Abstract
Femoral subcutaneous adipose tissue (SAT) appears to be cardioprotective compared with abdominal SAT, possibly through better triglyceride (TG) sequestration. We hypothesized that removal of femoral SAT would increase postprandial TG through a reduction in dietary fatty acid (FA) storage. Normal-weight (means ± SD; BMI 23.9 ± 2.6 kg/m2) women (n = 29; age 45 ± 6 yr) were randomized to femoral lipectomy (LIPO) or control (CON) and followed for 1 yr. Regional adiposity was measured by DEXA and CT. A liquid meal labeled with [14C]oleic acid was used to trace the appearance of dietary FA in plasma (6-h postprandial TG), breath (24-h oxidation), and SAT (24-h [14C]TG storage). Fasting LPL activity was measured in abdominal and femoral SAT. DEXA leg fat mass was reduced after LIPO vs. CON (Δ−1.4 ± 0.7 vs. 0.1 ± 0.5 kg, P < 0.001) and remained reduced at 1 yr (−1.1 ± 1.4 vs. −0.2 ± 0.5 kg, P < 0.05), as did CT thigh subcutaneous fat area (−39.6 ± 36.6 vs. 4.7 ± 14.6 cm2, P < 0.05); DEXA trunk fat mass and CT visceral fat area were unchanged. Postprandial TG increased (5.9 ± 7.7 vs. −0.6 ± 5.3 × 103 mg/dl, P < 0.05) and femoral SAT LPL activity decreased (−21.9 ± 22.3 vs. 10.5 ± 26.5 nmol·min−1·g−1, P < 0.05) 1 yr following LIPO vs. CON. There were no group differences in 14C-labeled TG appearing in abdominal and femoral SAT or elsewhere. In conclusion, femoral fat remained reduced 1 yr following lipectomy and was accompanied by increased postprandial TG and reduced femoral SAT LPL activity. There were no changes in storage of meal-derived FA or visceral fat. Our data support a protective role for femoral adiposity on circulating TG independent of dietary FA storage and visceral adiposity.
Keywords: adipose tissue, triglycerides, dietary fatty acids, liposuction
over the past 30 years, it has become increasingly apparent that regional (upper vs. lower body) adiposity is a better indicator of cardiometabolic risk than is overall obesity. Although abdominal obesity has been implicated as the primary player in increased risk for metabolic disease (5), less is known about the impact of femoral adiposity. We (36, 37) and others (27, 32, 33, 39) have demonstrated that lower body adiposity may not simply be benign with respect to cardiometabolic risk but may actually have protective properties. Previously, we observed lower fasting triglycerides (TG) in women with greater femoral subcutaneous adipose tissue (SAT), even after adjusting for abdominal adiposity (37). Although the hypertriglyceridemic waist phenotype has been well characterized and predicts postprandial lipemia (4), our data (37) suggested that a hypotriglyceridemic thigh phenotype also exists. Whether there is a causal relation between high femoral SAT and low fasting TG could not be determined from such observational studies, but it was theorized that femoral SAT might act as a metabolic sink to sequester TG, thereby keeping serum TG low (21). We aimed to prospectively test this possibility by measuring changes in plasma TG in response to femoral SAT removal (via lipectomy). We hypothesized that the usual daily flux of dietary fatty acids (FA) in the context of a relatively reduced femoral TG storage capacity would worsen circulating TG concentrations. Moreover, given that postprandial lipemia is associated with visceral adipose tissue accumulation (4), we expected that femoral lipectomy might be particularly concerning if accompanied by visceral reaccumulation. We additionally hypothesized that a reduction in net storage of dietary FA in SAT TG would account for the decreased postprandial TG clearance following lipectomy. To test these hypotheses, we measured 1-yr changes in postprandial TG clearance and net TG storage by abdominal and femoral SAT in women randomized to femoral liposuction surgery (LIPO) or control (CON).
MATERIALS AND METHODS
Subjects.
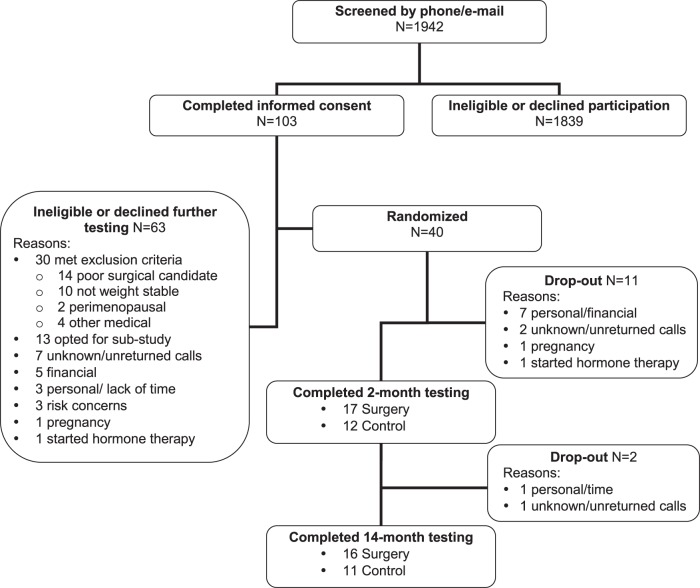
We studied healthy pre- and postmenopausal women aged 35–60 yr of similar total adiposity but seeking femoral liposuction surgery. Inclusion and exclusion criteria for the study population were reported previously (2). Briefly, all women were healthy, nonobese (BMI <30 kg/m2), nonsmokers, sedentary to moderately physically active (exercise ≤150 min/wk), weight stable (≥3 mo), and not using estrogen-based hormone, glucose, or lipid-lowering medication. None had hypertriglyceridemia, type 2 diabetes, impaired fasting glucose, or impaired glucose tolerance [as determined by oral glucose tolerance test (OGTT)]. None had evidence of body dysmorphic disorder. Prior to enrollment, each woman provided informed consent. The protocol was approved by the Colorado Multiple Institutional Review Board. Of the 1,942 women who inquired about the study, 103 gave informed consent, and of those, 40 were randomized into a 14-mo study with two arms (liposuction or control; Fig. 1).
Fig. 1.
Enrollment diagram.
Liposuction surgery.
Prior to randomization, all eligible women were assessed by the cosmetic surgeon and deemed to be good candidates for lipectomy isolated to the femoral region. Small volume (3,254 ± 1,139 ml of adipose tissue across both legs), suction-assisted lipectomy was performed as described previously (16). All participants (LIPO and CON) were counseled not to undergo major lifestyle changes during the duration of the study protocol. Participants paid out of pocket for the procedure at a reduced cost. Those in the control group were offered the surgery after completion of the study protocol.
Body composition assessment.
Total and regional fat mass (FM) and fat-free mass (FFM) were measured by dual-energy X-ray absorptiometry (DEXA; Hologic Discovery W, software version 11.2), as described previously (36). CT scans of the abdomen and midthigh were obtained to measure abdominal and femoral subcutaneous fat areas (cm2) as described previously (37). Visceral fat area (cm2) and intermuscular fat area was the difference between total area and subcutaneous fat areas in the abdominal and thigh regions, respectively. Circumferences were measured in triplicate at the midthigh, waist, and hip using a spring-loaded tape measure.
Physical activity assessment.
The Yale Physical Activity questionnaire (8) was administered at each time point to assess time and energy expenditure (EE) spent participating in a range of activities (kcal/day), as described previously (2).
Test meal visit.
Subjects consumed a 3-day standardized diet [kcal = (23.9 × FFM + 372) × 1.5, 50% carbohydrate, 34% fat (13% saturated/13.5% monunsaturated/7.5% polyunsaturated), and 16% protein] prior to admission to the Clinical Translational Research Center (CTRC) for a diet-matched liquid test meal labeled with 40 μCi of [1-14C]oleic acid (Moravek Biochemicals), as described previously (2). After baseline resting EE, breath, and blood samples were collected, subjects consumed the meal, and then blood was sampled at t = 30, 60, 90, 120, 180, 240, 300, and 360 min for the assessment of TG. Urine was collected throughout the 6-h postprandial period for N2 excretion. Subjects consumed a lunch and dinner matched to the lead-in diet, each consisting of one-third of their daily energy requirements, and then remained fasted overnight on the CTRC. Premenopausal women were studied during the follicular phase of their menstrual cycle (days 1–10).
EE and substrate oxidation.
Resting and postprandial EE (by indirect calorimetry) and breath CO2 samples (0.5 mM trapped in hyamine hydroxide) were collected at t = 0, 60, 120, 180, 240, 300, 360, 540, 840, and 1,440 min, as described previously (2). Total fat and carbohydrate oxidation were calculated from CO2 production, O2 consumption, and urinary N2 excretion using standard equations (13). 14CO2 in breath samples was counted via scintillation and multiplied by the CO2 production determined by indirect calorimetry to estimate the amount of [14C]oleic acid oxidized.
Separation of lipid fractions.
TG-rich lipoprotein (TRL) subfractions were isolated from fresh plasma collected at t = 0, 60, 120, 180, 240, and 360 min using a sequential floatation and ultracentrifugation method [large Svedberg flotation (Sf) >400 and medium Sf 20–400] (34), as described previously (2). [14C]radioactivity was assessed in 50- to 75-μl samples of each subfraction at each time point. The large subfraction consists primarily of chylomicron (CM) particles with small amounts of larger VLDL particles. The medium subfraction consists primarily of VLDL lipoproteins and some smaller CM particles.
Adipose tissue biopsies.
Twenty-four hours after the test meal, biopsies were taken from the abdominal and femoral SAT depots using a mini-liposuction technique, as described previously (2). Adipocyte cellularity and lipoprotein lipase (LPL) activity were determined on an aliquot of fresh SAT sample, and the remaining tissue was frozen for later analysis of 14C-specific activity. At the 2-mo visit, premeal biopsies were collected to account for background 14C.
Adipocyte size distribution.
Immediately after collection, 50 mg of SAT was used to determine adipocyte cell size with the Cell Counting Analysis Program (Mayo Clinic, Rochester, MN), as described previously (7, 17).
Adipose tissue LPL activity.
Immediately after collection, fasted heparin-releasable LPL activity (nmol FFA·min−1·g−1) was measured in fresh abdominal and femoral SAT samples, as described previously (30). Enzyme activity was measured as hydrolyzed [14C]- or [3H]oleic acid after incubation with a synthetic lipoprotein substrate.
14C in adipose tissue and breath.
Specific activity (SA; disintegrations·min−1·g−1) of 14C in SAT was determined by liquid scintillation counting (LS6000TA; Beckman Instruments), as described previously (2). Background SA was subtracted from all 14CO2 measurenents and from SAT at 2 mo. SA for abdominal and femoral SAT was multiplied by trunk and leg FM, respectively, to calculate upper body and lower body subcutaneous TG storage. 14CO2-SA was measured in breath samples to account for tracer expired during fat oxidation. Ectopic TG storage was the remaining 14C not accounted for in fat oxidation or SAT TG storage, as described previously (29).
Blood analyses.
Blood samples were stored at −80°C and analyzed in batches by the CTRC Core Laboratory. Glucose, total and HDL cholesterol, and TG were determined enzymatically (Beckman Coulter), and insulin, leptin, and adiponectin were determined by radioimmunoassay (EMD Millipore).
Statistics.
The areas under the curve (AUC) for postprandial outcomes (e.g, TG, CO2 production) were calculated by the trapezoidal method. Our primary objective was to determine whether removal of femoral FM results in an increase in postprandial lipemia (TG AUC) and a decrease in meal-derived TG-FA storage in SAT (SAT SA). Thus, our study was powered to detect significant between-group differences (LIPO vs. CON) in the changes (postlipectomy − baseline) in TG AUC and SAT SA based on previously reported variability in these outcomes (23, 35). Changes in outcomes over time (0, 2, and 14 mo) and by group (LIPO vs. CON) were evaluated by repeated-measures general linear model. Independent t-tests and Pearson correlation coefficients were used to compare groups for differences in baseline characteristics and to evaluate associations among outcomes, respectively. An additional a priori aim was to evaluate whether menopausal status impacted baseline differences and the trajectory of change following lipectomy. However, we were unable to recruit a sufficient number of postmenopausal women willing to undergo liposuction surgery, so we did not do these post hoc comparisons. Although we recently reported trends for baseline differences in TG AUC between pre- and postmenopausal women, there were no differences in the trafficking of dietary FA into SAT (2), suggesting that our current results would not have differed between menopausal groups. All statistical analyses were done in SPSS (version 22.0; IBM). Data are presented as means ± SD unless otherwise specified.
RESULTS
Baseline subject characteristics.
Compared with the control group, women randomized to femoral liposuction surgery were on average 5 years younger but, by design, had similar total adiposity (33 ± 6 vs. 34 ± 5% fat; Table 1). None of the women had impaired fasting glucose or glucose intolerance (determined by OGTT), hyperinsulinemia (fasting insulin <15 uU/ml), or hypertriglyceridemia (fasting TG <140 mg/dl). Self-reported daily physical activity EE did not differ between LIPO and CON at baseline (1,272 ± 1,065 vs. 1,208 ± 505 kcal/day) or change with time (2 mo: 1,040 ± 860 vs. 1,029 ± 528 kcal/day; 14 mo: 1,289 ± 1,120 vs. 1,119 ± 919 kcal/day). Leptin and adiponectin concentrations did not differ at baseline (Table 1) or change with time (data not shown).
Table 1.
Subject characteristics
| CON (n = 12) | LIPO (n = 17) | |
|---|---|---|
| Age, yr | 48 ± 7 | 43 ± 5* |
| Weight, kg | 62.8 ± 7.2 | 68.7 ± 8.9 |
| Height, cm | 163 ± 6.8 | 169 ± 6.1* |
| BMI, kg/m2 | 23.5 ± 2.7 | 24.1 ± 2.6 |
| Glucose, mg/dl | 84 ± 8 | 83 ± 8 |
| Insulin, μU/ml | 7 ± 3 | 8 ± 3 |
| Total cholesterol, mg/dl | 191 ± 27 | 167 ± 26 |
| HDL cholesterol, mg/dl | 59 ± 12 | 59 ± 11 |
| LDL cholesterol, mg/dl | 117 ± 20 | 94 ± 28 |
| TG, mg/dl | 62 ± 23 | 59 ± 16 |
| Leptin, ng/ml | 12 ± 5 | 14 ± 7 |
| Adiponectin, μg/ml | 17 ± 7 | 16 ± 5 |
Values are means ± SD.
CON, control; LIPO, liposuction surgery; TG, triglyceride.
P < 0.05.
Body composition changes.
By design, subcutaneous femoral fat (by DEXA and CT) was reduced in the LIPO group compared with CON after surgery (2 mo), whereas fat in the abdominal region was not changed (Table 2). At the 14-mo postsurgery followup, leg fat remained reduced in the LIPO group compared with CON (Table 2 and Fig. 2A). There were no increases in abdominal fat (by anthropometrics, DEXA, or CT).
Table 2.
Body composition at baseline, 2 mo, and 14 mo in women randomized to LIPO or CON
| Baseline |
2 Mo |
14 Mo |
P Value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | LIPO | CON | LIPO | CON | LIPO | Time | Time × group | |
| Anthropometric (cm) | ||||||||
| Waist | 75.6 ± 6.0 | 77.9 ± 5.6 | 76.0 ± 4.9 | 77.0 ± 4.4 | 75.9 ± 5.9 | 80.1 ± 7.8 | 0.266 | 0.293 |
| Hip | 102.5 ± 6.9 | 102.9 ± 7.6 | 101.1 ± 8.1 | 99.7 ± 7.2 | 100.1 ± 6.9 | 96.3 ± 9.8 | 0.001* | 0.185 |
| Thigh | 54.4 ± 4.9 | 58.2 ± 4.6 | 54.4 ± 4.0 | 55.5 ± 4.1 | 55.2 ± 3.9 | 54.9 ± 5.5 | 0.012* | 0.001* |
| DEXA (kg) | ||||||||
| FFM | 41.7 ± 4.6 | 44.8 ± 4.6 | 41.9 ± 5.1 | 45.7 ± 4.4 | 42.3 ± 4.8 | 45.2 ± 5.1 | 0.069 | 0.180 |
| FM | 21.4 ± 4.9 | 23.1 ± 5.5 | 21.5 ± 4.2 | 21.3 ± 5.3 | 20.7 ± 4.4 | 22.3 ± 7.6 | 0.240 | 0.128 |
| Trunk FM | 8.9 ± 2.5 | 9.4 ± 2.9 | 9.0 ± 2.1 | 8.9 ± 2.7 | 8.5 ± 2.2 | 9.6 ± 4.0 | 0.757 | 0.174 |
| Leg FM | 9.5 ± 2.3 | 10.6 ± 2.4 | 9.6 ± 2.0 | 9.2 ± 2.4 | 9.3 ± 2.1 | 9.4 ± 3.1 | 0.001* | 0.001* |
| CT (cm2) | ||||||||
| Ab SFA | 206 ± 64 | 225 ± 66 | 216 ± 66 | 219 ± 86 | 0.730 | 0.200 | ||
| Ab VFA | 39 ± 18 | 46 ± 32 | 41 ± 22 | 48 ± 33 | 0.301 | 0.917 | ||
| Fem SFA | 188 ± 50 | 211 ± 60 | 193 ± 54 | 171 ± 67 | 0.006* | 0.001* | ||
| Fem IMFA | 19 ± 10 | 15 ± 8 | 14 ± 10 | 16 ± 8 | 0.033* | 0.024* | ||
Values are means ± SD.
DEXA, dual-energy X-ray absorptiometry; Ab, abdominal; Fem, femoral (midthigh); FFM, fat-free mass; FM, fat mass; SFA, subcutaneous fat area; VFA, visceral fat area; IMFA, intramuscular fat area. P value for an effect of time and time-by-group interaction (general linear model, repeated measures).
P < 0.05.
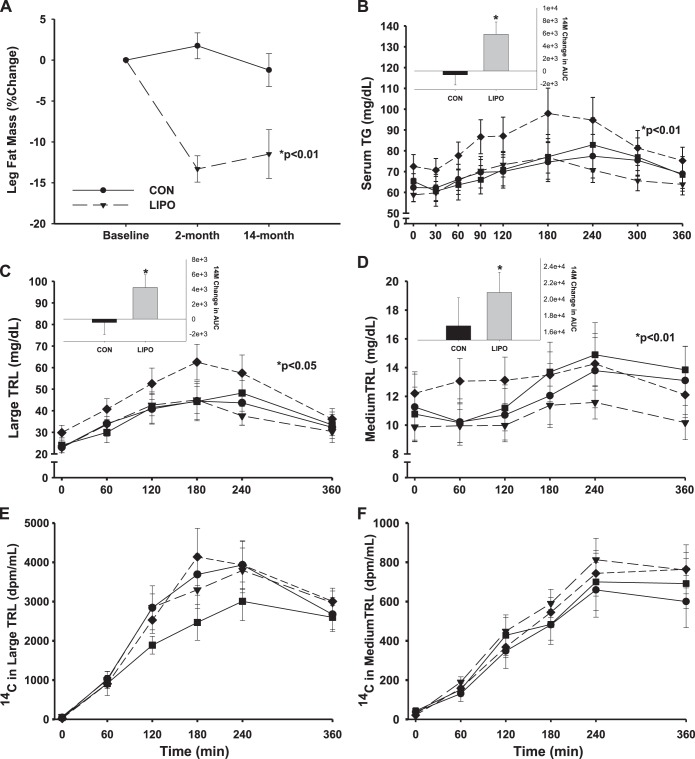
Fig. 2.
Change in postprandial (6-h) response from baseline to 14 mo after liposuction surgery (LIPO; n = 16) vs. nonsurgical control (CON; n = 12) for leg fat mass (A), total plasma triglyceride (TG; B), TG (C and D), and 14C (E and F) in the large (chylomicron; C and E) and medium (VLDL; D and F) TG-rich lipoprotein (TRL) subfractions. Solid lines are for CON (●, baseline; ■, 14 mo); dashed lines are for LIPO (▲, baseline; ⧫, 14 mo). P value for significant area under the curve (AUC) time-by-group interaction. Inset bar graphs (B–D) are mean (± SE) 14-mo change in AUC.
Postprandial triglyceride changes.
There were no changes in postprandial TG immediately following liposuction surgery or control (2 mo Δ: 1.4 ± 6.1 vs. 1.9 ± 5.6 × 103 mg/dl), but 1 yr later postprandial TG response was increased in the LIPO group compared with no change in the CON group (5.9 ± 7.7 vs. −0.6 ± 5.3 × 103 mg/dl, P < 0.05; Fig. 2B). The increase in total postprandial plasma TG was due to increases in TG appearing in the large buoyant (Fig. 2C) and medium dense (Fig. 2D) TRL subfractions, suggesting less clearance of CM-TG and increased appearance of VLDL-TG. There were no changes or group differences in the appearance of 14C tracer in the large (Fig. 2E) and medium (Fig. 2F) TRL fractions, suggesting that the source of the TG in these fractions was not from the labeled dietary FA.
Subcutaneous adipose tissue triglyceride storage.
The proportion of FA coming from the labeled test meal and appearing in the upper and lower body SAT did not differ between groups at baseline, 2 mo, or 14 mo (Table 3). There was a main effect of time on the proportion dietary FA being stored in SAT and the proportion being oxidized such that storage in SAT increased and oxidation decreased at 14 mo in both groups. There was also a main effect of time on absolute uptake of meal-derived FA by lower body but not upper body SAT. Premeal biopsies were not done at the 14-mo visit to account for background 14C, so the increase in 14C tracer in SAT over time may have been due to residual 14C from the 2-mo test meal visit.
Table 3.
Storage of dietary fatty acid as TG and LPL activity in SAT at baseline, 2 mo, and 14 mo in women randomized to LIPO or CON
| Baseline |
2 Mo |
14 Mo |
P value |
|||||
|---|---|---|---|---|---|---|---|---|
| CON | LIPO | CON | LIPO | CON | LIPO | Time | Time × group | |
| Meal-derived fatty acid trafficking (relative) | ||||||||
| Oxidized, % | 26 ± 8 | 24 ± 6 | 25 ± 6 | 24 ± 6 | 20 ± 4 | 20 ± 3 | 0.001* | 0.674 |
| Upper body SAT, % | 17 ± 13 | 11 ± 4 | 13 ± 5 | 13 ± 9 | 18 ± 5 | 21 ± 6 | 0.031* | 0.154 |
| Lower body SAT, % | 10 ± 7.2 | 12 ± 6 | 12 ± 11 | 8 ± 9 | 16 ± 6 | 16 ± 5 | 0.025* | 0.457 |
| Ectopic, % | 45 ± 23 | 56 ± 9 | 53 ± 20 | 54 ± 15 | 47 ± 7 | 43 ± 8 | 0.117 | 0.164 |
| Meal-derived fatty acid uptake (absolute) | ||||||||
| Upper body SAT (mg meal TG in trunk FM × 103) | 4.3 ± 3.1 | 2.8 ± 1.0 | 3.2 ± 1.3 | 3.5 ± 2.6 | 3.8 ± 1.3 | 4.4 ± 1.3 | 0.415 | 0.211 |
| Lower body SAT (mg meal TG in leg FM × 103) | 2.4 ± 1.9 | 3.1 ± 1.6 | 3.0 ± 2.8 | 2.0 ± 2.1 | 4.0 ± 1.3 | 3.9 ± 1.4 | 0.026* | 0.373 |
| LPL activity | ||||||||
| Abdominal SAT | ||||||||
| nmol·min−1·g−1 | 11.8 ± 10.0 | 17.3 ± 11.1 | 15.0 ± 5.9 | 21.3 ± 9.3 | 16.9 ± 12.7 | 12.4 ± 6.4 | 0.469 | 0.199 |
| nmol·min−1·106 cells | 0.16 ± 0.15 | 0.21 ± 0.21 | 0.18 ± 0.09 | 0.21 ± 0.18 | 0.25 ± 0.21 | 0.10 ± 0.06 | 0.940 | 0.250 |
| Femoral SAT | ||||||||
| nmol·min−1·g−1 | 27.1 ± 19.4 | 48.6 ± 8.5 | 33.3 ± 29.9 | 19.8 ± 8.8 | 37.6 ± 18.1 | 21.8 ± 15.9 | 0.152 | 0.005* |
| nmol·min−1·106 cells | 0.60 ± 0.50 | 0.65 ± 0.31 | 0.51 ± 0.51 | 0.27 ± 0.23 | 0.69 ± 0.64 | 0.13 ± 0.07 | 0.209 | 0.114 |
Values are means ± SD.
LPL, lipoprotein lipase; SAT, subcutaneous adipose tissue. P value for an overall effect of time and time-by-group interaction (general linear model, repeated measures).
P < 0.05.
SAT lipoprotein lipase activity.
There was a group-by-time interaction in femoral SAT LPL activity such that LPL activity was reduced in LIPO at 2 mo and remained reduced at 14 mo compared with CON; this was no longer significant when LPL was expressed per number of cells (Table 3). The 2-mo change in femoral SAT LPL activity was related to change in leg fat mass (r = 0.489, P < 0.05). LPL was correlated positively with lower body uptake of dietary FA (r = 0.318, P = 0.18) and inversely with change in postprandial TG AUC (r = −0.336, P = 0.16), but these did not reach statistical significance. There were no changes in abdominal SAT LPL activity with time or differences between groups.
Adipocyte size distribution.
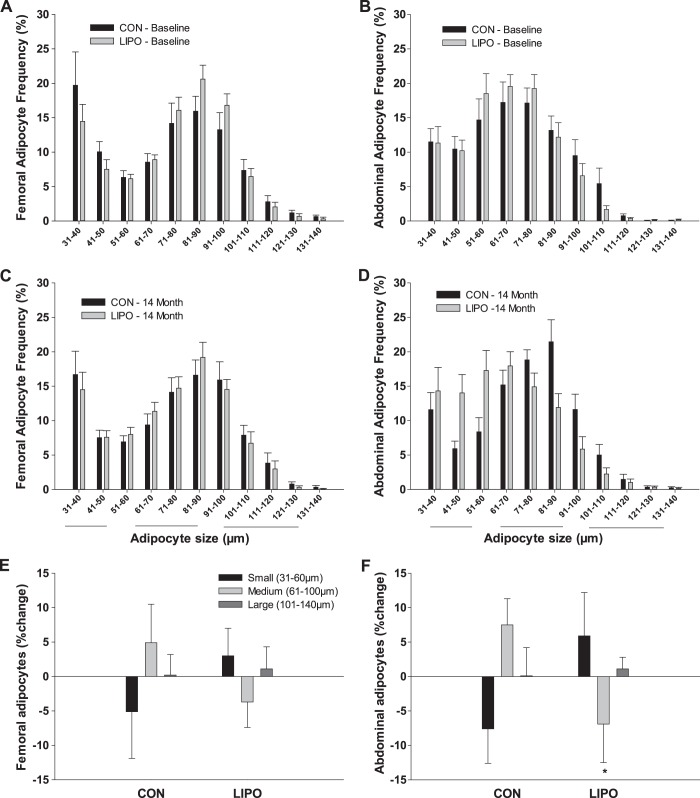
There were no group differences in mean cell size at baseline in abdominal (47 ± 9 vs. 43 ± 14 μm) or femoral (50 ± 13 vs. 49 ± 13 μm) adipocytes. Average abdominal but not femoral adipocyte size was correlated with abdominal SAT LPL activity (r = 0.512, P = 0.01) at baseline. There were shifts in cell size distribution at 14 mo, but not 2 mo, in abdominal SAT over time such that LIPO compared with CON tended to have an increase (P = 0.11) in the small (31–60 μm) and a significant decrease (P < 0.05) in the medium (61–100 μm) but no change (P = 0.80) in the large (101–140 μm) adipocyte fraction (Fig. 3). There were no group differences or changes over time in femoral SAT cell size distribution.
Fig. 3.
Adipocyte size frequency distribution curves after liposuction surgery (LIPO; n = 16) and nonsurgical control (CON; n = 12) in femoral and abdominal subcutaneous adipose tissue at baseline (A and B) and 14 mo (C and D). Change in the proportion of small (31–60 μm), medium (61–100 μm), and large (101–140 μm) adipocytes from baseline to 14 mo after LIPO vs. CON for femoral (E) and abdominal (F) subcutaneous adipose tissue.
DISCUSSION
This study is the first to demonstrate that removal of femoral SAT worsens postprandial triglycerides. One year after femoral lipectomy compared with control, leg fat mass had not returned, and there were no compensatory increases in abdominal (subcutaneous or visceral) adiposity, but postprandial TG worsened (in both the CM- and VLDL-TG fractions). Trafficking of 14C-labeled dietary FA did not differ between groups, suggesting that the mechanism for the increase in postprandial lipemia following femoral lipectomy was not through a reduced SAT storage of FA coming from the recent meal. Fasted femoral SAT LPL activity decreased following femoral lipectomy compared with control, indicating that basal lipolytic capacity was reduced, possibly defending against femoral fat regain.
Potential protective effect of leg fat.
Observational studies suggest a hierarchy among regional fat depots and their relation to disease risk such that lower body adiposity appears least harmful and upper body or abdominal (particularly visceral) adiposity most harmful. Although abdominal adipose tissue has been implicated as a primary player in obesity-related disease risk (22), epidemiological data suggest that lower body adiposity may in fact be protective against disease risk rather than simply less harmful. Early studies demonstrated an inverse correlation between thigh or hip girth and selected cardiometabolic risk (e.g., insulin sensitivity, dyslipidemia) (31, 33) as well as reduced risk for ischemic heart disease (19) and type 2 diabetes (14). Increasing hip size also appeared to attenuate the correlation between waist size and incidence of myocardial infarction (40). Consistent with these anthropometric data, we (36, 37) and others (27, 32) observed an inverse association between leg fat mass (measured by DEXA) and cardiometabolic risk in women. Although it might be argued that femoral adiposity is not inherently protective but simply a marker of reduced abdominal adiposity, our preliminary studies suggested that women with greater femoral adiposity consistently had lower fasting serum TG concentrations independent of visceral adiposity (37), but whether the association was causal remained unknown.
Femoral adipose tissue as a sink for TG.
It has been hypothesized that SAT acts as a buffer for the daily flux of FA in the circulation, particularly during the postprandial period, when energy supply from the gut far exceeds immediate energy requirements (12). Although liver and skeletal muscles accumulate some TG, SAT is the major TG-storing tissue in the postprandial period. It has been suggested that femoral SAT is a better sink for the TG derived from dietary FA than abdominal SAT (9). This could be mediated by increased uptake of FA (TG synthesis) into femoral SAT and/or decreased FA mobilization (TG hydrolysis) from that depot. The latter possibility appears likely, as early in vivo studies have observed less uptake of meal-derived FA into femoral compared with abdominal SAT, whereas the half-life of TG (assimilation and mobilization over months) in SAT was 50% longer in femoral compared with abdominal (3, 23). Lower basal FA release in femoral compared with abdominal AT suggests that basal lipolysis may be reduced (24). Under postprandial conditions, women with lower body obesity suppress leg FA release to a greater extent than women with upper body obesity (15). Moreover, femoral adipose tissue appears to extract less TG in the postprandial period, preferentially utilizing VLDL-TG over CM-TG (26). Lesser uptake and release of FA after a meal in femoral compared with abdominal SAT (23, 29) is consistent with relatively greater energy conservation by femoral SAT in the face of excess energy availability. Taken together, the reduced TG hydrolysis and slower turnover in femoral SAT are consistent with this depot being a relatively more effective sink for TG than abdominal SAT.
Impact of femoral adipose tissue removal.
We tested directly the hypothesis that femoral SAT is an effective sink for TG by evaluating changes in postprandial TG and SAT storage of dietary FA in response to femoral SAT removal. The increase in postprandial TG 1 yr following femoral SAT removal supports a causal association between leg fat mass and circulating TG. The postprandial increases were observed in both the CM-TG and VLDL-TG fractions but were not accompanied by the increased appearance of 14C label in these fractions, suggesting that the source of the augmented TG from the gut and liver were from previous stores and not from the most recent meal. This is consistent with the observation that more than half of the TG entering the circulation in the postprandial period come from delayed release from a previous meal or de novo lipogenesis (18). Thus, at the whole body level, femoral lipectomy did not alter the oxidation or storage of dietary FA by SAT but appeared to increase the net flux of FA from gut and liver. At the tissue level femoral SAT LPL activity was reduced, and abdominal SAT adipocytes were shifted toward smaller cell size, consistent with decreased potential for TG hydrolysis in the femoral region and increased potential for TG storage in the abdominal region. However, there were no apparent compensatory increases in abdominal subcutaneous or visceral fat accrual or in the amount of labeled TG not accounted for in SAT (i.e., going to ectopic depots). Thus, the increases in postprandial TG were independent of changes in body fat distribution per se.
Comparison with previous liposuction studies.
Lipectomy is a useful model to study defense of fat mass and the direct role of fat on metabolic outcomes. Other methods of reducing fat (e.g., caloric restriction and exercise) not only invoke many systemic metabolic adaptations but reduce fat throughout the body; i.e., they cannot isolate just one fat depot. In rodents, lipectomy is followed by rapid reaccumulation of adipose tissue (10, 25) and has been shown to worsen metabolic outcomes, including hypertriglyceridemia (11, 38). Recent human studies similarly suggest that lipectomy is followed by reaccumulation, at least in the abdominal region (16), but that compensatory increases in visceral fat can be countered by increased physical activity (1). There have been a number of studies that have evaluated the short-term (within 6 mo) effect of abdominal lipectomy on insulin sensitivity and markers of inflammation with mixed results (6, 20, 28). To our knowledge, there have been no studies of isolated femoral fat removal despite the apparent cardioprotective benefits of lower body adiposity. Those studies that did remove femoral fat did so in conjunction with removal of abdominal fat, and none were designed to study the impact on postprandial lipid trafficking. Moreover, almost all previous liposuction studies, with few exceptions (1, 16), studied obese women. Thus, our study was the first to remove femoral fat from normal-weight women with a propensity to store their fat in the lower body. In contrast to previous studies that found reaccumulation of abdominal fat (1, 16), we did not observe reaccumulation of femoral fat following lipectomy, nor was there any apparent redistribution of fat to the abdominal region. Consistent with studies of abdominal lipectomy that measured insulin sensitivity via the hyperinsulinemic euglycemic clamp (16, 20), we did not observe any effect of femoral lipectomy on insulin sensitivity as estimated (using Matsuda index) from an OGTT (data not shown).
Study limitations.
The generalizability of our studies is limited by the inclusion of only normal-weight women with a propensity to store fat in the femoral region. It is not known whether obese women and those with a propensity to store fat in the abdominal region would respond similarly. We also do not know whether the results would differ by menopausal status. It is possible that older and more abdominally obese women with hypertriglyceridemia at baseline would have an even greater lipemic response to femoral lipectomy. However, our previous comparisons of pre- and postmenopausal women suggest that our lipid trafficking results would not have been impacted by menopausal status (2). Our studies are also limited by the fact that we did not collect a premeal biopsy prior to the 14-mo test meal visit. Based on previous studies in men, we expected no residual 14C remaining from the 2-mo testing. However, the increase in tracer in both groups over time suggested that background 14C was likely present at 14 mo. Given that the femoral adipose tissue LPL activity was reduced in the liposuction group compared with control, we cannot rule out the possibility that retention of the 14C tracer from the 2-mo to the 14-mo visit was greater in the LIPO group, thereby increasing background and reducing our ability to detect group differences in dietary FA uptake. Lastly, biopsies were only collected in the fasted and not the fed state, so we do not know whether postprandial LPL activity was altered following lipectomy.
Conclusions.
Our studies are the first to demonstrate that removal of femoral SAT by liposuction worsens postprandial lipemia, consistent with a protective role for femoral SAT on circulating TG. Femoral adiposity remained reduced 1 yr after the surgery and was not accompanied by compensatory increases in abdominal adiposity. There were no decreases in postprandial incorporation of dietary FA into the adipose tissue TG pool to account for the increased lipemia. Reductions in fasted LPL activity in femoral SAT and adipocyte size in abdominal SAT may set these depots up for preferential TG storage in abdominal compared with femoral SAT in the context of positive energy balance. Together, our data suggest that femoral SAT in healthy normal-weight women reduces mobilization rather than increases assimilation of lipid stores, extending what we know about this effective sink for TG.
GRANTS
The following awards from the National Institutes of Health supported this research: DK-077992, DK-002935, AG-000279, DK-038088, HD-057022, P30-DK-048520, and UL1-TR-000154
DISCLOSURES
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS
T.L.H., D.H.B., and R.E.V.P. conception and design of research; T.L.H., D.H.B., K.A.C.-Y., C.B.E., C.K.L., M.K.A., H.W., M.R.J., and R.E.V.P. performed experiments; T.L.H., D.H.B., K.A.C.-Y., C.B.E., H.W., M.R.J., and R.E.V.P. interpreted results of experiments; T.L.H., D.H.B., K.A.C.-Y., and R.E.V.P. drafted manuscript; T.L.H., D.H.B., K.A.C.-Y., C.B.E., and R.E.V.P. edited and revised manuscript; T.L.H., D.H.B., K.A.C.-Y., C.B.E., C.K.L., M.K.A., H.W., M.R.J., and R.E.V.P. approved final version of manuscript; K.A.C.-Y., C.B.E., H.W., M.R.J., and R.E.V.P. analyzed data; R.E.V.P. prepared figures.
ACKNOWLEDGMENTS
We thank the staffs of the University of Colorado Anschutz Medical Campus CTRC, Department of Radiology, and Energy Balance Core of the Nutrition and Obesity Research Unit for their assistance in conducting this study. We also like to thank the members of their research group for carrying out the day-to-day activities of the project and the study volunteers for their time and efforts.
REFERENCES
- 1.Benatti F, Solis M, Artioli G, Montag E, Painelli V, Saito F, Baptista L, Costa LA, Neves R, Seelaender M, Ferriolli E, Pfrimer K, Lima F, Roschel H, Gualano B, Lancha A Jr. Liposuction induces a compensatory increase of visceral fat which is effectively counteracted by physical activity: a randomized trial. J Clin Endocrinol Metab 97: 2388–2395, 2012. [DOI] [PubMed] [Google Scholar]
- 2.Bessesen DH, Cox-York KA, Hernandez TL, Erickson CB, Wang H, Jackman MR, Van Pelt RE. Postprandial triglycerides and adipose tissue storage of dietary fatty acids: impact of menopause and estradiol. Obesity (Silver Spring) 23: 145–153, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Björntorp P. The regulation of adipose tissue distribution in humans. Int J Obes 20: 291–302, 1997. [PubMed] [Google Scholar]
- 4.Blackburn P, Lamarche B, Couillard C, Pascot A, Bergeron N, Prud'homme D, Tremblay A, Bergeron J, Lemieux I, Després JP. Postprandial hyperlipidemia: another correlate of the “hypertriglyceridemic waist” phenotype in men. Arteriosclerosis 171: 327–336, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, Van Pelt RE, Wang H, Eckel RH. The metabolic syndrome. Endocr Rev 29: 777–822, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Andrea F, Grella R, Rizzo MR, Grella E, Nicoletti G, Barbieri M, Paolisso G. Changing the metabolic profile by large-volume liposuction: a clinical study conducted with 123 obese women. Aesthetic Plast Surg 29: 472–478, 2005. [DOI] [PubMed] [Google Scholar]
- 7.DiGirolamo M, Mendlinger S, Fertig JW. A simple method to determine fat cell size and number in four mamalian species. Am J Physiol 221: 850–858, 1971. [DOI] [PubMed] [Google Scholar]
- 8.Dipietro L, Caspersen CJ, Ostfeld AM, Nadel ER. A survey for assessing physical activity among older adults. Med Sci Sports Exerc 25: 628–642, 1993. [PubMed] [Google Scholar]
- 9.Evans K, Burdge GC, Wootton SA, Clark ML, Frayn KN. Regulation of dietary fatty acid entrapment in subcutaneous adipose tissue and skeletal muscle. Diabetes 51: 2684–2690, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Faust IM, Johnson PR, Hirsch J. Adipose tissue regeneration following lipectomy. Science 197: 391–393, 1977. [DOI] [PubMed] [Google Scholar]
- 11.Forger NG, Dark J, Stern JS, Wade GN, Zucker I. Lipectomy influences white adipose tissue lipoprotein lipase activity and plasma triglyceride levels in ground squirrels. Metabolism 37: 782–786, 1988. [DOI] [PubMed] [Google Scholar]
- 12.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia 45: 1201–1210, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol 55: 628–634, 1983. [DOI] [PubMed] [Google Scholar]
- 14.Freedman DS, Rimm AA. The relation of body fat distribution, as assessed by six girth measurements, to diabetes mellitus in women. Am J Public Health 79: 715–720, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Z, Hensrud DD, Johnson CM, Jensen MD. Regional postprandial fatty acid metabolism in different obesity phenotypes. Diabetes 48: 1586–1592, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hernandez TL, Kittelson JM, Law CK, Ketch LL, Stob NR, Lindstrom RC, Scherzinger A, Stamm ER, Eckel RH. Fat redistribution following suction lipectomy: defense of body fat and patterns of restoration. Obesity (Silver Spring) 19: 1388–1395, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Jackman MR, Steig A, Higgins JA, Johnson GC, Fleming-Elder BK, Bessesen DH, MacLean PS. Weight regain after sustained weight reduction is accompanied by suppressed oxidation of dietary fat and adipocyte hyperplasia. Am J Physiol Regul Integr Comp Physiol 294: R1117–R1129, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Jacome-Sosa MM, Parks EJ. Fatty acid sources and their fluxes as they contribute to plasma triglyceride concentrations and fatty liver in humans. Curr Opin Lipidol 25: 213–220, 2014. [DOI] [PubMed] [Google Scholar]
- 19.Kahn HS, Austin H, Williamson DF, Arensberg D. Simple anthropometric indices associated with ischemic heart disease. J Clin Epidemiol 49: 1017–1024, 1996. [DOI] [PubMed] [Google Scholar]
- 20.Klein S, Fontana L, Young L, Coggan AR, Kilo C, Patterson BW, Mohammed BS. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med 350: 2549–2557, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lemieux I. Energy partitioning in gluteal-femoral fat: does the metabolic fate of triglycerides affect coronary heart disease risk? Arterioscler Thromb Vasc Biol 24: 795–797, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Lemieux S, Després JP, Moorjani S, Nadeau A, Thériault G, Prud'homme D, Tremblay A, Bouchard C, Lupien PJ. Are gender differences in cardiovascular disease risk factors explained by the level of visceral adipose tissue? Diabetologia 37: 757–764, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Marin P, Rebuff-Scrive M, Björntorp P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur J Clin Invest 20: 158–165, 1990. [DOI] [PubMed] [Google Scholar]
- 24.Martin ML, Jensen MD. Effects of body fat distribution on regional lipolysis in obesity. J Clin Invest 88: 609–613, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mauer M, Bartness TJ. Temporal changes in fat pad mass and cellularity after lipectomy in Siberian hamsters. Physiol Behav 62: 1029–1036, 1997. [DOI] [PubMed] [Google Scholar]
- 26.McQuaid SE, Humphreys SM, Hodson L, Fielding BA, Karpe F, Frayn KN. Femoral adipose tissue may accumulate the fat that has been recycled as VLDL and nonesterified fatty acids. Diabetes 59: 2465–2473, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okura T, Nakata Y, Yamabuki K, Tanaka K. Regional body composition changes exhibit opposing effects of coronary heart disease risk factors. Arterioscler Thromb Vasc Biol 24: 923–929, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Rizzo MR, Paolisso G, Grella R, Barbieri M, Grella E, Ragno E, Nicoletti G, D'Andrea F. Is dermolipectomy effective in improving insulin action and lowering inflammatory markers in obese women? Clin Endocrinol (Oxf) 63: 253–258, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Romanski SA, Nelson RM, Jensen MD. Meal fatty acid uptake in adipose tissue: gender effects in nonobese humans. Am J Physiol Endocrinol Metab 279: E455–E462, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Sadur CN, Eckel RH. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. J Clin Invest 69: 1119–1125, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seidell JC, Perusse L, Despres JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr 74: 315–321, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, Heine RJ, Nijpels G, Seidell JC; Hoorn study. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care 27: 372–377, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Terry RB, Stefanick ML, Haskell WL, Wood PD. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism 40: 733–740, 1991. [DOI] [PubMed] [Google Scholar]
- 34.Traber MG, Kayden HJ, Rindler MJ. Polarized secretion of newly synthesized lipoproteins by the Caco-2 human intestinal cell line. J Lipid Res 28: 1350–1363, 1987. [PubMed] [Google Scholar]
- 35.van Beek AP, de Ruijter-Heijstek FC, Erkelens DW, de Bruin TW. Menopause is associated with reduced protection from postprandial lipemia. Arterioscler Thromb Vasc Biol 19: 2737–2741, 1999. [DOI] [PubMed] [Google Scholar]
- 36.Van Pelt RE, Evans EM, Schechtman KB, Ehsani AA, Kohrt WM. Contributions of total and regional fat mass to risk for cardiovascular disease in older women. Am J Physiol Endocrinol Metab 282: E1023–E1028, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Van Pelt RE, Jankowski CM, Gozansky WS, Schwartz RS, Kohrt WM. Lower-body adiposity and metabolic protection in postmenopausal women. J Clin Endocrinol Metab 90: 4573–4578, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber RV, Buckley MC, Fried SK, Kral JG. Subcutaneous lipectomy causes a metabolic syndrome in hamsters. Am J Physiol Regul Integr Comp Physiol 279: R936–R943, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr 65: 855–860, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, Tanomsup S, Wangai P Jr, Razak F, Sharma AM, Anand SS. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet 366: 1640–1649, 2005. [DOI] [PubMed] [Google Scholar]