Abstract
Na+/H+ exchange by Na+/H+ exchanger 3 (NHE3) is a major route of sodium absorption in the intestine and kidney. We have shown previously that lysophosphatidic acid (LPA), a small phospholipid produced ubiquitously by all types of cells, stimulates NHE3 via LPA5 receptor. Stimulation of NHE3 activity by LPA involves LPA5 transactivating EGF receptor (EGFR) in the apical membrane. EGFR activates proline-rich tyrosine kinase 2 (Pyk2) and ERK, both of which are necessary for NHE3 regulation. However, Pyk2 and ERK are regulated by EGFR via independent pathways and appear to converge on an unidentified intermediate that ultimately targets NHE3. The p90 ribosomal S6 kinase (RSK) family of Ser/Thr protein kinases is a known effector of EGFR and ERK. Hence, we hypothesized that RSK may be the convergent effector of Pyk2 and ERK although it is not known whether Pyk2 regulates RSK. In this study, we show that Pyk2 is necessary for the maintenance of phosphoinositide-dependent kinase 1 (PDK1) autophosphorylation, and knockdown of Pyk2 or PDK1 mitigated LPA-induced phosphorylation of RSK and stimulation of NHE3 activity. Additionally, we show that RSK2, but not RSK1, is responsible for NHE3 regulation. RSK2 interacts with NHE3 at the apical membrane domain, where it phosphorylates NHE3. Alteration of S663 of NHE3 ablated LPA-induced phosphorylation of NHE3 and stimulation of the transport activity. Our study identifies RSK2 as a new kinase that regulates NHE3 activity by direct phosphorylation.
Keywords: Na+/H+ exchanger 3, lysophosphatidic acid, phosphorylation, ribosomal S6 kinase
lysophosphatidic acid (LPA) is a bioactive phospholipid that mediates a broad range of effects that alter cell fates, cytokine secretion, neural retraction, pain perception, and embryo implantation (6). LPA acts on a family of G protein-coupled receptors, LPA1–6 (6). Most cells express multiple LPA receptors, and at least five LPA receptors are expressed in mouse intestinal epithelial cells with LPA1 and LPA5 being most abundant (20). Increasing evidence shows that oral application of LPA exerts significant effects on the gastrointestinal system (7, 17, 21). Recent studies have revealed a novel function of LPA in regulation of electrolyte transport processes. Naren and coworkers (18) have shown that LPA acting via LPA2 attenuates cholera toxin-induced Cl− secretion in mouse intestine by inhibiting the cystic fibrosis transmembrane conductance regulator (CFTR). Recently, we have shown that LPA activates Na+/H+ exchanger 3 (NHE3), which is the major Na+ transporter in the intestine (22, 45). The activity of NHE3 directly contributes to diarrhea, and the means to stimulate NHE3 is a potential treatment strategy for diarrheal diseases. In addition to the generation of LPA in situ, LPA is present in several types of foodstuff, implying that foodborne LPA may have an antidiarrheal effect by regulation of CFTR and NHE3 (2, 39).
Unlike CFTR that is inhibited by LPA2, stimulation of NHE3 by LPA is mediated by the LPA5 receptor (22). The activation of NHE3 by LPA5 requires transactivation of EGF receptor (EGFR) expressed in the apical membrane of Caco-2bbe cells (45). Transactivation of EGFR results in the activation of the RhoA-Rho-associated kinase-proline-rich tyrosine kinase 2 (Pyk2) cascade and the MEK-ERK pathway. These pathways do not appear to work in series but work in parallel because inhibition of one does not alter the other. Both Pky2 and ERK are necessary for the regulation of NHE3 because inhibition of either pathway blocks the stimulatory effect of LPA. These results suggest that Pyk2 and ERK1/2 may converge on the same effector, but the common downstream effector of Pyk2 and ERK1/2 remains unknown.
The p90 ribosomal S6 kinase (RSK) family of kinase is activated in response to several growth factors and mitogens, including EGF, insulin, and insulin-like growth factor-1 (1). Multiple targets, including transcription factors, glycogen synthase kinase 3β, and cyclin-dependent kinase inhibitor, whose activities are associated with regulation of cell proliferation, protein synthesis, cell mobility, and survival, are phosphorylated by RSK (1, 32). Moreover, it has been shown previously that RSK regulates NHE1 in fibroblasts and neurons (23, 38). In addition to being a target of EGFR and ERK, the diverse functions of RSK appear to correlate with a similarly broad range of effects by LPA. Therefore, we postulated that RSK is a potential kinase regulating NHE3. In this study, we demonstrate that RSK2 is the convergent effector of Pky2 and ERK in the signaling cascade elicited by LPA5. Our study shows that RSK2 interacts with NHE3 and phosphorylates NHE3.1
MATERIALS AND METHODS
Chemical and materials.
LPA (18:1, 1-oleoyl-2-hydroxy-sn-glycero-3-phosphate) was purchased from Avanti Polar Lipids (Alabaster, AL) and prepared in PBS, pH 7.2, containing 0.1% BSA. For all experiments, LPA was used at the final concentration of 1 μM unless otherwise specified, and the equal volume of PBS containing 0.1% BSA was added as a control. Rabbit anti-RSK1 and anti-p-RSK(S221) antibodies were obtained from R&D Systems (Minneapolis, MN). Antibodies against RSK2, p-RSK(S380), p-RSK(T573), phosphoinositide-dependent kinase 1 (PDK1), and p-PDK1 were from Cell Signaling (Danvers, MA). Antibody to p-Ser was from Abcam (Cambridge, MA). All other antibodies were obtained from Cell Signaling or Sigma (St. Louis, MO). All chemicals were obtained from Sigma or EMD Millipore (Billerica, MA).
RT-PCR.
RT-PCR was performed using PCR Master Mix (Roche, Indianapolis, IN) as previously described (22). The primers used are as follows: RSK1: GAA GAA GGC AAC GCT GAA AG (forward) and CTC CTC CGT GAA CAT CAC CT (reverse); RSK2: CTC AGG CTC TGA TGC TAG GC (forward) and CTC CTC CCC TGA GAA AAT CC (reverse); RSK3: GGA CCG AGT GAG ATC GAA GA (forward) and CCA GCT CAG CCA GGT AGA AC (reverse); and RSK4: GCC CCA ATG ATA CT CTG AA (forward) and TGG CAA CTG GTC TCT GTG AG (reverse).
Gene silencing.
Lentiviral vector (pLKO.1-puro) containing small hairpin (sh)RNA-targeting Pyk2 (shPyk2), RSK1 (shRSK1), or RSK2 (shRSK2) was obtained from Sigma. Lentiviral shRNA for PDK1 was obtained from Open Biosystems (Huntsville, AL). pLKO.1-puro/nontarget shRNA control (shCon) was from Sigma.
Site-directed mutagenesis and sequencing analysis.
pcDNA with human NHE3 with a vesicular stomatitis virus glycoprotein (VSVG) epitope tag at the COOH terminus has been described previously (22). Site-directed mutagenesis of S663 or S693 of NHE3 to Ala was performed using the QuickChange site-directed mutagenesis kit according to the recommendation by the manufacturer (Stratagene, La Jolla, CA). The presence of S663A or S693A mutation was confirmed by nucleotide sequencing.
Cell cultures.
Caco-2bbe cells were grown in DMEM supplemented with 10% FBS, 50 U/ml penicillin, 50 μg/ml streptomycin, 1 mM sodium pyruvate, 15 mM HEPES, and 1× nonessential amino acids. Caco-2bbe cells were transfected with pcDNA-NHE3, pcDNA-NHE3/S663A, or pcDNA-NHE3/S693A, Caco-2bbe/NHE3 using a Neon Transfection System (Life Technologies, Grand Island, NY) as previously described (22). Transfected cells were cultured in the presence of puromycin and subjected to acid suicide for three consecutive passages to select stable expression of NHE3 or its variant. Caco-2bbe/NHE3 cells were infected with lentiviral pCDH containing NH2-terminal hemagglutinin (HA)-tagged human LPA5 and were selected with puromycin, resulting in Caco-2bbe/NHE3/LPA5 (22). pCDH was used as a control.
Na+-dependent intracellular pH recovery.
The Na+-dependent changes in intracellular pH (pHi) by NHE3 were determined using the ratio-fluorometric, pH-sensitive dye 2′,7′-bis-(2-carboxyethyl)-5-carboxyfluorescein acetoxymethyl ester (BCECF-AM) as described previously (9). Briefly, cells were washed in Na+ buffer [130 mM NaCl, 20 mM HEPES, 5 mM KCl, 1 mM tetramethylammonium-PO4 (TMA-PO4), 2 mM CaCl2, 1 mM MgSO4, and 25 mM glucose] and then were dye loaded by incubation with 6.5 μm BCECF-AM in the same solution for 10 min. The coverslips were mounted on a perfusion chamber mounted on an inverted microscope and were superfused with NH4+ buffer (40 mM NH4Cl, 90 mM NaCl, 20 mM HEPES, 5 mM KCl, 1 mM TMA-PO4, 2 mM CaCl2, 1 mM MgSO4, and 25 mM glucose) for 5 min, followed by perfusion with TMA+ buffer (130 mM TMA-Cl, 20 mM HEPES, 5 mM KCl, 1 mM TMA-PO4, 2 mM CaCl2, 1 mM MgSO4, and 25 mM glucose) for 2 min, and subsequently with Na+ buffer. When necessary, Na+ buffer was supplemented with 50 μM HOE694 to inhibit NHE1 and NHE2 activities. The rate of Na+-dependent pH recovery was calculated by determining slopes along the early stage of pH recovery by linear least-squares analysis over a minimum of 9 s. Calibration of the fluorescence signal was performed using the K+/H+ ionophore nigericin as previously described (9). Comparisons of Na+/H+ exchange were made between measurements made on the same day. The microfluorometry was performed on an inverted fluorescence microscope, and photometric data were acquired using the Metafluor software (Molecular Devices, Sunnyvale, CA).
Immunoprecipitation and Western blot.
Immunoprecipitation was performed as previously described with a modification (10). Briefly, Caco-2/NHE3/LPA5 cells were washed twice in cold PBS, scraped, and lysed in 1× cell lysis buffer (Cell Signaling) containing protease inhibitors (Roche). The crude lysate was sonicated for 2 × 15 s and spun at 14,000 g for 15 min. Protein concentration was determined by the bicinchoninic acid assay (Sigma). Lysate (300 μg) was precleared by incubation with 30 μl of protein G-Sepharose beads for 1 h, and the supernatant was then incubated overnight with anti-VSVG antibody. Immunocomplex was purified by incubation with 50 μl of protein G-Sepharose beads for 1.5 h, followed by three washes in lysis buffer and two washes in PBS. All the above steps were performed at 4°C or on ice. The bound immunocomplex was eluted by incubating the protein A beads in Laemmli sample buffer for 10 min at 95°C and was then separated by SDS-PAGE. The proteins were then transferred to nitrocellulose membrane for Western immunoblotting as previously described (45). For in vivo phosphorylation of NHE3, anti-p-Ser antibody was used to determine NHE3 phosphorylation levels. The blot was stripped and blotted with anti-VSVG antibody to quantify the amount of immunoprecipitated NHE3. For preparation of the intestinal lysates, C57BL/6 mice were euthanized using isofluorane under approval by the Institutional Animal Care and Use Committee of Emory University and in accordance of the NIH Guide for the Care and Use of Laboratory Animals. The proximal small intestine was removed from the mouse, flushed with cold PBS, and cut open longitudinally to expose the epithelial layer. The epithelial layer was lightly scraped with a glass coverslip and lysed as described above.
Surface biotinylation.
Surface biotinylation of NHE3 was performed as previously described (10). Briefly, cells were rinsed twice in PBS and incubated in borate buffer (154 mM NaCl, 7.2 mM KCl, 1.8 mM CaCl2, and 10 mM H3BO3, pH 9.0) for 10 min. Cells were then incubated for 40 min with 0.5 mg/ml sulfo-NHS-LC-biotin (Pierce, Rockford, IL) in borate buffer. Unbound sulfo-NHS-LC-biotin was quenched with Tris buffer (20 mM Tris and 120 mM NaCl, pH 7.4). Cells were lysed in lysis buffer and sonicated for 2 × 15 s. Lysate was agitated for 30 min and spun at 14,000 g for 30 min at 4°C to remove the insoluble cell debris. An aliquot was retained as the total fraction representing the total cellular NHE3. Protein concentration was determined, and 1 mg of lysate was then incubated with streptavidin-agarose beads (Pierce) for 2 h. The streptavidin-agarose beads were washed three times in lysis buffer and twice in PBS. All the above procedures were performed at 4°C or on ice. Biotinylated surface proteins were then eluted by boiling the beads at 95°C for 10 min. Dilutions of the total and surface NHE3 were resolved by SDS-PAGE and immunoblotted with an anti-VSVG antibody. Densitometric analysis was performed using ImageJ software (National Institutes of Health).
Confocal immunofluorescence microscopy.
Caco-2bbe cells grown 7 days postconfluence on Transwells were washed twice with cold PBS, fixed in 4% paraformaldehyde in PBS for 10 min at room temperature, permeabilized in 0.2% Triton X-100 in PBS for 5 min, and blocked in PBS containing 5% normal goat serum for 30 min at room temperature. Cells were then stained with anti-VSVG or anti-p-RSK antibody for 1 h at room temperature. Following three washes, 10 min each, with PBS, the cells were incubated with Alexa 488-conjugated donkey anti-mouse IgG or Alexa 555-conjugated goat anti-rabbit IgG (Invitrogen, Carlsbad, CA) for 1 h at room temperature. After 3 × 10 min washes with PBS, the excised Transwells were mounted with ProLong Gold Antifade Reagent (Invitrogen) and observed under a Zeiss LSM510 laser confocal microscope (Zeiss Microimaging, Thornwood, NY) coupled to a Zeiss Axioplane2 with ×63 Pan-Apochromat oil lenses.
Statistical analysis.
Statistical analyses were performed by Student's t-test for paired comparison. Results were presented as means ± SE. A P value of <0.05 was considered significant.
RESULTS
RSK is activated by LPA.
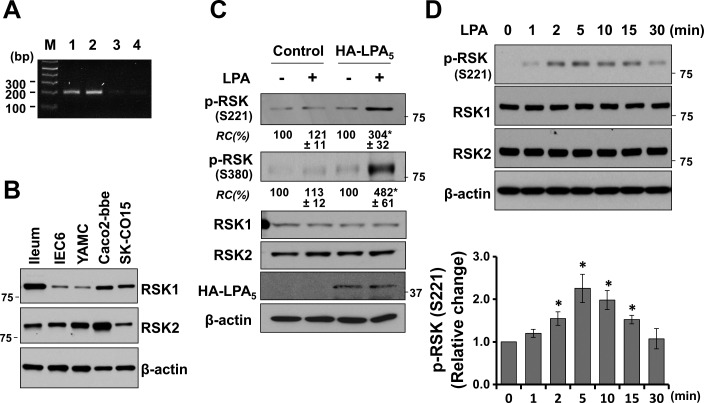
There are four RSK isoforms (1), and we initially assessed the expression levels of RSK isoforms in Caco-2bbe cells by RT-PCR. RSK1 and RSK2 mRNA was abundant compared with RSK3 or RSK4, suggesting that RSK1 and RSK2 are the major RSK isoforms in these cells (Fig. 1A). The presence of RSK1 and RSK2 was confirmed by Western blot (Fig. 1B), which showed the presence of RSK1 and RSK2 in mouse ileal epithelium and several other intestinal cell lines, although the relative expression levels of RSK1 and RSK2 vary. Henceforth, we assume that intestinal epithelial cells mainly express RSK1 and RSK2. We next sought to determine whether LPA regulates RSK by determining RSK phosphorylation. RSK is characteristically phosphorylated at multiple sites (1, 32). LPA increased phosphorylation of RSK at S221 (a.a. numbering is based on RSK1) and S380 in Caco-2bbe cells stably transfected with LPA5 (Fig. 1C). However, no change in RSK phosphorylation was observed in control transfected Caco-2bbe cells, consistent with the previous studies that LPA5 expression in Caco-2 cells is low (22, 45). The time course of the effect shows that LPA acutely phosphorylates RSK without altering RSK expression (Fig. 1D).
Fig. 1.
The p90 ribosomal S6 kinase (RSK) is phosphorylated by lysophosphatidic acid (LPA). A: RSK1-4 mRNA levels in Caco-2bbe cells were determined by RT-PCR. M, molecular markers. B: expression of RSK1 and RSK2 protein was determined in enterocytes isolated from mouse ileum, intestinal epithelial cell 6, young adult mouse colon, Caco-2bbe, and SK-CO15 cells. C: hemagglutinin (HA)-LPA5 or pCDH control plasmid transfected Caco2-bbe/Na+/H+ exchanger 3 (NHE3) cells were treated with 1 μM LPA (+) or PBS (-) for 5 min. Phosphorylation of RSK (p-RSK) and the expression levels of RSK1 and RSK2 were determined. Expression of HA-LPA5 was determined using anti-HA antibody. β-Actin was used as a loading control. RC, relative change normalized to the control condition (- LPA) for each cell line. D: Caco2-bbe/NHE3/HA-LPA5 cells were treated with LPA for the indicated time durations. Changes in RSK phosphorylation were normalized to the sum of RSK1 and RSK2 and quantified in the bar graph. All results are representatives of 3 independent experiments. *P < 0.05.
NHE3 regulation by LPA requires PDK1.
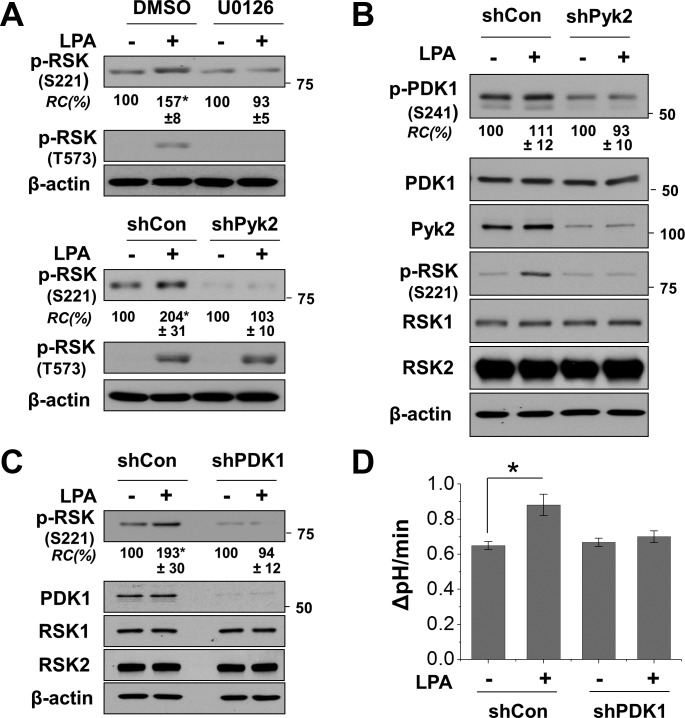
Full activation of RSK involves sequential phosphorylation of RSK by ERK and PDK1 (1). However, to our best knowledge there is no precedence where Pyk2 is involved in RSK phosphorylation. Hence, we assessed whether LPA-induced phosphorylation of RSK is dependent on Pyk2. The present model of RSK activation depicts that ERK phosphorylates RSK at T573 and S363, which is followed by autophosphorylation at S380. Phosphorylation at S380 creates a docking site for PDK1, which phosphorylates S221, leading to full activation of RSK (1, 32). Inhibition of MEK by U0126 ablated LPA-induced phosphorylation of RSK at S221 and at T573 (Fig. 2A). Similarly, knockdown of Pyk2 blocked phosphorylation at S221 but did not affect phosphorylation at T573, consistent with previous studies that show that phosphorylation of T573 is dependent on ERK. These results suggest that Pyk2 modulates phosphorylation of RSK and also confirm that Pyk2 and ERK differentially target RSK. Although the role of Pyk2 in RSK regulation is uncertain, it has been shown that Pyk2 mediates activation of PDK1 by angiotensin II (40). Hence, we examined whether LPA activates PDK1 in Caco-2bbe cells. Figure 2B shows that LPA slightly increased phosphorylation of PDK1 at S241, which is the site of autophosphorylation within the catalytic domain. The small effect was not surprising because PDK1 is often thought to be constitutively active (25). On the contrary, knockdown of Pyk2 markedly decreased the phosphorylation level of PDK1 under basal and LPA-treated conditions without altering PDK1 expression. These data suggest that Pyk2 is necessary for the maintenance of PDK1 autophosphorylation at S241.
Fig. 2.
NHE3 activation by LPA requires phosphoinositide-dependent kinase 1 (PDK1). A: Caco2-bbe/NHE3/HA-LPA5 cells were pretreated with U0126 or DMSO (top) or were transfected with small hairpin (sh) proline-rich tyrosine kinase 2 (Pyk2) or control shRNA (shCon) (bottom). Cells were treated with LPA (+) or PBS (-) for 5 min, and phosphorylation of RSK at S221 or T573 was determined. Representative Western blots of 3 independent experiments are shown. *P < 0.01 compared with the control. B: effect of Pyk2 knockdown on PDK1 phosphorylation was determined by using shPyk2 or control shCon. Cells were treated with LPA (+) or PBS (-) for 5 min to determine p-PDK1 and PDK1 expression. C: effect of PDK1 knockdown on RSK phosphorylation by LPA was determined. Middle: efficacy of PDK1 knockdown. D: effect of PDK1 knockdown on NHE3 activity in Caco2-bbe/NHE3/HA-LPA5 cells was determined. NHE3 activity was determined fluorometrically by measuring Na+-dependent intracellular pH (pHi) recovery as described in materials and methods. The activity is expressed as the rate of change in pHi. Results are presented as means ± SE; n ≥ 6. *P < 0.01 compared with shCon.
To ascertain that PDK1 is involved in LPA-mediated regulation of NHE3, we assessed RSK phosphorylation and NHE3 activity in cells with PDK1 knockdown. In line with the finding that Pyk2 regulates PDK1, silencing of PDK1 abated LPA-induced phosphorylation of RSK (Fig. 2C). In addition, LPA-mediated stimulation of NHE3 activity was blocked by PDK1 knockdown (Fig. 2D). These results indicate that Pyk2-PDK1 and ERK cooperatively regulate RSK, which in turn stimulates NHE3 activation.
RSK2 interacts with NHE3.
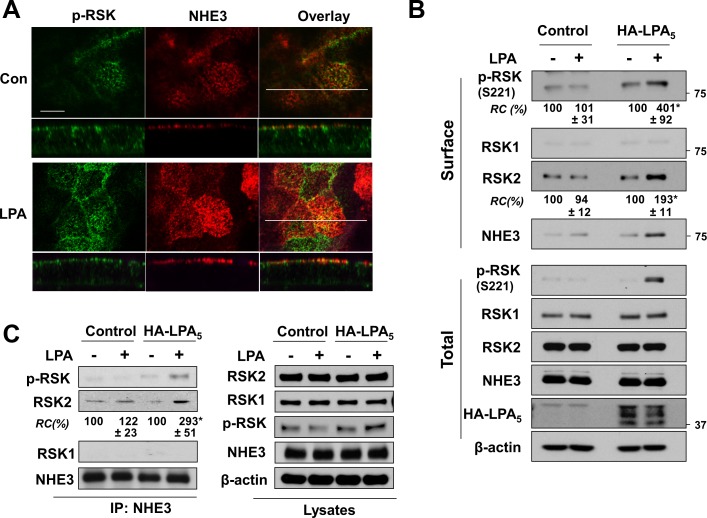
Although our results suggest that RSK is the converging point of the RhoA-Pky2-PDK1 and MEK-ERK pathways, how RSK regulates NHE3 remains to be determined. NHE3 regulation is mostly mediated by trafficking of NHE3 proteins between the brush-border membrane and intracellular pools in intestinal cells although not in kidney in vivo (24). As we have reported previously (22, 45), LPA increased NHE3 abundance in the surface membrane as evidenced by the increased NHE3 fluorescence level at the apical membrane of Caco-2bbe cells (Fig. 3A). RSK is normally present in the cytoplasm of quiescent cells, but upon activation p-RSK translocates to the plasma membrane (30). Consistently, we observed an increased level of p-RSK in the apical membrane, where it colocalized with NHE3. These results were corroborated by surface biotinylation, which depicted increased surface abundance of NHE3 and p-RSK in Caco-2bbe/NHE3 cells only when LPA5 was coexpressed (Fig. 3B). Interestingly, RSK2 was increased in the surface fraction. On the contrary, the abundance of RSK1 at the cell surface was low, and no change in RSK1 was observed in response to LPA. To further ascertain the possibility that RSK directly targets NHE3, we performed coimmunoprecipitation of NHE3 and RSK from cell lysates. Figure 3C shows that an increased amount of p-RSK2 coimmunoprecipitated with NHE3 in response to LPA. Moreover, we found RSK2 coimmunoprecipitated with NHE3 was significantly increased in Caco-2bbe/NHE3/LPA5 cells treated with LPA. On the contrary, RSK1 presence was not observed under all conditions tested. These results imply that LPA induces interaction of RSK2 with NHE3.
Fig. 3.
LPA enhances the interaction of NHE3 with RSK. A: cellular expression of NHE3 (red) and p-RSK (green) in Caco2-bbe/NHE3/HA-LPA5 cells was determined by confocal immunofluorescence microscopy. Cells grown on Transwells were treated with LPA or PBS for 5 min and labeled with anti-vesicular stomatitis virus glycoprotein (VSVG) and anti-p-RSK(S221) antibodies. Top: horizontal views at the cell surface. Bottom: cross section (x–z) perpendicular to the confocal focal plane. Scale bar = 10 μm. B: surface biotinylation was performed to determine expression of NHE3 and RSK at the apical membrane as described in materials and methods. RSK and NHE3 expression in the surface membrane (top) and total lysate (bottom) were determined by immunoblotting and quantified relative to control conditions (- LPA). C: interaction between NHE3 and RSK was determined by coimmunoprecipitation. NHE3 was immunoprecipitated using anti-VSVG antibody, followed by Western blotting to determine the presence of RSK1 and RSK2 in the immunoprecipitates. 300 μg of cell lysates was used for immunoprecipitation. Right: relative expression levels of RSK1 and RSK2 in the cell lysate; n = 3. *P < 0.05.
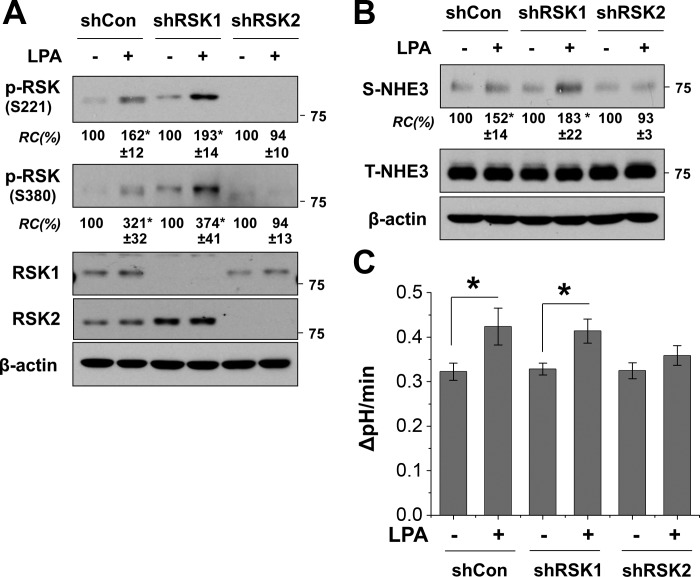
Antibodies to p-RSK cannot distinguish p-RSK1 from p-RSK2. To determine whether LPA activates RSK1, RSK2, or both, we knocked down RSK1 or RSK2 before LPA treatment. Silencing of RSK1 did not appear to have a significant effect on LPA-induced phosphorylation at S221 or S380 (Fig. 4A). On the contrary, RSK2 knockdown lessened the RSK phosphorylation level under basal conditions and importantly ablated LPA-induced change. These results suggest that RSK2, but not RSK1, is activated by the LPA-LPA5 signaling cascade. To ascertain the role of RSK2 in regulation of NHE3, we compared the effect of RSK1 or RSK2 depletion on NHE3 surface expression and activity. Silencing of RSK1 did not alter LPA-induced changes in NHE3 surface expression or Na+/H+ transport activity (Fig. 4, B and C). RSK2 depletion, however, completely blocked the changes by LPA, further demonstrating the critical role of RSK2 in the regulation of NHE3 by LPA.
Fig. 4.
Activation of NHE3 by LPA is RSK2 dependent. A: Caco2-bbe/NHE3/HA-LPA5 cells transfected with shCon, shRSK1, or shRSK2 were treated with LPA to determine RSK phosphorylation at S221 and S380. RSK phosphorylation levels were quantified relative to the control condition (-) for each cell line. Bottom: knockdown efficiency of RSK1 and RSK2. B: surface expression of NHE3 in cells with knockdown of RSK1 or RSK2 was determined as described in materials and methods. The amounts of surface NHE3 were quantified relative to control conditions. C: effect of RSK knockdown on NHE3 activity was determined. NHE3 activities are expressed as the rate of Na+-dependent intracellular pH recovery. Results are presented as means ± SE; n ≥ 6. *P < 0.05.
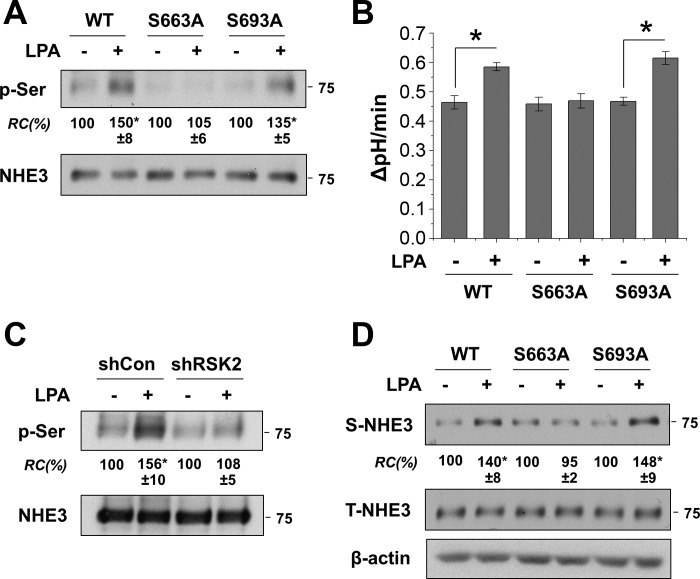
LPA increases NHE3 phosphorylation at S663 through RSK2.
The minimum consensus phosphorylation target sequence of RSK is R/K-X-R-X-X-pS/T, which is also shared by other kinases of the AGC (PKA, PKG, and PKC) family (1, 28). NHE3 contains the R/K-X-R-X-X-pS/T motif at S663 (RKRLGS) and S693 (KRRNSS). To determine whether NHE3 is phosphorylated by LPA-mediated signaling, we immunoprecipitated NHE3 in Caco-2bbe/NHE3/HA-LPA5 cells treated or untreated with LPA, followed by immunoblotting using anti-p-Ser antibody. Figure 5A demonstrates that LPA increased NHE3 phosphorylation in vivo. On the other hand, LPA did not alter phosphorylation of NHE3-S663A, whereas S693A mutation did not affect LPA-mediated phosphorylation of NHE3. Consistent with the phosphorylation pattern, S663A, but not S693A, ablated LPA-induced NHE3 activation (Fig. 5B). We showed above that LPA specifically increases RSK2 phosphorylation. To determine that RSK2 is responsible for NHE3 phosphorylation, NHE3 phosphorylation was determined in cells with RSK2 knockdown. Unlike control cells, LPA-induced phosphorylation of NHE3 was abrogated by knockdown of RSK2 (Fig. 5C). These results indicate that the LPA-induced phosphorylation of NHE3 is mediated by RSK2.
Fig. 5.
Phosphorylation at S663 is necessary for the activation of NHE3 by LPA. A: NHE3 and NHE3 mutants were immunoprecipitated from cells treated with or without LPA for 5 min. Immunocomplex was resolved by SDS-PAGE, and immunoblotting was performed using anti-p-Ser antibody to determine phosphorylation levels of NHE3 (p-Ser). Bottom: amount of immunoprecipitated NHE3. RC, p-Ser normalized to NHE3; WT, wild-type. *P < 0.05 compared with PBS-treated control. Western blots are representative of 3 independent experiments. B: NHE3 activity was determined in cells expressing NHE3, NHE3/S663A, or NHE3/S693A. NHE3 activities are expressed as the rate of Na+-dependent pHi recovery; n ≥ 6. *P < 0.01. C: NHE3 phosphorylation was determined in cells transfected with shCon or shRSK2. D: surface expression of NHE3 variants was determined by surface biotinylation. *P < 0.05 compared with PBS-treated control.
It has been anticipated that NHE3 is phosphorylated by a number of protein kinases, but it remains to be determined how phosphorylation of NHE3 protein regulates NHE3 activity. In this context, we questioned whether phosphorylation of NHE3 at S663 by RSK2 is required for trafficking of NHE3 to the plasma membrane. To address this problem, we performed surface biotinylation of NHE3/S663A and NHE3/S693A. Figure 5D shows that LPA increased wild-type and S693A NHE3 abundance in the plasma membrane. However, no significant change was observed with NHE3/S663A. These results suggest that phosphorylation at S663 is critical for apical trafficking of NHE3 by LPA.
DISCUSSION
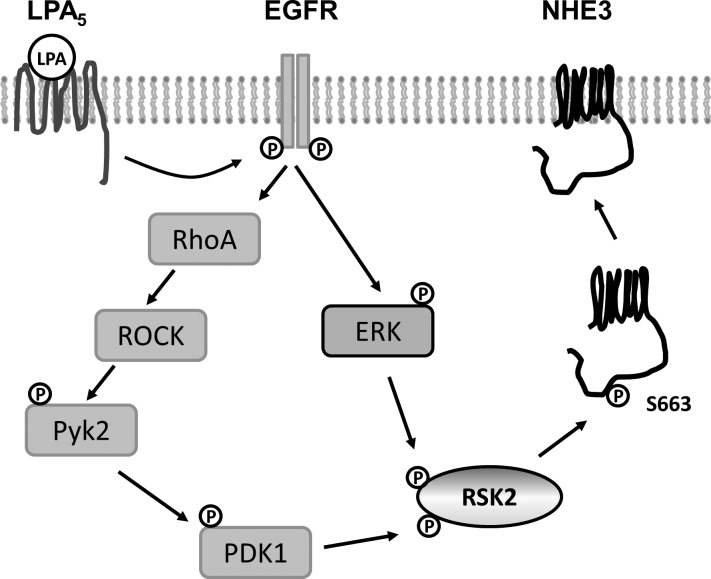
Recent studies have shown that LPA inhibits secretory diarrhea by inhibition of CFTR and stimulation of NHE3 in the intestine (18, 22, 37). Stimulation of NHE3 by LPA is dependent on the LPA5 receptor that is highly expressed in the intestinal tract (16, 22). NHE3 regulation is dependent on both Pyk2 and ERK, but how these two kinases ultimately regulate NHE3 is unclear. The study reported here identifies RSK2 as the signaling node where Pyk2 and ERK intersect to regulate NHE3. We also show that RSK2 phosphorylates NHE3 and that the phosphorylation of NHE3 by RSK2 is essential for apical trafficking of NHE3 (Fig. 6).
Fig. 6.
Activation of NHE3 by LPA is mediated by RSK2. This schematic model depicts how LPA regulates NHE3 in Caco-2bbe cells. Transactivation of EGF receptor (EGFR) by LPA5 activates RhoA- RhoA-Rho associated kinase (ROCK)-Pyk2 and ERK (45). The present study shows that Pyk2 and ERK phosphorylate RSK2, which in turn phosphorylates NHE3 at S663. This phosphorylation of NHE3 by RSK2 appears to be necessary for trafficking NHE3 to the apical membrane.
Previous studies have shown that NHE3 is regulated by various second messengers that turn on protein kinases, such as PKA (12), PKC (11), PKG (4), calmodulin-dependent kinase II (49), and serum/glucocorticoid-inducible kinase (SGK) (46). However, NHE3 regulation by these kinases is not known to be dependent on ERK, and, hence, these are unlikely targets of the LPA-LPA5-EGFR cascade. Our expectation that RSK may be the converging effector of Pyk2 and ERK was based on the fact that RSK is a known effector of ERK. RSK is activated by sequential phosphorylation by ERK and PDK1 (1, 32). PDK1 is a pivotal regulator involved in the activation of multiple members of the AGC superfamily of Ser/Thr protein kinases, including Akt, PKC, SGK, and RSK (26). Our previous study showed that PDK1 is involved in stimulation of NHE3 by SGK1 (9). In addition, the PDK1 hypomorphic mice display a marked decrease in intestinal NHE3 activity without changing NHE3 expression, demonstrating that PDK1 is a pivotal kinase for NHE3 regulation (33). Although a role of Pyk2 in activation of RSK has not been reported, a previous study by Taniyama et al. (40) has shown that Pyk2 acts as a scaffold for phosphorylation of PDK1 by Src. Similarly, IGF-1 forms a complex consisting of SHP2, Pyk2, Src, and Grb2 that recruits and phosphorylates PDK1 (35). Although PDK1 is thought to be constitutively active with autophosphorylation at S241 located in the catalytic domain (3), previous studies have shown that PDK1 activity can be regulated by phosphorylation at sites other than S241 (3, 27). For instance, angiotensin II stimulates phosphorylation of PDK1 at Tyr-373/376 in vascular smooth muscle cells (40). In addition, insulin can further increase phosphorylation levels at S244 in mouse hypothalamic neuronal cells while phosphorylating PDK1 at other sites, suggesting that PDK1 can be regulated in certain cell types (31). The effect of LPA on PDK1 phosphorylation at S241 was not robust, but knockdown of Pyk2 markedly decreased autophosphorylation of PDK1 at S241. Our results indicate that Pyk2 plays a critical role in maintenance of PDK1 autophosphorylation and demonstrate that PDK1 is necessary for the regulation of NHE3 by LPA. However, whether Pyk2 phosphorylates PDK1 at Tyr and the mechanistic basis for PDK1 regulation by Pky2 are not known.
RSK1 and RSK2 are closely related with 78% identity, and the isoform selectivity of RSKs is incompletely understood (1, 32). RSK1 knockout mice appear viable although no phenotype has been reported (8). On the other hand, human mutation in RSK2 is linked to Coffin-Lowry syndrome, an X-lined mental retardation, and RSK2-deficient mice exhibit impaired learning and coordination (29). Other studies have supported the specificity of RSK1 and RSK2. RSK1 but not RSK2 induces neurite outgrowth, whereas RSK2 uniquely induces IL-2 and IL-15 in T lymphocytes (19, 36). These studies demonstrate that RSK1 and RSK2 do not have redundant functions. In the present study, we focused on RSK1 and RSK2 based on their relative abundant mRNA expression. Both RSK1 and RSK2 proteins were present in mouse enterocytes and several intestinal epithelial cell lines. Because the efficacy of anti-RSK1 and anti-RSK2 antibody is expected to differ, it is not possible to directly compare the abundance of these two RSK isoforms. Nonetheless, several lines of evidence show that RSK2, but not RSK1, is involved in NHE3 regulation. First, RSK2 interacts with NHE3 in response to LPA. Second, LPA induced translocation of RSK2 to the brush-border membrane of Caco-2bbe cells where NHE3 is located, and lastly knockdown of RSK2, but not RSK1, ablated stimulation of NHE3 by LPA. A question remains whether RSK3 or RSK4 may take part in NHE3 regulation. However, knockdown of RSK2 abolished the effects of LPA on NHE3 phosphorylation, trafficking, and activity. Hence, the possibility of RSK3 or RSK4 altering NHE3 is low.
The classical paradigm of acute regulation of NHE3 models around reversible phosphorylation of NHE3. Previous studies have shown that phosphorylation of NHE3 at S552 and S605 is essential for the inhibition of NHE3 by PKA (14, 48). Additionally, phosphorylation at S719 by casein kinase 2 is important for the basal NHE3 activity (34). On the other hand, phorbol ester inhibits NHE3 in PS120 fibroblasts without a change in NHE3 phosphorylation although this finding has been disputed in AP-1 cells (43, 44). Our results demonstrate phosphorylation of NHE3 at S663, which is critical for the stimulation of NHE3 activity. The S663 residue was previously shown to be essential for acute stimulation of NHE3 by glucocorticoids and SGK (9, 42). Hence, S663 emerges as an important phosphorylation site associated with elevated NHE3 activity. However, how phosphorylation alters NHE3 is not yet clear. A recent study by Kocinsky et al. (13) has shown that increased phosphorylation of NHE3 at S552 and S605 was not accompanied by an immediate decrease in NHE3 activity, but rather the phosphorylation of NHE3 preceded functional inhibition. Although the mechanism by which phosphorylation contributes to NHE3 regulation remains unclear, it is generally accepted that phosphorylation of NHE3 is a functionally important step. In the present study, we found that the S663A mutation was sufficient to block trafficking of NHE3 to the apical membrane. Therefore, the primary role of the posttranslational modification appears to be the translocation of NHE3 to the cell surface rather than NHE3 protein turnover. However, it is not apparent how phosphorylation at S663 modulates apical trafficking of NHE3 by LPA. In the case of NHE3 regulation by PKA or SGK1, phosphorylation of NHE3 is reported to require the presence of Na+/H+ exchanger regulator factor 1 (NHERF1) or NHERF2, which is linked to the actin cytoskeleton via ezrin (9, 15). In this context, it is noteworthy that RSK contains a PDZ binding motif in the COOH terminus, and the interaction of RSK2 with the PDZ domain proteins of the Shank family has been shown to modulate the synaptic transmission by amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor (41). We have shown previously that LPA-dependent regulation is dependent on NHERF2, such that NHERF2 interaction is important for the optimal signaling by LPA5 (22). Because NHERF2 also interacts with NHE3 (47), NHERF2 may have a dual function in direct regulation of NHE3. However, NHERF2 alone is insufficient to address the specificity of RSK2, and it is likely that phosphorylation of NHE3 by RSK2 may enhance its interaction with other protein, such as ezrin, that can link NHE3 to the apical membrane (5). Additional studies are needed to determine whether phosphorylation at S663 affects NHE3 interaction with NHERF2 or the cytoskeletal network.
In summary, this study identifies RSK2 as a new protein kinase that regulates NHE3. This study also shows the role of Pyk2 in stimulation of RSK2 through its effect on PDK1. Stimulation of NHE3 by LPA requires phosphorylation of NHE3 at S663, which is important for trafficking of NHE3 to the apical membrane.
GRANTS
This work was supported by National Institutes of Health grants DK061418 (C. Yun) and T32DK007771 (B. Yoo) and American Heart Association grant 13SDG1623001 (P. He).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.R.N., P.H., and B.K.Y. performed experiments; Y.R.N., P.H., and C.C.Y. interpreted results of experiments; Y.R.N. and P.H. prepared figures; Y.R.N. and P.H. edited and revised manuscript; C.C.Y. conception and design of research; C.C.Y. analyzed data; C.C.Y. drafted manuscript; C.C.Y. approved final version of manuscript.
Footnotes
This article is the topic of an Editorial Focus by Jada C. Domingue and Mrinalini C. Rao (7a).
REFERENCES
- 1.Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol 9: 747–758, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Aoki J, Inoue A, Okudaira S. Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781: 513–518, 2008. [DOI] [PubMed] [Google Scholar]
- 3.Casamayor A, Morrice NA, Alessi DR. Phosphorylation of Ser-241 is essential for the activity of 3-phosphoinositide-dependent protein kinase-1: identification of five sites of phosphorylation in vivo. Biochem J 342: 287–292, 1999. [PMC free article] [PubMed] [Google Scholar]
- 4.Cha B, Kim JH, Hut H, Hogema BM, Nadarja J, Zizak M, Cavet M, Lee-Kwon W, Lohmann SM, Smolenski A, Tse CM, Yun C, de Jonge HR, Donowitz M. cGMP inhibition of Na+/H+ antiporter 3 (NHE3) requires PDZ domain adapter NHERF2, a broad specificity protein kinase G-anchoring protein. J Biol Chem 280: 16642–16650, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Cha B, Tse M, Yun C, Kovbasnjuk O, Mohan S, Hubbard A, Arpin M, Donowitz M. The NHE3 juxtamembrane cytoplasmic domain directly binds ezrin: dual role in NHE3 trafficking and mobility in the brush border. Mol Biol Cell 17: 2661–2673, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi JW, Herr DR, Noguchi K, Yung YC, Lee CW, Mutoh T, Lin ME, Teo ST, Park KE, Mosley AN, Chun J. LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50: 157–186, 2010. [DOI] [PubMed] [Google Scholar]
- 7.Deng W, ES, Tsukahara R, Valentine WJ, Durgam G, Gududuru V, Balazs L, Manickam V, Arsura M, Vanmiddlesworth L, Johnson LR, Parrill AL, Miller DD, Tigyi G. The lysophosphatidic acid type 2 receptor is required for protection against radiation-induced intestinal injury. Gastroenterology 132: 1834–1851, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7a.Domingue JC, Rao MC. Pyk and ERK your way to the hub by taking a RSK 2. Focus on “Regulation of NHE3 by lysophosphatidic acid is mediated by phosphorylation of NHE3 by RSK2.” Am J Physiol Cell Physiol (May 13, 2015). doi: 10.1152/ajpcell.00128.2015. [DOI] [PubMed] [Google Scholar]
- 8.Dumont J, Umbhauer M, Rassinier P, Hanauer A, Verlhac MH. p90Rsk is not involved in cytostatic factor arrest in mouse oocytes. J Cell Biol 169: 227–231, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He P, Lee SJ, Lin S, Seidler U, Lang F, Fejes-Toth G, Naray-Fejes-Toth A, Yun CC. Serum- and glucocorticoid-induced kinase 3 in recycling endosomes mediates acute activation of Na+/H+ exchanger NHE3 by glucocorticoids. Mol Biol Cell 22: 3812–3825, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He P, Zhang H, Yun CC. IRBIT, inositol 1,4,5-triphosphate (IP3) receptor-binding protein released with IP3, binds Na+/H+ exchanger NHE3 and activates NHE3 activity in response to calcium. J Biol Chem 283: 33544–33553, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janecki AJ, Montrose MH, Tse CM, de Medina FS, Zweibaum A, Donowitz M. Development of an endogenous epithelial Na+/H+ exchanger (NHE3) in three clones of Caco-2 cells. Am J Physiol Gastrointest Liver Physiol 277: G292–G305, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Kandasamy RA, Yu FH, Harris R, Boucher A, Hanrahan JW, Orlowski J. Plasma membrane Na+/H+ exchanger isoforms (NHE-1, -2, and -3) are differentially responsive to second messenger agonists of the protein kinase A and C pathways. J Biol Chem 270: 29209–29216, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Kocinsky HS, Dynia DW, Wang T, Aronson PS. NHE3 phosphorylation at serines 552 and 605 does not directly affect NHE3 activity. Am J Physiol Renal Physiol 293: F212–F218, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Kurashima K, Yu FH, Cabado AG, Szabo EZ, Grinstein S, Orlowski J. Identification of sites required for down-regulation of Na/H exchanger NHE3 activity by cAMP-dependent protein kinase. J Biol Chem 272: 28672–28679, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Lamprecht G, Weinman EJ, Yun CC. The role of NHERF and E3KARP in the cAMP-mediated inhibition of NHE3. J Biol Chem 273: 29972–29978, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Lee CW, Rivera R, Gardell S, Dubin AE, Chun J. GPR92 as a new G12/13- and Gq-coupled lysophosphatidic acid receptor that increases cAMP, LPA5. J Biol Chem 281: 23589–23597, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Lee SJ, Leoni G, Neumann PA, Chun J, Nusrat A, Yun CC. Distinct phospholipase C-β isozymes mediate lysophosphatidic acid receptor 1 effects on intestinal epithelial homeostasis and wound closure. Mol Cell Biol 33: 2016–2028, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li C, Dandridge KS, Di A, Marrs KL, Harris EL, Roy K, Jackson JS, Makarova NV, Fujiwara Y, Farrar PL, Nelson DJ, Tigyi GJ, Naren AP. Lysophosphatidic acid inhibits cholera toxin-induced secretory diarrhea through CFTR-dependent protein interactions. J Exp Med 202: 975–986, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin JX, Spolski R, Leonard WJ. Critical role for Rsk2 in T-lymphocyte activation. Blood 111: 525–533, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin S, Lee SJ, Shim H, Chun J, Yun CC. The absence of LPA receptor 2 reduces the tumorigenesis by ApcMin mutation in the intestine. Am J Physiol Gastrointest Liver Physiol 299: G1128–G1138, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin S, Wang D, Iyer S, Ghaleb AM, Shim H, Yang VW, Chun J, Yun CC. The absence of LPA2 attenuates tumor formation in an experimental model of colitis-associated cancer. Gastroenterology 136: 1711–1720, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin S, Yeruva S, He P, Singh AK, Zhang H, Chen M, Lamprecht G, de Jonge HR, Tse M, Donowitz M, Hogema BM, Chun J, Seidler U, Yun CC. Lysophosphatidic acid stimulates the intestinal brush border Na+/H+ exchanger 3 and fluid absorption via LPA5 and NHERF2. Gastroenterology 138: 649–658, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo J, Kintner DB, Shull GE, Sun D. ERK1/2-p90RSK-mediated phosphorylation of Na+/H+ exchanger isoform 1. J Biol Chem 282: 28274–28284, 2007. [DOI] [PubMed] [Google Scholar]
- 24.McDonough AA, Biemesderfer D. Does membrane trafficking play a role in regulating the sodium/hydrogen exchanger isoform 3 in the proximal tubule? Curr Opin Nephrol Hypertens 12: 533–541, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol 15: 161–170, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Newton AC. Regulation of the ABC kinases by phosphorylation: protein kinase C as a paradigm. Biochem J 370: 361–371, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park J, Hill MM, Hess D, Brazil DP, Hofsteenge J, Hemmings BA. Identification of tyrosine phosphorylation sites on 3-phosphoinositide-dependent protein kinase-1 and their role in regulating kinase activity. J Biol Chem 276: 37459–37471, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 11: 9–22, 2010. [DOI] [PubMed] [Google Scholar]
- 29.Poirier R, Jacquot S, Vaillend C, Soutthiphong AA, Libbey M, Davis S, Laroche S, Hanauer A, Welzl H, Lipp HP, Wolfer DP. Deletion of the Coffin-Lowry syndrome gene Rsk2 in mice is associated with impaired spatial learning and reduced control of exploratory behavior. Behav Genet 37: 31–50, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Richards SA, Dreisbach VC, Murphy LO, Blenis J. Characterization of regulatory events associated with membrane targeting of p90 ribosomal S6 kinase 1. Mol Cell Biol 21: 7470–7480, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Riojas RA, Kikani CK, Wang C, Mao X, Zhou L, Langlais PR, Hu D, Roberts JL, Dong LQ, Liu F. Fine tuning PDK1 activity by phosphorylation at Ser163. J Biol Chem 281: 21588–21593, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Romeo Y, Zhang X, Roux PP. Regulation and function of the RSK family of protein kinases. Biochem J 441: 553–569, 2012. [DOI] [PubMed] [Google Scholar]
- 33.Sandu C, Artunc F, Palmada M, Rexhepaj R, Grahammer F, Hussain A, Yun C, Alessi DR, Lang F. Impaired intestinal NHE3 activity in the PDK1 hypomorphic mouse. Am J Physiol Gastrointest Liver Physiol 291: G868–G876, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Sarker R, Gronborg M, Cha B, Mohan S, Chen Y, Pandey A, Litchfield D, Donowitz M, Li X. Casein kinase 2 binds to the C terminus of Na+/H+ exchanger 3 (NHE3) and stimulates NHE3 basal activity by phosphorylating a separate site in NHE3. Mol Biol Cell 19: 3859–3870, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen X, Xi G, Radhakrishnan Y, Clemmons DR. PDK1 recruitment to the SHPS-1 signaling complex enhances insulin-like growth factor-I-stimulated AKT activation and vascular smooth muscle cell survival. J Biol Chem 285: 29416–29424, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Silverman E, Frodin M, Gammeltoft S, Maller JL. Activation of p90 Rsk1 is sufficient for differentiation of PC12 cells. Mol Cell Biol 24: 10573–10583, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh AK, Riederer B, Krabbenhoft A, Rausch B, Bonhagen J, Lehmann U, de Jonge HR, Donowitz M, Yun C, Weinman EJ, Kocher O, Hogema BM, Seidler U. Differential roles of NHERF1, NHERF2, and PDZK1 in regulating CFTR-mediated intestinal anion secretion in mice. J Clin Invest 119: 540–550, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi E, Abe J, Gallis B, Aebersold R, Spring DJ, Krebs EG, Berk BC. p90RSK is a serum-stimulated Na+/H+ exchanger isoform-1 kinase: regulatory phosphorylation of serine 703 of Na+/H+ exchanger isoform-1. J Biol Chem 274: 20206–20214, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka T, Kassai A, Ohmoto M, Morito K, Kashiwada Y, Takaishi Y, Urikura M, Morishige J, Satouchi K, Tokumura A. Quantification of phosphatidic acid in foodstuffs using a thin-layer-chromatography-imaging technique. J Agric Food Chem 60: 4156–4161, 2012. [DOI] [PubMed] [Google Scholar]
- 40.Taniyama Y, Weber DS, Rocic P, Hilenski L, Akers ML, Park J, Hemmings BA, Alexander RW, Griendling KK. Pyk2- and Src-dependent tyrosine phosphorylation of PDK1 regulates focal adhesions. Mol Cell Biol 23: 8019–8029, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas GM, Rumbaugh GR, Harrar DB, Huganir RL. Ribosomal S6 kinase 2 interacts with and phosphorylates PDZ domain-containing proteins and regulates AMPA receptor transmission. Proc Natl Acad Sci USA 102: 15006–15011, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang D, Sun H, Lang F, Yun CC. Activation of NHE3 by dexamethasone requires phosphorylation of NHE3 at Ser663 by SGK1. Am J Physiol Cell Physiol 289: C802–C810, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiederkehr MR, Zhao H, Moe OW. Acute regulation of Na/H exchanger NHE3 activity by protein kinase C: role of NHE3 phosphorylation. Am J Physiol Cell Physiol 276: C1205–C1217, 1999. [DOI] [PubMed] [Google Scholar]
- 44.Yip JW, Ko WH, Viberti G, Huganir RL, Donowitz M, Tse CM. Regulation of the epithelial brush border Na+/H+ exchanger isoform 3 stably expressed in fibroblasts by fibroblast growth factor and phorbol esters is not through changes in phosphorylation of the exchanger. J Biol Chem 272: 18473–18480, 1997. [DOI] [PubMed] [Google Scholar]
- 45.Yoo BK, He P, Lee SJ, Yun CC. Lysophosphatidic acid 5 receptor induces activation of Na+/H+ exchanger 3 via apical epidermal growth factor receptor in intestinal epithelial cells. Am J Physiol Cell Physiol 301: C1008–C1016, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yun CC, Chen Y, Lang F. Glucocorticoid activation of Na(+)/H(+) exchanger isoform 3 revisited. The roles of SGK1 and NHERF2. J Biol Chem 277: 7676–7683, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Yun CHC, Lamprecht G, Forster DV, Sidor A. NHE3 kinase A regulatory protein E3KARP binds the epithelial brush border Na+/H+ exchanger NHE3 and the cytoskeletal protein ezrin. J Biol Chem 273: 25856–25863, 1998. [DOI] [PubMed] [Google Scholar]
- 48.Zhao H, Wiederkehr MR, Fan L, Collazo RL, Crowder LA, Moe OW. Acute inhibition of Na/H exchanger NHE-3 by cAMP. Role of protein kinase a and NHE-3 phosphoserines 552 and 605. J Biol Chem 274: 3978–3987, 1999. [DOI] [PubMed] [Google Scholar]
- 49.Zizak M, Chen T, Bartonicek D, Sarker R, Zachos NC, Cha B, Kovbasnjuk O, Korac J, Mohan S, Cole R, Chen Y, Tse CM, Donowitz M. Calmodulin kinase II constitutively binds, phosphorylates, and inhibits brush border Na+/H+ exchanger 3 (NHE3) by a NHERF2 protein-dependent process. J Biol Chem 287: 13442–13456, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]