, Rao G, et al. Severe obesity in children and adolescents: identification, associated health risks, and treatment approaches: A scientific statement from the American Heart Association. Circulation. 2013;128:1689–1712. [DOI] [PubMed] [Google Scholar]
- 16. Gulati AK, Kaplan DW, Daniels SR. Clinical tracking of severely obese children: A new growth chart. Pediatrics. 2012;130:1136–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004;114:555–576. [PubMed] [Google Scholar]
- 18. American Diabetes Association. Standards of medical care in diabetes—2014. Diabetes Care. 2014;37 Suppl 1:S14–80. [DOI] [PubMed] [Google Scholar]
- 19. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: Comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 20. de Simone G, Daniels SR, Devereux RB, et al. Left ventricular mass and body size in normotensive children and adults: Assessment of allometric relations and impact of overweight. J Am Coll Cardiol. 1992;20:1251–1260. [DOI] [PubMed] [Google Scholar]
- 21. De Boeck BW, Cramer MJ, Oh JK, van der Aa RP, Jaarsma W. Spectral pulsed tissue Doppler imaging in diastole: A tool to increase our insight in and assessment of diastolic relaxation of the left ventricle. Am Heart J. 2003;146:411–419. [DOI] [PubMed] [Google Scholar]
- 22. Kasner M, Westermann D, Steendijk P. Utility of Doppler echocardiography and tissue Doppler imaging in the estimation of diastolic function in heart failure with normal ejection fraction: A comparative Doppler-conductance catheterization study. Circulation. 2007;116:637–647. [DOI] [PubMed] [Google Scholar]
- 23. Urbina EM, Dabelea D, D'Agostino RB., Jr Effect of type 1 diabetes on carotid structure and function in adolescents and young adults: The SEARCH CVD study. Diabetes Care. 2013;36:2597–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. London GM, Guerin AP. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am Heart J. 1999;138:220–224. [DOI] [PubMed] [Google Scholar]
- 25. Urbina EM1, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Dolan Cardiac and vascular consequences of pre-hypertension in youth. J Clin Hypertens (Greenwich). 2011;13:332–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Urbina EM, Kieltkya L, Tsai J, Srinivasan SR, Berenson GS. Impact of multiple cardiovascular risk factors on brachial artery distensibility in young adults: The Bogalusa Heart Study. Am J Hypertens. 2005;18:767–771. [DOI] [PubMed] [Google Scholar]
- 27. Tsimihodimos V, Gazi I, Kostara C, Tselepis AD, Elisaf M. Plasma lipoproteins and triacylglycerol are predictors of small, dense LDL particles. Lipids 2007;42:403–409. [DOI] [PubMed] [Google Scholar]
- 28. Mackinnon AD, Jerrard-Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS. Rates and determinants of site-specific progression of carotid artery intima-media thickness: The carotid atherosclerosis progression study. Stroke. 2004;35:2150–2154. [DOI] [PubMed] [Google Scholar]
- 29. Solberg LA, Eggen DA. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 1971;43:711–724. [DOI] [PubMed] [Google Scholar]
- 30. Mancia G, De Backer G, Dominiczak A, et al. 2007 Guidelines for the Management of Arterial Hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2007;25:1105–1187. [DOI] [PubMed] [Google Scholar]
- 31. Urbina EM, Williams RV, Alpert BS, et al. Noninvasive assessment of subclinical atherosclerosis in children and adolescents: recommendations for standard assessment for clinical research: a scientific statement from the American Heart Association. Hypertension. 2009;54:919–950. [DOI] [PubMed] [Google Scholar]
- 32. London GM, Blacher J, Pannier B, Guérin AP, Marchais SJ, Safar ME. Arterial wave reflections and survival in end-stage renal failure. Hypertension. 2001;38:434–438. [DOI] [PubMed] [Google Scholar]
- 33. Williams B, Lacy PS, Thom SM, et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: Principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 34. Urbina EM, Brinton TJ, Elkasabany A, Berenson GS. Brachial artery distensibility and relation to cardiovascular risk factors in healthy young adults (The Bogalusa Heart Study). Am J Cardiol. 2002;89:946–951. [DOI] [PubMed] [Google Scholar]
- 35. Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114:345–352. [DOI] [PubMed] [Google Scholar]
- 36. Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: The Bogalusa Heart Study. J Pediatr 2007;150:12–17.e12. [DOI] [PubMed] [Google Scholar]
- 37. Baker JL, Olsen LW, Sorensen TI. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120:340–345. [DOI] [PubMed] [Google Scholar]
- 40. Tirosh A, Shai I, Afek A. Adolescent BMI trajectory and risk of diabetes versus coronary disease. N Engl J Med. 2011;364:1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]