Abstract
Context:
Molecular testing for oncogenic mutations or gene expression in fine-needle aspirations (FNAs) from thyroid nodules with indeterminate cytology identifies a subset of benign or malignant lesions with high predictive value.
Objective:
This study aimed to evaluate a novel diagnostic algorithm combining mutation detection and miRNA expression to improve the diagnostic yield of molecular cytology.
Setting:
Surgical specimens and preoperative FNAs (n = 638) were tested for 17 validated gene alterations using the miRInform Thyroid test and with a 10-miRNA gene expression classifier generating positive (malignant) or negative (benign) results.
Design:
Cross-sectional sampling of thyroid nodules with atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS) or follicular neoplasm/suspicious for a follicular neoplasm (FN/SFN) cytology (n = 109) was conducted at 12 endocrinology centers across the United States. Qualitative molecular results were compared with surgical histopathology to determine diagnostic performance and model clinical effect.
Results:
Mutations were detected in 69% of nodules with malignant outcome. Among mutation-negative specimens, miRNA testing correctly identified 64% of malignant cases and 98% of benign cases. The diagnostic sensitivity and specificity of the combined algorithm was 89% (95% confidence interval [CI], 73–97%) and 85% (95% CI, 75–92%), respectively. At 32% cancer prevalence, 61% of the molecular results were benign with a negative predictive value of 94% (95% CI, 85–98%). Independently of variations in cancer prevalence, the test increased the yield of true benign results by 65% relative to mRNA-based gene expression classification and decreased the rate of avoidable diagnostic surgeries by 69%.
Conclusions:
Multiplatform testing for DNA, mRNA, and miRNA can accurately classify benign and malignant thyroid nodules, increase the diagnostic yield of molecular cytology, and further improve the preoperative risk-based management of benign nodules with AUS/FLUS or FN/SFN cytology.
Cytopathology on ultrasound-guided fine-needle aspiration (FNA) biopsies has dramatically improved the clinical management of patients with solid thyroid nodules greater than 1 cm. In routine clinical practice, this procedure can identify approximately 50% of malignant nodules (50% sensitivity) and 70% of benign nodules (70% specificity) without the need to perform a diagnostic surgery (1–4). Because cytology has both high positive predictive value (PPV) (PPV > 98%) and high negative predictive value (NPV) (NPV > 95%), it allows accurate, preoperative, risk-based classification of thyroid nodules. However, the relatively low diagnostic yield of cytology also results in a large fraction of nodule aspirates, up to 35%, without a definitive benign or malignant diagnosis (Bethesda categories II or VI) (1–4). In addition, the residual risk of thyroid cancer in nodules with indeterminate cytology varies significantly across institutions, in particular for nodules with a preoperative diagnosis of atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS, Bethesda category III) or follicular neoplasm/suspicious for a follicular neoplasm (FN/SFN, Bethesda category IV) (1, 4, 5).
To overcome these limitations, novel diagnostic procedures have been developed and optimized to generate clinically actionable information in FNAs with indeterminate cytology. Qualitative molecular tests for specific gene mutations or RNA fusion transcripts associated with thyroid carcinogenesis can detect malignant nodules with high PPV, from 70–100% for different markers (6–11). Alternatively, a microarray-based test combining seven distinct classifiers and interrogating the expression levels of 167 genes can detect benign nodules with a high NPV of 94% (12). The broad availability of these methods has undoubtedly increased the diagnostic yield of conventional cytopathology and improved the personalized, risk-based management of thyroid patients with cancer (13, 14). Yet, they are not without their own limitations. For example, optimized mutation panels can only identify approximately 65% of malignant nodules (65% sensitivity) and the absence of a known oncogenic gene alteration cannot be used to rule out cancer (7, 9). The gene expression classifier only identifies approximately 50% of benign nodules (50% specificity) and most the nodules classified as “suspicious” by the test will go on to be diagnosed as benign (12). Thus, a large number of potentially avoidable diagnostic surgeries are still performed on patients with benign nodules and AUS/FLUS or FN/SFN cytology. Furthermore, when a malignant nodule is diagnosed by surgical histopathology following hemithyroidectomy, a second surgery associated with a higher risk of complication is often required to complete the thyroidectomy (2, 3).
Advances in genome-scale technologies have recently accelerated the discovery of novel biomarker candidates. miRNAs are small, highly conserved RNA molecules that are involved in the pathology of thyroid cancer by regulating key cellular processes such as cell-cycle progression or cell differentiation, proliferation, and survival (15, 16). The differential expression of miRNAs in distinct histopathological tumor types and at various stages of tumor differentiation or progression has been reported in more than 100 publications (17–27). There is currently no validated miRNA test available; however, information derived from miRNA analyses may complement existing molecular diagnostic procedures. Several studies have reported the potential diagnostic utility of miRNAs in preoperative thyroid nodules FNAs (18, 22, 23, 26, 27). Others have shown that miRNA expression changes in papillary thyroid carcinoma are not necessarily correlated with the presence of oncogenic gene mutations (17, 24, 25). In a recent study, we further showed that miRNA expression levels in preoperative FNAs can identify a subset of malignant nodules that are negative for a panel of well-known oncogenic gene alterations (9). In the present work, we took advantage of this characteristic to design a miRNA gene expression classifier that efficiently complements molecular testing for DNA mutations and RNA fusion transcripts. The multianalyte combination test was evaluated in a cohort of thyroid nodules with AUS/FLUS or FN/SFN cytology to establish its diagnostic sensitivity and specificity in preoperative FNAs and model its potential clinical effect on surgery rates. We put forth that this novel strategy can further increase the diagnostic yield of molecular cytology and significantly improve the preoperative risk-based diagnosis of benign thyroid nodules with indeterminate cytology.
Materials and Methods
Algorithm development
miRNA classifiers were developed using miRNA expression data determined by RT-qPCR, diagonal linear discriminant analysis as training classification models (28), miRNA candidate selection based on effect size, and built-in model normalization enforced by constraints on model coefficients. The case-control training set consisted of 240 surgical specimens collected under a research protocol approved by the University of Michigan Institutional Review Board. Two independent case-control specimen sets were also used for algorithm optimization: 54 resected tissues acquired from Asterand and 235 remnant nucleic acids samples from preoperative FNAs archived by Asuragen's clinical laboratory for research purpose.
Cohort study
We studied consecutive thyroid nodule FNAs submitted to Asuragen's clinical laboratory for evaluation with the miRInform Thyroid Test (9, 29) by endocrinology centers across the United States between January 2011 and October 2013 and for whom nucleic acids isolated from the FNAs were available for molecular testing. The study was noninterventional, samples and clinical information were deidentified, and no protected health information or other information identifying study subjects was collected. The protocol was approved by a central investigational review board, the requirement for inform consent waived according to 45 CFR §46.116(d), and all participating sites signed the study agreement. At the close of the study on January 31 2014, 282 AUS/FLUS or FN/SFN cytology reports had been received from 20 physicians at 14 sites. Among those 282 aspirations, 113 nodules (40%) had a traceable surgical pathology outcome with a documented histological diagnosis of benign or malignant primary thyroid lesion. Four cases with known history of cancer or previous radioiodine therapy were excluded before performance assessment, resulting in a final set of 109 specimens from 16 physicians at 12 sites for statistical analysis. All specimens and study subjects were distinct from the 529 cases used for development activities.
Molecular analyses
Surgically resected thyroid lesions and thyroid nodule FNAs were collected, processed, and tested for the presence of genetic alterations in the BRAF, RAS, RET, or PAX8 genes as previously described (9, 29). miRNA expression in residual total nucleic acid (TNA) samples was measured by RT-qPCR using the miRCURY LNA Universal RT microRNA PCR system (Exiqon) and custom-designed Pick-&-Mix microRNA PCR Panels (Exiqon). Samples were tested in 384-well plates containing primer sets specific for each target miRNA and for a 22-mer, synthetic RNA spiked in every TNA sample as an additional internal control to assess the quality of the TNA preparation, RT step and qPCR step. During the cohort study, all runs further included three known TNA sample controls generating positive or negative miRNA classifier results.
Data analyses
Diagnostic sensitivity and specificity were determined using standard 2 × 2 contingency tables comparing qualitative, binary molecular test results (positive or negative) relative to the reference standard diagnoses determined by pathology (benign or malignant). Post-test probability metrics for prevalence values ranging from 0–100% were calculated using sensitivity, specificity, and Bayes theorem for PPV and NPV. The numbers of true positive, true negative, false positive, and false negative molecular results for a given total number of specimens were computed using sensitivity, specificity, and prevalence. All calculations assumed conservation of intrinsic test performance in distinct test populations. Unless indicated, 95% confidence intervals (CI) were calculated using the Clopper-Pearson exact method for proportions and P values were calculated using the Fisher exact test for categorical variables. Graphic and statistical analyses were performed in Excel (Microsoft) or R version 3.1 (http://www.r-project.org/).
Additional information is available in the Supplemental Materials and Methods.
Results
Test algorithm
The multiplatform mutation and miRNA test (MPT) consisted of distinct molecular assays interrogating DNA, RNA, or miRNA markers in total nucleic acids samples. The miRNA gene expression classifier reported a qualitative positive or negative result based on the expression levels of 10 miRNA genes, miR-29b-1–5p, miR-31–5p, miR-138–1-3p, miR-139–5p, miR-146b-5p, miR-155, miR-204–5p, miR-222–3p, miR-375, and miR-551b-3p. The other qualitative tests detected the presence of known oncogenic gene alterations, either DNA mutations in the BRAF, HRAS, KRAS, and NRAS genes, or the PAX8-PPARG, RET-PTC1, and RET-PTC3 fusion transcripts. Samples positive with either assay were scored as positive and samples negative with all assays were scored as negative. The miRNA classifier was initially trained using well-characterized, surgically-resected, benign or malignant thyroid lesions (n = 240) with the classification thresholds then optimized based on performance evaluated in the original training set alongside independent resected thyroid tissues (n = 54) and preoperative thyroid FNAs (n = 235). Specimens' characteristics and estimates of MPT performance are summarized in Table 1 and Supplemental Figure 1.
Table 1.
Specimens' Characteristics and Point Estimates of Sensitivity and Specificity for the Multiplatform Mutation and miRNA Test in Three Independent Case-control Specimen Sets
| Training Set | Fine-needle Aspiration | Resected Tissue | |
|---|---|---|---|
| No. of cases | 240 | 235 | 54 |
| Age, y, range [average] | 14–85 [49] | 19–88 [52] | 19–81 [50] |
| Female/male, % | 75/25 | 72/28 | 77/23 |
| Malignant/benign, % | 55/45 | 40/60 | 59/41 |
| Sensitivity/specificity, % | 84/84 | 84/90 | 84/100 |
Cross-sectional cohort
To sample a representative cohort of thyroid nodules with indeterminate cytology, consecutive FNAs with known mutation status collected from 12 distinct clinical sites in the United States were tested for miRNA expression. The set consisted of 109 nodules with AUS/FLUS or FN/SFN cytology and a traceable surgical outcome of primary benign or malignant thyroid lesion (Table 2). The histopathological reference standard diagnoses were based solely on local pathology expertise and pathologists at each participating site who evaluated the surgically removed thyroid nodules were not aware of the results of molecular testing. There were a total of 74 nodules classified as benign, including 17 Hurthle cell adenomas and 12 follicular adenomas. The 35 malignant nodules consisted of 18 follicular variant of papillary thyroid carcinomas, 10 papillary carcinomas, 5 follicular carcinomas, and two Hurthle cell carcinomas (32% thyroid cancer prevalence). All specimens generated valid miRNA gene expression classifier results.
Table 2.
Cohort Characteristics and Summary of Molecular Results in 109 Preoperative Aspirates
| Surgical Histology | Malignant | Benign |
|---|---|---|
| No. of cases | 35 | 74 |
| Age, y, range [average] | 24–79 [52] | 21–89 [58] |
| Female/male, % | 79/21 | 72/28 |
| Mutation positive, n | 24 | 10 |
| miRNA positive, n | 20 | 6 |
| Positive by either test, n | 31 | 11 |
| Negative by both tests, n | 4 | 63 |
Distribution of molecular results by surgical outcome
Among the 35 nodules with a confirmed malignant outcome, 24 were positive by mutation testing and 20 were positive by miRNA classifier (Table 2). Seven (7) of the 11 mutation-negative cases were positive by miRNA classifier resulting in an overall cancer detection rate of 89% (31/35) for the MPT. The four malignant cases negative by mutation testing and by miRNA gene expression classifier were three follicular variants of papillary thyroid carcinoma, 0.8–3.0 cm in size, and a 3.0-cm follicular thyroid carcinoma (Supplemental Table 1). Among the 74 nodules with confirmed benign outcome, 10 were positive by mutation testing and six were positive by miRNA classifier. Five (5) of the six miRNA-positive cases were also positive for a RAS mutation resulting in an overall benign detection rate of 85% (63/74) for the MPT. The single benign nodule negative by mutation testing but positive by miRNA gene expression was from a study subject who had undergone total thyroidectomy and had a 0.9-cm follicular variant of papillary thyroid carcinoma in the opposite thyroid lobe (Supplemental Table 1).
Diagnostic performance
The performance characteristics of the MPT and corresponding 95% CI obtained in the cross-sectional cohort study are summarized in Table 3. The MPT accurately classified both the malignant nodules and the benign nodules. Among the cases with a positive MPT result, the post-test probability of a malignant nodule (PPV) was 74% (31/42) and among the MPT-negative cases, the post-test probability of a benign nodule (NPV) was 94% (63/67). The overall odds of a correct molecular result were 44 times higher than the odds of an incorrect result (P < .01). Importantly, the rate of negative calls generated by the MPT was high (67/109 or 61% of all nodules evaluated), ie, the MPT identified 67 nodules with a low residual risk of thyroid cancer (4/67 or 6%). In the clinical setting, those nodules would be candidates for active surveillance without surgery. At 32% prevalence, the MPT had a true negative call rate of 58% (63/109) and would efficiently reduce the number of surgical procedures from 74 diagnostic surgeries in the absence of molecular testing (68% of all nodules evaluated) to 11 surgeries after reclassification by the MPT (10% of all nodules evaluated).
Table 3.
Performance of the Multiplatform miRNA and Mutation Test
| Cohort, % (95% CI) | AUS/FLUS, % (95% CI) | FN/SFN, % (95% CI) | |
|---|---|---|---|
| No. of cases | 109 | 58 | 51 |
| Sensitivity | 89 (73–97) | 94 (73–100) | 82 (57–96) |
| Specificity | 85 (75–92) | 80 (64–91) | 91 (76–98) |
| PPV | 74 (58–86) | 68 (46–85) | 82 (57–96) |
| NPV | 94 (85–98) | 97 (84–100) | 91 (76–98) |
| Odds ratio | 44 (13–151) | 68 (8–590) | 48 (9–269) |
Clinical relevance at different prevalence
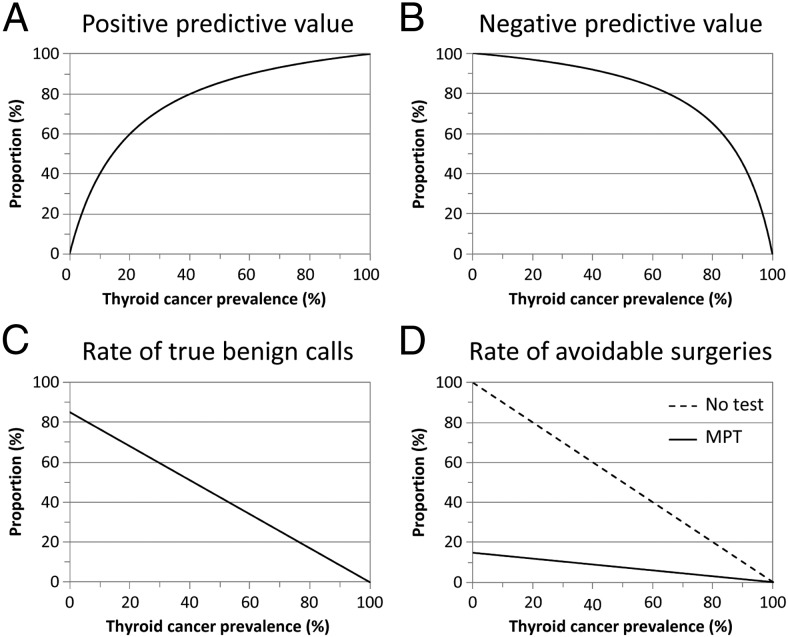
The predictive value of any diagnostic procedure, whether based on pathology review or molecular testing, varies according to the pretest probability of the condition of interest in the evaluated population. As the prevalence of thyroid cancer in nodules with AUS/FLUS or FN/SFN cytology can be different at various institutions and pathology practices (1, 4), we next calculated the PPV and NPV of the MPT in populations with different pretest cancer probability using the Bayes' theorem and the sensitivity and specificity observed in the present study. The PPV was predicted to be greater than 50% for any prevalence greater than 15% and the NPV would range from 93–98% for cancer probability greater than 15% and less than 35% (Figure 1, A and B). Because the rate of true benign calls generated by the MPT would be high in the relevant range of 15–35% prevalence (72–55%; Figure 1C), the MPT was predicted to significantly reduce the number of avoidable surgeries (Figure 1D). Independently of potential variations in thyroid cancer prevalence in the clinical setting, the use of the MPT would result in a constant 6.7-fold or 85% decrease in the number of diagnostic surgeries that may be performed in the absence of molecular testing (Figure 1D, dashed line).
Figure 1.
Expected diagnostic performance of the MPT in thyroid nodules with AUS/FLUS or FN/SFN cytopathology according to thyroid cancer prevalence in a given test population. The graphs show the PPV (A), NPV (B), rate of true benign molecular calls (C), and rate of potentially avoidable surgeries (D) calculated using the sensitivity and specificity obtained in the present study and pretest probabilities of cancer ranging from 0–100%. D, the rate of potentially avoidable surgeries when no molecular testing is performed (no test, dashed line) is the proportion of benign cases by histopathology after diagnostic surgery, ie, [1-prevalence].
Discussion
In the present study, we showed that a molecular test combining miRNA expression and gene mutation detection can increase the diagnostic yield of molecular cytology by accurately classifying thyroid nodules with AUS/FLUS or FN/SFN cytology into benign or malignant categories. Our results underscore the value of this novel diagnostic algorithm and highlight key performance metrics that are important to improve the management of patients with thyroid nodules.
Cytopathology and molecular testing on indeterminate nodules are routinely combined in the clinical setting to identify different subsets of benign or malignant nodules prior to thyroid surgery. Molecular testing itself is often a combination of multiple assays with distinct analytical and/or clinical performance characteristics (6–9). For example, mutation testing includes BRAF c.1799T>A, a mutation exclusively associated with thyroid carcinomas (100% specificity), as well as other oncogenic alterations, such as RAS mutations or PAX8-PPARG fusion transcripts, to identify different subsets of BRAF-negative carcinomas (10, 11). Similarly, the miRNA classifier interrogates well-known miRNAs associated with thyroid carcinogenesis (17–22, 24–26) to identify a subset of malignant nodules that are negative by mutation testing. One key feature of the miRNA classifier, however, is that it was designed and optimized to have high specificity to increase the sensitivity of the MPT without significantly affecting its specificity. In our cohort study, greater than 90% (10/11) of the misclassified benign specimens were positive for a gene alteration and the single apparent false positive by miRNA expression alone was from the left thyroid lobe of a study subject with multiple bilateral circumscribed follicular epithelial cell lesions and a papillary carcinoma in the right lobe. Among the mutation-negative cases, the specificity of the miRNA classifier was extremely high, 98% in both the cohort study (63/64) and the three case-control specimen sets (240/246).
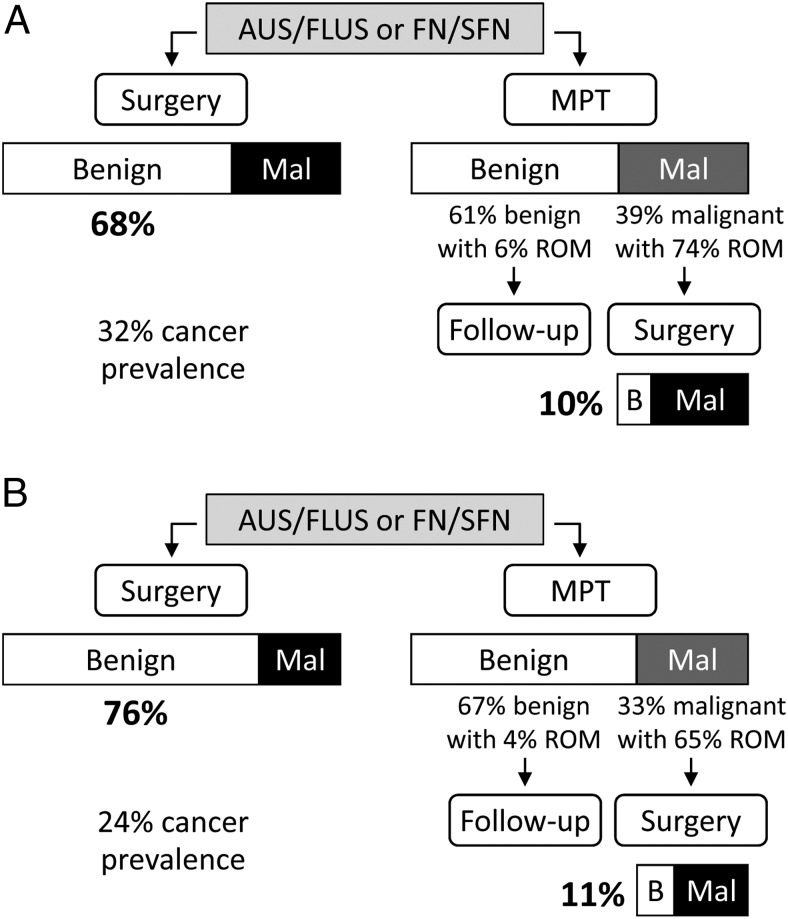
The relatively high specificity of the MPT is a critical characteristic not only because it enables high and actionable PPV (less false positive) but also because it increases the benign call rate (more true negative). In a simplified clinical management algorithm in which all patients with AUS/FLUS or FN/SFN nodules would be sent to diagnostic surgery, the proportion of patients with a benign nodule who would undergo a potentially avoidable surgery is [1-prevalence], ie, 68% for the 32% thyroid cancer prevalence observed in our cohort study (Figure 2A). Following molecular reclassification with the MPT, 61% of the patients would have a negative MPT result with a low residual risk of malignancy (ROM) of 6% and only 39% of the patients might be referred to surgery because of a positive MPT result. With a PPV of 74%, only 10% of all the patients evaluated would have undergone surgery to remove a nodule later diagnosed as benign by histopathology (0.39 × [1–0.74] = 0.1). The relative decrease in the rate of avoidable surgery from 68% to 10% is the specificity of the MPT ([0.68–0.1]/0.68 = 0.85; see also algebraic demonstration in Supplemental Materials and Methods). Thus, use of the MPT in the clinical setting could potentially result in a 6.7-fold reduction in the number of unnecessary diagnostic surgeries, independently of variations in cancer prevalence.
Figure 2.
Expected surgical histopathology outcomes in thyroid nodules with AUS/FLUS or FN/SFN cytopathology after diagnostic surgery or after preoperative molecular testing. The size of each box and the associated percentages represent the expected proportions of benign/malignant outcomes (white/black boxes) or molecular results (white/dark gray boxes) at 32% (A) or 24% (B) thyroid cancer prevalence. The residual risk of malignancy (ROM) in nodules with negative/benign molecular results is [1-NPV] and the ROM in nodules with positive/malignant molecular results is PPV. The rate of unnecessary or potentially avoidable surgeries is the proportion of surgeries performed on nodules subsequently classified as benign by surgical histology. Abbreviations: B, benign; Mal, malignant.
Molecular reclassification of benign thyroid nodules to reduce the number of surgeries and associated healthcare costs has gained considerable attention in the past few years with the commercial availability of the Afirma gene expression classifier (AGEC). Although our study was not designed to compare different molecular methods, post-test probability metrics such as predictive values or surgery rates can easily be modeled using estimates of diagnostic sensitivity and specificity determined in representative clinical populations. In a recent multicenter cross-sectional cohort study, Alexander and colleagues (12) reported a sensitivity of 90% and a specificity of 52% for the AGEC in thyroid nodules with AUS/FLUS or FN/SFN cytology. With a specificity of 52%, the AGEC would indeed reduce by 52% the rate of avoidable surgeries, from 76% of all nodules evaluated without molecular testing (24% cancer prevalence observed) to 37% after AGEC testing. This rate would be significantly improved to 11% with the MPT (P < .01), in part because of the higher PPV and mainly because of the higher benign call rate (Figure 2B). At 24% prevalence, 41% of the calls generated by the AGEC were “benign” with a 6% ROM (39% true benign call rate) whereas 67% of the MPT calls would be “benign” with a 4% ROM (65% true benign call rate). The relative increase in true benign call rate from 39% to 65% (1.65-fold or 65%) and the relative decrease in the rate of avoidable surgeries from 37% to 11% (3.3-fold or 69%) are both directly proportional to the specificity of the two molecular methods and are therefore independent of thyroid cancer prevalence (see algebraic demonstration in Supplemental Materials and Methods).
The relatively high sensitivity of the MPT (89%) is also an important characteristic as it contributes to PPV (more true positive) and is critical to reach actionable NPV (less false negative). The NPV observed in our study (94% at 32% prevalence) was not statistically different from the NPV of the AGEC (P = 1.0) whereas the large differences in PPV and true benign call rate between the two methods were significant (P < .01). Notably, the widths of the 95% CI for NPV and true benign call rate were similar with both methods, indicating similar precision in the estimation of performance (Supplemental Figure 2, A–C). Statistical analyses performed either on nodules with AUS/FLUS cytology or on nodules with FN/SFN cytology showed similar patterns for NPV, PPV, and true benign call rate, but with broader 95% CI as the number of computed features was smaller for each independent cytology category (Table 3 and Supplemental Figure 2, D–I).
Recent advances in the understanding of the molecular pathogenesis of thyroid cancer and in next generation sequencing (NGS) technologies have exponentially increased the number of genetic alterations that can be interrogated in a single nodule aspirate. Nikiforov and colleagues (30) recently reported a high sensitivity (90%) and specificity (93%) for the ThyroSeq v2 NGS panel in nodules with FN/SFN cytology at a single institution. Thus, NGS with extended mutation panels could further increase the sensitivity of the MPT by detecting additional malignant cases. However, NGS could also decrease specificity (and therefore the benign call rate) by detecting germline or low-level somatic mutations of unknown clinical significance in nodules classified as benign by surgical pathology. The clinical utility of this novel technology will require careful consideration of study design- and institution-specific parameters that can dramatically affect test performance such as FNA collection methods and sites, pathology review procedures, or histopathology features and cutoffs used to establish the final benign/malignant classification.
Cross-sectional cohort studies are designed to sample consecutive specimens in the source population and then retrospectively assess the history of exposures and outcomes that are available at the end of the study (7, 9, 12–14, 30). Estimation of diagnostic performance is therefore restricted to thyroid nodules with available surgical histology. Variations in local clinical practices and pathology classification may affect the calculation and extrapolation of performance at different cancer prevalence and limit the comparison across studies performed in distinct populations. In addition, our study was designed to evaluate clinical cases that had been submitted to molecular testing as part of their diagnostic work-up at diverse endocrinology centers. Although this design guaranteed that only FNAs truly representative of the target clinical population were interrogated, we do acknowledge that it may have caused a study bias by increasing the number of mutation-positive cases with available surgical outcome. This in turn may have artificially decreased the specificity of mutation testing and increased its sensitivity (69% observed). Regardless, the miRNA classifier correctly identified 64% of the mutation-negative malignant cases. Even with a sensitivity of 55–60% for mutation testing alone, ie, lower than the range of 61–75% reported in the literature (6–9), the sensitivity of the MPT would be 83–86%. With specificity at 85%, the NPV of the MPT would still be high (94–95% NPV at 24% prevalence) and similar to other methods routinely used in the clinical setting.
In summary, we have demonstrated that a diagnostic algorithm combining miRNA expression and gene mutation detection yields clinically actionable molecular information in thyroid nodules with AUS/FLUS or FN/SFN cytology. Based on the high PPV and NPV of the MPT, it is reasonable to propose that patients with positive (malignant) MPT results may be sent to surgery while patients with negative (benign) MPT results may benefit from a more conservative management, ie, active followup without surgery (Figure 2). On the one hand, knowledge of the mutational status would provide valuable diagnostic, prognostic, and theranostic information for the selection of optimal and personalized therapeutic strategies. On the other hand, the MPT would significantly increase the benign call rate and decrease the rate of unnecessary diagnostic surgeries, thus further reducing the number of two-step total thyroidectomies and the total number of surgeries performed. Our data and predictive models underscore substantial improvements to thyroid cancer patient management and collateral cost saving opportunities.
Note added in proof: The two molecular tests used in this study are currently marketed as ThyGenX Thyroid Oncogene Panel and ThyraMIR Thyroid miRNA Classifier by Interpace Diagnostics, a subsidiary of PDI, Inc.
Acknowledgments
We thank Professor Thomas J. Giordano from the Department of Pathology at the University of Michigan Medical School for his expert pathology review of the surgical cases used for miRNA classifier training as well as the personnel from Asuragen's molecular laboratory and from every participating clinical site for their respective contribution.
Disclosure Summary: B.A. is an employee of Asuragen Inc. E.L., D.W., and S.B.H. were employees of Asuragen Inc. at the time of the study. E.L. is a consultant for PDI Inc. A.S., A.E.B., M.A.L., and M.L.M. have no conflicts of interest to declare.
Footnotes
- AGEC
- Afirma gene expression classifier
- AUS/FLUS
- atypia of undetermined significance/follicular lesion of undetermined significance
- CI
- confidence interval
- FN/SFN
- follicular neoplasm/suspicious for a follicular neoplasm
- FNA
- fine-needle aspiration
- MPT
- multiplatform mutation and miRNA test
- NGS
- next generation sequencing
- NPV
- negative predictive value
- PPV
- positive predictive value
- ROM
- risk of malignancy
- TNA
- total nucleic acid.
References
- 1. Bongiovanni M, Spitale A, Faquin WC, Mazzucchelli L, Baloch ZW. The Bethesda System for Reporting Thyroid Cytopathology: A meta-analysis. Acta Cytol. 2012;56:333–339. [DOI] [PubMed] [Google Scholar]
- 2. Cooper DS, Doherty GM, Haugen BR, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 3. Gharib H, Papini E, Paschke R, et al. American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and EuropeanThyroid Association Medical Guidelines for Clinical Practice for the Diagnosis and Management of Thyroid Nodules. Endocr Pract. 2010;16:S1–S43. [DOI] [PubMed] [Google Scholar]
- 4. Wang CC, Friedman L, Kennedy GC, et al. A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid. 2011;21:243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Banks ND, Kowalski J, Tsai HL, et al. A diagnostic predictor model for indeterminate or suspicious thyroid FNA samples. Thyroid. 2008;18:933–941. [DOI] [PubMed] [Google Scholar]
- 6. Cantara S, Capezzone M, Marchisotta S, et al. Impact of proto-oncogene mutation detection in cytological specimens from thyroid nodules improves the diagnostic accuracy of cytology. J Clin Endocrinol Metab. 2010;95:1365–1369. [DOI] [PubMed] [Google Scholar]
- 7. Nikiforov YE, Ohori NP, Hodak SP, et al. Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: A prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab. 2011;96:3390–3397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nikiforov YE, Steward DL, Robinson-Smith TM, et al. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab. 2009;94:2092–2098. [DOI] [PubMed] [Google Scholar]
- 9. Beaudenon-Huibregtse S, Alexander EK, Guttler RB, et al. Centralized molecular testing for oncogenic gene mutations complements the local cytopathologic diagnosis of thyroid nodules. Thyroid. 2014;24:1479–1487. [DOI] [PubMed] [Google Scholar]
- 10. Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab. 2003;88:5399–5404. [DOI] [PubMed] [Google Scholar]
- 11. Nikiforova MN, Lynch RA, Biddinger PW, et al. RAS point mutations and PAX8-PPAR gamma rearrangement in thyroid tumors: Evidence for distinct molecular pathways in thyroid follicular carcinoma. J Clin Endocrinol Metab. 2003;88:2318–2326. [DOI] [PubMed] [Google Scholar]
- 12. Alexander EK, Kennedy GC, Baloch ZW, et al. Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med. 2012;367:705–715. [DOI] [PubMed] [Google Scholar]
- 13. Alexander EK, Schorr M, Klopper J, et al. Multicenter clinical experience with the Afirma gene expression classifier. J Clin Endocrinol Metab. 2014;99:119–125. [DOI] [PubMed] [Google Scholar]
- 14. Yip L, Wharry LI, Armstrong MJ, et al. A clinical algorithm for fine-needle aspiration molecular testing effectively guides the appropriate extent of initial thyroidectomy. Ann Surg. 2014;260:163–168. [DOI] [PubMed] [Google Scholar]
- 15. Pallante P, Battista S, Pierantoni GM, Fusco A. Deregulation of microRNA expression in thyroid neoplasias. Nat Rev Endocrinol. 2014;10:88–101. [DOI] [PubMed] [Google Scholar]
- 16. Yuan ZM, Yang ZL, Zheng Q. Deregulation of microRNA expression in thyroid tumors. J Zhejiang Univ Sci B. 2014;15:212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Swierniak M, Wojcicka A, Czetwertynska M, et al. In-depth characterization of the microRNA transcriptome in normal thyroid and papillary thyroid carcinoma. J Clin Endocrinol Metab. 2013;98:E1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen YT, Kitabayashi N, Zhou XK, Fahey TJ, 3rd, Scognamiglio T. MicroRNA analysis as a potential diagnostic tool for papillary thyroid carcinoma. Mod Pathol. 2008;21:1139–1146. [DOI] [PubMed] [Google Scholar]
- 19. Dettmer M, Perren A, Moch H, Komminoth P, Nikiforov YE, Nikiforova MN. Comprehensive MicroRNA expression profiling identifies novel markers in follicular variant of papillary thyroid carcinoma. Thyroid. 2013;23:1383–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yip L, Kelly L, Shuai Y, et al. MicroRNA signature distinguishes the degree of aggressiveness of papillary thyroid carcinoma. Ann Surg Oncol. 2011;18:2035–2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. He H, Jazdzewski K, Li W, et al. The role of microRNA genes in papillary thyroid carcinoma. Proc Natl Acad Sci U S A. 2005;102:19075–19080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE. MicroRNA expression profiling of thyroid tumors: Biological significance and diagnostic utility. J Clin Endocrinol Metab. 2008;93:1600–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stokowy T, Wojta B, Krajewska J, et al. A two miRNA classifier differentiates follicular thyroid carcinomas from follicular thyroid adenomas. Mol Cell Endocrinol. 2015;399:43–49. [DOI] [PubMed] [Google Scholar]
- 24. Sheu SY, Grabellus F, Schwertheim S, Worm K, Broecker-Preuss M, Schmid KW. Differential miRNA expression profiles in variants of papillary thyroid carcinoma and encapsulated follicular thyroid tumours. Br J Cancer. 2010;102:376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Keutgen XM, Filicori F, Crowley MJ, et al. A panel of four miRNAs accurately differentiates malignant from benign indeterminate thyroid lesions on fine needle aspiration. Clin Cancer Res. 2012;18:2032–2038. [DOI] [PubMed] [Google Scholar]
- 27. Kitano M, Rahbari R, Patterson EE, et al. Evaluation of candidate diagnostic microRNAs in thyroid fine-needle aspiration biopsy samples. Thyroid. 2012;22:285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dudoit S, Fridlyand J, Speed TP. Comparison of Discrimination Methods for the Classification of Tumors Using Gene Expression Data. J Amer Statist Assoc. 2002;97:77–87. [Google Scholar]
- 29. Giordano TJ, Beaudenon-Huibregtse S, Shinde R, et al. Molecular testing for oncogenic gene mutations in thyroid lesions: A case-control validation study in 413 postsurgical specimens. Hum Pathol. 2014;45:1339–1347. [DOI] [PubMed] [Google Scholar]
- 30. Nikiforov YE, Carty SE, Chiosea SI, et al. Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer. 2014;120:3627–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]