Abstract
Hydrogen sulfide (H2S) can act as a signaling molecule for various ion channels and/or transporters; however, little is known about its potential involvement in Ca2+ balance. Using developing zebrafish (Danio rerio) as an in vivo model system, the present study demonstrated that acute exposure to H2S donors increased Ca2+ influx at 4 days postfertilization, while chronic (3-day) exposure caused a rise in whole body Ca2+ levels. The mRNA expression of Ca2+-transport-related genes was unaffected by H2S exposure, suggesting that posttranscriptional modifications were responsible for the altered rates of Ca2+ uptake. Indeed, treatment of fish with the protein kinase A inhibitor H-89 abolished the H2S-mediated stimulation of Ca2+ influx, suggesting that H2S increased Ca2+ influx by activating cAMP-protein kinase A pathways. Cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE) are two key enzymes in the endogenous synthesis of H2S. Using an antisense morpholino knockdown approach, we demonstrated that Ca2+ influx was reduced in CBS isoform b (CBSb)- but not in CSE-deficient fish. Interestingly, the reduction in Ca2+ influx in CBSb-deficient fish was observed only in fish that were acclimated to low-Ca2+ water (i.e., 25 μM Ca2+; control: 250 μM Ca2+). Similarly, mRNA expression of cbsb but not cse was increased in fish acclimated to low-Ca2+ water. Results from whole-mount immunohistochemistry further revealed that CBSb was expressed in Na+-K+-ATPase-rich cells, which are implicated in Ca2+ uptake in zebrafish larvae. Collectively, the present study suggests a novel role for H2S in promoting Ca2+ influx, particularly in a low-Ca2+ environment.
Keywords: calcium, ECaC, hydrogen sulfide, protein kinase A, zebrafish, ionocyte, NaR cell
hydrogen sulfide (h2s) is a signaling molecule that can modulate various physiological functions in vertebrates (17). Biosynthesis of H2S is mediated primarily by the enzymes cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), which are expressed in various tissues in mammals, including the kidney, ileum, brain, and endothelium (6, 21). Although it has been reported that H2S concentration in the plasma or blood is typically in the micromolar range (measured as total sulfide) in mammals and fish (26), critical measurements of intracellular H2S are lacking. Previous studies suggested that H2S is involved in cardiorespiratory control, oxygen sensing, and Na+ transport in mammals and fish (1, 13, 25, 27, 31, 33). In mammalian systems, it was also demonstrated that H2S exposure increased cytosolic Ca2+ levels (15, 22, 24). However, the mechanisms by which H2S modulates Ca2+ fluxes, and the physiological importance of H2S in regulating whole body Ca2+ content, remain unknown.
In typical freshwater ecosystems, the environmental Ca2+ levels range from 0.01 to 3 mM, yet plasma Ca2+ levels are remarkably constant, regardless of the ambient Ca2+ levels. To achieve Ca2+ homeostasis, freshwater fish modulate their Ca2+ transport functions in response to changing external Ca2+ levels (3, 23). In zebrafish, Ca2+ uptake is thought to be localized to a subset of ionocytes. The Ca2+-transporting ionocytes are Na+-K+-ATPase-rich cells (NaRCs), which express epithelial Ca2+ channels (ECaC) at the apical membrane, and plasma membrane Ca2+-ATPase (PMCA) and Na+/Ca2+ exchanger (NCX) at the basolateral membrane (7). It has been documented that acclimation to low-Ca2+ water increases the capacity for Ca2+ uptake in zebrafish (14, 19, 29). Various mechanisms for chronic modulation of Ca2+ uptake have been identified, primarily involving regulated expression of ECaC (7). However, little is known about the mechanisms that promote acute regulation of Ca2+ transport. The ECaC contains several putative protein kinase A (PKA) and protein kinase C (PKC) phosphorylation sites, and in mammalian systems activation of these sites can increase the activity of ECaC (2, 4). The activity of PKA is highly dependent on the intracellular levels of cAMP, and treatment with the cAMP-elevating agent forskolin increased the activity of ECaC in a mammalian kidney cell line (4). Exposure to H2S also is known to increase cAMP levels in primary cultures of rat brain cells (10). However, evidence that H2S can interact with the cAMP-PKA pathways and modulate Ca2+ transport is lacking.
In the present study, we examined the effects of exposure to H2S donors on whole body Ca2+ fluxes and evaluated the physiological role of endogenous H2S on Ca2+ balance in developing zebrafish. We demonstrated that H2S exposure increases Ca2+ influx via posttranslational activation of cAMP-PKA pathways and found that CBS isoform b (CBSb)-generated H2S promotes Ca2+ influx when fish are maintained in a low-Ca2+ environment.
MATERIALS AND METHODS
Zebrafish maintenance.
Adult zebrafish (Danio rerio) were maintained in aerated, dechloraminated City of Ottawa tap water at 28°C (in mM; 0.25 [Ca2+], 0.78 [Na+], 0.02 [K+], 0.15 [Mg2+], where brackets denote concentration; pH 7.6). Fish were subjected to a constant 14:10-h light-dark photoperiod and fed daily until satiation with no. 1 crumble-Zeigler (Aquatic Habitats, Apopka, FL). Embryos were collected and reared in 50-ml petri dishes containing dechloraminated City of Ottawa tap water supplemented with 0.05% methylene blue. The petri dishes were kept in incubators at 28°C. The experiments were conducted in compliance with guidelines of the Canadian Council of Animal Care and after the approval of the University of Ottawa Animal Care Committee (protocol BL-226).
Acclimation experiments.
Control (normal) and low-Ca2+ water were prepared with double deionized water supplemented with CaSO4·2H2O, MgSO4·7H2O, NaCl, K2HPO4, and KH2PO4. The [Ca2+] of the normal and low-Ca2+ water were 250 and 25 μM, respectively; the levels were verified using flame emission spectrophotometry. All other ion concentrations were kept constant (in mM; 0.8 Na+, 0.16 Mg2+, and 0.3 K+). Fish were transferred to control or low-Ca2+ water at 1 day postfertilization (dpf) and were sampled for subsequent experiments (detailed below) at 4 dpf, unless stated otherwise.
Measurement of Ca2+ influx.
Influx of Ca2+ was measured using a radiotracer method, as described previously (14). In brief, fish were exposed to 0.2 μCi/ml 45Ca2+ (as CaCl2; Perkin Elmer) for 1 h. At the end of the flux period, fish were killed with an overdose (i.e., 4 g/l) of tricaine methanesulfonate (MS-222) and rinsed in isotope-free water; two fish were pooled as one sample (n = 1). Fish were digested with a tissue solubilizer (Solvable; Perkin Elmer) and later neutralized using glacial acetic acid. The radioactivity of the digest and the water samples was measured using a liquid scintillation counter (LS-6500; Beckman Coulter) following the addition of a scintillation cocktail (BioSafe-II; Research Products International). The Ca2+ influx (Jin; pmol·fish−1·h−1) was determined using the formula: Jin = F/(SA × n × t), where F is the total radioactivity counted in the fish (counts/min), SA is the specific activity of the water (cpm/nmol), n is the number of fish, and t is the duration of the experiment in hours.
Treatment with H2S donors.
To evaluate the acute effects of H2S exposure on Ca2+ influx, normal or low-Ca2+ water acclimated zebrafish were preexposed to the H2S donor sodium sulfide (Na2S) (1 or 10 μM) for 30 min. The chronic effects of H2S exposure on Ca2+ balance were examined using a stable and slow-releasing H2S donor GYY4137 (Sigma-Aldrich) (16). Fish were exposed to 100 or 200 μM GYY4137 starting at 1 dpf. Water was changed daily with the addition of GYY4137. To examine whether exposure to H2S donors affect the capacity for Ca2+ uptake, influx of Ca2+ at 4 dpf (N = 6) was measured in normal Ca2+ water (250 μM). The concentrations of GYY4137 used in the present study (100 and 200 μM) are estimated to yield stable H2S levels of ∼5 and 10 μM, respectively (16).
Whole body Ca2+ content also was measured in fish exposed to GYY4137. At 4 dpf, control or GYY4137-treated fish were killed with an overdose of MS-222 and then briefly rinsed in double-deionized water. Ten fish were pooled as one sample, and a total of six samples (N = 6) were analyzed in this experiment. The fish were digested with 5 N HNO3 at 70°C for 24 h, and diluted appropriately with deionized water. The total [Ca2+] was measured by flame emission spectrophotometry (Spectra AA 220FS; Varian) and verified using certified Ca2+ standards (Fisher Scientific).
Treatment with PKA and PKC inhibitors.
The potential role of protein kinases in H2S-stimulated Ca2+ influx was evaluated using selective blockers for PKA and PKC. Fish at 4 dpf were exposed to 10 μM Na2S together with either 10 μM H-89 or bisindolylmaleimide I (BIS-1) (Cayman Chemical) for 30 min, and Ca2+ influx was measured as described above. H-89 and BIS-1 are reversible competitive inhibitors for PKA and PKC, respectively. Both H-89 and BIS-1 inhibit PKA or PKC activity by acting on the ATP binding sites on the kinase catalytic subunit. To test whether Ca2+ influx could also be directly modulated by cAMP, fish were exposed to a cAMP-elevating agent forskolin or 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP) (both at 10 μM) for 30 min before Ca2+ uptake measurements. Coexposure to H-89 and forskolin also was performed to evaluate the effects of PKA inhibition on forskolin-stimulated Ca2+ influx. Additionally, influx of Ca2+ was examined following 30-min exposure to 100 μM isobutylmethylxanthine (IBMX). IBMX is a selective inhibitor of phosphodiesterase, which is an enzyme responsible for degrading cAMP. All influxes (N = 6) were performed in normal Ca2+ water (250 μM).
Pharmacological inhibition of H2S biosynthesis.
To examine the involvement of endogenous H2S on basal Ca2+ influx, fish acclimated to normal (250 μM) or low-Ca2+ (25 μM) water were exposed to either 100 μM propargylglycine (PPG; an inhibitor of CSE) or 100 μM aminooxyacetic acid (AOA; an inhibitor of CBS) at 3 dpf. The water pH was adjusted to 7.6 with 0.1 N NaOH. Influx of Ca2+ was measured in the same exposure water at 4 dpf (N = 6).
Gene knockdown of H2S-biosynthesis enzymes.
The involvement of endogenous H2S in regulating basal Ca2+ influx was examined further using a gene knockdown approach. Two H2S-biosynthesis enzymes, CSE and CBSb, were knocked down using antisense oligonucleotide morpholinos (GeneTools). The CSE morpholino (5′-CGC ACA AGA GTG AAC AGC TCT CTG T-3′) was designed to splice out exon 1 (57–219 bp of NC_007117.5), and the CBSb morpholino (5′-TTG TCC TGT GAG AAA AAG CTG CAT T-3′) was designed to splice out exon 3 (385–491 bp of NM_001014345.2). The effectiveness of these morpholinos was confirmed previously (13, 33). The morpholinos were diluted in Danieau buffer [58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM HEPES (pH 7.6)] plus 0.05% phenol red, and 4 ng were injected into one-cell stage embryos. A “sham” group was injected with a standard control morpholino (5′-CCT CTT ACC TCA GTT ACA ATT TAT A-3′). Influx of Ca2+ was measured in the same acclimation water (250 or 25 μM [Ca2+]) at 4 dpf (N = 6).
Western blot analysis.
Methods for Western blot have been described previously (14). Twenty fish were pooled as one sample, and three samples (N = 3) were analyzed after extraction using a RIPA buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM Tris·HCl, 1 mM EDTA, and 1 mM phenylmethanesulfonyl fluoride) plus protease inhibitor cocktail (ThermoScientific). The extracted protein was loaded on a 10% SDS-PAGE and transferred to polyvinylidene difluoride membrane (Bio-Rad). After transfer, the membrane was blocked with 5% skimmed milk in Tris buffer plus 0.05% Tween 20 (TBST) for 2 h at room temperature. The membrane was then probed with CBSb antibody (1:1,000; peptide sequence = YKKAGLKEFGTMEKC; GenScript) in TBST with 2% skimmed milk at 4°C overnight. After being washed with TBST (three times and 5 min each; 3 × 5 min), the membrane was probed with 1:5,000 goat anti-rabbit antibodies (Invitrogen) for 2 h at room temperature. The membrane was then washed (5 × 5 min), and the bands were detected using enhanced chemiluminescence (SuperSignal West femto chemiluminescent substrate; Pierce) with a ChemiDoc system (Bio-Rad). Subsequently, the membrane was reprobed with β-actin antibodies (1:4,000; Sigma) after being striped with a reblot solution (ThermoScientific).
PCR analysis.
Methods for RNA extraction, cDNA synthesis, and PCR analysis were described previously (14). In brief, total RNA from different tissues of adult zebrafish, or from larval zebrafish following experimental treatments (described below), were extracted using RNeasy kit (Qiagen). After treatment with DNase I (Biolabs), cDNA was synthesized with 1 μg of RNA using RevertAid H-minus reverse transcriptase (Thermo) and random hexamer primers. To evaluate the relative abundance of cse and cbsb in different tissues of adult zebrafish, 150 ng of cDNA template were used and amplified for 30 or 40 cycles. Potential changes in mRNA levels of ecac, NCX isoform 1b (ncx1b), and PMCA isoform 2 (pmca2) following GYY4137 exposure or CBSb knockdown were also examined using real-time PCR (N = 6). Both ncx1b and pmca2 are known to be expressed in ecac-positive cells (18). Real-time PCR analysis was performed using a Bio-Rad CFX96 qPCR system, as described previously (14); 18S RNA was used as an internal control. Primer pairs used in the present study are provided in Table 1.
Table 1.
Primers used in the present study
| Gene | Sequence | Reference No. |
|---|---|---|
| RT-PCR | ||
| cse | FWD: 5′-CGT CTT TCA GTG GGT CTG GA-3′ | Present study |
| REV: 5′-CAC TGC TGT TCC TCA TCC GT-3′ | ||
| cbsb | FWD: 5′-TTG ACC AGT ACC GCA ATC CC-3′ | Present study |
| REV: 5′-CCT GCG ACC AGC ATG TCT AT-3′ | ||
| Real-time PCR | ||
| ecac | FWD: 5′-TCC TTT CCC ATC ACC CTC T-3′ | 19 |
| REV: 5′- GCA CTG TGG CAA CTT TCG T-3′ | ||
| pmca2 | FWD: 5′-AAG CAG TTC AGG GGT TTA C-3′ | 19 |
| REV: 5′-CAG ATC ATT GCC TTG TAT CA-3′ | ||
| ncx1b | FWD: 5′-TAA AGT GGC AGC GAT ACA GGT-3′ | 19 |
| REV: 5′-CAG ATC AAG GCG AAG ATG G-3′ | ||
cse, Cystathionine γ-lyase; cbsb, cystathionine β-synthase isoform b; ecac, epithelial Ca2+ channels; pmca2, plasma membrane Ca2+-ATPase isoform 2; ncx1b, Na+/Ca2+ exchanger isoform 1b; FWD, forward; REV, reverse.
Immunohistochemistry and confocal imaging.
Fish were fixed overnight in a 4% paraformaldehyde solution in PBS at 4°C. After fixation, the fish were rinsed briefly with PBS containing 0.1% Tween (PBST) and then dehydrated using methanol. Following rehydration with PBST, the fish were subjected to antigen retrieval, as described previously (8). Fish were then blocked with 3% BSA in PBS plus 0.8% Triton X for 2 h and then incubated with CBSb and Na+-K+-ATPase (labels NaRCs; Developmental Studies Hybridoma Bank, University of Iowa, Ames, IA) antibodies overnight at 4°C. Subsequently, fish were rinsed with PBST and then incubated with secondary antibodies (Alexa-488 conjugated anti-rabbit IgG and Alexa-596 conjugated anti-mouse IgG; Invitrogen) at 1:500 dilution in PBST for 2 h at room temperature. To label H+-ATPase-rich cells (HRCs), a vital dye concanavalin A (ConA; Invitrogen) was used, as described previously (13). The images were acquired using a confocal laser scanning microscopy (A1R+; Nikon Instruments).
Statistical analysis.
All statistical analyses were performed using SigmaPlot (version 11.2; Systat Software). Data were analyzed using either Student's t-test, or one-way ANOVA followed by a post hoc Holm-Sidak test. Data are reported as means ± SE, and P ≤ 0.05 was taken as the level of significance.
RESULTS
H2S stimulates Ca2+ influx in a low-Ca2+ environment.
The effects of Na2S on Ca2+ influx were examined in fish acclimated to either normal (250 μM) or low-Ca2+ (25 μM) water. Exposure to Na2S at concentrations of 1 or 10 μM had no effect on Ca2+ influx in fish acclimated to normal Ca2+ water (Fig. 1A). In contrast, Na2S exposure significantly increased Ca2+ influx in fish acclimated to low-Ca2+ water (Fig. 1B). A fivefold increase in mRNA levels of ecac was observed after acclimation to low-Ca2+ water (data not shown). Similarly, the density of NaRCs increased significantly in fish acclimated to low-Ca2+ water (19.8 ± 0.9 cells/100 μm2) compared with that in normal Ca2+ water (9.4 ± 0.8 cells/100 μm2).
Fig. 1.
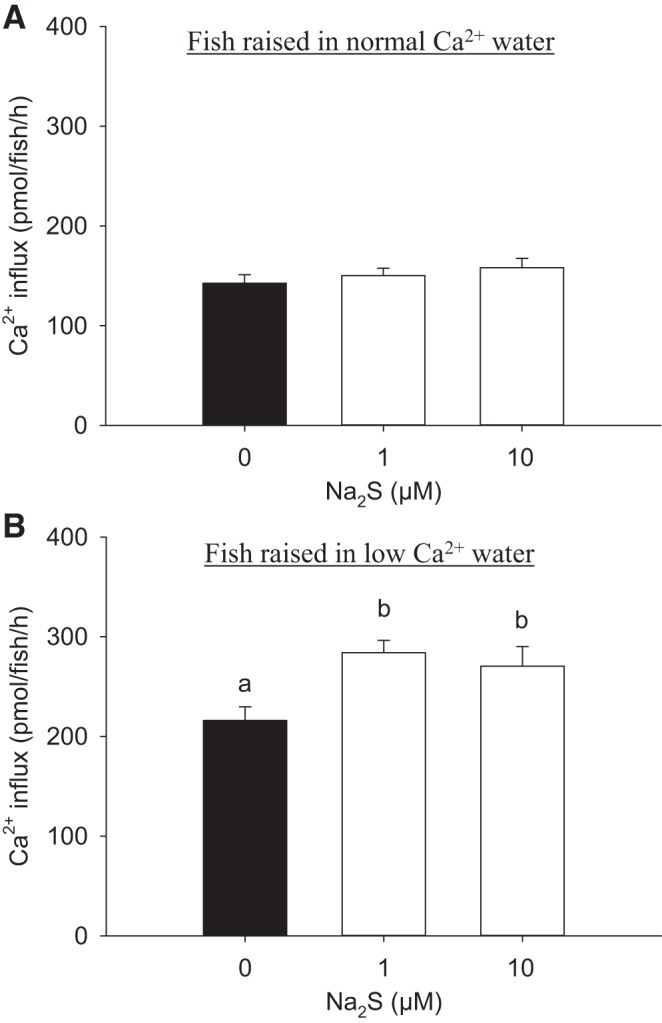
Acute exposure to sodium sulfide (Na2S) increases Ca2+ influx in fish acclimated to low-Ca2+ concentration ([Ca2+]) of water. The effects of Na2S exposure on Ca2+ influx in larval zebrafish [4 days postfertilization (dpf)] acclimated to normal [Ca2+] (250 μM; A) or low-[Ca2+] (25 μM; B) water for 3 days, beginning at 1 dpf, are shown. Influx of Ca2+ was measured in normal [Ca2+] water for both experiments. Values are means ± SE; N = 6. a,b Different letters represent a statistical difference from each other (one-way ANOVA, followed by a Holm-Sidak post hoc test; P < 0.05).
H2S stimulates Ca2+ influx through its interaction with PKA.
Exposure to 10 μM Na2S significantly increased Ca2+ influx at 4 dpf, a response that was abolished in the presence of the PKA inhibitor H-89 (Fig. 2A). In contrast, treatment of fish with a PKC inhibitor BIS-1 did not reduce the Na2S-stimulated Ca2+ influx (Fig. 2B). Similarly, exposure to the cAMP-elevating agent forskolin significantly increased Ca2+ influx, which was reduced substantially by H-89 (Fig. 3A). Fish treated with forskolin exhibited a significant increase in cAMP levels by ∼40-fold compared with the controls (forskolin treatment: 917.4 ± 114.2 pmol/ml, control: 24. 2 ± 2.1 pmol/ml, N = 5; Y. Kumai and M. Tresguerres, personal communications). Influx of Ca2+ also was increased during exposure to 8-Br-cAMP (Fig. 3B) and the phosphodiesterase inhibitor IBMX (Fig. 3C). However, exposure to Na2S did not further increase the 8-Br-cAMP stimulated Ca2+ influx (data not shown).
Fig. 2.
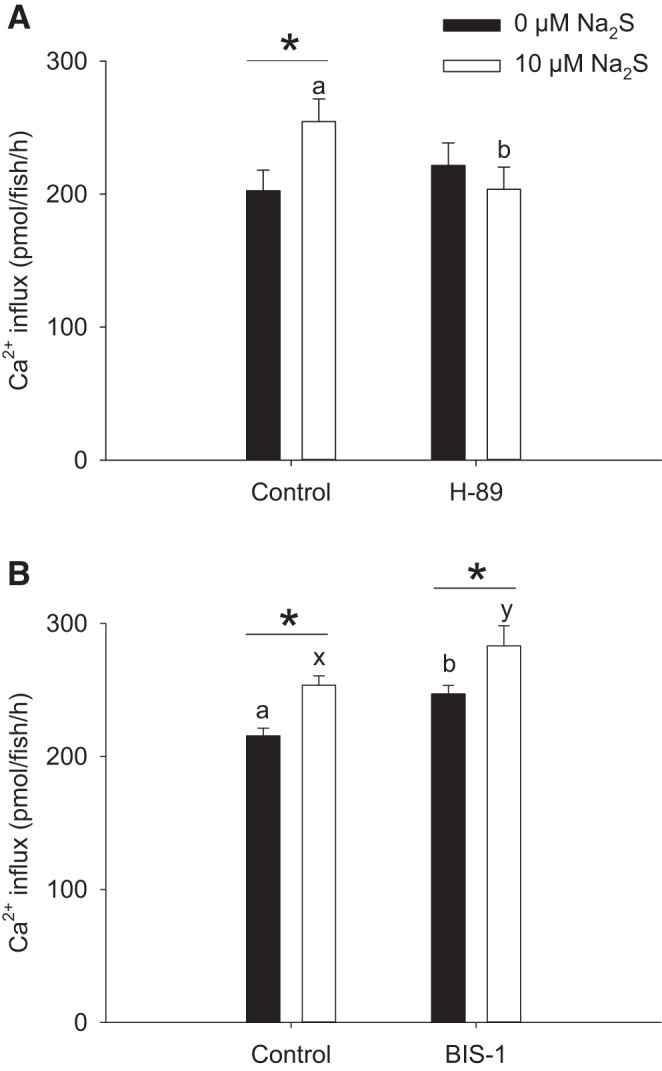
Protein kinase A (PKA) inhibition abolishes the effects of Na2S on increasing Ca2+ influx. The effects of the PKA inhibitor H-89 (A) or the protein kinase C (PKC) inhibitor bisindolylmaleimide I (BIS-1; B) on Na2S-treated larval zebrafish are shown. In both experiments, fish were acclimated to low-[Ca2+] (25 μM) water beginning at 1 day postfertilization (dpf), and influx was performed in normal [Ca2+] (250 μM) water at 4 dpf. Values are means ± SE; N = 6. *Statistical difference between 0 and 10 μM Na2S exposure. a,b,x,y Different letters represent a statistical difference between control and H-89/BIS-1 treatment within the same Na2S exposure group (two-way ANOVA, followed by a Holm-Sidak post hoc test; P < 0.05).
Fig. 3.
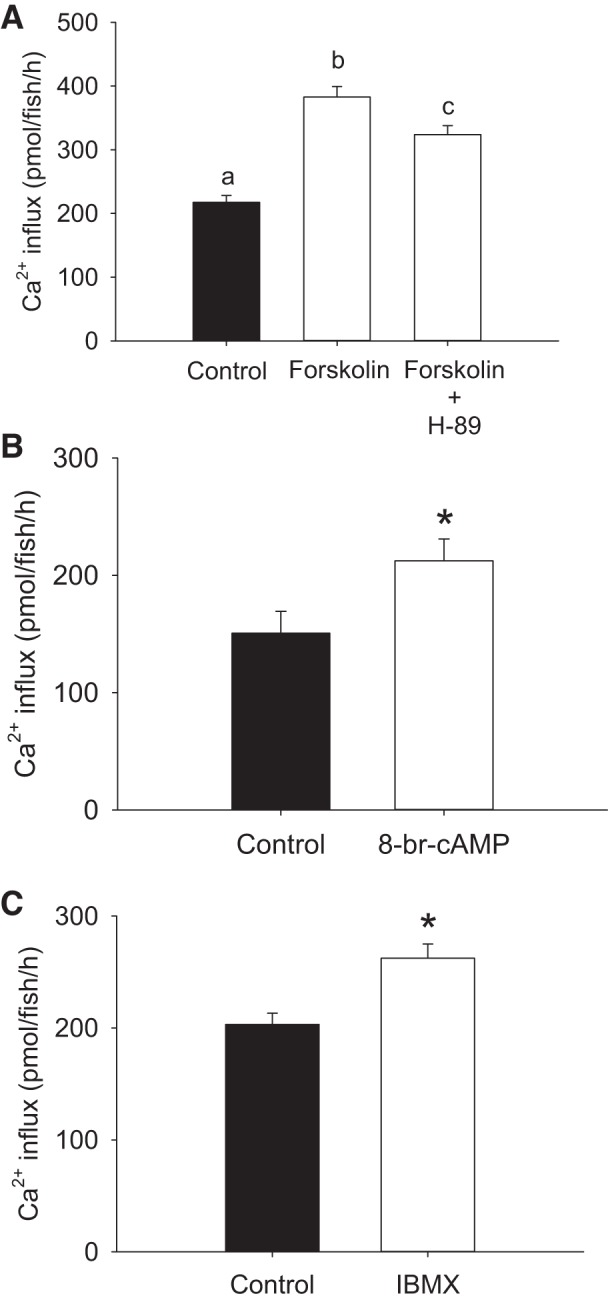
Treatment with cAMP-elevating agents increases Ca2+ influx. A: the effects of forskolin/H-89 treatment on Ca2+ influx in larval zebrafish. B: the effects of 8-bromoadenosine 3′,5′-cyclic monophosphate (8-Br-cAMP) treatment on Ca2+ influx in larval zebrafish. C: the effects of isobutylmethylxanthine (IBMX) treatment on Ca2+ influx in larval zebrafish. In all experiments, fish were acclimated to low-[Ca2+] (25 μM) water beginning at 1 day postfertilization (dpf), and influx was performed in normal [Ca2+] (250 μM) water at 4 dpf. Values are means ± SE; N = 6. a,b,c Different letters represent a statistical difference from each other (one-way ANOVA, followed by a Holm-Sidak post hoc test; P < 0.05). *Statistical difference (Student's t-test, P < 0.05).
Chronic exposure to H2S donor GYY4137 increases Ca2+ influx and whole body Ca2+ levels.
The chronic effects of H2S exposure on Ca2+ fluxes were evaluated using a stable H2S donor GYY4137. Fish exposed to 200 μM GYY4137 exhibited a significant increase in Ca2+ influx at 4 dpf (Fig. 4A). An increase in whole body Ca2+ levels also was observed in fish exposed to 200 μM GYY4137 (Fig. 4B). Results from real-time PCR experiments demonstrated that exposure to 200 μM GYY4137 did not affect mRNA expression of ecac, ncx1b, or pmca2 (Fig. 4C).
Fig. 4.
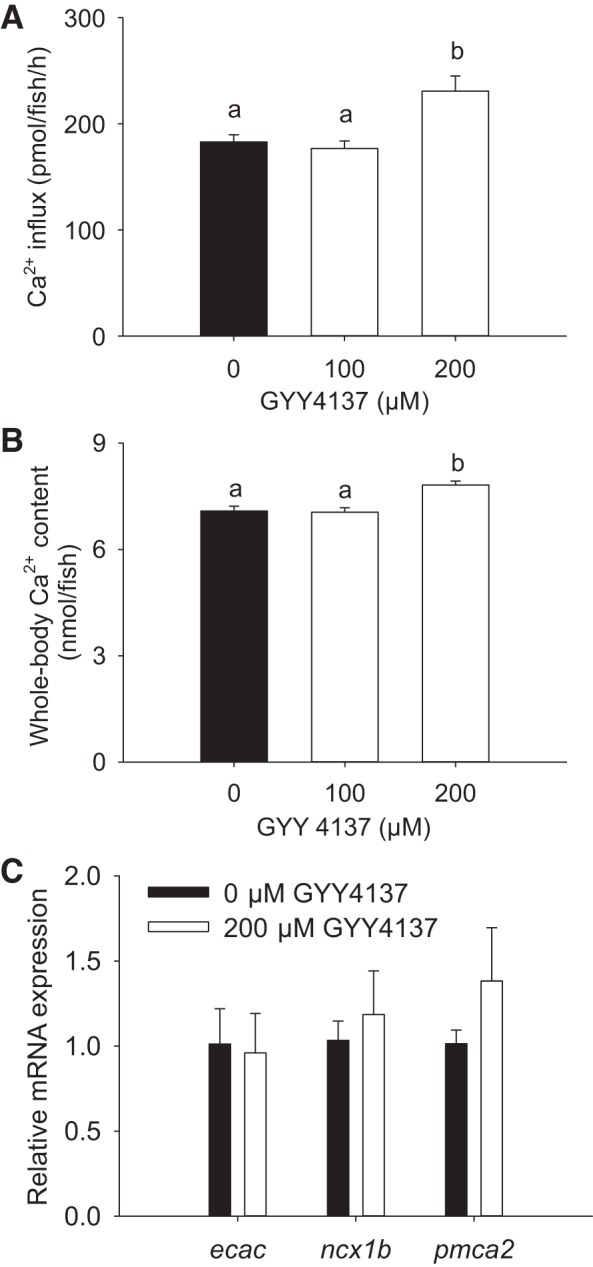
Chronic exposure to GYY4137 increases Ca2+ influx and whole body Ca2+ levels. The effects of GYY4137 exposure on Ca2+ influx (A) and whole body Ca2+ levels (B) in larval zebrafish are shown. Fish were exposed to GYY4137 and low-[Ca2+] (25 μM) water for 3 days, beginning at 1 day postfertilization (dpf), and influx was performed in normal [Ca2+] (250 μM) water at 4 dpf. C: the effects of exposure to GYY4137 (200 μM) on mRNA expression levels of epithelial Ca2+ channel (ecac), Na+/Ca2+ exchanger isoform 1b (ncx1b), and plasma membrane Ca2+-ATPase isoform 2 (pmca2) in larval zebrafish. The fish were exposed to GYY4137 and low-[Ca2+] (25 μM) water starting at 1 dpf, and mRNA levels were measured at 4 dpf. Data are expressed relative to control (0 μM GYY4137) within the same gene, and 18S was used as an internal control. Values are means ± SE; N = 6. a,b Different letters represent a statistical difference from each other (one-way ANOVA, followed by a Holm-Sidak post hoc test; P < 0.05).
Tissue-specific distribution of cse and cbsb in adults and their expression levels in larvae acclimated to low-Ca2+ water.
Figure 5A shows representative images of cse and cbsb mRNA expression in different tissues of adult zebrafish using RT-PCR. Tissues particularly abundant in cse mRNA were kidney, muscle, and intestine. For cbsb, mRNA appeared to be expressed primarily in the kidney and muscle with lower levels in the brain, gill, and heart. Acclimation to low-Ca2+ water did not affect mRNA expression of cse in larval zebrafish at 4 dpf (Fig. 5B). In contrast, mRNA expression of cbsb was significantly increased in fish acclimated to low-Ca2+ water (Fig. 5C).
Fig. 5.
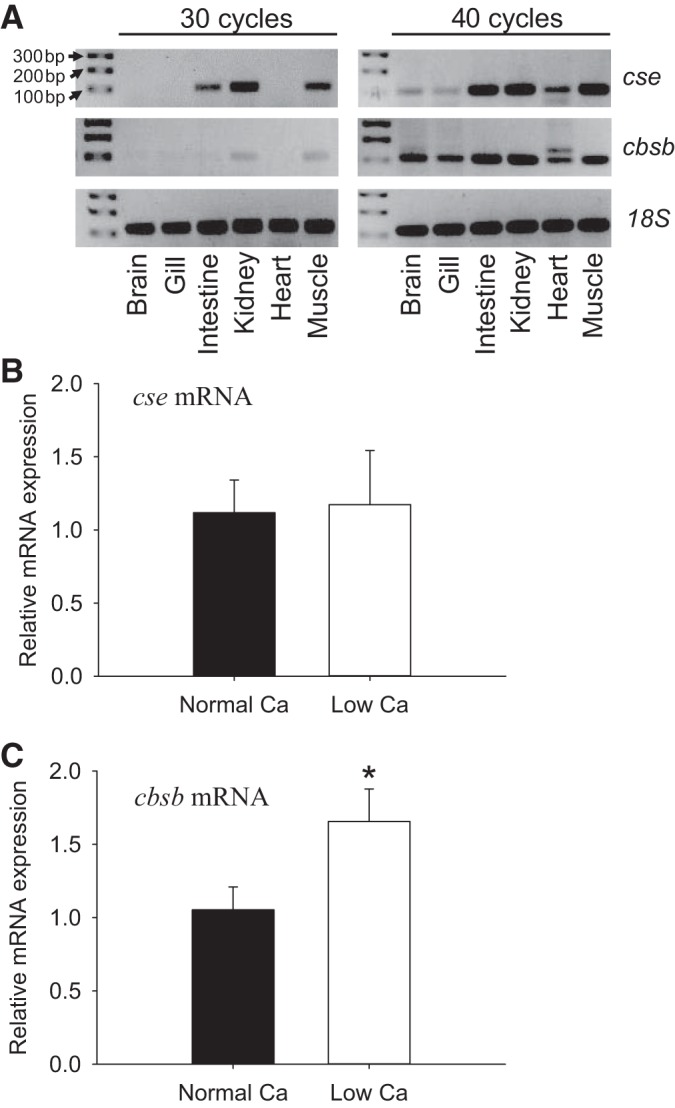
Tissue-specific mRNA expression of cystathionine γ-lyase (cse) and cystathionine β-synthase isoform b (cbsb) in adults, and their expression levels in larvae following acclimation to low-[Ca2+] water. A: the mRNA expression of cse and cbsb in different tissues of adult zebrafish. The expression profile was evaluated using RT-PCR, and 18S was used as an internal control. The PCR reactions were performed for 30 or 40 cycles. Changes in mRNA expression levels of cse (B) and cbsb (C) in larval zebrafish acclimated to normal (250 μM) or low-[Ca2+] (25 μM) water are shown. Fish were acclimated to the normal or low-[Ca2+] water beginning at 1 day postfertilization (dpf), and mRNA levels were measured at 4 dpf using real-time PCR. Data are expressed relative to mRNA levels in fish acclimated to normal [Ca2+] water; 18S was used as an internal control. Values are means ± SE; N = 6. *Statistical differences (Student's t-test, P < 0.05).
The H2S-biosynthesis enzyme CBSb is expressed in ionocytes.
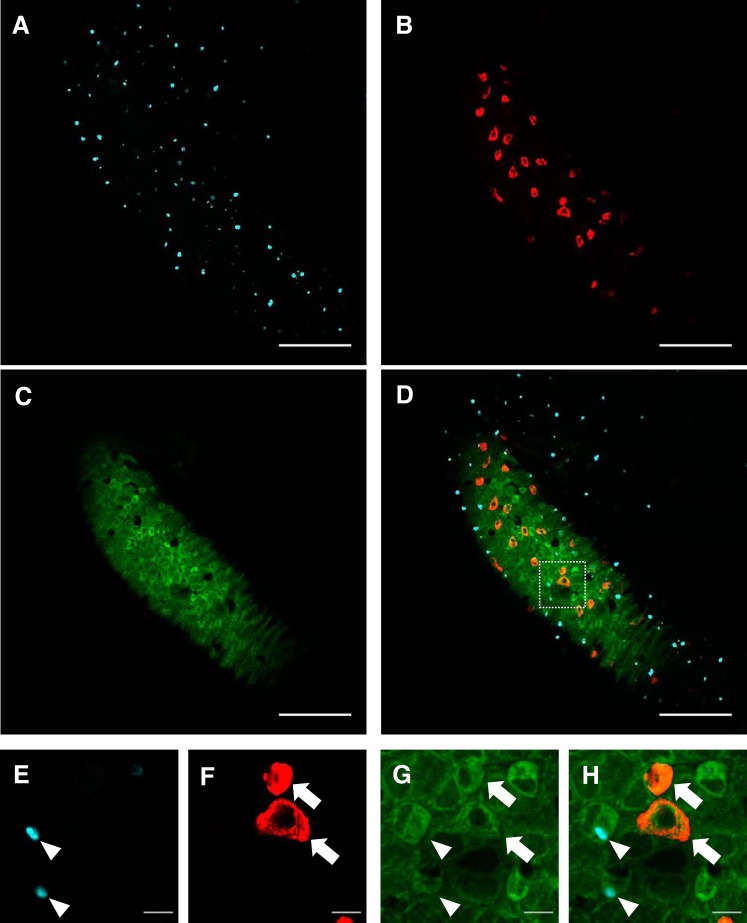
Whole-mount immunohistochemistry and confocal microscopy were performed to examine the localization of CBSb in larval zebrafish at 4 dpf (Fig. 6). Immunostaining of HRCs (purple; Fig. 6A) and NaRCs (red; Fig. 6B) was performed using a vital dye concanavalin A and Na+-K+-ATPase antibody, respectively. Immunostaining of CBSb (green; Fig. 6C) was observed in the skin covering the yolk sac. Both HRCs and NaRCs were found to express CBSb (Fig. 6D). Figure 6, E–H, are higher magnification images showing CBSb in HRCs and NaRCs.
Fig. 6.
CBSb is expressed in ionocytes on the skin of larval zebrafish. Whole-mount immunohistochemistry and confocal microscopy of CBSb in larval zebrafish at 4 day postfertilization (dpf) are shown. The fish were stained with concanavalin A [marker for H+-ATPase-rich cells (HRCs); A and E], Na+-K+-ATPase antibody [marker for Na+-K+-ATPase-rich cells (NaRCs); B and F], and CBSb antibody (C and G). D and H: merged images showing expression of CBSb in both HRCs and NaRCs. Scale bars in A–D are 100 μm; scale bars in E–H are 10 μm.
Pharmacological inhibition of CBS activity reduces Ca2+ influx in a low-Ca2+ environment.
The physiological importance of endogenous H2S in regulating Ca2+ influx was evaluated by pharmacological inhibition of H2S-biosythesis enzymes. Treatment of fish with either PPG (an inhibitor of CSE) or AOA (an inhibitor of CBS) did not affect Ca2+ influx in fish acclimated to normal Ca2+ water (Fig. 7A). Exposure to AOA, but not PPG, significantly reduced Ca2+ influx in fish acclimated to low-Ca2+ water (Fig. 7B).
Fig. 7.
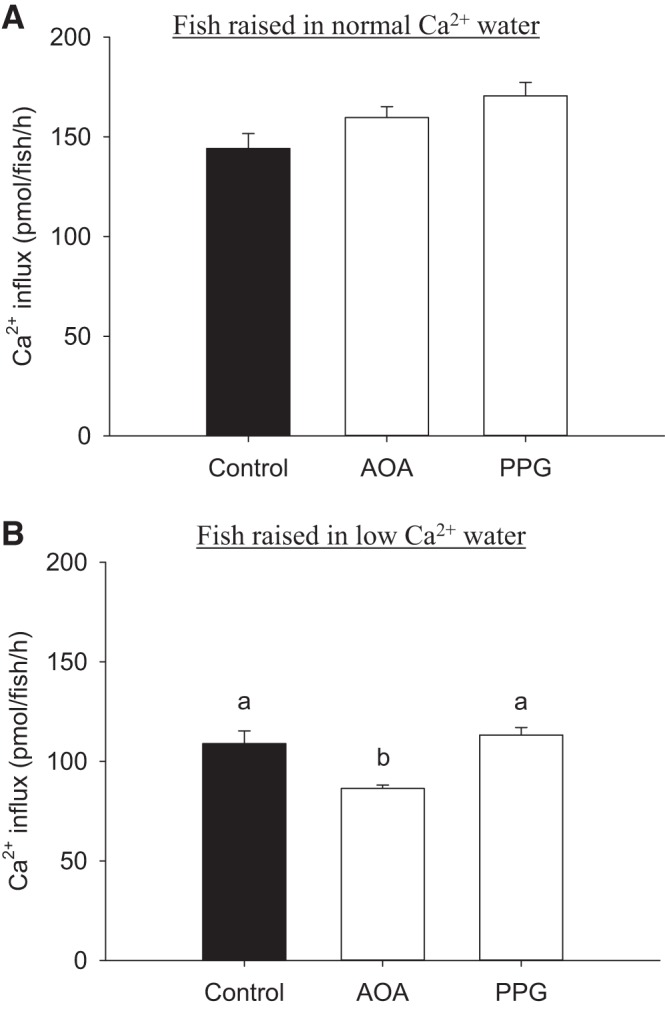
Inhibition of CBS reduces Ca2+ influx in fish acclimated to low-[Ca2+] water. The effects of aminooxyacetic acid (AOA; an inhibitor of CBS) or propargylglycine (PPG; an inhibitor of CSE) treatment on Ca2+ influx in larval zebrafish acclimated to normal (250 μM; A) or low-[Ca2+] (25 μM; B) water are shown. Fish were acclimated to the normal or low-[Ca2+] water beginning at 1 day postfertilization (dpf), and influx was measured at 4 dpf in the same water. Values are means ± SE; N = 6. a,b Different letters represent a statistical difference from each other (one-way ANOVA, followed by a Holm-Sidak post hoc test; P < 0.05).
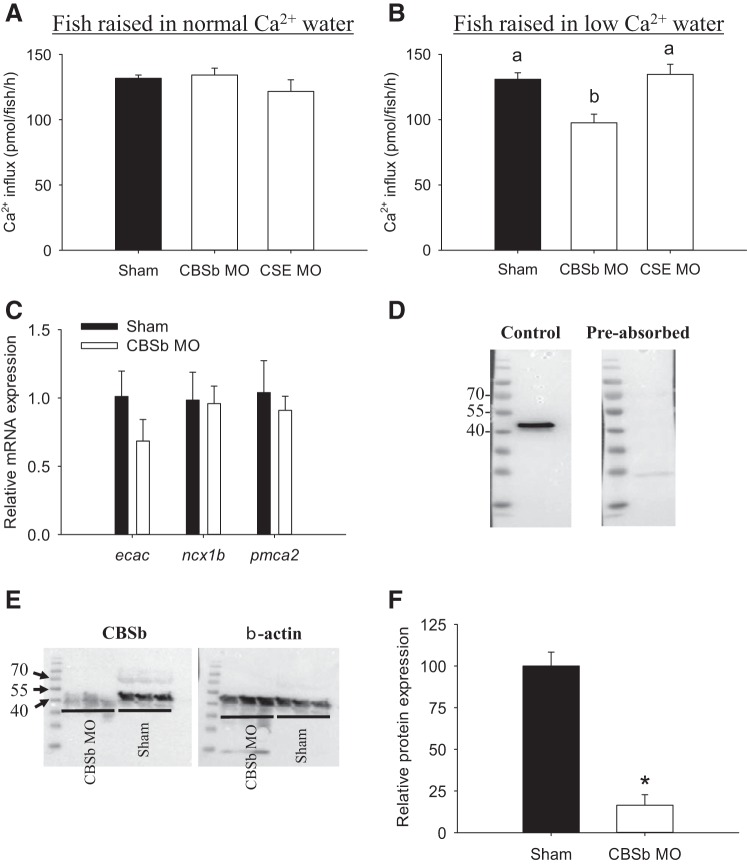
Knockdown of CBSb reduces Ca2+ influx in a low-Ca2+ environment.
The potential role of CSE and CBSb in regulating Ca2+ influx was further evaluated using a morpholino knockdown approach. Knockdown of CSE or CBSb did not affect Ca2+ influx in fish acclimated to normal Ca2+ water (Fig. 8A). A significant reduction in Ca2+ influx was observed in CBSb-deficient fish following acclimation to low-Ca2+ water (Fig. 8B). Results from real-time PCR suggested that mRNA expression of ecac, ncx1b, and pmca2 was not altered in 4 dpf fish experiencing CBSb knockdown (Fig. 8C).
Fig. 8.
Influx of Ca2+ is decreased in CBSb-deficient fish acclimated to low-[Ca2+] water. The effects of CBSb or CSE knockdown on Ca2+ influx in larval zebrafish acclimated to normal (250 μM; A) or low-[Ca2+] (25 μM; B) water are shown. Fish were acclimated to the normal or low-[Ca2+] water beginning at 1 day postfertilization (dpf), and influx was measured at 4 dpf in the same water. a,b Different letters represent a statistical difference from each other (one-way ANOVA, followed by a Holm-Sidak post hoc test; P < 0.05). C: the effects of CBSb knockdown on mRNA expression levels of ecac, ncx1b, and pmca2 in larval zebrafish. Shams and CBSb morphants (MO) were acclimated to low-Ca2+ water starting at 1 dpf, and mRNA levels were measured at 4 dpf using real-time PCR. Data are expressed relative to shams within the same gene, and 18S was used as an internal control. D: Western blot with CBSb antibody yielded a band at ∼50 kDa, and preabsorption of the CBSb antibody with corresponding immunizing peptide eliminated the immunoreactivity. E: compared with shams, the intensity of the band at ∼50 kDa was substantially reduced in CBSb MO. F: quantitative analysis showing that protein expression levels of CBSb was decreased by 85% in the CBSb MO. Values are means ± SE; N = 6 (A–C) or 3 (F). *Statistical differences (Student's t-test, P < 0.05).
As reported previously [Kumai et al. (13); Porteus et al. (33)], the CBSb morpholino was originally designed to splice out exon 3, but it appeared to reduce total CBSb mRNA levels, likely by premature mRNA degradation. In the present study, we further confirmed the effectiveness of the CBSb knockdown by Western blot using a specific antibody against the zebrafish CBSb. Western blot analysis demonstrated that the CBSb antibody detected a band at ∼50 kDa, and preabsorption of the CBSb antibody with corresponding immunizing peptide eliminated the immunoreactivity (Fig. 8D). Notably, the size of the CBSb protein appeared to be smaller than its predicted size (65 kDa). This smaller size possibly reflected posttranslational cleavage of CBS as reported in mammalian systems (9). Consistent with previous observations, the reduced cbsb mRNA expression by morpholino knockdown [Kumai et al. (13); Porteus et al. (33)] also resulted in a reduction in its protein expression (Fig. 8E). Quantitative analysis suggested that knockdown of CBSb led to 85% reduction in the protein expression levels of CBSb (Fig. 8F).
DISCUSSION
Overview.
The gasotransmitter H2S is known to regulate the central nervous system, cardiorespiratory function, and epithelial Na+ transport in vertebrates (1, 13, 17, 25, 40). Using developing zebrafish as a model system, we demonstrated a novel stimulatory role for H2S in promoting Ca2+ influx, which appeared to involve rapid activation of the cAMP-PKA pathways. Production of endogenous H2S via the H2S-biosynthesis enzyme CBSb was found to be critically involved in increasing Ca2+ influx, particularly in fish acclimated to a low-Ca2+ environment.
The effects of exposure to H2S donors on Ca2+ fluxes and the role of cAMP/PKA pathways.
In zebrafish, it is proposed that transepithelial Ca2+ uptake occurs predominantly through a subset of NaRCs, which express ECaC at the apical membrane, and NCX and PMCA at the basolateral membrane (7). It is also largely assumed that absorption of Ca2+ via ECaC is the rate-limiting step (32). Here we demonstrated that exposure to H2S donors Na2S or GYY4137 increased Ca2+ influx in larval zebrafish at 4 dpf. Additionally, whole body Ca2+ levels were increased following chronic exposure to GYY4137. Interestingly, the H2S-stimulated Ca2+ influx was observed only in fish that were acclimated to low-Ca2+ water. Several previous studies have demonstrated that acclimation to low-Ca2+ water increases the levels of ecac mRNA expression and numbers of ecac-expressing NaRCs in larval zebrafish (14, 19, 29). Thus it seems possible that the capacity for H2S to stimulate Ca2+ uptake is higher when the preexisting levels of ECaC expression and/or the numbers of ECaC-expressing cells are high. In isolated rat colonic epithelial cells, H2S exposure was found to promote Ca2+ extrusion via NCX (34). Similarly, exposure to GYY4137 increased the expression and activity of NCX in a human HeLa cell line (22). In the present study, we observed that mRNA expression of ecac, pmca2, and ncx1b was unaffected following chronic exposure to GYY4137. Considering that Ca2+ influx also was elevated after acute exposure (30 min) to H2S donors, it seems likely that H2S increased the affinity for Ca2+ uptake in larval zebrafish through rapid activation of the preexisting Ca2+ channels, likely via posttranslational modification (discussed below).
To examine the potential for cross talk between H2S and nitric oxide (NO) on modulating Ca2+ influx (41), Ca2+ influx was also evaluated following exposure to NO donors (S-nitroso-N-acetylpenicillamine) or knockdown of neuronal NO synthase. S-nitroso-N-acetylpenicillamine exposure did not affect Ca2+ influx in larval zebrafish. Similarly, influx of Ca2+ remained unchanged in fish experiencing neuronal NO synthase knockdown (K. McGregor, R. W. M. Kwong, and S. F. Perry, personal observations). These findings suggested that NO has little role in stimulating Ca2+ influx in larval zebrafish.
ECaC is known to contain several putative PKA and PKC phosphorylation sites, and in mammalian systems acute activation of these sites was shown to increase the activity of ECaC (2, 4). It has also been suggested that activation of the cAMP-dependent PKA signaling pathways promotes phosphorylation at the COOH-terminus of ECaC, thereby increasing its conductance to Ca2+ (4, 39). To examine whether stimulation of Ca2+ influx by H2S was dependent on PKA and/or PKC activity, Ca2+ influx was evaluated during coexposure to Na2S with selective PKA or PKC inhibitors. The results demonstrated that treatment of fish with the PKA inhibitor H-89 prevented the effects of H2S on increasing Ca2+ influx. In contrast, exposure of the PKC inhibitor BIS-1 did not affect H2S-stimulated Ca2+ influx. These findings suggest that the stimulatory effects of H2S on Ca2+ influx were probably associated with activation of PKA. Because the activity of PKA is highly dependent on the cellular levels of cAMP, we also determined whether Ca2+ influx could be stimulated by the cAMP-elevating agent forskolin, 8-Br-cAMP or IBMX (i.e., a specific inhibitor of cAMP-degrading enzyme phosphodiesterase). The results suggested that exposure to these cAMP-elevating agents significantly increased Ca2+ influx in larval zebrafish. Interestingly, we also observed that the effects of forskolin on stimulating Ca2+ influx was substantially lower when the fish were acclimated to normal Ca2+ water; exposure to forskolin resulted in a 1.25-fold increase in Ca2+ influx in fish acclimated to normal Ca2+ water (data not shown), while it caused a 2-fold increase in Ca2+ uptake in fish acclimated to low-Ca2+ water. This finding reinforces our contention that the prevailing level of ECaC expression is probably a limiting factor in cAMP-stimulated Ca2+ influx. Taken together, the findings of the present study demonstrated that activation of the cAMP/PKA signaling pathways, either by H2S donors or by cAMP-elevating agents, acutely stimulates Ca2+ uptake in larval zebrafish.
H2S is known to have diverse physiological effects on various ion channels/transporters. For examples, H2S inhibits voltage-dependent Ca2+ channels in cardiomyocytes (36), whereas it activates transient receptor potential vanilloid channels (e.g., TRPV1) in urinary tract (35) and airway smooth muscle (38). Our laboratory previously demonstrated that exposure to H2S donors reduced Na+ influx in larval zebrafish (13). Similarly, morpholino knockdown of H2S-biosynthesis enzymes CSE or CBSb increased Na+ influx, suggesting that endogenously produced H2S inhibits Na+ uptake (13). H2S was found to reduce Na+ uptake via its interaction with HRCs, but not Na+/Cl−-cotransporter-expressing cells, which are two major types of ionocytes responsible for Na+ uptake in zebrafish (13). Interestingly, treatment with forskolin was shown to increase Na+ influx via both HRCs and Na+/Cl−-cotransporter-expressing cells, indicating that Na+ uptake by these ionocytes is stimulated by activation of the cAMP/PKA signaling cascade (12). The physiological significance of H2S reducing Na+ influx in HRCs, while simultaneously promoting Ca2+ influx in NaRCs, remains unclear. However, these observations suggest that H2S may regulate Na+ and Ca2+ uptake through different mechanisms in different cell types. Although the precise mechanisms by which H2S inhibits Na+ influx is not clear, in airway epithelial cell models, it has been shown that H2S exposure reduces Na+ absorption by inhibiting the activity of Na+-K+-ATPase at the basolateral membrane, thereby reducing the gradient for driving Na+ influx through the apical epithelial Na+ channels (1, 5). It has also been demonstrated that H2S exposure suppresses the production of angiotensin II (20), a hormone that was shown to be important in promoting Na+ uptake in larval zebrafish (11). The biological significance of the differential regulation of Na+ and Ca2+ influx by H2S has yet to be addressed. It will be crucial in future studies to identify the effects of H2S on different ion channels/transporters in various tissues and cell types.
The physiological involvement of H2S-biosynthesis enzymes in the regulation of Ca2+ uptake.
In mammals, the two major H2S-biosynthesis enzymes, CSE and CBS, are expressed in a tissue-specific manner, with CBS being expressed primarily in the brain and CSE in peripheral tissues (30). In rainbow trout (Oncorhynchus mykiss), cse and cbs mRNA are expressed in various tissues, including gill, liver, brain, heart, and skeletal muscle (28). Here we observed that, in adult zebrafish, cse mRNA was expressed abundantly in the intestine, kidney, and muscle. cbsb mRNA appeared to be expressed primarily in the kidney and muscle, with lower levels in the brain, gill, and heart. In larval zebrafish, CSE is expressed in both NaRCs and HRCs on the skin covering the yolk sac (13). In the present study, we also observed that CBSb was expressed in NaRCs and HRCs on the skin of the yolk sac, suggesting that these ionocytes have the capacity to produce H2S via either CSE (13) or CBSb (this study).
To further examine the physiological role of endogenous H2S, the activity/expression of CSE or CBSb was suppressed using pharmacological or gene knockdown approaches. We observed that treatment of fish with the CSE inhibitor PPG or morpholino knockdown of CSE did not affect Ca2+ influx. In contrast, treatment with the CBS inhibitor AOA or morpholino knockdown of CBSb significantly reduced Ca2+ influx. Notably, the reduction of Ca2+ influx by AOA exposure or by CBSb knockdown only was observed in fish that were acclimated to low-Ca2+ water. These findings suggest that H2S produced by CBSb is important in promoting Ca2+ influx in a low-Ca2+ environment. The precise mechanism(s) by which endogenous H2S promotes Ca2+ influx is not clear. Because the mRNA levels of ecac, ncx1b, and pmca2 remained unchanged in fish experiencing CBSb knockdown, it suggests that H2S stimulated Ca2+ influx through posttranscriptional regulation, presumably involving PKA signaling. Nevertheless, mRNA expression of cbsb was substantially increased in fish acclimated to low-Ca2+ water. The elevated cbsb expression may contribute to an increased H2S synthesis, which may promote ECaC phosphorylation to increase Ca2+ conductance via cAMP-dependent PKA signaling pathways (4, 39).
Concluding remarks.
In summary, the present study demonstrated that exposure to H2S donors increased Ca2+ influx and whole body Ca2+ content. H2S stimulated Ca2+ influx by activating the cAMP-PKA pathways, which likely modified the conductance of ECaC to Ca2+. Furthermore, we showed that H2S generated by CBSb is important in promoting Ca2+ influx, particularly under a low-Ca2+ environment. Interestingly, a recent study showed that H2S exposure reduced Na+ influx in larval zebrafish (13), suggesting that H2S may have divergent physiological functions in regulating ion movements in fish. Further investigation is required to fully elucidate the cell- and tissue-specific regulation of ion transport by H2S. The precise molecular targets for endogenous H2S in modulating Na+ and Ca2+ uptake have yet to be examined. Future experiments should also examine the effects of H2S and cAMP on intracellular Ca2+ levels in ionocytes. Although the biochemistry and physiology of H2S remain poorly understood, there is a growing interest in using H2S inhibitors or donors as potential therapeutic options for various diseases (37). The present study suggests that the zebrafish could be a useful model to examine the actions and physiological consequences of H2S in vivo.
GRANTS
This study was supported by the National Sciences and Engineering Research Council (NSERC) Discovery and NSERC Research Tools and Instrumentation Grants to S. F. Perry.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: R.W.K. and S.F.P. conception and design of research; R.W.K. performed experiments; R.W.K. and S.F.P. analyzed data; R.W.K. and S.F.P. interpreted results of experiments; R.W.K. prepared figures; R.W.K. and S.F.P. drafted manuscript; R.W.K. and S.F.P. edited and revised manuscript; R.W.K. and S.F.P. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank J. Pollack, A. Ochalski, V. Saxena, and B. Fletcher for technical assistance.
REFERENCES
- 1.Althaus M, Urness KD, Clauss WG, Baines DL, Fronius M. The gasotransmitter hydrogen sulphide decreases Na+ transport across pulmonary epithelial cells. Br J Pharmacol 166: 1946–1963, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cha SK, Wu T, Huang CL. Protein kinase C inhibits caveolae-mediated endocytosis of TRPV5. Am J Physiol Renal Physiol 294: F1212–F1221, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Chen Yy Lu Fi, Hwang Pp. Comparisons of calcium regulation in fish larvae. J Exp Zool 295A: 127–135, 2003. [DOI] [PubMed] [Google Scholar]
- 4.De Groot T, Lee K, Langeslag M, Xi Q, Jalink K, Bindels RJM, Hoenderop JGJ. Parathyroid hormone activates TRPV5 via PKA-dependent phosphorylation. J Am Soc Nephrol 20: 1693–1704, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erb A, Althaus M. Actions of hydrogen sulfide on sodium transport processes across native distal lung epithelia (Xenopus laevis). PLos One 9: e100971, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Hwang PP, Chou MY. Zebrafish as an animal model to study ion homeostasis. Pflügers Arch 465: 1233–1247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inoue D, Wittbrodt J. One for All-A highly efficient and versatile method for fluorescent immunostaining in fish embryos. PLos One 6: e19713, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kery V, Poneleit L, Kraus JP. Trypsin cleavage of human cystathionine β-synthase into an evolutionarily conserved active core: Structural and functional consequences. Arch Biochem Biophys 355: 222–232, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Kimura H. Hydrogen sulfide induces cyclic AMP and modulates the NMDA receptor. Biochem Biophys Res Commun 267: 129–133, 2000. [DOI] [PubMed] [Google Scholar]
- 11.Kumai Y, Bernier NJ, Perry SF. Angiotensin-II promotes Na+ uptake in larval zebrafish, Danio rerio, in acidic and ion-poor water. J Endocrinol 220: 195–205, 2014. [DOI] [PubMed] [Google Scholar]
- 12.Kumai Y, Kwong RWM, Perry SF. The role of cAMP-mediated intracellular signaling in regulating Na+ uptake in zebrafish larvae. Am J Physiol Regul Integr Comp Physiol 306: R51–R60, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumai Y, Porteus C, Kwong RM, Perry S. Hydrogen sulfide inhibits Na+ uptake in larval zebrafish, Danio rerio. Pflugers Arch: 1–14, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Kwong RWM, Auprix D, Perry SF. Involvement of the calcium-sensing receptor in calcium homeostasis in larval zebrafish exposed to low environmental calcium. Am J Physiol Regul Integr Comp Physiol 306: R211–R221, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee SW, Hu YS, Hu LF, Lu Q, Dawe GS, Moore PK, Wong PT, Bian JS. Hydrogen sulphide regulates calcium homeostasis in microglial cells. Glia 54: 116–124, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Lee ZW, Zhou J, Chen CS, Zhao Y, Tan CH, Li L, Moore PK, Deng LW. The slow-releasing hydrogen sulfide donor, GYY4137, exhibits novel anti-cancer effects in vitro and in vivo. PLoS One 6: e21077, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li L, Rose P, Moore PK. Hydrogen sulfide and cell signaling. Annu Rev Pharmacol Toxicol 51: 169–187, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Liao BK, Deng AN, Chen SC, Chou MY, Hwang PP. Expression and water calcium dependence of calcium transporter isoforms in zebrafish gill mitochondrion-rich cells. BMC Genomics 8: 354, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin CH, Tsai IL, Su CH, Tseng DY, Hwang PP. Reverse effect of mammalian hypocalcemic cortisol in fish: Cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLos One 6: e23689, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu M, Liu YH, Goh HS, Wang JJX, Yong QC, Wang R, Bian JS. Hydrogen sulfide inhibits plasma renin activity. J Am Soc Nephrol 21: 993–1002, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Y, O'Dowd BF, Orrego H, Israel Y. Cloning and nucleotide sequence of human liver cDNA encoding for cystathionine γ-lyase. Biochem Biophys Res Commun 189: 749–758, 1992. [DOI] [PubMed] [Google Scholar]
- 22.Markova J, Hudecova S, Soltysova A, Sirova M, Csaderova L, Lencesova L, Ondrias K, Krizanova O. Sodium/calcium exchanger is upregulated by sulfide signaling, forms complex with the β1 and β3 but not β2 adrenergic receptors, and induces apoptosis. Pflügers Arch 466: 1329–1342, 2014. [DOI] [PubMed] [Google Scholar]
- 23.McCormick SD, Hasegawa S, Hirano T. Calcium uptake in the skin of a freshwater teleost. Proc Natl Acad Sci U S A 89: 3635–3638, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moccia F, Bertoni G, Pla AF, Dragoni S, Pupo E, Merlino A, Mancardi D, Munaron L, Tanzi F. Hydrogen sulfide regulates intracellular Ca2+ concentration in endothelial cells from excised rat aorta. Curr Pharm Biotechnol 12: 1416–1426, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Olson KR. Hydrogen sulfide and oxygen sensing: implications in cardiorespiratory control. J Exp Biol 211: 2727–2734, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Olson KR. Is hydrogen sulfide a circulating “gasotransmitter” in vertebrate blood? Biochim Biophys Acta 1787: 856–863, 2009. [DOI] [PubMed] [Google Scholar]
- 27.Olson KR. Mitochondrial adaptations to utilize hydrogen sulfide for energy and signaling. J Comp Physiol B 182: 881–897, 2012. [DOI] [PubMed] [Google Scholar]
- 28.Olson KR, Healy MJ, Qin Z, Skovgaard N, Vulesevic B, Duff DW, Whitfield NL, Yang G, Wang R, Perry SF. Hydrogen sulfide as an oxygen sensor in trout gill chemoreceptors. Am J Physiol Regul Integr Comp Physiol 295: R669–R680, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Pan TC, Liao BK, Huang CJ, Lin LY, Hwang PP. Epithelial Ca2+ channel expression and Ca2+ uptake in developing zebrafish. Am J Physiol Regul Integr Comp Physiol 289: R1202–R1211, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012. [DOI] [PubMed] [Google Scholar]
- 31.Peng YJ, Nanduri J, Raghuraman G, Souvannakitti D, Gadalla MM, Kumar GK, Snyder SH, Prabhakar NR. H2S mediates O2 sensing in the carotid body. Proc Natl Acad Sci U S A 107: 10719–10724, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perry SF, Flik G. Characterization of branchial transepithelial calcium fluxes in freshwater trout, Salmo gairdneri. Am J Physiol Regul Integr Comp Physiol 254: R491–R498, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Porteus CS, Abdallah SJ, Pollack J, Kumai Y, Kwong RWM, Yew HM, Milsom WK, Perry SF. The role of hydrogen sulphide in the control of breathing in hypoxic zebrafish (Danio rerio). J Physiol 592: 3075–3088, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pouokam E, Diener M. Mechanisms of actions of hydrogen sulphide on rat distal colonic epithelium. Br J Pharmacol 162: 392–404, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Streng T, Axelsson HE, Hedlund P, Andersson DA, Jordt SE, Bevan S, Andersson KE, Hogestatt ED, Zygmunt PM. Distribution and function of the hydrogen sulfide-sensitive TRPA1 ion channel in rat urinary bladder. Eur Urol 53: 391–399, 2008. [DOI] [PubMed] [Google Scholar]
- 36.Sun YG, Cao YX, Wang WW, Ma SF, Yao T, Zhu YC. Hydrogen sulphide is an inhibitor of L-type calcium channels and mechanical contraction in rat cardiomyocytes. Cardiovasc Res 79: 632–641, 2008. [DOI] [PubMed] [Google Scholar]
- 37.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov 6: 917–935, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Trevisani M, Patacchini R, Nicoletti P, Gatti R, Gazzieri D, Lissi N, Zagli G, Creminon C, Geppetti P, Harrison S. Hydrogen sulfide causes vanilloid receptor 1-mediated neurogenic inflammation in the airways. Br J Pharmacol 145: 1123–1131, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.van der Hagen EAE, Tudpor K, Verkaart S, Lavrijsen M, van der Kemp A, van Zeeland F, Bindels RJM, Hoenderop JGJ. β1-Adrenergic receptor signaling activates the epithelial calcium channel, transient receptor potential vanilloid type 5 (TRPV5), via the protein kinase A pathway. J Biol Chem 289: 18489–18496, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshida T, Inoue R, Morii T, Takahashi N, Yamamoto S, Hara Y, Tominaga M, Shimizu S, Sato Y, Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat Chem Biol 2: 596–607, 2006. [DOI] [PubMed] [Google Scholar]