Subjects and household members performed longitudinal sampling for methicillin-resistant Staphylococcus aureus colonization. The duration of colonization was shorter than previously reported. Treatment with clindamycin was associated with more rapid clearance of colonization, and older age with longer duration of colonization.
Keywords: methicillin-resistant Staphylococcus aureus (MRSA), MRSA colonization, MRSA skin and soft-tissue infection, MRSA decolonization
Abstract
Background. The duration of colonization and factors associated with clearance of methicillin-resistant Staphylococcus aureus (MRSA) after community-onset MRSA skin and soft-tissue infection (SSTI) remain unclear.
Methods. We conducted a prospective cohort study of patients with acute MRSA SSTI presenting to 5 adult and pediatric academic hospitals from 1 January 2010 through 31 December 2012. Index patients and household members performed self-sampling for MRSA colonization every 2 weeks for 6 months. Clearance of colonization was defined as negative MRSA surveillance cultures during 2 consecutive sampling periods. A Cox proportional hazards regression model was developed to identify determinants of clearance of colonization.
Results. Two hundred forty-three index patients were included. The median duration of MRSA colonization after SSTI diagnosis was 21 days (95% confidence interval [CI], 19–24), and 19.8% never cleared colonization. Treatment of the SSTI with clindamycin was associated with earlier clearance (hazard ratio [HR], 1.72; 95% CI, 1.28–2.30; P < .001). Older age (HR, 0.99; 95% CI, .98–1.00; P = .01) was associated with longer duration of colonization. There was a borderline significant association between increased number of household members colonized with MRSA and later clearance of colonization in the index patient (HR, 0.85; 95% CI, .71–1.01; P = .06).
Conclusions. With a systematic, regular sampling protocol, duration of MRSA colonization was noted to be shorter than previously reported, although 19.8% of patients remained colonized at 6 months. The association between clindamycin and shorter duration of colonization after MRSA SSTI suggests a possible role for the antibiotic selected for treatment of MRSA infection.
(See the Editorial Commentary by Calderwood on pages 1497–9.)
Staphylococcus aureus is one of the most common causes of infection in community and healthcare settings [1]. Historically, infection with methicillin-resistant S. aureus (MRSA) has been restricted almost exclusively to hospitalized and chronically ill patients [2]. However, recently, MRSA infections have been increasingly reported in the community [3].
The prevalence of MRSA colonization in the community is estimated at between 0.2% and 7.4% [3], but rates as high as 67% have been reported in household members of patients with recent MRSA infection [4, 5]. Household interactions may influence the duration of MRSA colonization, presumably in relation to the frequency and duration of exposure [6]. Failure to identify and interrupt colonization within the household may serve as a barrier to preventing persistent colonization or repeated infections [7, 8].
Prior studies have estimated that duration of MRSA colonization in the community ranges from 6 to 9 months [9, 10]. However, the populations studied were not representative of the typical community-dwelling patient with MRSA skin and soft-tissue infection (SSTI) in the United States. In addition, factors associated with clearance of MRSA colonization in the community remain unclear. Therefore, we sought to determine the duration of MRSA colonization and determinants of clearance of colonization in ambulatory patients presenting with an acute MRSA SSTI.
METHODS
Study Design and Study Subjects
We conducted a prospective cohort study between 1 January 2010 and 31 December 2012 at 5 academic medical centers: The Hospital of the University of Pennsylvania, a 782-bed urban adult acute care hospital; Penn Presbyterian Medical Center, a 300-bed urban adult acute care hospital; Pennsylvania Hospital, a 500-bed urban adult community hospital; Children's Hospital of Philadelphia, a 535-bed urban children's hospital; and Penn State Milton S. Hershey Medical Center, a 551-bed urban adult and pediatric hospital. Adults and children presenting to the emergency departments and primary care settings at any of the study sites with an acute SSTI for which a sample was sent for microbiologic culture were approached for entry. Hospitalized patients were approached if an acute SSTI was identified and a lesion swab sample was sent for culture within 48 hours of admission. Eligible subjects were those whose culture subsequently revealed MRSA. To be enrolled, a study subject (ie, index patient) and all members of his or her household were required to agree to participate. Informed consent or assent was obtained from all index patients and household members. This study was approved by the institutional review boards of all participating institutions.
Longitudinal Follow-up and Data Collection
Index patients and household members performed self-sampling for MRSA from 3 anatomic sites (nares, axillae, and groin) every 2 weeks for 6 months from enrollment to assess for MRSA colonization, for a total of 14 potential sampling periods. Self-collection of swabs has proved highly sensitive compared with those collected by research staff [11]. Multiple anatomic sites were chosen to maximize the sensitivity of detection of MRSA colonization [11–13]. Previous studies have shown that the throat [14] and rectum [15] are also sites of MRSA colonization, but self-sampling of these sites was considered infeasible. The ESwab System (Copan Diagnostics) was used for sample collections. Subjects obtained specimens by placing a single swab in both nares and then placing a second swab in both axillae followed by the groin. If the initial skin lesion was present, that site was sampled with a third swab. Subjects then mailed swabs to the study laboratory. At the initial household visit, research staff demonstrated the sampling method. Parents or guardians were instructed to perform the sampling for children, if necessary
Demographic data, medical history, including comorbid conditions and medications, number of persons in the household, and antibiotic use were collected via self-report at the initial home interview and updates requested at each sampling period. These data were confirmed or expanded with medical record review, including prescription records.
Laboratory Testing
Swab samples were plated on BBL ChromAgar MRSA (BD) medium and processed according to manufacturer's instructions [16]. Testing for in vitro susceptibility of S. aureus to oxacillin, penicillin, erythromycin, clindamycin, levofloxacin, chloramphenicol, gentamicin, trimethoprim-sulfamethoxazole, rifampin, and vancomycin was performed using the Vitek 2 automated identification and susceptibility testing system with the Advanced Expert System (bioMerieux) and interpreted according to established criteria [17]. Isolates that were erythromycin resistant but clindamycin susceptible were tested for inducible macrolide-lincosamide-streptogramin resistance by the disk diffusion method (D-test) [17].
Data Analysis
Only index patients who returned samples for at least the first 2 consecutive sampling periods were included in analyses. Subjects were assumed to be colonized at the time of infection [18]. Index patients were censored at clearance of MRSA colonization or at the end of follow-up, whichever came first. Clearance of colonization was defined as 2 consecutive sampling periods with no positive MRSA surveillance cultures; the clearance date was identified as the midpoint between the date of the last positive surveillance culture and that of the first negative culture. The median duration of colonization was determined using a Kaplan–Meier estimate. In a second, exploratory analysis, we included only those index patients with ≥1 positive swab sample during the initial 2 visits.
Antibiotic exposure in the index patient was assessed in 4 time periods: the year before diagnosis of SSTI (pretreatment), the 14 days after diagnosis (treatment), and day 15 after enrollment through the end of follow-up (posttreatment). Antibiotic use in the 14 days before diagnosis was not included because this was assumed to be empiric treatment for the SSTI. The presence of colonization in household members was analyzed in 2 ways: colonization at baseline (ie, first sampling period) and colonization status at each sampling period (treated as a time-varying covariate). Three measures of household member MRSA colonization were defined: ≥1 household member positive, number of household members positive, and proportion of household members positive. Bivariable and multivariable analyses were performed using Cox proportional hazards regression analysis. Multivariable models were developed to determine the association between factors and clearance of MRSA colonization in index patients. Variables were included in the model if they were associated with time to clearance at bivariable analysis (P value ≤.20) [19], and they were maintained in the final model if they remained significantly associated with the outcome when manual backward deletion was used. Age and number of household members with MRSA colonization at each sampling period (time-varying covariate) were identified a priori as potential important factors and so were maintained in the model. A hazard ratio (HR) and 95% confidence interval (CI) were calculated to evaluate the strength of associations.
For all calculations, differences were considered significant at P < .05 (2 tailed). Statistical calculations were performed using commercially available software (SAS 9.3; SAS Institute).
RESULTS
A total of 349 households provided informed consent. Of these, 243 index patients (69.6%) returned samples for the first 2 sampling periods (permitting a calculation of duration of colonization) and were included in the analysis (Figure 1). The only significant difference between included and excluded subjects was the proportion of white subjects (42.8% of included subjects vs 26.4% of excluded subjects; P = .004). The 243 households included 803 household members. The median duration of follow-up for index patients and household members was 156 days (interquartile range [IQR], 70–193 days) and 171 days (103–193 days), respectively. Household sampling was completed for a median of 10 episodes (IQR 5–14).
Figure 1.
Flow chart for study subjects. Abbreviation: MRSA, methicillin-resistant Staphylococcus aureus.
The median age of index patients was 18.9 years (IQR, 3.9–44.4 years), and 152 (62.6%) were female. The median age of household members was 21.9 years (IQR, 8.7–36.5 years), and included 451 (56.2%) females. Fifty-three (29.1%) of the index patients reported a history of prior MRSA infection; however, 61 (25.1%) did not respond to this question.
Antibiotic treatment for the SSTI was prescribed to 223 index patients (91.8%) , most commonly clindamycin (110 subjects; 45.3%) and trimethoprim-sulfamethoxazole (105 subjects; 43.2%). Complete antibiotic susceptibility data were available for 190 patients (85.2%), revealing that 176 (92.6%) received an antibiotic to which the organism was susceptible. Approximately 20% of subjects received a prescription at initial presentation for an intervention to eradicate colonization: 53 (21.8%) were prescribed topical nasal mupirocin, and 47 (19.3%) were prescribed bleach baths/body wipes or chlorhexidine. Prescription of mupirocin and chlorhexidine/bleach baths did not differ significantly between those who reported prior MRSA infection and those who did not (P = .97 and P = .57, respectively).
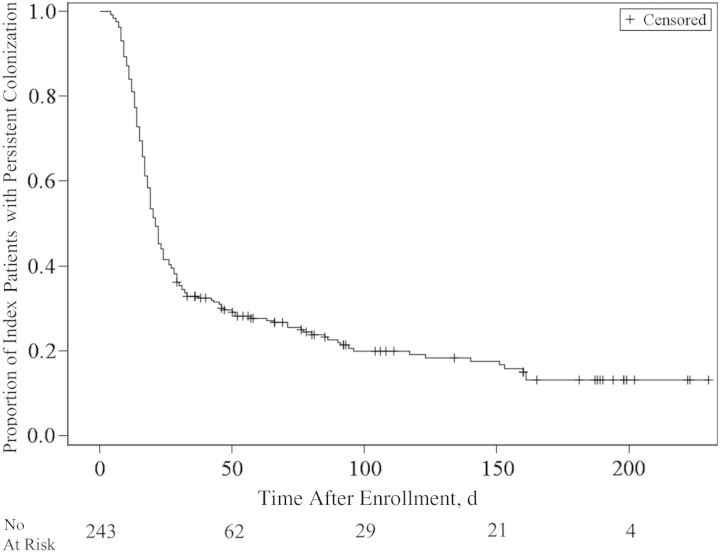
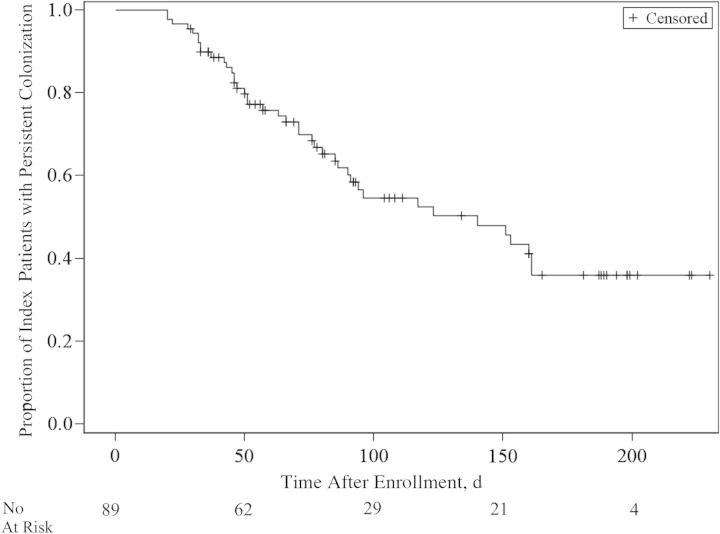
Figure 2 shows the Kaplan–Meier curve of MRSA colonization over time. The median duration of colonization was 21 days (95% CI, 19–24 days). Forty-eight (19.8%) of the index patients remained colonized at the end of the study. If only index patients with MRSA colonization confirmed at the first sampling period were analyzed (89 patients; 36.6%), the duration of MRSA colonization was longer, with a median of 140 days (95% CI, 86–161 days) (Figure 3).
Figure 2.
Kaplan–Meier curve of duration of colonization with methicillin-resistant Staphylococcus aureus (MRSA) after diagnosis and treatment of MRSA skin and soft-tissue infection.
Figure 3.
Kaplan–Meier curve of duration of colonization with methicillin-resistant Staphylococcus aureus (MRSA) after confirmation of MRSA carriage.
In bivariable analyses (Table 1), subjects with later clearance of MRSA colonization were more likely to be older, be white, have a higher proportion of household members <18 years old, or have a diagnosis of diabetes mellitus, cancer, or prior MRSA infection. Table 2 shows the unadjusted associations between duration of MRSA colonization and drug prescriptions in 3 discrete time periods. Prescription of clindamycin in the 14 days after diagnosis was associated with earlier clearance of colonization. Clindamycin was more commonly prescribed in index patients <18 years old (P < .001). Exposure to any antibiotics from day 15 after enrollment to the end of follow-up was associated with later clearance of colonization. Receipt of mupirocin or chlorhexidine/bleach baths or wipes after SSTI diagnosis had no association with the duration of MRSA colonization.
Table 1.
Unadjusted Hazard Ratios for Clearance of MRSA Colonization by Baseline Characteristics
| Characteristic | Index Patients, No (%)a | HR (95% CI) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 25.9 (23.3) | 0.99 (.98–.99) | <.001 |
| Age group, y | |||
| <18 | 119 (49.0) | 1.00 (Reference) | Reference |
| 18–44 | 65 (26.8) | 0.68 (.49–.96) | .03 |
| 45–65 | 42 (17.3) | 0.53 (.35–.80) | .002 |
| >65 | 17 (7.0) | 0.68 (.20–.70) | .002 |
| Proportion of household members aged <18 y | … | 1.01 (1.00–1.01) | .02 |
| Female sex | 152 (62.6) | 1.26 (.94–1.69) | .12 |
| Race | |||
| White | 104 (42.8) | 0.61 (.46–.83) | .001 |
| Nonwhite | 139 (57.2) | 1.00 (Reference) | Reference |
| Black/African American | 123 (50.6) | … | … |
| Hispanic | 3 (1.2) | … | … |
| Asian | 3 (1.2) | … | … |
| Mixed/other | 5 (2.1) | … | … |
| Refused to answer | 5 (2.1) | … | … |
| Enrollment site | |||
| HUP | 64 (26.3) | 1.00 (Reference) | Reference |
| CHOP | 105 (43.2) | 1.95 (1.37–2.79) | <.001 |
| HMC | 38 (15.6) | 1.08 (.68–1.71) | .75 |
| PPMC | 34 (12.5) | 1.19 (.73–1.93) | .49 |
| PAH | 5 (2.1) | 0.86 (.27–2.76) | .80 |
| Medical setting | |||
| Emergency department | 167 (68.7) | 1.00 (Reference) | Reference |
| Primary care | 61 (25.1) | 0.92 (.66–1.26) | .59 |
| Inpatient | 15 (6.2) | 0.76 (.42–1.37) | .36 |
| Comorbid conditionb | |||
| Hepatic dysfunction | 12 (5.0) | 0.76 (.39–1.49) | .42 |
| Renal dysfunction | 6 (2.5) | 0.28 (.07–1.13) | .07 |
| Diabetes mellitus | 24 (9.9) | 0.52 (.30–.88) | .01 |
| Cancer | 18 (6.2) | 0.35 (.17–.72) | .004 |
| Organ transplant | 4 (1.7) | 0.43 (.11–1.73) | .23 |
| Prior MRSA infectionc | 53 (29.1) | 0.58 (.40–.83) | .003 |
| Household size, No. of persons | |||
| 1 | 23 (9.5) | 1.00 (Reference) | Reference |
| 2 | 32 (13.2) | 0.72 (.38–1.37) | .32 |
| 3 | 41 (16.9) | 1.15 (.65–2.05) | .63 |
| 4 | 51 (21.0) | 1.42 (.82–2.45) | .21 |
| 5 | 38 (15.6) | 1.02 (.57–1.84) | .95 |
| >5 | 58 (23.9) | 1.40 (.82–2.40) | .22 |
Abbreviations: CHOP, Children's Hospital of Philadelphia; CI, confidence interval; HMC, Penn State Milton S. Hershey Medical Center; HR, hazard ratio; HUP, Hospital of the University of Pennsylvania; MRSA, methicillin-resistant Staphylococcus aureus; PAH, Pennsylvania Hospital; PPMC, Penn Presbyterian Medical Center; SD, standard deviation.
a Data represent No. (%) unless otherwise specified.
b Proportion calculated using available data (available for 242 index patients).
c Proportion calculated using available data (available for 182 index patients).
Table 2.
Unadjusted Hazard Ratios for Clearance of MRSA Colonization by Drug Treatment
| Treatment by Perioda | Index Cases, No. (%) | HR (95% CI) | P Value |
|---|---|---|---|
| Pretreatment period | |||
| Any antibiotic | 39 (16.1) | 0.79 (.53–.17) | .23 |
| Amoxicillin | 12 (4.9) | 1.11 (.59–2.10) | .74 |
| Amoxicillin-clavulanate | 7 (2.9) | 0.73 (.30–1.78) | .49 |
| Azithromycin | 5 (2.1) | 0.33 (008–1.34) | .12 |
| Cephalexin | 3 (1.2) | 4.24 (1.34–13.44) | .01 |
| Clindamycin | 9 (3.7) | 1.36 (.70–2.65) | .37 |
| Doxycycline | 2 (0.8) | 0.78 (.19–3.16) | .73 |
| Trimethoprim-sulfamethoxazole | 10 (4.1) | 1.06 (.52–2.14) | .880 |
| Mupirocin | 3 (1.2) | 0.19 (.03–1.34) | .10 |
| Bleach bath/chlorhexidine | 1 (0.4) | 0.00 (0–∞) | .98 |
| Treatment period | |||
| Any antibiotic | 223 (91.8) | 1.01 (.99–1.03) | .34 |
| Amoxicillin-clavulanate | 11 (4.5) | 0.94 (.48–1.84) | .86 |
| Cephalexin | 15 (6.2) | 0.87 (.47 1.60) | .65 |
| Clindamycin | 110 (45.3) | 1.95 (1.46–2.59) | <.001 |
| Doxycycline | 13 (5.4) | 0.67 (.36–1.28) | .23 |
| Levofloxacin | 4 (1.7) | 0.48 (.15–1.50) | .21 |
| Trimethoprim-sulfamethoxazole | 105 (43.2) | 0.84 (.63–1.12) | .24 |
| Mupirocin | 53 (21.8) | 0.93 (.65–1.32) | .68 |
| Bleach bath/chlorhexidine | 47 (19.3) | 0.81 (.56–1.18) | .28 |
| Nasal steroid | 12 (4.9) | 0.85 (.44–1.66) | .64 |
| Posttreatment period | |||
| Any antibiotic | 42 (17.3) | 0.53 (.36–.80) | .002 |
| Clindamycin | 9 (3.7) | 0.89 (.44–1.81) | .75 |
| Doxycycline | 6 (2.5) | 0.57 (.23–1.39) | .21 |
| Levofloxacin | 4 (1.7) | 0.26 (.06–1.06) | .06 |
| Trimethoprim-sulfamethoxazole | 16 (6.6) | 0.53 (.28–1.01) | .05 |
| Mupirocin | 8 (3.3) | 0.41 (.15–1.09) | .07 |
| Bleach bath/chlorhexidine | 6 (2.5) | 0.17 (.02–1.23) | .08 |
| Nasal steroid | 13 (5.4) | 0.76 (.39–1.48) | .42 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MRSA, methicillin-resistant Staphylococcus aureus.
a The pretreatment period was defined as the year before study enrollment, not including the 14 days prior diagnosis of skin and soft-tissue infection (SSTI); the treatment period, the 14 days after diagnosis of MRSA SSTI; and the posttreatment period, from 15 days after diagnosis of MRSA SSTI until the end of follow-up.
In multivariable analyses (Table 3), treatment with clindamycin in the 14 days after diagnosis was associated with earlier clearance of MRSA colonization in the index patient (HR, 1.7; 95% CI, 1.27–1.30; P < .001). Conversely, age (HR, 0.99; 95% CI, .98–1.00; P = .004) was associated with longer duration of colonization. Given that index patients <18 years old were more likely to receive clindamycin, we looked for effect modification of treatment with clindamycin by age and found no significant interaction (P = .79). Although white race was significantly associated with longer duration of colonization, we found that it had significant correlation with cancer (Pearson's correlation coefficient, 0.252; P < .001). Based on biologic plausibility, cancer was maintained in the final model. The point estimate for increased number of household members colonized with MRSA reflects a borderline association with later clearance (HR, 0.85; 95% CI, .71–1.01; P = .06). Exclusion of subjects who received an antibiotic regimen to which their infection was not susceptible did not change the findings (data not shown). The presence of colonization in household members, at baseline and at each sampling period, after adjustment for age, cancer, and treatment with clindamycin, was not significantly associated with time to clearance of MRSA colonization in the index patient using the various measures of colonization status (Table 4).
Table 3.
Multivariable Cox Proportional Hazards Regression Model of Variables Associated With Clearance of MRSA Colonization
| Variable | HR (95% CI) | P Value |
|---|---|---|
| Treatment with clindamycin | 1.72 (1.28–2.30) | <.001 |
| Agea | 0.99 (.98–1.00) | .010 |
| Cancer | 0.54 (.26–1.15) | .112 |
| No. of household members colonized with MRSAa | 0.85 (.71–1.01) | .064 |
Abbreviations: CI, confidence interval; HR, hazard ratio; MRSA, methicillin-resistant Staphylococcus aureus.
a HRs for these variables represent hazards for each unit increase.
Table 4.
Unadjusted and Adjusted HRs for Clearance of MRSA Colonization by Presence of MRSA Colonization in Household Members at Baseline and at Each Sampling Period
| Variable | No. (%) or Mean (SD) | Duration of Colonization, Median (IQR), d | HR (95% CI) | P Value (Unadjusted) | aHR (95% CI) | P Value (Adjusted) |
|---|---|---|---|---|---|---|
| Baseline findings | ||||||
| Patients with no household members colonized, No. (%) | 154 (63.4) | 20.5 (18–23) | 1.00 (Reference) | Reference | 1.00 (Reference) | Reference |
| Patients with ≥1 household member colonized, No. (%) | 89 (36.6) | 22 (18–30) | 0.89 (.67–1.20) | .452 | 0.81 (.60–1.09) | .16 |
| Proportion of household members colonized, mean (SD), %a | 15.1 (25.5) | … | 0.99 (.99–1.00) | .044 | 1.00 (.99–1.00) | .09 |
| Household members colonized, mean (SD), No.b | 0.54 (0.89) | … | 0.88 (.74–1.04) | .123 | 0.84 (.71–1.00) | .06 |
| Findings at each sampling periodc | ||||||
| ≥1 household member colonized | … | … | 0.87 (.65–1.17) | .355 | 0.78 (.58–1.05) | .10 |
| Proportion of household members colonizeda | … | … | 0.99 (.99–1.00) | .056 | 1.00 (.99–1.00) | .09 |
| No. of household members colonizedb,d | … | … | 0.89 (.75–1.05) | .167 | 0.85 (.71–1.01) | .06 |
Abbreviations: aHR, adjusted hazard ratio (adjusted for age, cancer, and treatment with clindamycin); CI, confidence interval; HR, hazard ratio; IQR, interquartile range; MRSA, methicillin-resistant Staphylococcus aureus; SD, standard deviation.
a HRs represent hazards of clearance for each 1% increase in household members colonized.
b HRs represent hazards of clearance for each 1-unit increase in household members colonized.
c Treated as time-varying covariates.
d Primary measure used in analysis.
DISCUSSION
We found that the median duration of MRSA colonization after diagnosis of SSTI was 21 days, but a significant proportion of subjects (19.8%) remained colonized at the end of the study. Treatment with clindamycin was the only factor associated with earlier clearance of colonization, whereas increased age was associated with longer duration of colonization. Presence of household members colonized with MRSA, household size, or use of mupirocin or chlorhexidine were not associated with duration of colonization.
The median duration of MRSA colonization is shorter than reported in prior studies [20–23]. However, most prior studies calculated duration of colonization according to colonization status at hospital readmission [20, 21, 23] rather than via systematic, longitudinal sampling, which precludes the precise measurement of duration of colonization. Moreover, studies conducted in the community setting included only subjects with confirmed MRSA carriage at study onset, whereas ours examined duration after diagnosis and treatment of MRSA SSTI. Two studies have followed up subjects longitudinally with regular sampling to determine MRSA colonization. Eveillard and colleagues [22] followed up healthcare workers colonized with MRSA, determining colonization status every 3 weeks until clearance or for 6 months, and they found a median duration of MRSA colonization of 83 days. Lucet et al [9] followed up subjects colonized with MRSA discharged from the hospital, checked for MRSA colonization every 3 months, and found a median duration of colonization of 282 days. A third study conducted by Larsson and colleagues [10] in Sweden, where MRSA infections are publicly reported and serial sampling for MRSA colonization is performed until clearance, revealed that the median duration of MRSA colonization was 179 days; however, these authors noted substantial variability, and 43% of subjects were colonized for <2 months.
These variable findings demonstrate the important effects of different study methods and populations. Indeed, when we included only subjects with confirmed MRSA colonization at the first sampling period after SSTI treatment, we found a median duration of colonization of 140 days. Our findings reveal the potential effect of treatment for MRSA SSTI on the duration of colonization, which more accurately reflects the clinical situation ambulatory providers typically encounter. In our study, 19.8% of subjects remained colonized at the end of sampling. This finding is similar to those seen in hospitalized patients, where 21%–40% of subjects had persistent colonization [20, 21]. Future studies should attempt to better identify this subset of subjects with persistent colonization to better target decolonization efforts.
Prescription of clindamycin in the 14 days after SSTI diagnosis was associated with earlier MRSA clearance. The vast majority of subjects received antibiotics to which the MRSA isolate was susceptible. Clindamycin was not associated with duration of colonization in the other time periods, but the proportion of index patients who were prescribed clindamycin during these time periods was small (3.7%), limiting the ability to demonstrate a significant association. A previous study showed that treatment of SSTI with antibiotics was associated with shorter duration of MRSA colonization [10].
To our knowledge, ours is the first study to identify the role of specific antibiotics on duration of colonization. Clindamycin has been used as a component of MRSA decolonization bundles owing to its activity against MRSA, with eradication rates of up to 90% [24, 25]. Doxycycline [25, 26] and trimethoprim-sulfamethoxazole [25] have also been studied as part of combination antibiotic treatment for MRSA colonization eradication. These antibiotics were not associated with earlier clearance of colonization in our study, however. The association between clindamycin and more rapid clearance remains unclear. No studies show higher concentrations of clindamycin in specific anatomic sites; in fact, both clindamycin and trimethoprim-sulfamethoxazole both achieve excellent tissue penetration in treatment of MRSA SSTI [27]. It is possible that clindamycin has a differential effect on the skin microbiome. Further studies to explain the association between clindamycin and more rapid clearance of colonization and a randomized controlled trial of clindamycin as part of a decolonization protocol may be warranted. Interestingly, receipt of agents used for decolonization (ie, topical mupirocin, bleach baths, or chlorhexidine) was not associated with duration of colonization in our study. Compliance with these measures was not determined, however, and prescription of these drugs may have been given to patients with a perceived higher risk of recurrence.
Increased age was associated with later clearance of MRSA colonization, with a 1% increase in hazards for each year of age. Several studies have found conflicting results in regard to the association between age and acquisition and duration of MRSA colonization. Lucet et al [9] determined that older age was a risk factor for both acquisition and transmission of MRSA, whereas Larsson and colleagues [10] found that younger community-dwelling subjects had longer duration of MRSA colonization. The association between age and MRSA colonization needs to be further studied in large populations of community-dwelling adults and children.
There was a borderline significant association between increased numbers of household members with MRSA colonization and longer duration of colonization. The optimal way to measure colonization in household members is unknown. We used several measures and found that increased numbers of colonized household members (at baseline or at each sampling period) yielded the strongest associations with longer duration of colonization, suggesting that an association probably does exist. Interestingly, household size was not associated with prolonged duration of MRSA colonization. It is possible that the more important factor is presence of a colonized household member rather than the “crowding” factor. Prior studies have shown that increased “colonization pressure” (ie, the proportion of patients colonized in a given time period) in hospital units increased the rate of MRSA transmission among hospitalized patients [28, 29]. More recently, Fritz et al [4] and Rodriguez et al [30] showed that this was also true in households of pediatric patients, and Larsson et al [10] demonstrated similar findings in adults in Sweden. Our study contributes to this knowledge by demonstrating this in subjects of all ages in the United States. Colonization with MRSA leads to subsequent infection in up to 38% of subjects [31–33]. It has been noted that up to two-thirds of household contacts of subjects with MRSA SSTI are subsequently colonized with MRSA [4, 5]. Disruption of colonization in household members may play a role in decreasing the burden associated with MRSA SSTI, as shown by Fritz and colleagues [34] in households of pediatric patients. Further studies are needed to determine whether decolonization of households decreases the rate of MRSA colonization and subsequent infection in adults as well as children.
This study has several limitations. Although onset of colonization with MRSA in the index patients was designated as the date of presentation with the SSTI, the onset was likely earlier. However, previous studies report a short time (ie, median 1–2 weeks) between new colonization with MRSA and subsequent MRSA infection [35, 36], so we suspect that our findings approximate the true duration of colonization. Furthermore, index patients may have truly been colonized but with false-negative sampling. However, swabbing of multiple anatomic sites and defining clearance of colonization as all samples negative for 2 consecutive sampling periods decreased the possibility of missing true clearance. Selection bias might have also occurred, but the 107 households that were excluded did not differ substantively from included subjects. Recall bias from survey data may have affected the ascertainment of important variables including antibiotic use and use of decolonization methods; this bias is probably nondifferential given that index patients were unaware of their colonization status. In addition, compliance with antibiotics and decolonization agents was not assessed. Finally, rates and patterns of antibiotic resistance may vary across regions, which may reflect differences in the distribution of risk factors. Nevertheless, this study was conducted at multiple sites comprising a geographically and racially diverse population of both adults and children, which should improve the generalizability of these findings.
In conclusion, we found that median duration of colonization was 21 days, and 19.8% of index patients remained colonized at the end of the 6-month study period. Increased age was associated with longer duration of MRSA colonization in index patients. Clindamycin treatment of MRSA SSTI, on the other hand, was associated with earlier clearance of colonization. There was also a borderline significant association between increased numbers of household members colonized with MRSA and longer duration of colonization. Future studies should examine the predictors of persistent colonization, the impact of prolonged duration of colonization on development of MRSA reinfection, and the potential role of total household decolonization in adults and children. In addition, the unique role of clindamycin in treatment of MRSA infections should be further elucidated.
Notes
Financial support. This work was supported by the Pennsylvania State Department of Health (Commonwealth Universal Research Enhancement Program grant to E. L.), the National Institutes of Health (NIH) (grant K24-AI080942 to E. L.), and the Centers for Disease Control and Prevention Epicenters Program (grant U54-CK000163 to E. L.).
Potential conflicts of interest. W. B. B. has received institutional grant support from the NIH. M. F. D. has received institutional grant support from the Johns Hopkins Center for a Livable Future, the Morris Anival Foundation, and the American College of Veterinary Dermatology, in addition to honorarium and travel support from the North American Veterinary Dermatology Forum. J. P. M., T. E. Z., K. G. J., J. E. H., L. J. G., and E. L. have received institutional grant support from the Pennsylvania Department of Health. J. S. G. has received institutional grant support from the Commonwealth of Pennsylvania (CURE grant) to support this research. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Pfaller MA, Jones RN, Doern GV, Kugler K. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997). Antimicrob Agents Chemother 1998; 42:1762–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zetola N, Francis JS, Nuermberger EL, Bishai WR. Community-acquired meticillin-resistant Staphylococcus aureus: an emerging threat. Lancet Infect Dis 2005; 5:275–86. [DOI] [PubMed] [Google Scholar]
- 3.Salgado CD, Farr BM, Calfee DP. Community-acquired methicillin-resistant Staphylococcus aureus: a meta-analysis of prevalence and risk factors. Clin Infect Dis 2003; 36:131–9. [DOI] [PubMed] [Google Scholar]
- 4.Fritz SA, Hogan PG, Hayek G, et al. Staphylococcus aureus colonization in children with community-associated Staphylococcus aureus skin infections and their household contacts. Arch Pediatr Adolesc Med 2012; 166:551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mollema FP, Richardus JH, Behrendt M, et al. Transmission of methicillin-resistant Staphylococcus aureus to household contacts. J Clin Microbiol 2010; 48:202–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang YC, Coxson P, Shen YM, Goldman L, Bibbins-Domingo K. A penny-per-ounce tax on sugar-sweetened beverages would cut health and cost burdens of diabetes. Health Aff 2012; 31:199–207. [DOI] [PubMed] [Google Scholar]
- 7.Calfee DP, Durbin LJ, Germanson TP, Toney DM, Smith EB, Farr BM. Spread of methicillin-resistant Staphylococcus aureus (MRSA) among household contacts of individuals with nosocomially acquired MRSA. Infect Control Hosp Epidemiol 2003; 24:422–6. [DOI] [PubMed] [Google Scholar]
- 8.Moran GJ, Amii RN, Abrahamian FM, Talan DA. Methicillin-resistant Staphylococcus aureus in community-acquired skin infections. Emerg Infect Dis 2005; 11:928–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med 2009; 169:1372–8. [DOI] [PubMed] [Google Scholar]
- 10.Larsson AK, Gustafsson E, Nilsson AC, Odenholt I, Ringberg H, Melander E. Duration of methicillin-resistant Staphylococcus aureus colonization after diagnosis: a four-year experience from southern Sweden. Scand J Infect Dis 2011; 43:456–62. [DOI] [PubMed] [Google Scholar]
- 11.Lautenbach E, Nachamkin I, Hu B, et al. Surveillance cultures for detection of methicillin-resistant Staphylococcus aureus: diagnostic yield of anatomic sites and comparison of provider- and patient-collected samples. Infect Control Hosp Epidemiol 2009; 30:380–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mermel LA, Cartony JM, Covington P, Maxey G, Morse D. Methicillin-resistant Staphylococcus aureus colonization at different body sites: a prospective, quantitative analysis. J Clin Microbiol 2011; 49:1119–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eveillard M, de Lassence A, Lancien E, Barnaud G, Ricard JD, Joly-Guillou ML. Evaluation of a strategy of screening multiple anatomical sites for methicillin-resistant Staphylococcus aureus at admission to a teaching hospital. Infect Control Hosp Epidemiol 2006; 27:181–4. [DOI] [PubMed] [Google Scholar]
- 14.Ringberg H, Petersson AC, Walder M, Hugo Johansson PJ. The throat: an important site for MRSA colonization. Scand J Infect Dis 2006; 38:888–93. [DOI] [PubMed] [Google Scholar]
- 15.Boyce JM, Havill NL, Maria B. Frequency and possible infection control implications of gastrointestinal colonization with methicillin-resistant Staphylococcus aureus. J Clin Microbiol 2005; 43:5992–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han Z, Lautenbach E, Fishman NO, Nachamkin I. Evaluation of mannitol salt agar, CHROMaga Staph aureus and CHROMaga MRSA for detection of methicillin-resistant Staphylococcus aureus from nasal swab specimens. J Med Microbiol 2007; 56:43–6. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: eighteenth informational supplement. M100-S18 Wayne, PA: Clinical and Laboratory Standards Institute, 2008. [Google Scholar]
- 18.von Eiff C, Becker K, Machka K, Stammer H, Peters G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N Engl J Med 2001; 344:11–6. [DOI] [PubMed] [Google Scholar]
- 19.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol 1993; 138:923–36. [DOI] [PubMed] [Google Scholar]
- 20.Robicsek A, Beaumont JL, Peterson LR. Duration of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis 2009; 48:910–3. [DOI] [PubMed] [Google Scholar]
- 21.Scanvic A, Denic L, Gaillon S, Giry P, Andremont A, Lucet JC. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis 2001; 32:1393–8. [DOI] [PubMed] [Google Scholar]
- 22.Eveillard M, Martin Y, Hidri N, Boussougant Y, Joly-Guillou ML. Carriage of methicillin-resistant Staphylococcus aureus among hospital employees: prevalence, duration, and transmission to households. Infect Control Hosp Epidemiol 2004; 25:114–20. [DOI] [PubMed] [Google Scholar]
- 23.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphylococcus aureus. Clin Infect Dis 1994; 19:1123–8. [DOI] [PubMed] [Google Scholar]
- 24.Tzermpos F, Kanni T, Tzanetakou V, et al. An algorithm for the management of Staphylococcus aureus carriage within patients with recurrent staphylococcal skin infections. J Infect Chemother 2013; 19:806–11. [DOI] [PubMed] [Google Scholar]
- 25.Ammerlaan HS, Kluytmans JA, Berkhout H, et al. Eradication of carriage with methicillin-resistant Staphylococcus aureus: effectiveness of a national guideline. J Antimicrob Chemother 2011; 66:2409–17. [DOI] [PubMed] [Google Scholar]
- 26.Simor AE, Phillips E, McGeer A, et al. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis 2007; 44:178–85. [DOI] [PubMed] [Google Scholar]
- 27.Frei CR, Miller ML, Lewis JS, II, et al. Trimethoprim-sulfamethoxazole or clindamycin for community-associated MRSA (CA-MRSA) skin infections. J Am Board Fam Med 2010; 23:714–9. [DOI] [PubMed] [Google Scholar]
- 28.Williams VR, Callery S, Vearncombe M, Simor AE. The role of colonization pressure in nosocomial transmission of methicillin-resistant Staphylococcus aureus. Am J Infect Control 2009; 37:106–10. [DOI] [PubMed] [Google Scholar]
- 29.Popoola VO, Carroll KC, Ross T, Reich NG, Perl TM, Milstone AM. Impact of colonization pressure and strain type on methicillin-resistant Staphylococcus aureus transmission in children. Clin Infect Dis 2013; 57:1458–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez M, Hogan PG, Krauss M, Warren DK, Fritz SA. Measurement and impact of colonization pressure in households. J Pediatr Infect Dis Soc 2013; 2:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Davis KA, Stewart JJ, Crouch HK, Florez CE, Hospenthal DR. Methicillin-resistant Staphylococcus aureus (MRSA) nares colonization at hospital admission and its effect on subsequent MRSA infection. Clin Infect Dis 2004; 39:776–82. [DOI] [PubMed] [Google Scholar]
- 32.Marschall J, Muhlemann K. Duration of methicillin-resistant Staphylococcus aureus carriage, according to risk factors for acquisition. Infect Control Hosp Epidemiol 2006; 27:1206–12. [DOI] [PubMed] [Google Scholar]
- 33.Ellis MW, Hospenthal DR, Dooley DP, Gray PJ, Murray CK. Natural history of community-acquired methicillin-resistant Staphylococcus aureus colonization and infection in soldiers. Clin Infect Dis 2004; 39:971–9. [DOI] [PubMed] [Google Scholar]
- 34.Fritz SA, Hogan PG, Hayek G, et al. Household versus individual approaches to eradication of community-associated Staphylococcus aureus in children: a randomized trial. Clin Infect Dis 2012; 54:743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pujol M, Peña C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strains. Am J Med 1996; 100:509–16. [DOI] [PubMed] [Google Scholar]
- 36.Garrouste-Orgeas M, Timsit JF, Kallel H, et al. Colonization with methicillin-resistant Staphylococcus aureus in ICU patients: morbidity, mortality, and glycopeptide use. Infect Control Hosp Epidemiol 2001; 22:687–92. [DOI] [PubMed] [Google Scholar]