Abstract
The role of the actin cytoskeleton in endothelial barrier function has been debated for nearly four decades. Our previous investigation revealed spontaneous local lamellipodia in confluent endothelial monolayers that appear to increase overlap at intercellular junctions. We tested the hypothesis that the barrier-disrupting agent histamine would reduce local lamellipodia protrusions and investigated the potential involvement of p38 mitogen-activated protein (MAP) kinase activation and actin stress fiber formation. Confluent monolayers of human umbilical vein endothelial cells (HUVEC) expressing green fluorescent protein-actin were studied using time-lapse fluorescence microscopy. The protrusion and withdrawal characteristics of local lamellipodia were assessed before and after addition of histamine. Changes in barrier function were determined using electrical cell-substrate impedance sensing. Histamine initially decreased barrier function, lamellipodia protrusion frequency, and lamellipodia protrusion distance. A longer time for lamellipodia withdrawal and reduced withdrawal distance and velocity accompanied barrier recovery. After barrier recovery, a significant number of cortical fibers migrated centrally, eventually resembling actin stress fibers. The p38 MAP kinase inhibitor SB203580 attenuated the histamine-induced decreases in barrier function and lamellipodia protrusion frequency. SB203580 also inhibited the histamine-induced decreases in withdrawal distance and velocity, and the subsequent actin fiber migration. These data suggest that histamine can reduce local lamellipodia protrusion activity through activation of p38 MAP kinase. The findings also suggest that local lamellipodia have a role in maintaining endothelial barrier integrity. Furthermore, we provide evidence that actin stress fiber formation may be a reaction to, rather than a cause of, reduced endothelial barrier integrity.
Keywords: endothelial permeability, actin cables, inflammation, lamellipodia, p38 MAPK
the endothelium plays a critical role in cardiovascular function, containing a relatively high-pressure closed-loop circulation while also permitting diffusive exchange between the blood and tissues through the capillaries and postcapillary venules. The mechanisms that control the permeability of microvessels, which permit significant leakage of plasma proteins during inflammation, have been debated for nearly a century (15). Evidence collected over the past few decades has highlighted the active role of endothelial cells in response to inflammatory stimuli, including remodeling of the actin cytoskeleton (26, 42, 45). One of the current prevailing theories from this line of investigation has been that endothelial cells adopt a contractile state during inflammation, which favors opening of junctions between cells, permitting increased paracellular flux of fluids and solutes (32, 33).
The contractile theory is supported by evidence that certain agents that elevate permeability also cause the development of centripetal tension generated by the actin cytoskeleton, which can place stress on the junctions and limit their strength (26, 29). Several inflammatory mediators also promote development of actin stress fibers in endothelial cells (7, 32). Actin-mediated contraction in endothelial cells is promoted by phosphorylation of myosin regulatory light chains (MLC) on Thr-18/Ser-19, which is determined by the activities of MLC kinase (MLCK) and MLC phosphatase (MLCP). Inhibition of MLCK attenuates hyperpermeability caused by activated neutrophils (46), histamine (34), and ethanol (21), and shortens the time course of the thrombin-induced barrier dysfunction (29) in cultured endothelial cell monolayers. Inhibition of Rho kinase (ROCK), an upstream regulator of MLCP (7, 32), also attenuates hyperpermeability caused by activated neutrophils (11), thrombin (13, 38, 43), histamine (43), and vascular endothelial growth factor (35) in endothelial cell monolayer models. Inhibition of MLCK or ROCK also decreases actin stress fiber formation, typically observed in fixed cells by labeling F-actin with a fluorochrome-bound phalloidin (5, 6, 10, 11, 13, 19, 34, 35, 38, 43, 46).
However, there is also evidence of histamine-induced increases in microvascular permeability that do not quite fit this paradigm. Histamine increases the permeability of postcapillary venules in the absence of actin stress fiber formation in endothelial cells (4). Interestingly, it was also noted that actin stress fibers did form after permeability had returned to normal (4). It was also apparent in early studies with cultured endothelial cells that histamine did not cause actin stress fiber formation within the time frame of elevated permeability in cultured endothelial cells. Rather, histamine decreased actin cables, which was postulated to be a cause of histamine-induced permeability of the endothelium (42). Likewise, histamine caused only mild phosphorylation of MLC compared with thrombin, and did not elicit an increase in the isometric tension of cultured endothelial cell monolayers (27–29). These findings indicate the importance of contraction-independent mechanisms in the control of the endothelial barrier (27, 43).
Recent work has highlighted the importance of cortical actin for maintaining endothelial barrier integrity (7, 33). The small GTPase Rac1 promotes cortical actin structures, thus stabilizing intercellular junctions (1, 40, 41), and RhoA activation localized to the cell periphery promotes barrier integrity (36). To better understand the dynamics of the actin cytoskeleton, we recently used time-lapse imaging of endothelial cells expressing green fluorescent protein (GFP)-actin. We discovered that endothelial cells had frequent, brief protrusions of the plasma membrane localized at intercellular junctions, termed local lamellipodia, which were reduced during increases in endothelial permeability and restored during the restoration of barrier function (12, 17). The local lamellipodia were dependent upon myosin II activity, and decreases in their protrusion frequency correlated with reduced Rac1 activity (12). These findings are relevant because maintenance of an optimal distance of the junctional cleft between endothelial cells is important for the normal permeability of postcapillary venules (14). Our previous study provided evidence that local lamellipodia may contribute to the maintenance of endothelial junctions. However, that study was limited to an investigation of thrombin and sphingosine-1-phosphate (S1P), and it remains unclear whether additional agents that alter microvascular permeability impact this mechanism.
To investigate the mechanism of action of histamine, we assessed the dynamic changes it induces in the actin cytoskeleton. We present evidence that histamine briefly reduced local lamellipodia formation. On the basis of our previous studies showing the importance of the p38 mitogen-activated protein (MAP) kinase in histamine-induced disruption of endothelial barrier integrity, we also determined the extent to which inhibition of p38 MAP kinase affects both histamine-induced changes in barrier function and lamellipodia protrusion/withdrawal. In addition, we developed a new understanding of the spatial mechanisms of stress fiber formation caused by histamine.
MATERIALS AND METHODS
Cell culture and transfection.
Human umbilical vein endothelial cells (HUVEC), endothelial growth medium (EGM2MV), and endothelial basal medium (EBM2) were obtained from Lonza (Basel, Switzerland). HUVEC were used at passages 2–5. The cells were transfected with plasmids encoding GFP-β-actin (generously provided by Dr. Wayne Orr, Louisiana State University Health Sciences Center, Shreveport, LA) using the Nucleofector II system (Lonza) under similar conditions as previously described (16, 17). Briefly, cells grown to 80% confluence were trypsinized and pelleted, and 5 × 105 cells were mixed with 0.2 μg of pCMV-GFP-β-actin and 100 μl of Nucleofector solution in a Nucleofection cuvette. Program A-34 on the Nucleofector II was used, and immediately after, 500 μl of warm EGM2MV was added and the cells were allowed to recover for 15 min at 37°C. The cells were then seeded onto gelatin-coated 35 × 22-mm glass no. 1 coverslips (Thermo Fisher Scientific, Waltham, MA) or MatTek 35-mm no. 1 glass-bottom plates (MatTek, Ashland, MA) for time-lapse imaging studies. The cells were used for experiments ∼24 h after transfection.
Time-lapse fluorescence microscopy protocols.
Experiments were performed with either a Nikon Eclipse TE-2000U inverted microscope, or an ASI RAMM inverted microscope system as detailed in our previous publications (16, 17). Briefly, the cell-covered coverslips or MatTek dishes were combined with open perfusion chamber inserts that were placed on a heated stage (Warner Instruments, Hamden, CT). The cells were bathed with 37°C physiological salt solution from a gravity reservoir at ∼0.5 ml/min. A confluent area of cells, with some of the cells expressing GFP-fusion protein of interest, was chosen for study. Optimal cells had sufficient expression to obtain good resolution of GFP-actin-containing structures. Areas with very high expression, which decreased resolution, were avoided. After a 30-min stabilization period, initial brightfield and fluorescent images (492 excitation, 510 emission) were obtained using a ×40 objective and Nikon Elements software on the Nikon system, or Micromanager software (18) on the ASI system. Time-lapse image sets were acquired to assess movement of GFP-actin in live cells, with 0.5- or 1-s exposures every 15 s for the duration of the time course. After a 20-min baseline period, histamine was added to both the bath and reservoir at a final concentration of 10 μM (20, 29, 44) and was not washed out for the remainder of the experiment (an additional 30 min). In another set of experiments, cells were pretreated for 20 min with the p38 MAP kinase inhibitor SB203580 at a concentration of 6 μM (24, 39) before the addition of histamine.
Time-lapse image analysis.
Analysis of local lamellipodia characteristics and stress fiber formation were performed in a similar fashion as in our previous publication (17). Briefly, time-lapse image stacks obtained in Nikon ND2 format were exported as TIF files for analysis, and image stacks obtained with MicroManager were obtained as TIF files. All analyses were performed using Fiji/ImageJ software. Brightness and contrast were adjusted for easier display without altering original pixel intensity data. To assess the dynamics of local lamellipodia over time, each lamellipodia event was marked using a region of interest (ROI) within the time stack. The time and location of each event was exported into an Excel spreadsheet, and protrusion frequency (PF) was determined as the number of protrusions every 5 min, normalized per 100 μm of the cell's perimeter. To assess the protrusion characteristics, kymographs of single-pixel lines over time, perpendicular to the edges of cells, were generated. In the kymographs, line ROIs were drawn, and bounding rectangle data were used to determine the protrusion distance (PD, μm), protrusion persistence (PP, min), protrusion velocity (PV, μm/min), withdrawal distance (WD, μm), withdrawal time (WT, min), and withdrawal velocity (WV, μm/min) of local lamellipodia.
Dynamics of actin stress fibers were also assessed using kymograph analysis. For this analysis, a line was drawn across the entire width of a cell. A kymograph was generated from each line in which the x-axis represented distance and the y-axis represented time. Lines formed by the presence of stress fibers were identified and annotated with ROIs, and the ImageJ Measure function was used to obtain bounding rectangle data. The lateral velocity of the actin fibers was calculated as distance/time, with movements toward the cell center assigned a positive value and movements toward the periphery assigned a negative value. The number of actin fibers within the kymograph during each time point was also obtained from these data.
Video files (AVI format) were generated from the time-lapse images using FIJI/ImageJ software using JPEG compression. Brightness and contrast were adjusted to optimize view of the structures containing GFP-actin. To reduce file size, every other frame was removed from the time-lapse stack so that the interval between frames was increased to 0.5 s.
Determination of barrier function.
An Electrical Cell-Substrate Impedance Sensor (ECIS) ZΘ System (Applied Biophysics, Troy, NY) was used for barrier function measurements as previously described (8, 16). Briefly, the cells were seeded at a density of 1.5 × 105 cells per well in gelatin-coated ECIS 8W1E arrays, and allowed to attach and form a confluent monolayer overnight. Each well within the array contains a single, small gold measuring electrode (250 μm diameter) and a large counter electrode. A 1-V, AC signal at 4 kHz was applied from an approximate constant current source (<1 μA). The ECIS instrument measured the voltage across the electrodes and its phase relative to the applied current, providing total impedance. Treating each cell-covered electrode as a series resistor–capacitor circuit, the impedance data was converted to transendothelial electrical resistance (TER) and capacitance of the cell monolayer, which represent barrier function and membrane capacitance, respectively. After the overnight “attach” period, the medium was changed to EBM, and the cells were allowed to stabilize for about 2 h to produce a steady baseline before the addition of histamine [10 μM (20, 29, 44)]. In some experiments, the cells were pretreated for 20 min with various MAP kinase inhibitors before the addition of histamine. These included the p38 MAP kinase inhibitor SB203580 [6 μM (24, 39)] and MEK-1/2 inhibitors PD98059 [10 μM (9, 44)] and U01226 [5 μM (44)].
Western blot analysis.
HUVEC were grown in 100-mm culture dishes to confluence. The day before protein collection, the medium was changed to EBM to reduce any phosphorylation of proteins due to growth factors present in the EGM2MV. After experimental treatments that were identical to those outlined in the previous section, protein was obtained by lysis of cells in ice-cold RIPA buffer (EMD Millipore, Billerica, MA) containing 1× HALT protease and phosphatase inhibitor cocktail (Pierce, Rockford, IL) for 15 min. Protein concentrations were determined with the Bradford assay, and equilibrated protein samples in 1× Novex Tris-Glycine SDS running buffer (Life Technologies, Carlsbad, CA) were loaded into Criterion 18-well 4–20% gels (Bio-Rad, Hercules, CA) and separated by SDS-PAGE. Proteins were electrically transferred from the gels to nitrocellulose for immunoblotting. Primary and secondary antibodies were diluted in RapidBlock Solution (Amresco, Solon, OH). The following primary antibodies were obtained from Cell Signaling Technology (Danvers, MA) and all were used at a dilution of 1:1,000: rabbit anti-phospho-p38 MAP kinase-Thr180/Tyr182 (catalog no. 9211); rabbit anti-p38 MAP kinase (catalog no. 9212); mouse anti-ERK-1/2-Thr202/Tyr204 (catalog no. 9106); and rabbit anti-ERK-1/2 (catalog no. 9102).
Secondary antibodies used were donkey 1:5,000 anti-rabbit IgG-HRP (catalog no. sc-2313; Santa Cruz Biotechnology, Santa Cruz, CA) or 1:5,000 donkey anti-mouse IgG-HRP (catalog no. ab97030; AbCam, Cambridge, UK). Precision Protein StrepTactin-HRP (catalog no. 161-0381; Bio-Rad, Hercules, CA; 1:5,000 dilution) was included with the secondary antibodies to allow visualization of molecular weight bands in the lane loaded with Precision Plus WesternC Standards (catalog no. 161-0376; Bio-Rad). Bands were visualized by chemiluminescence with SuperSignal West Femto Maximum Sensitivity Chemiluminescent Substrate (Thermo Fisher Scientific) in a GelLogic 2200 Pro Imaging System (Carestream Health, Rochester, NY). Blots were stripped with Restore Western Blot Stripping Buffer (Thermo Fisher Scientific) and reprobed with 1:1,000 mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) clone 0411 (catalog no. sc-47724; Santa Cruz Biotechnology) and the aforementioned donkey anti-mouse IgG-HRP secondary antibody to verify equivalent loading of samples. For GAPDH Western blots, the SuperSignal West Pico Chemiluminsecent Substrate (Thermo Fisher Scientific) was used to visualize bands.
Data analysis.
Quantitative data are presented as means ± SE. For time-lapse imaging studies, nine cells were studied from three to four separate experiments. To analyze these data, a two-way (nested) repeated-measures ANOVA was used to account for potential variability between both separate experiments and individual cells. When significance due to experimental treatments (over time) were observed, comparisons of individual time points to control (baseline) were performed using a Dunnett's test. For the ECIS experiments, ANOVA was used to compare the maximal change in TER between the four groups, followed by a Dunnett's test when appropriate to compare individual groups with the control. Significance was accepted at P < 0.05.
RESULTS
Histamine alters the protrusion and withdrawal of endothelial local lamellipodia.
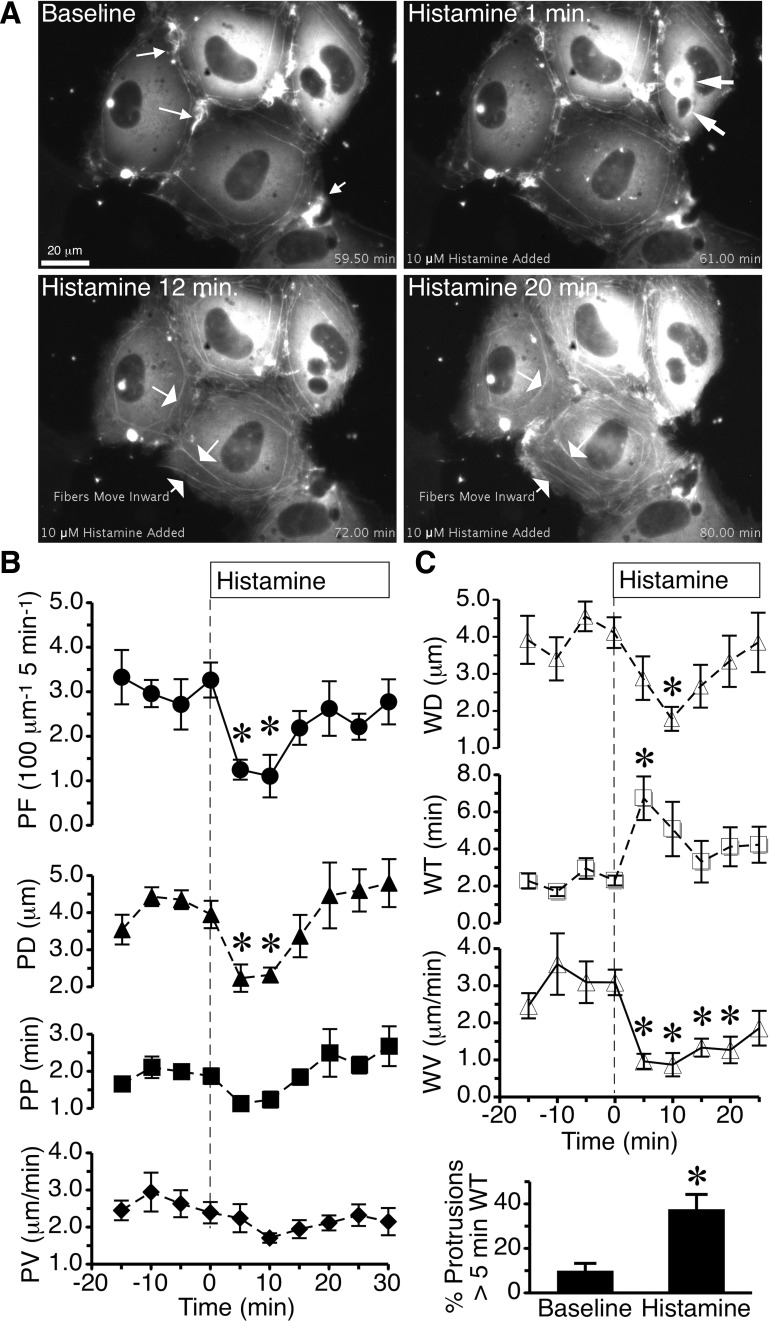
Dynamics of actin fibers before and after histamine challenge were studied using live cell imaging of GFP-actin in confluent HUVEC monolayers. Cells were typically stationary but displayed an ongoing formation and withdrawal of actin-rich protrusions, mainly local lamellipodia around the entire perimeter of most cells (Fig. 1A and Supplemental Video S1; supplemental material for this article is available online at the Journal website). When histamine was added to the cells, there was a significant decrease in protrusion frequency and protrusion distance at 5 and 10 min (Fig. 1B), but there was no significant impact on protrusion persistence or protrusion velocity. In addition, histamine significantly increased the lamellipodia withdrawal time at 5 min, decreased withdrawal distance at 10 min, and reduced withdrawal velocity throughout the duration of histamine (Fig. 1C). The percentage of protrusions with a withdrawal time over 5 min was significantly elevated in the 30-min period after histamine was added, compared with baseline (Fig. 1C). These data indicate that within 5 min, histamine disrupts the normal protrusion of normal local lamellipodia at junctions in confluent endothelial cell monolayers. However, a compensation for the reduced protrusions is that those local lamellipodia that do happen to form after the addition of histamine tend to withdraw much more slowly. This rapid response occurs within the time frame when histamine typically disrupts the endothelial barrier in HUVEC (2, 20, 27).
Fig. 1.
Histamine alters the dynamics of lamellipodia protrusion and withdrawal. A: time-lapse images show human umbilical vein endothelial cells (HUVEC) expressing green fluorescent protein (GFP)-actin during baseline and 1, 12, and 20 min after addition of 10 μM histamine. Cells either not expressing GFP-actin or expressing very low levels occupy the dark spaces around this cluster of cells. The small arrows in the baseline image point to local lamellipodia. Within 1 min after histamine was added, there was a shift in GFP-actin, with some cells developing very intense labeling around large vesicle-like structures (arrows). At 12 and 20 min, there was apparent movement of cortical actin fibers toward the center of the cell, eventually resembling actin stress fibers (arrows). The entire video is available in the supplemental material. B: time courses of histamine-induced changes in mean protrusion frequency (PF), distance (PD), persistence (PP), and velocity (PV) of endothelial local lamellipodia were obtained by kymograph analysis of n = 9 cells from three different experiments. C: withdrawal distance (WD), time (WT), and velocity (WV), and percentage of protrusions that had a WT greater than 5 min were also obtained from the same cells. Each time point represents an average of the previous 5-min period. *P < 0.05 vs. the 0-min time point, representing the period just before histamine addition.
Histamine causes delayed, central movement of cortical actin fibers.
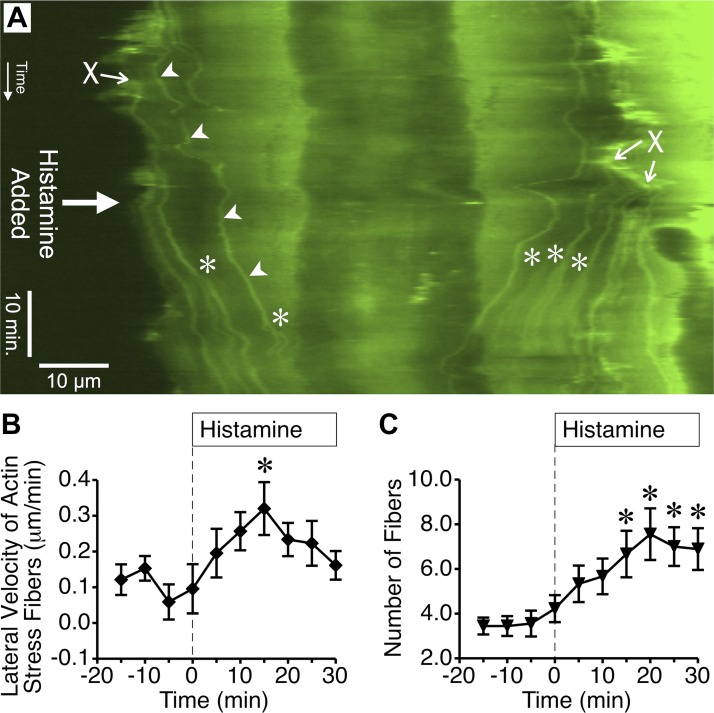
Many previous studies have reported actin stress fiber formation in endothelial cells after treatment with inflammatory mediators (5, 6, 11, 19). We observed that histamine caused cortical actin fibers near the edges of cells to migrate toward the center, after which they resembled actin stress fibers (documented in Supplemental Video S1 and summarized in Fig. 2). This process, visualized by kymograph analysis (Fig. 2A, arrowheads), revealed that histamine significantly increased the lateral velocity of these actin fibers toward the center of the cell (Fig. 2B) and also the number of actin stress fibers (Fig. 2C). However, these events occurred much later than the disruption of lamellipodia formation (Fig. 1). In addition, this elevated number of actin fibers occurred after the reported histamine-induced increases in permeability have already recovered to baseline (2, 27). Thus the central movement of cortical actin fibers to form structures resembling actin stress fibers does not appear to be important for histamine-induced endothelial hyperpermeability.
Fig. 2.
Histamine promotes lateral migration of cortical actin fiber bundles to the center of the cell. A: in this kymograph generated from time-lapse images of an endothelial cell expressing GFP-actin, the location where individual actin fibers cross the kymograph line over time can be observed. The beginning of the time-lapse series is at the top. Arrowheads denote where one individual actin fiber crosses the kymograph line (horizontal dimension) over time (vertical dimension). The fibers often form at the periphery of the cell (denoted by X) and travel toward the center. After 10 μM histamine was added, more fibers moved toward the center of the cell. Additional fibers also appeared due to the formation and movement of bifurcations. *Denotes where a stress fiber bifurcation crossed the kymograph line. B: mean lateral velocity of actin stress fibers over 5-min periods before and after treatment with 10 μM histamine was calculated using kymographs of nine different cells treated with histamine in three different experiments. C: mean number of actin fibers obtained from the same kymographs of histamine-treated cells for each 5-min period. In B and C, *P < 0.05 vs. baseline (0-min time point).
The p38 MAP kinase, but not ERK-1/2, mediates histamine-induced disruption of endothelial barrier integrity.
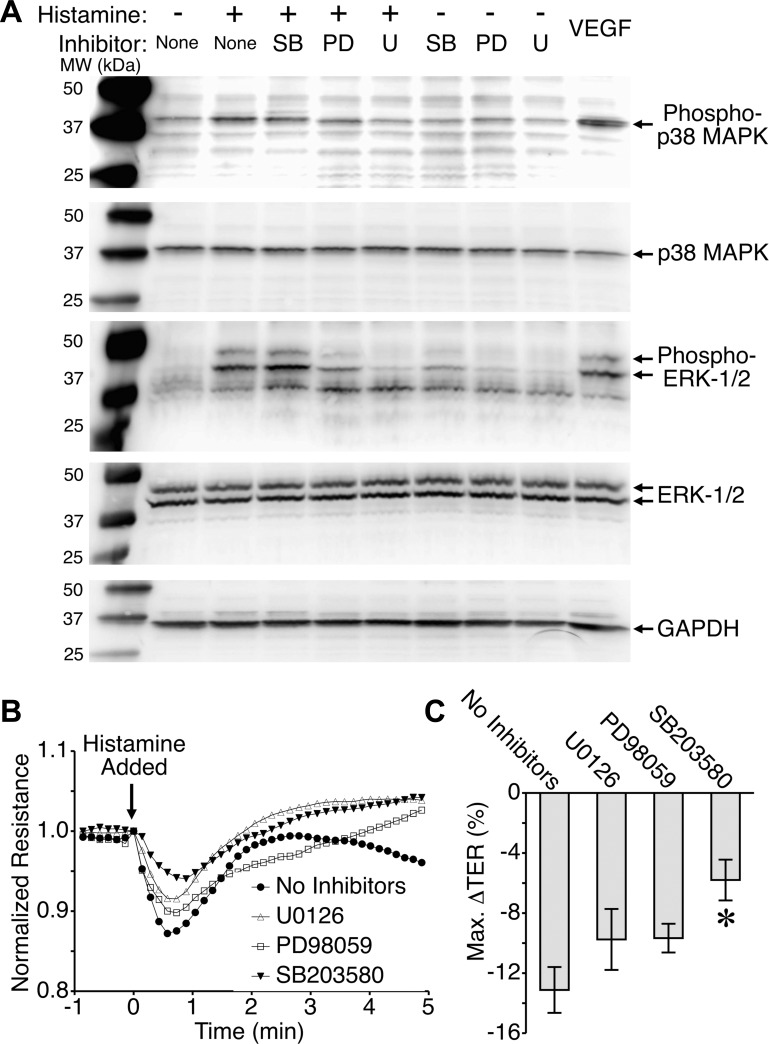
We recently demonstrated the importance of p38 MAP kinase for histamine-induced endothelial barrier dysfunction in both HUVEC and microvascular endothelial cell monolayers (2). In the current study we expanded this investigation and included the MAP kinase ERK-1/2. Histamine increased phosphorylation of both ERK and p38 (Fig. 3A). Pretreatment with the p38 MAP kinase inhibitor SB203580 did not significantly affect the histamine-induced phosphorylation of p38 MAP kinase or ERK-1/2 (Fig. 3A). This was expected because SB203580 does not impact phosphorylation of ERK-1/2, and its inhibitory mechanism for p38 MAP kinase is directed against the ATP-binding pocket to inhibit catalytic activity of the p38 MAP kinase without affecting phosphorylation by upstream kinases (23). In contrast, inhibitors of MEK-1/2 (PD98059 and U0126), the upstream activator of ERK-1/2, blunted phosphorylation of ERK-1/2 and also reduced phosphorylation of the p38 MAP kinase (Fig. 3A). VEGF was used as a positive control for MAP kinase phosphorylation, and GAPDH served as a loading control.
Fig. 3.
Inhibition of the p38 mitogen-activated protein (MAP) kinase, but not ERK-1/2, attenuates histamine-induced endothelial barrier disruption. A: histamine causes phosphorylation of the activation sites of both p38 MAP kinase and ERK-1/2 in endothelial cells. Histamine was applied at 10 μM for 2 min. Inhibitors were all applied for 30 min before histamine in the following concentrations: SB203580, 6 μM; PD98059, 10 μM; U0126, 5 μM. VEGF was used as a positive control and was applied for 5 min. The blots shown are representative of three separate experiments. B: time course of 10 μM histamine-induced decreases in transendothelial resistance (TER) in the absence of inhibitors or in the presence of 5 μM U0126, 10 μM PD98059, or 6 μM SB203580. C: maximal change in TER elicited by histamine in the presence of MAPK inhibitors. *P < 0.05 vs. no inhibitors. In B and C, n = 16 HUVEC monolayers in each group.
Next, we tested the impact of pretreatment with the same inhibitors on histamine-induced barrier disruption of HUVEC monolayers (Fig. 3, B and C). Histamine caused a brief drop in endothelial barrier function in a similar fashion as previously reported (2, 27), and this was partially inhibited with the p38 MAP kinase inhibitor SB203580. However, both of the MEK-1/2 inhibitors, PD98059 and U0126, failed to significantly affect the histamine-induced disruption of endothelial barrier integrity (Fig. 3, B and C). These data suggest that although histamine can activate both the p38 and ERK-1/2 pathways, only the p38 MAP kinase contributes histamine-induced disruption of the endothelial barrier.
Inhibition of p38 MAP kinase reduces the impact of histamine on local lamellipodia.
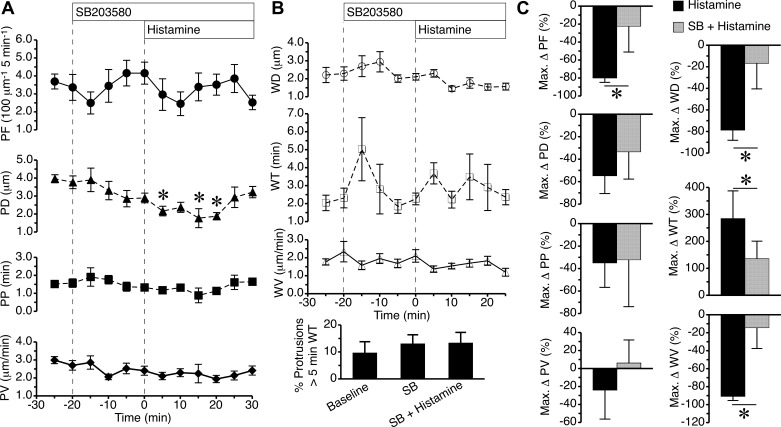
Pretreatment with the p38 MAP kinase inhibitor SB203580 did not significantly alter protrusion or withdrawal characteristics of local lamellipodia from baseline, but it blocked several effects of histamine on protrusion and withdrawal (Fig. 4). Whereas histamine alone (Fig. 1) could significantly reduce both protrusion frequency and distance, in the presence of SB203580, only the histamine-induced decrease in protrusion distance was significant (Fig. 4A). In addition, the histamine-induced changes in lamellipodia withdrawal characteristics shown in Fig. 1 failed to occur in the presence of SB203580 (Fig. 4B). When the maximum changes in the various lamellipodia characteristics were directly compared between cells treated with histamine alone versus SB203580 plus histamine, there was a significant difference in the histamine-induced decrease in protrusion frequency. SB203580 pretreatment also significantly inhibited histamine-induced decreases in withdrawal distance, withdrawal time, and withdrawal velocity (Fig. 4C). The data indicate that inhibition of the p38 MAP kinase reduces the impact of histamine on local lamellipodia protrusion and withdrawal.
Fig. 4.
Inhibition of p38 MAP kinase reduces the impact of histamine on lamellipodia protrusion and withdrawal. A: time courses of lamellipodia PF, PD, PP, and PV during baseline, addition of 6 μM SB203580, and addition of 10 μM histamine. B: time course changes in lamellipodia WD, WT, and WV, along with the percentage of protrusions with a WT greater than 5 min during baseline, SB203580 (SB) treatment both before and after histamine was added. C: maximum changes in the aforementioned protrusion and withdrawal parameters are directly compared between cells that were treated with histamine in the absence or presence of SB203580. *P < 0.05, in A compared with baseline (−20 min time point), and in C between the two groups. Measurements came from n = 9 cells from 3–4 different experiments in each group.
Inhibition of p38 MAP kinase reduces histamine-induced actin stress fiber formation.
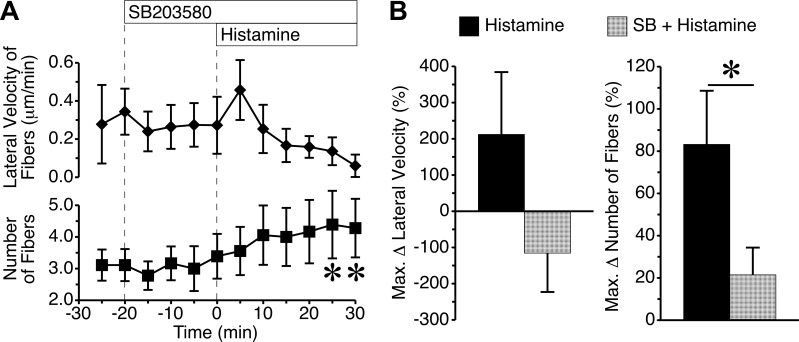
Blockade of p38 MAP kinase activity with SB203580 had no impact on the baseline number of actin fibers or their lateral velocity (Fig. 5). Upon addition of histamine, the SB203580-pretreated cells showed no significant change in the lateral velocity of fibers, but did display a significant increase or number of fibers at the 25- and 30-min time points (Fig. 5A). By comparing the cells treated with histamine alone versus the cells pretreated with SB203580 before histamine, we found no significant difference in the maximum change in the lateral velocity of the actin fibers. However, it is worth noting that the mean change in direction was reversed, so there was no net central movement (Fig. 5A). The maximum change in number of actin fibers was significantly reduced in the presence of SB203580 (Fig. 5B). The data indicate that inhibition of p38 MAP kinase markedly reduces histamine-induced actin stress fiber formation.
Fig. 5.
Inhibition of p38 MAP kinase attenuates histamine-induced actin stress fiber formation. A: time course of changes in lateral velocity and number of actin fiber cables during baseline, after addition of 6 μM SB203580, and after addition of 10 μM histamine. B: direct comparisons of maximum changes in lateral velocity and numbers of actin fibers are shown for histamine-treated cells with or without SB203580 pretreatment. *P < 0.05 between groups. Measurements came from n = 9 cells from 3–4 different experiments in each group.
DISCUSSION
Our study demonstrates that histamine reduces local lamellipodia protrusion activity in endothelial cells, and that p38 MAP kinase plays a key role in this process. This event occurs rapidly, during the time when histamine reduces endothelial barrier integrity. We were surprised to find that the endothelial cell cortical actin belt is a highly dynamic, fluid structure that gave rise to what appear to be actin stress fibers in response to histamine after the endothelial barrier had recovered. In combination, these findings support the novel idea that local lamellipodia have a role in maintaining endothelial barrier integrity (12, 31), in contrast to the popular notion that barrier function is supported primarily by cortical actin fiber stabilization (33).
We recently demonstrated that thrombin, which causes an even stronger and longer lasting disruption of endothelial monolayer barrier integrity, also rapidly and significantly decreases local lamellipodia protrusions (12, 17). In contrast, the bioactive lipid sphingosine-1-phosphate (S1P), which tightens the endothelial barrier, causes a corresponding increase in local lamellipodia. The previous study also identified that blockade of either Rac1 reduces local lamellipodia protrusions, concomitant with increases in endothelial permeability. Another key finding in the previous study was that the myosin II inhibitor blebbistatin could specifically inhibit protrusions of lamellipodia without affecting other visible structures containing actin. Blebbistatin also caused increases in permeability of both endothelial cell monolayers and intact venules in the time frame immediately after lamellipodia protrusion frequency began to decrease (12). An interesting finding with the current study was that the duration of the decreased protrusion frequency caused by histamine persisted longer than histamine-induced endothelial barrier dysfunction. This was different from our previous findings with thrombin, in which protrusion frequency returned to near baseline levels during the time when barrier function began to recover (12). This finding suggests that barrier restoration does not necessarily require resumption of the baseline protrusion frequency of local lamellipodia. Notably, histamine also slowed the withdrawal velocity, which means that any ground gained by the protrusion remained covered for a longer amount of time. This would help maintain contact or overlap with an adjacent cell, and may explain the ability of barrier function to recover in the presence of diminished local lamellipodia formation. It is also possible that local lamellipodia may be selectively stimulated to patch disrupted junctions. This concept is supported by elegant work from Martinelli and colleagues (25) who demonstrated that selective, mechanical wounding of endothelial junctions, or even microwounds of cell membrane away from junctions, could elicit local lamellipodia to fill the gaps.
Whereas several inflammatory mediators elicit increased actin stress fiber formation in conjunction with elevated endothelial permeability, it has been known for some time that this is not the case with histamine. Early studies utilizing cultured endothelial cells even showed that histamine decreased the number F-actin cables present, and that stabilizing F-actin with phalloidin prevented histamine-induced increases in permeability (3, 42). Actin stress fiber formation was detected in the endothelial cells of postcapillary venules after histamine treatment. However, this was at a later stage, after the peak increase in permeability, and was associated with decreased leakiness (37). Our findings are in agreement with these data obtained from intact microvessels despite some very different aspects of the two models; for example, the little or no fluid flow present in our culture model. In addition, our observation that histamine causes an increased lateral migration of cortical actin fibers to the central regions of endothelial cells provides the first identification of a possible mechanism of histamine-induced actin stress fiber formation. Three mechanisms or subtypes of actin stress fibers have been described from studies of cells migrating on two-dimensional surfaces: ventral fibers, dorsal fibers, and transverse arcs (22, 30). The histamine-induced lateral movement of cortical actin fibers toward the center of the endothelial cells is reminiscent of the transverse arc subtype.
Another important new finding from our study is that selective blockade of the p38 MAP kinase limits the impact of histamine on local lamellipodia. Additional evidence that local lamellipodia are important in endothelial barrier maintenance is found in the fact that p38 MAP kinase inhibitor simultaneously attenuates both histamine-induced disruption of endothelial barrier function and protrusion frequency of local lamellipodia. In addition, the histamine-induced migration of cortical actin cables resembling transverse arc stress fibers was also inhibited by blockade of p38 MAP kinase.
A combination of findings leads us to question the prevailing view about why actin stress fiber often accompanies endothelial barrier dysfunction. 1) Actin stress fibers do not form during histamine-induced increases in endothelial permeability, yet structures resembling actin stress fibers do form after recovery (4, 42). 2) Formation of these actin fibers appeared during recovery in our previous study with thrombin (12). 3) Experimentally stimulating actin stress fiber assembly with phalloidin in endothelial cells enhances endothelial barrier function (3). We conclude that actin stress fiber is not a stimulus of barrier disruption but is more likely a compensation mechanism to preserve barrier integrity.
In summary, we propose that local lamellipodia normally contribute to endothelial barrier integrity, and that disruption of these protrusions contributes to elevated microvascular permeability. In this paradigm, local lamellipodia contribute to maintaining the paracellular diffusion distance of the intercellular junctional cleft, keeping permeability to macromolecules relatively low. In addition, this model presumes that actin stress fibers and cortical actin fibers, which are both long, relatively thick and rigid actin cables, help preserve the thin shape of endothelial cells (and flat shape on two-dimensional surfaces). One key future area of study will be determining how local lamellipodia interact with other aspects of the endothelial barrier, such as adhesion proteins at junctions, and the glycocalyx. In addition, this study warrants additional investigation of the molecular mechanisms that govern lamellipodia protrusion and withdrawal.
GRANTS
Support for this study was provided by National Institutes of Health Grants R01 HL-098215 to J.W. Breslin and P20 GM-103424 to J.O. Olubadawo (for research scholarship), and by National Science Foundation Summer Research Award Grant 0928797 to C. Lawrence and E. Madonia. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.O.O. and J.W.B. conception and design of research; S.P.A., C.L., E.M., J.O.O., and J.W.B. performed experiments; S.P.A., C.L., and J.W.B. analyzed data; S.P.A., C.L., E.M., and J.W.B. interpreted results of experiments; C.L. and J.W.B. prepared figures; S.P.A. and J.W.B. drafted manuscript; S.P.A. and J.W.B. edited and revised manuscript; S.P.A., C.L., E.M., J.O.O., and J.W.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Peter Hickman for assistance with the Western blot analysis experiments.
REFERENCES
- 1.Adamson RH, Ly JC, Sarai RK, Lenz JF, Altangerel A, Drenckhahn D, Curry FE. Epac/Rap1 pathway regulates microvascular hyperpermeability induced by PAF in rat mesentery. Am J Physiol Heart Circ Physiol 294: H1188–H1196, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Adderley SP, Zhang XE, Breslin JW. Involvement of the H1 histamine receptor, p38 MAP kinase, MLCK, and Rho/ROCK in histamine-induced endothelial barrier dysfunction. Microcirculation 22: 237–248, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander JS, Hechtman HB, Shepro D. Phalloidin enhances endothelial barrier function and reduces inflammatory permeability in vitro. Microvasc Res 35: 308–315, 1988. [DOI] [PubMed] [Google Scholar]
- 4.Baldwin AL, Thurston G. Changes in endothelial actin cytoskeleton in venules with time after histamine treatment. Am J Physiol Heart Circ Physiol 269: H1528–H1537, 1995. [DOI] [PubMed] [Google Scholar]
- 5.Birukova AA, Alekseeva E, Mikaelyan A, Birukov KG. HGF attenuates thrombin-induced endothelial permeability by Tiam1-mediated activation of the Rac pathway and by Tiam1/Rac-dependent inhibition of the Rho pathway. FASEB J 21: 2776–2786, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Birukova AA, Zagranichnaya T, Alekseeva E, Bokoch GM, Birukov KG. Epac/Rap and PKA are novel mechanisms of ANP-induced Rac-mediated pulmonary endothelial barrier protection. J Cell Physiol 215: 715–724, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bogatcheva NV, Verin AD. The role of cytoskeleton in the regulation of vascular endothelial barrier function. Microvasc Res 76: 202–207, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breslin JW. ROCK and cAMP promote lymphatic endothelial cell barrier integrity and modulate histamine and thrombin-induced barrier dysfunction. Lymphat Res Biol 9: 3–11, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breslin JW, Pappas PJ, Cerveira JJ, Hobson RW 2nd, Durán WN. VEGF increases endothelial permeability by separate signaling pathways involving ERK-1/2 and nitric oxide. Am J Physiol Heart Circ Physiol 284: H92–H100, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Breslin JW, Sun H, Xu W, Rodarte C, Moy AB, Wu MH, Yuan SY. Involvement of ROCK-mediated endothelial tension development in neutrophil-stimulated microvascular leakage. Am J Physiol Heart Circ Physiol 290: H741–H750, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breslin JW, Yuan SY. Involvement of RhoA and Rho kinase in neutrophil-stimulated endothelial hyperpermeability. Am J Physiol Heart Circ Physiol 286: H1057–H1062, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Breslin JW, Zhang XE, Worthylake RA, Souza-Smith FM. Involvement of local lamellipodia in endothelial barrier function. PLoS One 10: e0117970, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbajal JM, Gratrix ML, Yu CH, Schaeffer RC Jr. ROCK mediates thrombin's endothelial barrier dysfunction. Am J Physiol Cell Physiol 279: C195–C204, 2000. [DOI] [PubMed] [Google Scholar]
- 14.Curry FR, Adamson RH. Vascular permeability modulation at the cell, microvessel, or whole organ level: towards closing gaps in our knowledge. Cardiovasc Res 87: 218–229, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dale HH, Laidlaw PP. Histamine shock. J Physiol 52: 355–390, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doggett TM, Breslin JW. Acute alcohol intoxication-induced microvascular leakage. Alcohol Clin Exp Res 38: 2414–2426, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doggett TM, Breslin JW. Study of the actin cytoskeleton in live endothelial cells expressing GFP-actin. J Vis Exp 57: 3187, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edelstein A, Amodaj N, Hoover K, Vale R, Stuurman N. Computer control of microscopes using microManager. Curr Protoc Mol Biol Chapter 14: Unit 14.20, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia JG, Liu F, Verin AD, Birukova A, Dechert MA, Gerthoffer WT, Bamberg JR, English D. Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J Clin Invest 108: 689–701, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo M, Breslin JW, Wu MH, Gottardi CJ, Yuan SY. VE-cadherin and beta-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am J Physiol Cell Physiol 294: C977–C984, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haorah J, Heilman D, Knipe B, Chrastil J, Leibhart J, Ghorpade A, Miller DW, Persidsky Y. Ethanol-induced activation of myosin light chain kinase leads to dysfunction of tight junctions and blood-brain barrier compromise. Alcohol Clin Exp Res 29: 999–1009, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Hotulainen P, Lappalainen P. Stress fibers are generated by two distinct actin assembly mechanisms in motile cells. J Cell Biol 173: 383–394, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound SB 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochem Biophys Res Commun 263: 825–831, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Lal BK, Varma S, Pappas PJ, Hobson RW 2nd, Durán WN. VEGF increases permeability of the endothelial cell monolayer by activation of PKB/akt, endothelial nitric-oxide synthase, and MAP kinase pathways. Microvasc Res 62: 252–262, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Martinelli R, Kamei M, Sage PT, Massol R, Varghese L, Sciuto T, Toporsian M, Dvorak AM, Kirchhausen T, Springer TA, Carman CV. Release of cellular tension signals self-restorative ventral lamellipodia to heal barrier micro-wounds. J Cell Biol 201: 449–465, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morel NM, Petruzzo PP, Hechtman HB, Shepro D. Inflammatory agonists that increase microvascular permeability in vivo stimulate cultured pulmonary microvessel endothelial cell contraction. Inflammation 14: 571–583, 1990. [DOI] [PubMed] [Google Scholar]
- 27.Moy AB, Blackwell K, Kamath A. Differential effects of histamine and thrombin on endothelial barrier function through actin-myosin tension. Am J Physiol Heart Circ Physiol 282: H21–H29, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Moy AB, Shasby SS, Scott BD, Shasby DM. The effect of histamine and cyclic adenosine monophosphate on myosin light chain phosphorylation in human umbilical vein endothelial cells. J Clin Invest 92: 1198–1206, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moy AB, Van Engelenhoven J, Bodmer J, Kamath J, Keese C, Giaever I, Shasby S, Shasby DM. Histamine and thrombin modulate endothelial focal adhesion through centripetal and centrifugal forces. J Clin Invest 97: 1020–1027, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci 120: 3491–3499, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Schnittler H, Taha M, Schnittler MO, Taha AA, Lindemann N, Seebach J. Actin filament dynamics and endothelial cell junctions: the Ying and Yang between stabilization and motion. Cell Tissue Res 355: 529–543, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Shen Q, Rigor RR, Pivetti CD, Wu MH, Yuan SY. Myosin light chain kinase in microvascular endothelial barrier function. Cardiovasc Res 87: 272–280, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spindler V, Schlegel N, Waschke J. Role of GTPases in control of microvascular permeability. Cardiovasc Res 87: 243–253, 2010. [DOI] [PubMed] [Google Scholar]
- 34.Srinivas SP, Satpathy M, Guo Y, Anandan V. Histamine-induced phosphorylation of the regulatory light chain of myosin II disrupts the barrier integrity of corneal endothelial cells. Invest Ophthalmol Vis Sci 47: 4011–4018, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Sun H, Breslin JW, Zhu J, Yuan SY, Wu MH. Rho and ROCK signaling in VEGF-induced microvascular endothelial hyperpermeability. Microcirculation 13: 237–247, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Szulcek R, Beckers CM, Hodzic J, de Wit J, Chen Z, Grob T, Musters RJ, Minshall RD, van Hinsbergh VW, van Nieuw Amerongen GP. Localized RhoA GTPase activity regulates dynamics of endothelial monolayer integrity. Cardiovasc Res 99: 471–482, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thurston G, Baldwin AL, Wilson LM. Changes in endothelial actin cytoskeleton at leakage sites in the rat mesenteric microvasculature. Am J Physiol Heart Circ Physiol 268: H316–H329, 1995. [DOI] [PubMed] [Google Scholar]
- 38.van Nieuw Amerongen GP, van Delft S, Vermeer MA, Collard JG, van Hinsbergh VW. Activation of RhoA by thrombin in endothelial hyperpermeability: role of Rho kinase and protein tyrosine kinases. Circ Res 87: 335–340, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Varma S, Breslin JW, Lal BK, Pappas PJ, Hobson RW 2nd, Durán WN. p42/44MAPK regulates baseline permeability and cGMP-induced hyperpermeability in endothelial cells. Microvasc Res 63: 172–178, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Waschke J, Drenckhahn D, Adamson RH, Barth H, Curry FE. cAMP protects endothelial barrier functions by preventing Rac-1 inhibition. Am J Physiol Heart Circ Physiol 287: H2427–H2433, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Waschke J, Drenckhahn D, Adamson RH, Curry FE. Role of adhesion and contraction in Rac 1-regulated endothelial barrier function in vivo and in vitro. Am J Physiol Heart Circ Physiol 287: H704–H711, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Welles SL, Shepro D, Hechtman HB. Vasoactive amines modulate actin cables (stress fibers) and surface area in cultured bovine endothelium. J Cell Physiol 123: 337–342, 1985. [DOI] [PubMed] [Google Scholar]
- 43.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci 114: 1343–1355, 2001. [DOI] [PubMed] [Google Scholar]
- 44.Wu MH, Yuan SY, Granger HJ. The protein kinase MEK1/2 mediate vascular endothelial growth factor- and histamine-induced hyperpermeability in porcine coronary venules. J Physiol 563: 95–104, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wysolmerski RB, Lagunoff D. Involvement of myosin light-chain kinase in endothelial cell retraction. Proc Natl Acad Sci USA 87: 16–20, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yuan SY, Wu MH, Ustinova EE, Guo M, Tinsley JH, De Lanerolle P, Xu W. Myosin light chain phosphorylation in neutrophil-stimulated coronary microvascular leakage. Circ Res 90: 1214–1221, 2002. [DOI] [PubMed] [Google Scholar]