Abstract
The inflammatory cytokine tumor necrosis factor-α (TNF-α) is a pathogenic factor in acute and chronic kidney disease. TNF-α is known to alter expression of epithelial tight junction (TJ) proteins; however, the underlying mechanisms and the impact of this effect on epithelial functions remain poorly defined. Here we describe a novel biphasic effect of TNF-α on TJ protein expression. In LLC-PK1 tubular cells, short-term (1–6 h) TNF-α treatment selectively elevated the expression of the channel-forming TJ protein claudin-2. In contrast, prolonged (>8 h) TNF-α treatment caused a marked downregulation in claudin-2 and an increase in claudin-1, -4, and -7. The early increase and the late decrease in claudin-2 expression involved distinct mechanisms. TNF-α slowed claudin-2 degradation through ERK, causing the early increase. This increase was also mediated by the EGF receptor and RhoA and Rho kinase. In contrast, prolonged TNF-α treatment reduced claudin-2 mRNA levels and promoter activity independent from these signaling pathways. Electric Cell-substrate Impedance Sensing measurements revealed that TNF-α also exerted a biphasic effect on transepithelial resistance (TER) with an initial decrease and a late increase. Thus there was a good temporal correlation between TNF-α-induced claudin-2 protein and TER changes. Indeed, silencing experiments showed that the late TER increase was at least in part caused by reduced claudin-2 expression. Surprisingly, however, claudin-2 silencing did not prevent the early TER drop. Taken together, the TNF-α-induced changes in claudin-2 levels might contribute to TER changes and could also play a role in newly described functions of claudin-2 such as proliferation regulation.
Keywords: tight junction, tumor necrosis factor, tubular epithelial cells, transepithelial resistance, ECIS
epithelial layers, such as the tubular epithelium in the kidney, mediate transcellular and paracellular transport of ions and various substances. The selective paracellular transport pathway is generated by transmembrane proteins in the tight junctions (TJs) (59). The kidney proximal tubules are adapted for large volume of transport and exhibit high paracellular permeability (leaky epithelia) (56). In contrast, the distal tubules have much lower paracellular permeability. These properties of the TJs are determined by members of the claudin family (20). Claudins are small molecular mass (21–28 kDa) tetraspan membrane proteins that are essential components of the paracellular pathway (61). The human claudin family consists of 27 members with unique permeability properties and expression profile (33, 41). Claudins can be divided into two main classes: those that form specific permeability pathways (pore-forming claudins) and those that reduce permeability (sealing or barrier-forming claudins) (56). TJs in various epithelial layers contain multiple claudin isoforms, the combination of which determines permeability and ion selectivity (3, 58, 62). Claudin-2 (Cldn-2) is one of the best studied pore-forming claudins. It was first identified as a TJ protein by the Tsukita group (16) and is now known to be present in layers with high paracellular permeability such as the proximal tubules (14). In contrast, tight epithelia have more sealing claudins, such as Cldn-1, -4, and -7. Pore-forming claudins are less abundant or absent in these layers.
TJs are dynamically regulated structures that are targeted by a multitude of stimuli, including inflammatory cytokines and hormones. Many of these exert their effects at least in part through the cytoskeleton and myosin-mediated contractility that in turn control endocytosis of TJ proteins (27, 31, 45). Thus the altered presence of various claudins at the TJs is likely an important mode of controlling permeability, but the underlying mechanisms are not fully understood.
One of the major inflammatory cytokines affecting epithelial junctions is tumor necrosis factor-α (TNF-α), a pleiotropic proinflammatory cytokine that is synthesized and released in response to inflammation, infection, and injury (Ref. 6 and reviewed in Ref. 68). In addition to its now well-recognized pathogenic role in chronic inflammatory diseases such as rheumatoid arthritis and inflammatory bowel disease (11), TNF-α has also emerged as a mediator in kidney diseases (46, 65). It is produced in the injured kidneys by infiltrating immune cells and activated resident cells including the tubular epithelium (28, 50). Importantly, inhibition of TNF-α production or the addition of neutralizing antibodies exerted protective effects in animal models of kidney disease (reviewed in Refs. 50, 55, 65). Similarly, TNF-receptor knockout mice had reduced kidney injury in an obstructive nephropathy model (21).
Despite its central role in kidney disease, the cellular mechanisms through which TNF-α affects kidney functions are incompletely understood. In our previous work we explored the effect of TNF-α on the cytoskeleton and permeability of tubular cells. We showed that in LLC-PK1 and MDCK cells TNF-α was a potent activator of the small GTPases RhoA and Rac, an effect mediated by the guanine nucleotide exchange factor GEF-H1 (29, 66). RhoA activation also required the epidermal growth factor receptor (EGFR) and ERK pathways. Moreover, TNF-α increased paracellular permeability for small uncharged molecules through RhoA/Rho kinase-dependent cytoskeleton remodeling and myosin phosphorylation (30). Interestingly, Mullin et al. (42) demonstrated that TNF-α elicits complex changes in transepithelial resistance (TER) in LLC-PK1 tubular cells. After a lag phase lasting ≈1 h, TNF-α caused an initial TER decrease, which was followed by an increase that lasted up to 72 h (36). Although these authors described some aspects of the signaling involved, overall the underlying molecular events remained poorly understood. In intestinal and lung epithelial cells inflammatory cytokines including TNF-α were shown to alter expression of various claudins; however, such changes in tubular cells are not established. Thus the underlying mechanisms that mediate TNF-α-induced junction remodeling are poorly characterized and whether altered expression of TJ proteins contributes to the described biphasic TER changes remain to be tested.
The aim of the current work was to gain new insight into the mechanisms of TNF-α-induced junction remodeling. Specifically, we aimed to explore changes in claudin expression, define the underlying mechanisms, and test the causal relationship with TER changes induced by the cytokine. We show that TNF-α caused significant, time-dependent alterations in claudin expression. Most notable, expression and surface localization of the channel-forming protein Cldn-2 showed an initial increase and a late decrease, which were mediated through distinct mechanisms. These changes coincided with an initial TER decrease and a late TER increase induced by TNF-α. Surprisingly, however, the initial TER effect was independent of Cldn-2. In contrast, the late TER increase was mediated by Cldn-2 downregulation.
MATERIALS AND METHODS
Materials and antibodies.
PD98059, AG1478, and Y27632 were from EMD Millipore (Billerica, MA). TNF-α was obtained from Sigma-Aldrich Chemical (St Louis, MO) and from IBI Scientific (Peosta, IA). The effects of TNF-α from the two sources were identical. Cycloheximide was from Sigma-Aldrich Chemical. Complete Mini Protease inhibitor tablet was from Roche Diagnostics (Laval, QC, Canada). Bovine serum albumin (BSA) was from BioShop (Burlington, ON, Canada). All other chemicals were from Sigma or BioShop. The following antibodies were used. Antibodies against Cldn-1, -4, and -7 were from Life Technologies (Invitrogen; Burlington, Ontario, Canada). We used three different antibodies against Cldn-2: a monoclonal mouse antibody (cat no. 325600) and a polyclonal rabbit antibody (cat. no. 51-6100) from Invitrogen and a polyclonal rabbit antibody (cat. no. 53032) from Abcam (Cambridge, MA). The occludin antibody was obtained from Lifespan (Seattle, WA); the E-cadherin antibody was from BD Transduction Laboratories (Mississauga, ON); the RhoA antibody was from Cell Signaling Technology (Danvers, MA); and the GAPDH antibody from Santa Cruz Biotechnology (Santa Cruz, CA). DAPI nucleic acid stain was from Life Technologies. Peroxidase- and Cy3-labeled secondary antibodies were from Jackson ImmunoResearch (West Grove, PA).
Cells.
LLC-PK1, a kidney proximal tubule epithelial cell line from the European Collection of Animal Cell Cultures (Wiltshire, UK), was used as in our earlier studies (38). LLC-PK1 cells were maintained in DMEM medium (Invitrogen) supplemented with 10% fetal bovine serum and 1% antibiotic suspension (penicillin and streptomycin) in an atmosphere containing 5% CO2. HT29, a human intestinal cell line, obtained from the American Type Culture Collection, was grown in DMEM/F12 medium supplemented with 10% fetal bovine serum and 1% antibiotic suspension. Tissue culture media and reagents were from Life Technologies.
Luciferase reporter assays.
The luciferase-coupled Cldn-2 promoter construct was a kind gift from Dr. J. F. Beaulieu (Sherbrooke, QC, Canada) and was previously described (15). The reporter assay was performed as described previously (40). Briefly, cells were plated onto 12-well plates. At ∼60% confluence cells were cotransfected with 0.5 μg/well Cldn-2 promoter plasmid and 0.125 μg/well normalizing vector pRL-TK (Renilla luciferase control plasmid; Promega, Madison, WI). Twenty-four hours later the cells were treated with TNF-α for 6 or 16 h. At the end of the treatment cells were lysed and luciferase activity was determined using the Dual-Luciferase Reporter Assay System kit (Promega) and a Berthold Lumat 9507 luminometer according to the manufacturers' instructions. For each condition treatments were done in duplicates in three independent experiments. From each sample the firefly luciferase activity corresponding to the Cldn-2 promoter construct was normalized to the renilla luciferase activity of the same sample. Results are expressed as fold changes compared with the mean firefly/renilla ratio of the untreated controls taken as unity.
Short interfering RNA.
Oligonucleotides were purchased from Applied Biosytems/Ambion (Austin, TX) and Dharmacon. The porcine RhoA short interfering (si)RNA targeted the following sequence: AAAGCAGGTAGAGTTGGCTTT. To silence Cldn-2 we used two different siRNAs against the following sequences in the porcine protein: CCAGAACUCUCGCGCCAAAUU (#1) and AACTCCTACAGCCTGACAGGG (#2). Data obtained using the two siRNAs were similar and were pooled. Cells were transfected with 100 nM siRNA oligonucleotide using the Lipofectamine RNAiMAX Transfection Reagent (Invitrogen) according to the manufacturer's instructions. Control cells were transfected with 100 nM Silencer siRNA negative control [nonrelated (NR) siRNA; Applied Biosystems/Ambion]. Unless otherwise indicated, experiments were performed 48 h after transfection. The levels of downregulated proteins were routinely checked using Western blotting.
Western blotting.
Following treatment, cells were lysed on ice using cold lysis buffer (100 mM NaCl, 30 mM HEPES pH 7.5, 20 mM NaF, 1 mM EGTA, 1% Triton X-100, supplemented with 1 mM Na3VO4, 1 mM PMSF, and protease inhibitors). Protein concentration was determined by the bicinchoninic acid assay (Pierce Biotechnology, Thermo Scientific, Mississauga, ON, Canada) with BSA used as standard. SDS-PAGE and Western blotting were performed as in Ref. 67. Blots were blocked in Tris-buffered saline containing either 5% BSA or 5% milk and incubated with the primary antibody overnight. Antibody binding was visualized with the corresponding peroxidase-conjugated secondary antibodies and the enhanced chemiluminescence method (kit from GE Healthcare Lifesciences). Where indicated, blots were stripped and reprobed using anti-GAPDH to demonstrate equal loading.
Densitometry.
Films with nonsaturated exposures were scanned and densitometry analysis was performed using a GS-800 calibrated densitometer and the Quantity One software (Bio-Rad, Hercules, CA).
Detecting surface Cldn-2 levels.
LLC-PK1 cells grown in 10-cm dishes were treated as indicated in the figure legends. At the end of the treatment, cells were placed on ice. Surface proteins were labeled by incubating with sulfo-NHS-SS-biotin (Pierce, Rockford, IL) on ice for 2 × 20 min. Next, cells were washed and lysed in the Triton-based lysis buffer used for Western blotting. Aliquots from each sample were taken to assess total Cldn-2 levels. The remaining lysates were rotated with streptavidin-agarose beads (Sigma) overnight to precipitate biotinylated proteins. The beads were washed extensively, and the bound proteins were eluted by sample buffer. Cldn-2 in the precipitates and total cell lysates was detected by Western blotting.
Immunofluorescence microscopy.
Confluent cells grown on coverslips were treated as indicated in the corresponding figure legends. At the end of the treatment, cells were fixed with methanol. The coverslips were blocked with 3% BSA in phosphate-buffered saline, followed by incubation with the Cldn-2 monoclonal mouse antibody from Invitrogen. Bound antibody was detected using fluorescent secondary antibody (1:1,000), and the nuclei were counterstained using DAPI (Life Technologies). The samples were viewed using an LSM 700 confocal microscope (Zeiss, Oberkochen, Germany), Plan-Apochromat ×63/numerical aperture 1.4 oil objective (Zeiss), and Zen 2010 software (Zeiss). Maximum-intensity projection images were generated from Z-sections (0.2-μm step size) by the Zen software. Image modifications were restricted to linear adjustments of contrast and brightness.
RT-PCR for mRNA analysis.
LLC-PK1 cells were treated as indicated in the corresponding figure legends. RNA was extracted using an RNeasy kit (Qiagen, Valencia, CA), and cDNA was synthesized from 1 μg total RNA using iScript reverse transcriptase (Bio-Rad Laboratories). SYBR green based real-time PCR was used to evaluate gene expression of Cldn-2, using GAPDH as the reference standard. Primer pairs designed against known pig sequences were as follows: Cldn-2: 5′-GCACTGGCATCACCCAGTGT-3′, 5′-GATGATACAGGCCAACGAGG-3′; and GAPDH: 5′-GCAAAGTGGACATGGTCGCCATCA-3′ and 5′-AGCTTCCCATTCTCAGCCTTGACT-3′.
Electric cell-substrate impedance sensing.
An electric cell-substrate impedance sensing (ECIS) Ztheta system (Applied Biophysics, Troy, NY) with the Transwell adapter was used to follow barrier functions. LLC-PK1 cells were seeded on Corning Transwell filters (0.4-μm pore size) at 1.5 × 105/well. In the studies where Cldn-2 was silenced, cells were transfected with a NR or Cldn-2-specific siRNA 24 h before trypsinizing, counting and seeding on the Transwell filters. The filters were placed into an ECIS trans-filter adapter and capacitance (C) and resistance (R) values were collected continuously using the frequency scan mode. After 48 h, the measurement was paused and TNF-α was added to the apical side of the filters in 20 μl medium to obtain a 20 ng/ml final concentration. Controls received 20 μl medium without TNF-α. R values of the filters without cells measured (referred to as empty filters) were determined at the beginning of each experiment and were subtracted from each point. For each condition measurements were performed in duplicates. For calculating the changes caused by TNF-α treatment, the curves were normalized to the last point before the addition of TNF-α. The difference between control and treated samples at the indicated times was determined in each experiment. Negative values indicate TER decrease. Efficient downregulation of Cldn-2 was verified at the end of experiments by lysing the cells on the filters and detecting Cldn-2 levels by Western blotting.
Statistical analysis.
All blots and immunofluorescent pictures are representatives of at least three similar experiments. Data are presented as means ± SE of the number of experiments indicated (n). Statistical significance was assessed by one-way ANOVA using the GraphPad Prism software, or Student's t-test, as appropriate.
RESULTS
TNF-α altered the expression of junctional proteins.
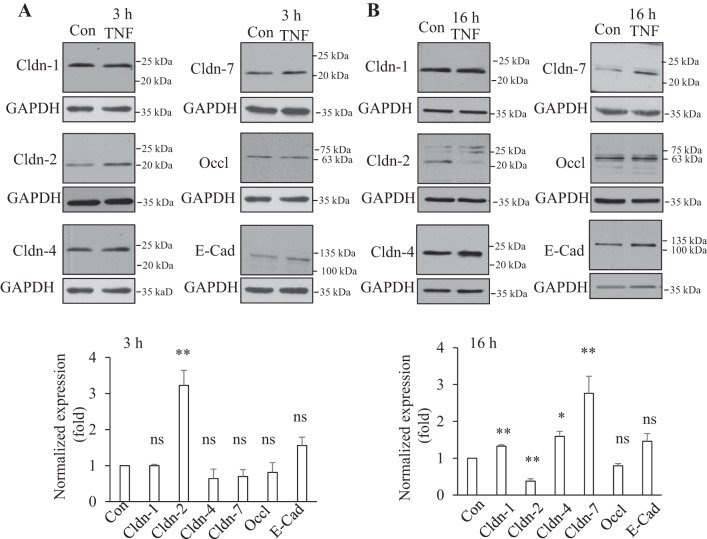
To gain insight into the mechanisms underlying TNF-α-induced changes in paracellular permeability, we explored how this cytokine affects expression of junctional proteins in renal tubular cells. LLC-PK1 proximal tubular cells were exposed to 10 ng/ml TNF-α for 3 or 16 h and proteins were detected by Western blotting. Expression of the investigated proteins was normalized using the housekeeping enzyme GAPDH. Since LLC-PK1 cells have been shown to express Cldn-1, -2, -4, and -7 (22) we focused on these claudins. We also assessed changes in occludin and the adherent junction protein E-cadherin. As shown on Fig. 1A, after 3 h of TNF-α treatment, expression of Cldn-1 and 4 and occludin was unaltered. In contrast, Cldn-2 levels showed a significant, approximately equal to threefold increase. TNF-α also caused a slight but not significant increase in E-cadherin. In some experiments we also detected a decrease in Cldn-7 levels. However, this observation was less consistent and the average over several experiments showed no significant change (Fig. 1A). A different picture emerged when cells were treated for 16 h with TNF-α, as the expression levels of all claudins tested were significantly altered (Fig. 1B). Cldn-2 levels showed a marked drop, while Cldn-1, -4, and -7 were elevated. In contrast, neither occludin nor E-cadherin was significantly altered. Taken together, this initial screen demonstrated that TNF-α changed the expression of various claudins in a differential and time-dependent manner.
Fig. 1.
Differential effect of short-term and long-term TNF-α treatment on tight junction (TJ) proteins. Confluent LLC-PK1 cells were treated with 10 ng/ml TNF-α for 3 (A) or 16 h (B). Cells were lysed and the levels of claudin (Cldn)-1, -2, -4, and -7, as well as occludin and E-cadherin were detected by Western blotting. The blots were redeveloped to detect GAPDH as loading control. In the densitometric analysis signals from the specific proteins were normalized using the GAPDH signal. In each experiment the normalized levels of TNF-α-treated samples were expressed as a fold change from the control taken as 1. The graphs show means ± SE (n ≥ 3). For statistical analysis each value was compared with the corresponding control using Student's t-test. *P < 0.05; **P < 0.01; ns: nonsignificant vs. control.
TNF-α caused a biphasic change in Cldn-2 expression.
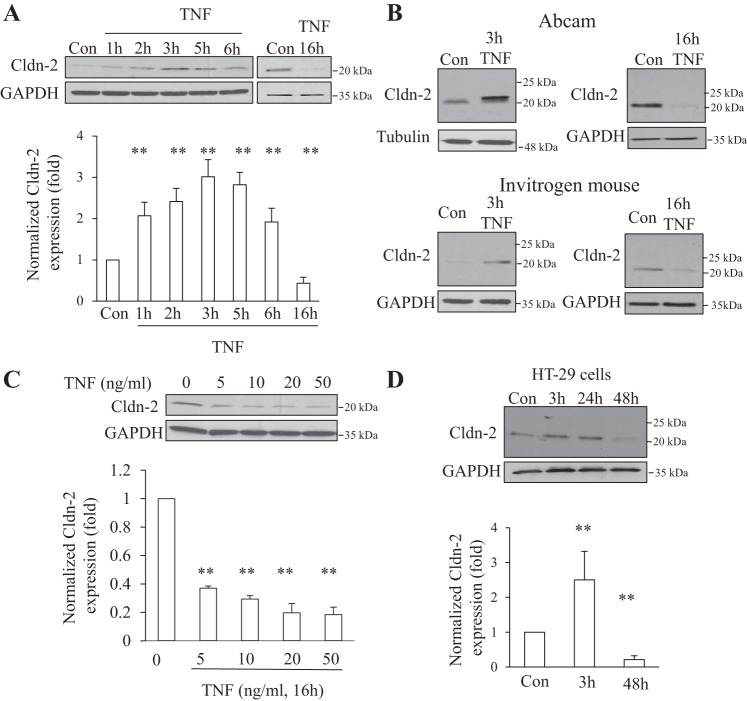
Cldn-2 is a channel forming protein with a central role in paracellular Na+ transport in the proximal tubules. Changes in the expression of this protein can have major implications on tubular transport. Having found that TNF-α has a differential effect on Cldn-2 expression depending on the time of exposure, in the next experiments we wished to further characterize this effect. First, we investigated the detailed kinetics of the TNF-α-induced effect. As shown on Fig. 2A, an increase in the level of Cldn-2 was detectable as early as 1 h after the addition of TNF-α. The expression peaked at 3 h. When cells were exposed to TNF-α for 16 h, a significant drop in Cldn-2 levels was detected (Figs. 1B and 2A). Based on this time course, we used 3- or 16-h TNF-α treatment throughout the study, referred to as short and long treatment, respectively.
Fig. 2.
TNF-α altered Cldn-2 expression causing an early increase and a late decrease. A: confluent LLC-PK1 cells were treated with 10 ng/ml TNF-α for the indicated times. Cldn-2 was detected using a rabbit polyclonal antibody from Invitrogen. Cldn-2 levels were normalized using GAPDH and quantified as in Fig 1. The graphs show means ± SE (n ≥ 3). **P < 0.01 vs. control. B: verification of Cldn-2 expression changes using different antibodies. Lysates from cells treated with 10 ng/ml TNF-α for 3 and 16 h as in A were tested by Western blotting with 2 different Cldn-2 antibodies, as indicated. Top blots show results using a polyclonal antibody from Abcam; bottom blots were developed with a monoclonal antibody from Invitrogen. The blots are representatives of n ≥ 3 independent experiments. C: concentration dependence of the TNF-α effect. LLC-PK1 cells were treated with the indicated concentration of TNF-α for 16 h. The levels of Cldn-2 were detected and quantified as in A. The graphs show means ± SE (n = 3). **P < 0.01 vs. control. D: TNF-α-induced early increase and late decrease in intestinal cells. Confluent HT-29 intestinal cells were treated with 10 ng/ml TNF-α for the indicated times. Cldn-2 was detected and quantified as in A. The graphs show means ± SE (n = 3). **P < 0.01 vs. control.
To avoid any confounding effects from nonspecific cross reaction of the Cldn-2 antibody with other claudins (a common problem with many claudin antibodies), we verified our findings using two additional antibodies. As shown on Fig. 2B, all Cldn-2 antibodies detected an increase in Cldn-2 protein levels after 3 h and a decrease after 16-h TNF-α treatment. Since the epitopes for the various antibodies used were different, the possibility that the detected changes in Cldn-2 levels were due to epitope masking/unmasking can also be excluded.
Next, we tested the TNF-α concentration dependence of the effects on Cldn-2 downregulation (Fig. 2C). Addition of 5 ng/ml TNF-α for 16 h was sufficient to cause a decrease, and the effect was only slightly larger at higher concentrations. Next we asked whether the biphasic effect of TNF-α on Cldn-2 is specific for tubular cells. Figure 2D shows that similar to its effects in LLC-PK1 cells, TNF-α also caused a readily detectable increase in Cldn-2 after 3 h in HT-29 cells, an intestinal cell line. In these cells the kinetics of the second phase was slightly different than in LLC-PK1 cells, since Cldn-2 levels were still high after 24-h TNF-α treatment and showed significant decreased only after 48-h TNF-α treatment. Thus the effect of TNF-α was overall similar in the two cell types, although the Cldn-2 decrease appeared with a slightly delayed kinetics in HT-29 cells and required longer TNF-α exposure.
TNF-α altered Cldn-2 levels at the cell surface.
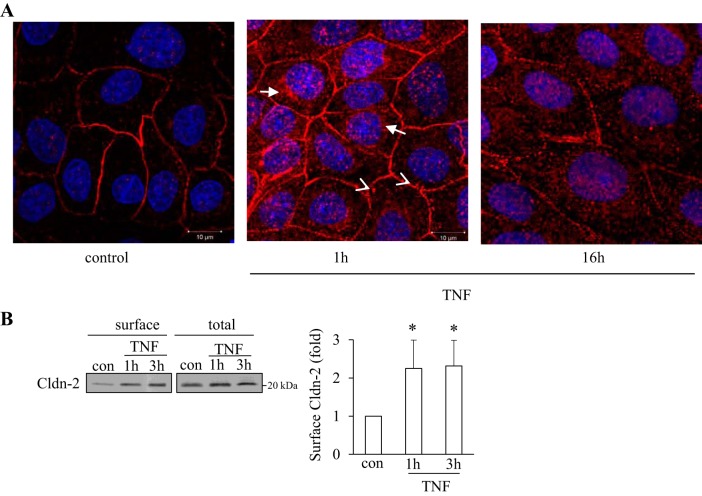
Next, we studied effects of TNF-α on the subcellular localization of Cldn-2. First, we visualized Cldn-2 using immunofluorescent staining. In control cells Cldn-2 was detectable both at the cell membrane and in cytosolic vesicular structures (Fig. 3A). When cells were exposed to TNF-α for 1 h, the overall staining became stronger (Fig. 3A, middle) consistent with an increase in Cldn-2 expression. Importantly, not only the membrane-localized Cldn-2 staining was enhanced, but a marked increase was also visible in the cytosolic vesicular staining. The vesicles were more numerous and larger, and in many cells they accumulated close to the nucleus (long arrows). In many areas, vesicles were also observed underneath the membrane (arrowheads). In contrast, when cells were treated with TNF-α for 16 h, the overall staining was weaker with discontinuous and punctate membrane staining. For a more quantitative assessment of the membrane localized protein, we used a biotin labeling assay to follow changes in cell surface Cldn-2 levels. Surface proteins in untreated or TNF-α-treated cells were biotinylated. Following lysis, all biotinylated proteins were captured using streptavidine-covered beads. The amount of biotinylated Cldn-2 that corresponds to cell surface Cldn-2 was quantified by Western blotting. As shown on Fig. 3B, surface levels of Cldn-2 increased after 1- and 3-h TNF-α treatment, verifying that the elevated expression of Cldn-2 induced by short-term TNF-α treatment resulted in an increase at the TJs.
Fig. 3.
TNF-α altered the levels of Cldn-2 at the cell surface. A: LLC-PK1 cells were grown on coverslips to confluence. Where indicated the cells were treated with 10 ng/ml TNF for 1 or 16 h. The cells were fixed with methanol, and endogenous Cldn-2 was visualized using an antibody from Invitrogen and a Cy3-labeled secondary antibody. The nuclei were stained using DAPI. The slides were visualized using a Zeiss LSM confocal microscope. Maximal Intensity Projection images are shown. The bar at right bottom corner = 10 μm for all. Cldn-2 was present both at the cell surface and in vesicular cytosolic structures. The abundance of Cldn-2 in vesicles is increased after TNF-α treatment. The images shown are representatives of n = 3 independent experiments. B: confluent LLC-PK1 cells were treated with 10 ng/ml TNF-α for the indicated times. The cells were placed on ice and surface proteins were biotinylated as described under materials and methods. Cells were lysed and biotinylated proteins precipitated using streptavidine-agarose beads. Cldn-2 in the precipitates (surface) and the cell lysates (total) was detected by Western blotting. The signal was quantified using densitometry. Levels of Cldn-2 in treated samples were expressed as fold change from control. The graph shows means ± SE from n = 4 experiments. *P < 0.05 vs. control.
Differential role of transcriptional regulation in the two phases of the TNF-α-induced Cldn-2 expression changes.
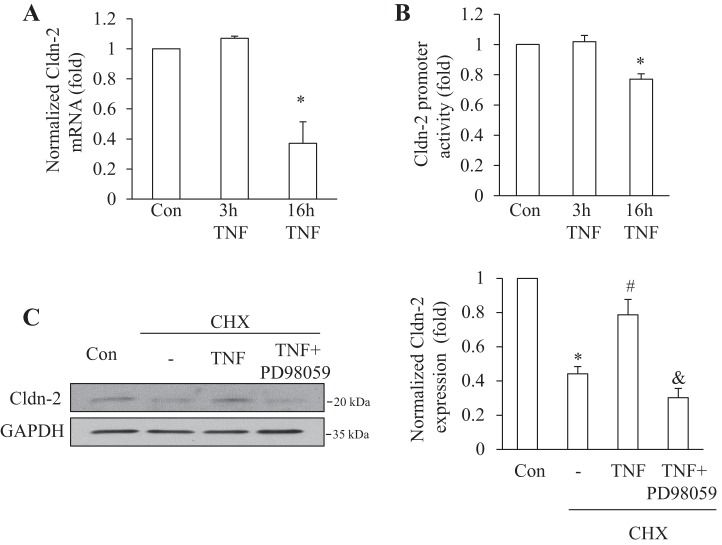
Having established the biphasic effect of TNF-α on the total and surface expression of Cldn-2, we next sought to determine the underlying mechanisms. To explore the role of altered synthesis, we measured Cldn-2 mRNA levels in untreated LLC-PK1 cells and in cells exposed to TNF-α for 3 or 16 h, using quantitative RT-PCR. Interestingly, after short-term treatment, when Cldn-2 protein expression was significantly elevated, no significant increase was detected in the mRNA levels (Fig. 4A). In contrast, after 16-h treatment, Cldn-2 mRNA expression dropped by ≈60%. To substantiate the differential role of altered protein synthesis in the biphasic effect of TNF-α, we used a luciferase-coupled Cldn-2 promoter construct to assess changes in gene transcription. Under basal conditions, the Cldn-2 promoter had a readily detectable activity. TNF-α added for 6 h did not alter this activity, supporting that the early rise in Cldn-2 protein was independent from changes in gene transcription (Fig. 4B). After 16-h TNF-α treatment, however, the promoter activity showed a significant decrease, verifying that reduced gene transcription contributed to the late decrease in Cldn-2.
Fig. 4.
Differential role of altered Cldn-2 transcription in the early and late TNF-α effects. A: LLC-PK1 cells were treated for the indicated times with 10 ng/ml TNF-α. RNA extraction, cDNA synthesis, and SYBR green based real-time PCR to determine Cldn-2 and GAPDH levels were performed as described under materials and methods. Data are means ± SE (n = 4). *P < 0.05 vs. control. B: LLC-PK1 cells were transfected with a firefly luciferase-coupled Cldn-2 promoter along with pRL-TK (Renilla luciferase, internal control). Twenty-four hours posttransfection cells were treated with 10 ng/ml TNF-α for 6 or 16 h. Luciferase activities were determined using the Dual Luciferase assay kit and normalized by dividing the Firefly luciferase activity with the Renilla luciferase activity. In each experiment mean from the three parallel measurements was calculated and values of treated samples were expressed as fold change from control taken as 1. The graph shows means ± SE (n = 4). *P < 0.05 vs. control. C: LLC-PK1 cells were treated with 100 μM cycloheximide (CHX) with or without 20 ng/ml TNF-α and 20 μM PD98059, as indicated, for 1 h. Cldn-2 levels were detected and quantified as in Fig 1. The graph shows means ± SE (n = 3). *P < 0.01 vs. control; #P < 0.01 vs. CHX treatment; &P < 0.01 vs. CHX + TNF treatment group.
Short-term TNF-α treatment reduced Cldn-2 degradation.
To define the mechanism through which TNF-α elevates Cldn-2 levels, we next explored the effects of the cytokine on Cldn-2 degradation. To this end, we inhibited protein synthesis using cycloheximide and detected changes in the level of Cldn-2. As shown on Fig. 4C, Cldn-2 levels rapidly decreased upon cycloheximide treatment, suggesting that the protein has fast turnover. In fact levels of Cldn-2 decreased by ≈50% after 1 h of cycloheximide treatment. Addition of TNF-α significantly mitigated this decrease, suggesting that TNF-α reduces the degradation of Cldn-2.
TNF-α increased Cldn-2 through the EGFR and ERK.
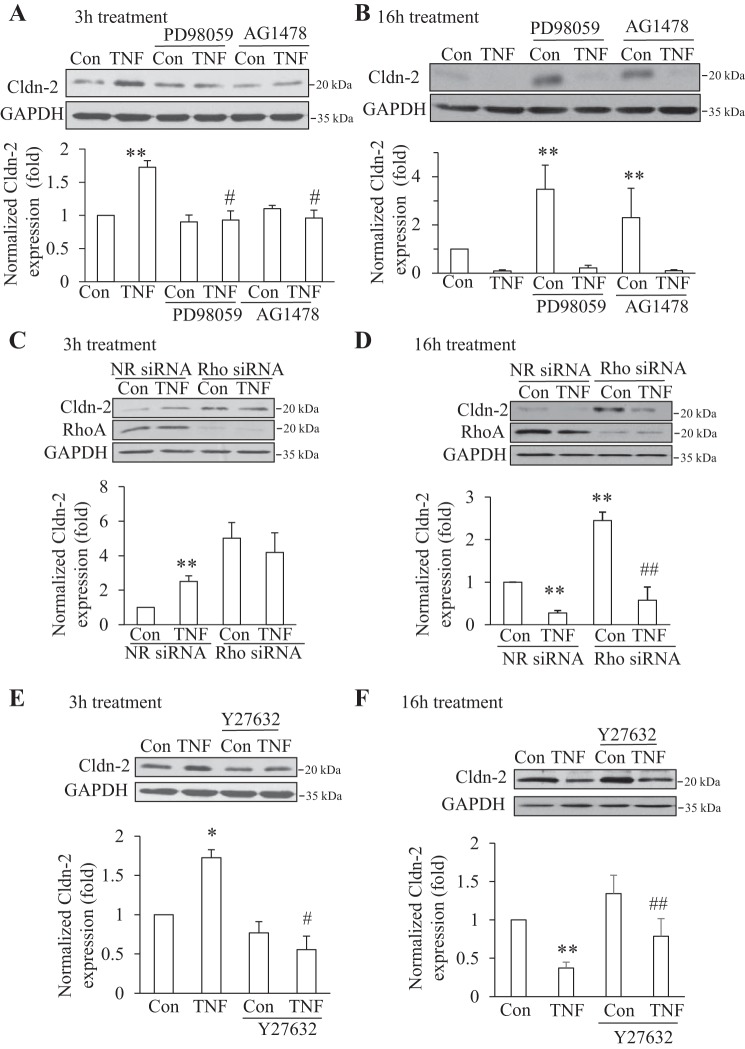
Next, we explored the signaling pathways through which TNF-α alters Cldn-2 expression. We have previously shown that TNF-α activates the EGFR, which in turn mediates ERK and RhoA activation. Since both the EGFR/ERK and the RhoA pathways have previously been implicated in the control of Cldn-2 expression (18, 23, 25, 54), we chose to study the role of these. Therefore, we used AG1478 and PD98059, inhibitors of EGFR and MEK1/2, respectively. Cells were exposed to TNF-α for 3 or 16 h with or without the inhibitors. As shown on Fig. 5A, both inhibitors prevented the TNF-α-induced increase in Cldn-2 protein expression. These data suggest that TNF-α might reduce Cldn-2 degradation through ERK. To test this, we asked whether inhibition of the ERK pathway might prevent the effect of TNF-α on Cldn-2 degradation. Indeed, as shown in Fig. 4C, while TNF-α reduced Cldn-2 degradation in cycloheximide treated cells, PD98059 prevented this effect. Thus these data suggest that short-term TNF-α treatment elevates Cldn-2 levels through an ERK-dependent reduction of its degradation.
Fig. 5.
TNF-α-induced early rise in Cldn-2 required ERK, EGF receptor, and RhoA, while the late decrease was independent of these. A and B: confluent LLC-PK1 cells were treated with 20 μM PD98059 or 10 μM AG1478 for 15 min, followed by addition of 10 ng/ml TNF-α for 3 h (A) or 16 h (B) in the presence of the inhibitor. At the end of the treatment cells were lysed and Cldn-2 levels detected and quantified as in Fig 1. The graphs show means ± SE (n = 3) **P < 0.05 vs. control; #P < 0.01 vs. TNF treatment alone. C and D: LLC-PK1 cells were transfected with nonrelated (NR) siRNA or an siRNA against porcine RhoA. Forty-eight h later the cells were treated with 10 ng/ml TNF-α for 3 h (C) or 16 h (D) and Cldn-2 levels were detected and quantified as in Fig 1. Graphs show means ± SE (n = 3). **P < 0.01 vs. control, ##P < 0.01 vs. Rho siRNA control. E and F: cells were treated with 20 μM Y27632 for 30 min, followed by 20 ng/ml TNF-α for 3 h (E) or 16 h (F), and Cldn-2 levels were detected as above. The graphs show means ± SE from n = 3 (E) and 6 (F) independent experiments. **P < 0.01 vs. control; #P < 0.05 vs. TNF treatment alone; ##P < 0.01 vs. Y27632 alone.
We next asked whether Cldn-2 degradation following prolonged TNF-α treatment also required EGFR and ERK. Interestingly, extended (16 h) treatment of the cells with PD98059 or AG1478 alone caused a marked increase in Cldn-2 expression (Fig. 5B). These data suggest that the EGFR and ERK might suppress Cldn-2 synthesis in resting cells and contribute to maintaining relatively low expression levels of the protein. Despite this marked effect on the basal Cldn-2 levels, long-term TNF-α treatment reduced Cldn-2 expression in the presence of the inhibitors, suggesting that TNF-α suppresses Cldn-2 expression independent of the EGFR and ERK pathways (Fig. 5B).
RhoA and Rho kinase were required for the short-term but not the long-term TNF-α effect on Cldn-2.
RhoA is a central regulator of the cytoskeleton and has been implicated in the control of resting Cldn-2 expression (19). This raised the possibility that it might also play a role in mediating the TNF-α-induced changes in Cldn-2. Indeed, we found that TNF-α activates RhoA in tubular cells and this effect is mediated by the EGFR and ERK (29). To address the role of RhoA, we silenced it using an siRNA, as in our earlier studies (66). Transfection of LLC-PK1 cells with a nonrelated siRNA had no effect on the TNF-α-induced early increase and late decrease in Cldn-2 (Fig. 5, C and D). Interestingly, similar to the long-term inhibition of the EGFR and ERK, transfection with a RhoA siRNA for 48 h caused a marked increase in the expression of Cldn-2 (Fig. 5, C and D). However, in cells where RhoA was downregulated, short-term TNF-α treatment did not further elevate the expression of Cldn-2 (Fig. 5C). In contrast, TNF-α added for 16 h induced a marked decrease in Cldn-2 both in cells transfected with control and with RhoA siRNA (Fig. 5D). These findings suggested that RhoA might be involved in the early but not the late effect of TNF-α. However, the fact that RhoA downregulation by itself elevated Cldn-2 is a confounding factor in exploring its role in the early increase. Therefore, we used a pharmacological approach to inhibit the Rho effector Rho kinase. As anticipated, the short-term addition of the Rho kinase inhibitor Y27632 did not alter resting Cldn-2 levels (Fig. 5E). Importantly, Y27632 prevented the increase in Cldn-2 expression induced by TNF-α. When added for 16 h, Rho kinase inhibition also caused a marked elevation in Cldn-2 expression, similar to the effects of EGFR and ERK inhibition and RhoA silencing (Fig. 5F). However, TNF-α treatment for 16 h effectively reduced Cldn-2 levels in the presence of Rho kinase inhibition. Combined, these data substantiate that the early and late effects of TNF-α on Cldn-2 were mediated through distinct mechanisms. The early increase in Cldn-2 levels required EGFR, ERK, RhoA, and Rho kinase, while the late Cldn-2 decrease was independent of all of these.
TNF-α caused an early decrease and late increase in TER.
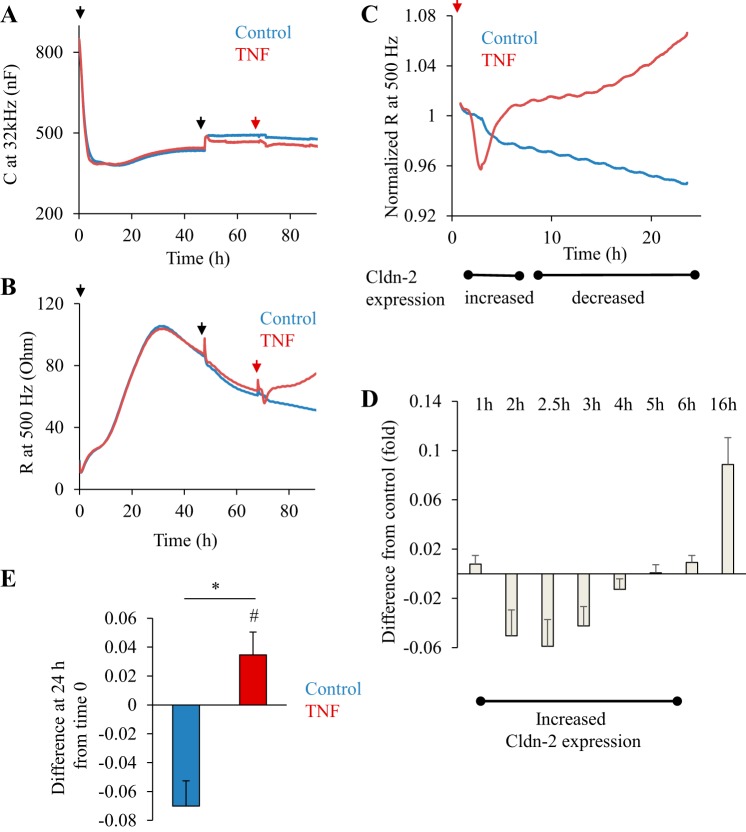
The presence of Cldn-2 enhances paracellular Na+ permeability (2), suggesting that the TNF-α-induced TER changes might at least in part be due to altered expression of this claudin. To correlate Cldn-2 changes and TER, we followed the latter using ECIS. ECIS is an impedance-based assay that provides real-time measurement of various parameters of a cell layer, including TER (69). Cells grown on electrodes or filters are exposed to biologically inert alternating currents at different frequencies and changes in impedance and capacitance (C) are continuously measured. Using these parameters, resistance (R) is calculated. C at high frequencies correlates with cell confluence and R measured at low frequencies is indicative of the resistance across the layer (TER) (57). In the following experiments we used the newly developed filter-based ECIS system. Growing LLC-PK1 cells on filters allowed them to completely polarize and prevented dome formation by the confluent layer that could interfere with the measurement. Cells were seeded on semipermeable filters that were placed in the ECIS filter adapter. The cells were grown for 48 h to reach confluence and to allow time for the junctions to mature. The establishment of a confluent layer causes a decline in C (indicative of confluence) (Fig. 6A) and an increase in R (indicative of the development of junctions) (Fig. 6B). After 24 h, the cells have reached confluence as shown by the fact that C reached a minimum and remained stable (Fig. 6A). R also reached a maximum by 24 h; however, after this a continuous slow decline was observed. The rate of this slow drop stabilized by 48 h (≈10% drop/24 h; Fig. 6B). After 72-h growth on the filter, TNF-α or vehicle was added to the cells. The addition itself caused a slight drop in R and increase in C that was identical in all samples in all measurements (see red arrows on Fig. 6, A and B), indicating that this was an artifact. During the analysis we corrected for this effect. A typical measurement is shown on Fig. 6B. Figure 6C shows the same measurement, with the curves normalized to the last point before the addition of TNF-α. This representation allows easier comparisons. These measurements verify the complex effects of TNF-α on TER reported earlier (36, 42). An initial lag phase of about 60–90 min was observed, followed by a fast TER decrease. TER remained below the control values between 2 and 6 h after TNF-α addition. Interestingly, this was followed by a gradual rise in TER that stabilized at higher values than control and remained elevated for up to 48 h. To quantify these changes, we calculated the difference between the TER in the control and TNF-α-treated samples at given time points using normalized curves from several measurements (Fig. 6D). This analysis allowed us to reveal and quantify differences while taking into account the continuous small drop in the control values that was present in all experiments. The graph on Fig. 6D shows the difference between the control and the TNF-α treated samples. Negative values indicate that TER was lower in the treated samples than in control. The analysis verified that TNF-α induced a significant decrease in TER between 2 and 4 h after addition. In contrast, long-term (16 h) TNF-α treatment caused a significant elevation in TER. At 24 TER was significantly higher in TNF-α-treated cells than in control. Moreover, these values were higher than the values before TNF-α addition. Taken together, these data reveal that TNF-α caused a biphasic effect in TER, with an early decrease and a late increase above the resting values.
Fig. 6.
A: transepithelial resistance (TER) measurements in LLC-PK1 cells during the development of a confluent layer. TER of LLC-PK1 cells grown on filters was followed using electric cell-substrate impedance sensing (ECIS). At the beginning of each measurement filters with medium alone were placed in the ECIS filter holder. Capicitance (C) and resistance (R) were monitored for 15 min using the frequency scan mode to establish the R values of the empty filters. Next, the measurement was paused, and LLC-PK1 cells (1.5 × 105 cells/filter) were plated on the filter (1st black arrow), and then the measurement was restarted. A shows C measured at 32 kHz, and B shows the corresponding R at 500 Hz. Note that at 24 h C reached a minimum, indicating confluence. At 48 h, the medium was changed (2nd black arrow). After 72 h, 20 μl medium alone (control, blue curve) or medium containing TNF-α (20 ng/ml final concentration, red curve) was added (second arrow). The curves are the average of 2 parallel measurements and are representatives of n = 6 independent experiments. C and D: biphasic effect of TNF-a on TER. The part of the curves in B following TNF-α addition is shown (the artifact caused by the addition was removed from the curve). The data were normalized to the last point before addition of TNF-α taken as 1. The x-axis shows time after TNF-α addition. The timing of Cldn-2 level changes induced by TNF-α is shown under the graph. D: differences in TER between control and TNF-α-treated cells were calculated using the normalized curves (see materials and methods). Graph shows means ± SE (n = 6 independent measurements performed in duplicates). E: change in TER induced by 24-h TNF-α treatment. The difference between the last point before treatment (taken as 1) and the normalized TER value at 24 h after treatment was determined in n = 6 independent experiments performed in duplicates. The positive value (TNF treatment, red column) indicates an increase in TER, while the negative value (control, blue column) indicates decrease. *P < 0.01 vs. control; #P < 0.05 vs. 0 (one-tailed t-test).
Cldn-2 downregulation by TNF-α caused late TER elevation.
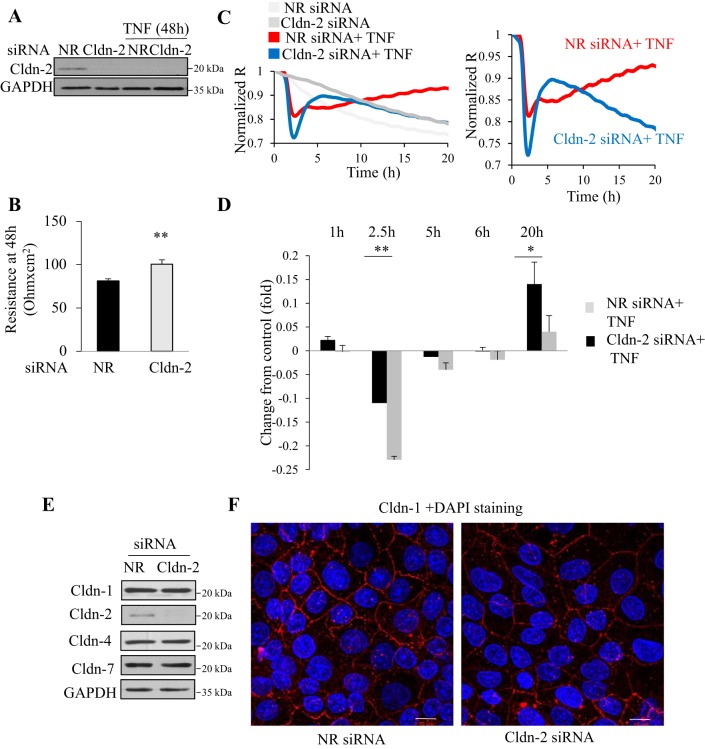
The biphasic effect of TNF-α on TER was reminiscent of its effects on Cldn-2 levels, with a good temporal correlation (Figs. 2A and 6C). Indeed, since Cldn-2 is a high permeability junction protein, its increased expression could contribute to elevated permeability (i.e., lower TER). Conversely, reduced Cldn-2 levels might cause an increase in TER. Therefore, we hypothesized that Cldn-2 expression changes induced by TNF-α contributed to its biphasic effect on TER. To test this possibility, we silenced Cldn-2 using an siRNA. Western blotting verified efficient downregulation of Cldn-2 throughout the time of the full measurement (Fig. 7A). As expected from its role as a high permeability protein, Cldn-2 silencing significantly elevated basal TER in confluent LLC-PK1 cells grown on filters (Fig. 7B). In fact, these data mimicked the changes observed after long-term TNF-α treatment, when Cldn-2 levels were reduced (Fig. 6C). Next, we tested the effects of TNF-α under conditions where Cldn-2 was downregulated before the addition of the cytokine. Figure 7, C and D, depicts the TNF-α-induced TER changes in cells transfected with control or Cldn-2-specific siRNA. For easier comparisons the curves in C were normalized to express fold changes in TER, thereby eliminating the difference in basal TER induced by Cldn-2 silencing. Figure 7C, left, shows a typical measurement, and in the right graph only the TNF-treated samples are shown for easier comparison. The effects were quantified using three separate measurements (done in duplicates). Accordingly, Fig. 7D shows the difference in TER between the control and TNF-α-treated samples at the indicated time points. Surprisingly, when Cldn-2 was downregulated before TNF-α addition, the early effect of TNF-α was preserved and, in fact, was significantly augmented. In fact, at 2.5 h after TNF-α addition, the decrease in TER was more than twice as big in Cldn-2 silenced cells (Fig. 7, C and D). Thus the early TER decrease caused by TNF-α was independent of Cldn-2. Moreover, the augmented effect of TNF-α suggests an indirect role for Cldn-2 in controlling junctions, independent of its role as a channel-forming protein. Since alterations in one claudin often affects expression of other claudins, we showed that Cldn-2 siRNA did not alter the levels of Cldn-1, -4 and -7 (Fig. 7E). Cldn-2 silencing also did not interfere with polarization of the layer and general junction development: Cldn-1 showed similar membrane localization in cells transfected with NR and Cldn-2-specific siRNA (Fig. 7F). Thus the early effect of TNF-α in Cldn-2 downregulated cells showed a surprising augmentation. In contrast, the late TER increase induced by TNF-α was essentially abolished. While in the control TNF-α treatment resulted in elevated TER at 24 h, silencing Cldn-2 before the addition of TNF-α resulted in an almost absent further elevation in TER at 24 h. In fact, after 24-h TNF-α treatment, TER in the NR and Cldn-2 siRNA-transfected cells became similar (not shown). Thus the late TER increase induced by TNF-α was at least in part due to downregulation of Cldn-2.
Fig. 7.
Effect of Cldn-2 downregulation on TNF-α-induced TER changes. A–D: LLC-PK1 cells were transfected with NR or Cldn-2-specific siRNA. Twenty four hours later the cells were trypsinized, and seeded on Corning permeable filters that were placed in the ECIS filter adapter, as in Fig 6. R was continuously measured at 500 Hz. A: effective and sustained downregulation of Cldn-2. At the end of the ECIS measurement (5 days posttransfection; 2 days after TNF addition), cells were lysed and Cldn-2 and GAPDH were detected by Western blotting. The blot is representative of n = 3 independent experiments. B: Cldn-2 silencing elevated basal TER. The graph shows R values at 48 h. R of the empty filter was subtracted. Graph shows means ± SE (n = 6 obtained in 3 independent measurements) **P < 0.01 vs. control. C and D: effect of TNF-α on TER in cells transfected with control and Cldn-2 siRNA. Cells were transfected with control (black curve) or Cldn-2 specific siRNA (grey). ECIS was performed as in Fig 6. C: at 48 h after seeding the cells 20 ng/ml TNF-α was added (taken as time 0). The curves are averages of 2 parallel measurements in a typical experiment and are representatives of n = 6 independent experiments. The values were normalized as in Fig 6. The left shows the full measurement. For easier comparison the 2 curves with TNF addition are also shown separately at right. D: differences in TER between cells transfected with NR siRNA and control siRNA were calculated as in Fig 6. The times indicate the time after TNF-α treatment. The graph shows means ± SE (n = 6 from 3 independent experiments). *P < 0.05; **P < 0.01 vs. control. E and F: Cldn-2 downregulation does not affect expression of other claudins or cell polarization. LLC-PK1 cells were transfected with NR or Cldn-2 specific siRNA. In E, expression of Cldn-1, -2, -4, and -7 was detected by Western blotting as in Fig 1. In F, Cldn-1 was stained with an antibody from Invitrogen and visualized with a Cy3-labeled anti-rabbit secondary antibody. This claudin was selected, since it is a “housekeeping claudin” the presence of which indicates polarization and intact TJs. The nuclei were stained using DAPI. Images were captured as in Fig 3. Maximum Intensity Projections are shown. The size bar in the right bottom corner = 10 μm. The images are representatives of n = 3 independent experiments.
DISCUSSION
In this study we demonstrated a previously unrecognized two-phase effect of TNF-α on Cldn-2 and showed that the two phases involve distinct mechanisms and signaling pathways. Although these changes show a good temporal correlation with the biphasic effect of TNF-α on TER, suggesting a causal relationship, silencing experiments revealed that Cldn-2 plays a role only in the TNF-α-induced elevation of TER.
Cldn-2 is expressed in leaky epithelia such as the kidney proximal tubules and the intestine (14, 15, 37, 56), where it elevates transepithelial permeability and reduces TER. These properties make Cldn-2 an excellent target for permeability modifying stimuli, including inflammatory cytokines (10). Here we show that TNF-α exerts a biphasic effect on Cldn-2 expression both in tubular and intestinal cells, suggesting that this might be a general response. Similar to our finding, Mankertz et al. (35) reported that exposure of HT-29/B6 intestinal cells to TNF-α induced a marked increase in Cldn-2. However, in contrast to our findings, these authors did not observe a late decrease in Cldn-2. This discrepancy could be due to cell type-specific differences, since Mankertz et al. used a subtype of HT-29 cells. Increased Cldn-2 expression is also well documented in inflammatory bowel disease, a condition that involves chronic elevation of cytokines (e.g., Refs. 1, 35, 71). In contrast, in MDCK tubular cells combined treatment with TNF-α and interferon-γ for 24 h markedly decreased Cldn-2 levels (47). Interestingly, in tubular cells a large variety of stimuli can downregulate Cldn-2 expression, including metabolic acidosis (5), hyperosmolarity (24), H2O2 (17), EGF (25, 54), and the immunosuppressant drugs sirolimus and cyclosporine A (38). It will be of interest to test whether these stimuli also cause a biphasic change in Cldn-2.
We found that the initial TNF-α-induced elevation in Cldn-2 was not accompanied by changes in mRNA levels and promoter activity. Instead, TNF-α reduced the degradation of the protein. Since Cldn-2 is a high turnover protein, this effect could likely account for the increase in Cldn-2 levels. Cldn-2 was shown to dynamically cycle between the membrane and intracellular pools (13). Indeed, using immunofluorescent staining in LLC-PK1 cells, we found Cldn-2 both at the plasma membrane and in intracellular vesicles. Moreover, upon short-term TNF-α treatment not only the total but also the membrane-associated Cldn-2 levels increased. Membrane retention of Cldn-2 is controlled by its posttranslational modifications. For example, phosphorylation on serine 208 affects both membrane retention and degradation (64). Accordingly, a nonphosphorylatable mutant had reduced plasma membrane and enhanced lysosomal localization. Sumoylation also controls membrane localization (63). Cldn-2 can also form heteromers with other claudins (60), and in the presence or absence of specific binding partners, a cell-type specific parameter could also affect its turnover. Interestingly, Cldn-8 and Cldn-4 were both shown to affect TJ localization of Cldn-2 (4, 9). Our ongoing studies are aimed at exploring whether TNF-α induces Cldn-2 posttranslational modifications and/or alters its interactions.
The EGFR, ERK, and RhoA have been implicated in control of Cldn-2 expression and localization (7, 12, 18, 23, 25, 32, 34, 39, 53, 70). Since TNF-α activates these pathways (29, 66), we asked whether they also mediate TNF-α-induced Cldn-2 expression changes. Our experiments yielded two interesting findings. First, the initial TNF-α-induced Cldn-2 increase required the EGFR, ERK, RhoA, and Rho kinase, but the long-term effect was independent from these. Moreover, we also showed that TNF-α reduced Cldn-2 degradation through ERK. Thus our current and previous data (29) suggest that the EGFR/ERK/RhoA/Rho kinase pathway mediates the TNF-α-induced early Cldn-2 elevation by decreasing its degradation. This regulation might involve effects on Cldn-2 membrane localization through acto-myosin (27, 31, 51). Second, prolonged inhibition of the EGFR/ERK and RhoA pathways caused a prominent elevation in Cldn-2 levels, suggesting that under resting conditions these pathways suppress Cldn-2 expression in LLC-PK1 cells. Previous studies yielded contradictory data on the role of EGFR and ERK in regulating resting Cldn-2 levels. Indeed, both inhibition and stimulation of Cldn-2 expression were reported through these pathways (12, 23, 25, 32, 34, 39, 53, 70). While cell type specificity may account for some of the discrepancies, our current study also highlights the importance of treatment time.
Long-term TNF-α treatment reduced Cldn-2 mRNA levels and promoter activity. The human Cldn-2 promoter contains binding sites for a number of transcription factors including cdx homeodomain proteins, the hepatocyte nuclear factors, and GATA-4 (15, 49). Since some of these are tissue specific, it will be important to explore regulation by these factors specifically in tubular cells.
To assess the functional implications of TNF-α-induced Cldn-2 changes, we first assessed the role of Cldn-2 in altered barrier functions. TJ permeability is determined by the combination of claudin isoforms at the TJs, and stimulus-induced permeability changes might involve selective removal or addition of specific claudins. In its “classic” role as a TJ permeability protein, Cldn-2 generates a paracellular Na+ and water channel (2, 22, 48). Accordingly, in Cldn-2 knockout mice transepithelial reabsorption of Na+, Cl−, and water in the proximal tubules was significantly decreased and paracellular shunt resistance increased (43). Thus we hypothesized that the initial increase in Cldn-2 could cause TER decrease (permeability increase) and the late decrease in Cldn-2 could elevate TER. Unexpectedly, however, in cells where Cldn-2 was downregulated, the initial TNF-α-provoked TER drop was not only preserved, but in fact it was significantly augmented. This finding strongly implies that the direct, permeability modifying function of Cldn-2 is not required for the early TER decrease induced by TNF-α. We currently do not have an explanation for the surprising augmentation of the TER drop that could point to an indirect effect of Cldn-2 on TJs. In support of this hypothesis, myosin light chain kinase expression was elevated in colon cells derived from Cldn-2 knockout animals. These cells also had augmented inflammatory responses (44). Since myosin-dependent contractility is a central regulator of TJ permeability (31, 52), augmented myosin phosphorylation in the absence of Cldn-2 might contribute to an increased response to TNF-α.
Consistent with previous reports (36), and with our hypothesis that Cldn-2 decrease contributes to TER elevation, silencing Cldn-2 in LLC-PK1 cells increased TER. Importantly, when Cldn-2 was downregulated before TNF-α addition, the TNF-α-induced late increase in TER was largely prevented. This suggests that in contrast to the early TER drop, Cldn-2 downregulation contributes to the late TER elevation. However, Cldn-2 is not the only claudin altered by TNF-α, since the sealing claudins Cldn-1, -4, and -7 were also elevated by prolonged TNF-α treatment. Indeed, the remaining TER change in the Cldn-2 siRNA-transfected cells points to a possible role for those claudins. It also has to be noted, that in this study we focused only a few claudins and did not address the role of other claudins that could also play a role.
Altered Cldn-2 expression by TNF-α treatment could also affect cell functions through the recently described “nonclassical” functions of the protein. Cldn-2 was suggested to affect proliferation in several cell types (8, 12, 26). Thus the initial increase in Cldn-2 levels might contribute to TNF-α-induced proliferation (29). However, the late decrease in the protein likely exerts a negative feedback. Our ongoing studies are aimed at testing the role of Cldn-2 in TNF-α-induced changes in cell proliferation.
Taken together, our study shows that TNF-α modifies claudin expression in tubular cells and this can have both short-term and long-term effects on transport and barrier functions. Chronic exposure to TNF-α might induce a phenotype in the tubular epithelium that has lower resting paracellular permeability but is primed for a larger immediate permeability change upon repeated stimulation. In addition, Cldn-2 expression changes could also affect epithelial cell proliferation and repair of the injured layer. Together these effects could contribute to the pathogenesis of chronic kidney disease.
GRANTS
This work was supported by grants from the Natural Sciences and Engineering Research Council of Canada (NSERC Grant RGPIN 327407) and the Kidney Foundation of Canada. K. Szászi is a recipient of an Early Researcher Award from the Ontario Ministry of Research and Innovation. F. Waheed was supported by a Li Ka Shing Scholarship and a University of Toronto open scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.A., Q.D., J.X., and F.W. performed experiments; Y.A., Q.D., and K.S. analyzed data; Y.A. and K.S. interpreted results of experiments; Y.A. and Q.D. prepared figures; K.S. conception and design of research; K.S. drafted manuscript; K.S. edited and revised manuscript; K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Andras Kapus for valuable discussions throughout the project and during the preparation of the manuscript. We also thank Dr. Caterina DiCiano-Oliveira for carefully reading the manuscript and the Keenan Research Center Core Facility for help.
REFERENCES
- 1.Amasheh M, Fromm A, Krug SM, Amasheh S, Andres S, Zeitz M, Fromm M, Schulzke JD. TNFalpha-induced and berberine-antagonized tight junction barrier impairment via tyrosine kinase, Akt and NFkappaB signaling. J Cell Sci 123: 4145–4155, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Amasheh S, Meiri N, Gitter AH, Schoneberg T, Mankertz J, Schulzke JD, Fromm M. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J Cell Sci 115: 4969–4976, 2002. [DOI] [PubMed] [Google Scholar]
- 3.Angelow S, Ahlstrom R, Yu AS. Biology of claudins. Am J Physiol Renal Physiol 295: F867–F876, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Angelow S, Schneeberger EE, Yu AS. Claudin-8 expression in renal epithelial cells augments the paracellular barrier by replacing endogenous claudin-2. J Membr Biol 215: 147–159, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Balkovetz DF, Chumley P, Amlal H. Downregulation of claudin-2 expression in renal epithelial cells by metabolic acidosis. Am J Physiol Renal Physiol 297: F604–F611, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol 11: 372–377, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol 287: C327–C335, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Buchert M, Papin M, Bonnans C, Darido C, Raye WS, Garambois V, Pelegrin A, Bourgaux JF, Pannequin J, Joubert D, Hollande F. Symplekin promotes tumorigenicity by up-regulating claudin-2 expression. Proc Natl Acad Sci USA 107: 2628–2633, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capaldo CT, Farkas AE, Hilgarth RS, Krug SM, Wolf MF, Benedik JK, Fromm M, Koval M, Parkos C, Nusrat A. Proinflammatory cytokine-induced tight junction remodeling through dynamic self-assembly of claudins. Mol Biol Cell 25: 2710–2719, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Capaldo CT, Nusrat A. Cytokine regulation of tight junctions. Biochim Biophys Acta 1788: 864–871, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clark IA. How TNF was recognized as a key mechanism of disease. Cytokine Growth Factor Rev 18: 335–343, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Dhawan P, Ahmad R, Chaturvedi R, Smith JJ, Midha R, Mittal MK, Krishnan M, Chen X, Eschrich S, Yeatman TJ, Harris RC, Washington MK, Wilson KT, Beauchamp RD, Singh AB. Claudin-2 expression increases tumorigenicity of colon cancer cells: role of epidermal growth factor receptor activation. Oncogene 30: 3234–3247, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dukes JD, Whitley P, Chalmers AD. The PIKfyve inhibitor YM201636 blocks the continuous recycling of the tight junction proteins claudin-1 and claudin-2 in MDCK cells. PLoS One 7: e28659, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol 281: F966–F974, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Escaffit F, Boudreau F, Beaulieu JF. Differential expression of claudin-2 along the human intestine: implication of GATA-4 in the maintenance of claudin-2 in differentiating cells. J Cell Physiol 203: 15–26, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539–1550, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez JE, DiGeronimo RJ, Arthur DE, King JM. Remodeling of the tight junction during recovery from exposure to hydrogen peroxide in kidney epithelial cells. Free Radic Biol Med 47: 1561–1569, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guillemot L, Citi S. Cingulin regulates claudin-2 expression and cell proliferation through the small GTPase RhoA. Mol Biol Cell 17: 3569–3577, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guillemot L, Paschoud S, Jond L, Foglia A, Citi S. Paracingulin regulates the activity of Rac1 and RhoA GTPases by recruiting Tiam1 and GEF-H1 to epithelial junctions. Mol Biol Cell 19: 4442–4453, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gunzel D, Fromm M. Claudins and other tight junction proteins. Compr Physiol 2: 1819–1852, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Guo G, Morrissey J, McCracken R, Tolley T, Klahr S. Role of TNFR1 and TNFR2 receptors in tubulointerstitial fibrosis of obstructive nephropathy. Am J Physiol Renal Physiol 277: F766–F772, 1999. [DOI] [PubMed] [Google Scholar]
- 22.Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem 281: 36117–36123, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Ikari A, Sato T, Watanabe R, Yamazaki Y, Sugatani J. Increase in claudin-2 expression by an EGFR/MEK/ERK/c-Fos pathway in lung adenocarcinoma A549 cells. Biochim Biophys Acta 1823: 1110–1118, 2012. [DOI] [PubMed] [Google Scholar]
- 24.Ikari A, Takiguchi A, Atomi K, Sato T, Sugatani J. Decrease in claudin-2 expression enhances cell migration in renal epithelial Madin-Darby canine kidney cells. J Cell Physiol 226: 1471–1478, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Ikari A, Takiguchi A, Atomi K, Sugatani J. Epidermal growth factor increases clathrin-dependent endocytosis and degradation of claudin-2 protein in MDCK II cells. J Cell Physiol 226: 2448–2456, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Ikari A, Watanabe R, Sato T, Taga S, Shimobaba S, Yamaguchi M, Yamazaki Y, Endo S, Matsunaga T, Sugatani J. Nuclear distribution of claudin-2 increases cell proliferation in human lung adenocarcinoma cells. Biochim Biophys Acta 1843: 2079–2088, 2014. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci 13: 6662–6681, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Jevnikar AM, Brennan DC, Singer GG, Heng JE, Maslinski W, Wuthrich RP, Glimcher LH, Kelley VE. Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int 40: 203–211, 1991. [DOI] [PubMed] [Google Scholar]
- 29.Kakiashvili E, Dan Q, Vandermeer M, Zhang Y, Waheed F, Pham M, Szaszi K. The epidermal growth factor receptor mediates tumor necrosis factor-alpha-induced activation of the ERK/GEF-H1/RhoA pathway in tubular epithelium. J Biol Chem 286: 9268–9279, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kakiashvili E, Speight P, Waheed F, Seth R, Lodyga M, Tanimura S, Kohno M, Rotstein OD, Kapus A, Szaszi K. GEF-H1 mediates tumor necrosis factor-alpha-induced Rho activation and myosin phosphorylation: role in the regulation of tubular paracellular permeability. J Biol Chem 284: 11454–11466, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapus A, Szaszi K. Coupling between apical and paracellular transport processes. Biochem Cell Biol 84: 870–880, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Kim TI, Poulin EJ, Blask E, Bukhalid R, Whitehead RH, Franklin JL, Coffey RJ. Myofibroblast keratinocyte growth factor reduces tight junctional integrity and increases claudin-2 levels in polarized Caco-2 cells. Growth Factors 30: 320–332, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lal-Nag M, Morin PJ. The claudins. Genome Biol 10: 235, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipschutz JH, Li S, Arisco A, Balkovetz DF. Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J Biol Chem 280: 3780–3788, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Mankertz J, Amasheh M, Krug SM, Fromm A, Amasheh S, Hillenbrand B, Tavalali S, Fromm M, Schulzke JD. TNFalpha up-regulates claudin-2 expression in epithelial HT-29/B6 cells via phosphatidylinositol-3-kinase signaling. Cell Tissue Res 336: 67–77, 2009. [DOI] [PubMed] [Google Scholar]
- 36.Marano CW, Laughlin KV, Russo LM, Peralta Soler A, Mullin JM. Long-term effects of tumor necrosis factor on LLC-PK1 transepithelial resistance. J Cell Physiol 157: 519–527, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Markov AG, Veshnyakova A, Fromm M, Amasheh M, Amasheh S. Segmental expression of claudin proteins correlates with tight junction barrier properties in rat intestine. J Comp Physiol B 180: 591–598, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Martin-Martin N, Dan Q, Amoozadeh Y, Waheed F, McMorrow T, Ryan MP, Szaszi K. RhoA and Rho kinase mediate cyclosporine A and sirolimus-induced barrier tightening in renal proximal tubular cells. Int J Biochem Cell Biol 44: 178–188, 2012. [DOI] [PubMed] [Google Scholar]
- 39.Martin-Martin N, Ryan G, McMorrow T, Ryan MP. Sirolimus and cyclosporine A alter barrier function in renal proximal tubular cells through stimulation of ERK1/2 signaling and claudin-1 expression. Am J Physiol Renal Physiol 298: F672–F682, 2010. [DOI] [PubMed] [Google Scholar]
- 40.Masszi A, Speight P, Charbonney E, Lodyga M, Nakano H, Szaszi K, Kapus A. Fate-determining mechanisms in epithelial-myofibroblast transition: major inhibitory role for Smad3. J Cell Biol 188: 383–399, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mineta K, Yamamoto Y, Yamazaki Y, Tanaka H, Tada Y, Saito K, Tamura A, Igarashi M, Endo T, Takeuchi K, Tsukita S. Predicted expansion of the claudin multigene family. FEBS Lett 585: 606–612, 2011. [DOI] [PubMed] [Google Scholar]
- 42.Mullin JM, Laughlin KV, Marano CW, Russo LM, Soler AP. Modulation of tumor necrosis factor-induced increase in renal (LLC-PK1) transepithelial permeability. Am J Physiol Renal Fluid Electrolyte Physiol 263: F915–F924, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Muto S, Hata M, Taniguchi J, Tsuruoka S, Moriwaki K, Saitou M, Furuse K, Sasaki H, Fujimura A, Imai M, Kusano E, Tsukita S, Furuse M. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA 107: 8011–8016, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nishida M, Yoshida M, Nishiumi S, Furuse M, Azuma T. Claudin-2 regulates colorectal inflammation via myosin light chain kinase-dependent signaling. Dig Dis Sci 58: 1546–1559, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Nusrat A, Turner JR, Madara JL. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol 279: G851–G857, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Pascher A, Klupp J. Biologics in the treatment of transplant rejection and ischemia/reperfusion injury: new applications for TNFalpha inhibitors? Biodrugs 19: 211–231, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Patrick DM, Leone AK, Shellenberger JJ, Dudowicz KA, King JM. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma modulate epithelial barrier function in Madin-Darby canine kidney cells through mitogen activated protein kinase signaling. BMC Physiol 6: 2, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosenthal R, Milatz S, Krug SM, Oelrich B, Schulzke JD, Amasheh S, Gunzel D, Fromm M. Claudin-2, a component of the tight junction, forms a paracellular water channel. J Cell Sci 123: 1913–1921, 2010. [DOI] [PubMed] [Google Scholar]
- 49.Sakaguchi T, Gu X, Golden HM, Suh E, Rhoads DB, Reinecker HC. Cloning of the human claudin-2 5′-flanking region revealed a TATA-less promoter with conserved binding sites in mouse and human for caudal-related homeodomain proteins and hepatocyte nuclear factor-1alpha. J Biol Chem 277: 21361–21370, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Nino MD, Benito-Martin A, Goncalves S, Sanz AB, Ucero AC, Izquierdo MC, Ramos AM, Berzal S, Selgas R, Ruiz-Ortega M, Egido J, Ortiz A. TNF superfamily: a growing saga of kidney injury modulators. Mediators Inflamm 2010: 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shen L. Tight junctions on the move: molecular mechanisms for epithelial barrier regulation. Ann NY Acad Sci 1258: 9–18, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shen L, Black ED, Witkowski ED, Lencer WI, Guerriero V, Schneeberger EE, Turner JR. Myosin light chain phosphorylation regulates barrier function by remodeling tight junction structure. J Cell Sci 119: 2095–2106, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Singh AB, Harris RC. Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J Biol Chem 279: 3543–3552, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Singh AB, Sugimoto K, Dhawan P, Harris RC. Juxtacrine activation of EGFR regulates claudin expression and increases transepithelial resistance. Am J Physiol Cell Physiol 293: C1660–C1668, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR. Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol 36: 261–270, 2012. [DOI] [PubMed] [Google Scholar]
- 56.Szaszi K, Amoozadeh Y. New insights into functions, regulation, and pathological roles of tight junctions in kidney tubular epithelium. Int Rev Cell Mol Biol 308: 205–271, 2014. [DOI] [PubMed] [Google Scholar]
- 57.Szulcek R, Bogaard HJ, van Nieuw Amerongen GP. Electric cell-substrate impedance sensing for the quantification of endothelial proliferation, barrier function, and motility. J Vis Exp 85: 51300, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsukita S, Furuse M. The structure and function of claudins, cell adhesion molecules at tight junctions. Ann NY Acad Sci 915: 129–135, 2000. [DOI] [PubMed] [Google Scholar]
- 59.Van Itallie CM, Anderson JM. Architecture of tight junctions and principles of molecular composition. Semin Cell Dev Biol 36: 157–165, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Itallie CM, Anderson JM. Claudin interactions in and out of the tight junction. Tissue Barriers 1: e25247, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Itallie CM, Anderson JM. Claudins and epithelial paracellular transport. Annu Rev Physiol 68: 403–429, 2006. [DOI] [PubMed] [Google Scholar]
- 62.Van Itallie CM, Anderson JM. The role of claudins in determining paracellular charge selectivity. Proc Am Thorac Soc 1: 38–41, 2004. [DOI] [PubMed] [Google Scholar]
- 63.Van Itallie CM, Mitic LL, Anderson JM. SUMOylation of claudin-2. Ann NY Acad Sci 1258: 60–64, 2012. [DOI] [PubMed] [Google Scholar]
- 64.Van Itallie CM, Tietgens AJ, LoGrande K, Aponte A, Gucek M, Anderson JM. Phosphorylation of claudin-2 on serine 208 promotes membrane retention and reduces trafficking to lysosomes. J Cell Sci 125: 4902–4912, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vielhauer V, Mayadas TN. Functions of TNF and its receptors in renal disease: distinct roles in inflammatory tissue injury and immune regulation. Semin Nephrol 27: 286–308, 2007. [DOI] [PubMed] [Google Scholar]
- 66.Waheed F, Dan Q, Amoozadeh Y, Zhang Y, Tanimura S, Speight P, Kapus A, Szaszi K. Central role of the exchange factor GEF-H1 in TNF-alpha-induced sequential activation of Rac, ADAM17/TACE, and RhoA in tubular epithelial cells. Mol Biol Cell 24: 1068–1082, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waheed F, Speight P, Kawai G, Dan Q, Kapus A, Szaszi K. Extracellular signal-regulated kinase and GEF-H1 mediate depolarization-induced Rho activation and paracellular permeability increase. Am J Physiol Cell Physiol 298: C1376–C1387, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wajant H, Pfizenmaier K, Scheurich P. Tumor necrosis factor signaling. Cell Death Differ 10: 45–65, 2003. [DOI] [PubMed] [Google Scholar]
- 69.Wegener J, Sieber M, Galla HJ. Impedance analysis of epithelial and endothelial cell monolayers cultured on gold surfaces. J Biochem Biophys Methods 32: 151–170, 1996. [DOI] [PubMed] [Google Scholar]
- 70.Yasuda T, Saegusa C, Kamakura S, Sumimoto H, Fukuda M. Rab27 effector Slp2-a transports the apical signaling molecule podocalyxin to the apical surface of MDCK II cells and regulates claudin-2 expression. Mol Biol Cell 23: 3229–3239, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zeissig S, Burgel N, Gunzel D, Richter J, Mankertz J, Wahnschaffe U, Kroesen AJ, Zeitz M, Fromm M, Schulzke JD. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn's disease. Gut 56: 61–72, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]